Abstract
Histoplasmosis is an important systemic fungal infection, particularly among immunocompromised individuals, who may develop a progressive disseminated form which is often fatal if it is untreated. In such patients, the detection of antibody responses for both diagnosis and follow-up may be of limited use, whereas the detection of Histoplasma capsulatum var. capsulatum antigens may provide a more practical approach. We have recently described an inhibition enzyme-linked immunosorbent assay (ELISA) for the detection in patients’ sera of a 69- to 70-kDa H. capsulatum var. capsulatum-specific antigen which appears to be useful in diagnosis. To investigate its potential for the follow-up of histoplasmosis patients during treatment, antigen titers in the sera of 16 patients presenting with different clinical forms of histoplasmosis were monitored at regular intervals for up to 80 weeks. Sera from four of five patients with the acute form of the disease showed rapid falls in antigenemia, becoming antigen negative by week 14 (range, weeks 10 to 16). Sera from four patients with disseminated histoplasmosis showed falls in antigen levels; three of them became antigen negative by week 32; the fourth patient became negative by week 48. In contrast, antigen titers in four of six AIDS patients with the disseminated form of the disease remained positive throughout follow-up. Sera from only one patient who presented with the chronic form of the disease were analyzed, and this individual’s serum became antigen negative by week 9. The inhibition ELISA is shown to be of particular use in the monitoring of non-AIDS patients with the acute and disseminated forms of the disease and may complement existing means of follow-up.
Histoplasmosis is a systemic mycosis with a world wide distribution and is one of the most common systemic mycoses in North, Central, and South America (1, 12, 15, 16, 17, 20). The clinical spectrum varies from asymptomatic infection to severe, disseminated, and often fatal forms of disease (3, 8, 11, 22). Progressive disease is particularly severe in immunocompromised individuals (2, 8, 21). The definitive diagnosis of histoplasmosis relies on the isolation and identification of Histoplasma capsulatum var. capsulatum by culture or the direct visualization of the fungus (3, 19). However, both approaches may be problematic (10, 21). Antibody detection methods offer a rapid alternative to microbiological techniques, and the detection of antibodies to H. capsulatum var. capsulatum by immunodiffusion and the complement fixation (CF) test is often used (3, 13, 14, 17). However, the use of such tests for the follow-up of patients is problematic since anti-H. capsulatum var. capsulatum antibody titers remain elevated months or even years after successful therapy (3, 7, 8). In addition, false-negative results are often obtained for immunocompromised patients, since antibody titers may be low or absent in such individuals (24). The same may also be true in certain patients with the chronic, disseminated forms of disease (20, 21).
A more practical approach to both the diagnosis and the follow-up of patients with histoplasmosis may be the detection of H. capsulatum var. capsulatum antigen in body fluids. H. capsulatum var. capsulatum polysaccharide antigen has successfully been detected by radioimmunoassay (RIA) (4, 21, 25, 27), particularly in patients with AIDS, who develop disseminated histoplasmosis (21, 22, 24). In these patients, it has been shown that falls in antigenuria correlate well with effective therapy, making it feasible for clinicians to monitor treatment responses (21–23). However, there are problems associated with the use of RIA, notably relating to cross-reactivity with other dimorphic fungi such as Blastomyces dermatitidis (5, 21), Coccidioides immitis, Paracoccidioides brasiliensis, and Penicillium marneffei (26).
We have recently developed a novel H. capsulatum var. capsulatum antigen detection test (6) using a species-specific murine monoclonal antibody in an inhibition enzyme-linked immunosorbent assay (ELISA) system. We used this test to monitor the follow-up of 16 patients under treatment for different clinical forms of histoplasmosis, and it appears to be useful in this context.
MATERIALS AND METHODS
Patients and serum samples.
Sixteen histoplasmosis patients with different forms of the disease were studied. Five patients presented with the acute pulmonary form, one patient presented with the chronic form, and four patients presented with the disseminated form; six of the patients had AIDS and the disseminated form of histoplasmosis (Table 1). The diagnosis for all individuals was confirmed either by cultural identification of H. capsulatum var. capsulatum or by direct identification of intracellular yeast cells in samples from the patients. CF and immunodiffusion (ID) tests were also performed. A total of 86 serum samples taken both at the time of diagnosis and subsequently at regular intervals were analyzed; samples were collected between November 1991 and March 1998 at the Mycology Laboratory, Corporación para Investigaciones Biológicas, Medellín, Colombia. Details relating to the time of follow-up, medication, and length of therapy are included in Table 1. The ages of the patients varied from 1 to 62 years, with a mean age of 27.8 years; there were 12 males and 4 females. Fifty serum samples from healthy volunteers (normal human sera[NHS]) from areas where histoplasmosis is endemic were included as negative controls. All sera were aliquoted and stored at −20°C until use.
TABLE 1.
Characteristics of the 16 patients with histoplasmosis according to clinical classification
| Form of histoplasmosis and patient no. | Age (yr) | Sexa | No. of samples tested | Follow-up time (wk) | Treatment | Length of therapy (mo) | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Acute form | ||||||||
| 1 | 40 | M | 4 | 26 | ITZb | 3 | Clinically cured | |
| 2 | 39 | M | 10 | 124 | ITZ | 7 | Clinically cured | |
| 3 | 29 | F | 5 | 42 | ITZ | 5 | Clinically cured | |
| 4 | 27 | M | 8 | 55 | ITZ | 3 | Clinically cured | |
| 5 | 10 | M | 7 | 53 | ITZ | 11 | Sequelae | |
| Disseminated form | ||||||||
| 6 | 20 | F | 4 | 20 | ITZc | 11 | Clinically cured | |
| 7 | 2 | F | 4 | 56 | ITZ | 12 | Clinically cured | |
| 8 | 1 | F | 5 | 164 | ITZ | 3 | Clinically cured | |
| 9 | 7 | F | 6 | 68 | ITZ | 6 | Clinically cured | |
| Disseminated form and AIDS | ||||||||
| 10 | 43 | M | 6 | 24 | ITZ | 6 | Maintenance treatment | |
| 11 | 26 | M | 5 | 23 | ITZc | 5d | Late-stage AIDS | |
| 12 | 28 | M | 4 | 12 | ITZ | 4d | Late-stage AIDS | |
| 13 | 38 | M | 3 | 15 | ITZ | 4 | Maintenance treatment | |
| 14 | 43 | M | 4 | 19 | ITZ | 5d | Symptomatic | |
| 15 | 30 | M | 7 | 52 | ITZ | 12 | Death | |
| Chronic form | ||||||||
| 16 | 17 | M | 4 | 26 | ITZ | 6 | Clinically cured |
M, male; F, female.
ITZ, itraconazole.
Patients with induction therapy with amphotericin B.
Patients still undergoing treatment.
Clinical evaluation.
Patients were evaluated clinically and radiographically at the time of diagnosis and subsequently at each follow-up visit. Clinical symptoms and signs were recorded, as were the results of a physical examination and chest X rays. The serological tests described below were also carried out at similar intervals.
Antigen detection by inhibition ELISA.
The inhibition ELISA was performed as described previously (6). In brief, inhibition standards were produced by adding increasing concentrations (from 4 to 66.5 μg) of H. capsulatum var. capsulatum Hc 1980 cytoplasmic yeast antigen (CYA) to a pool of NHS (6). Constant aliquots of monoclonal antibody H1C were mixed with the inhibition standards, sera from patients with histoplasmosis, and control NHS, and these were incubated overnight at 4°C on a previously blocked microtiter plate (inhibition plate) to allow the occurrence of the inhibition reaction. The reaction plates were coated with H. capsulatum var. capsulatum Hc 1980 CYA and were incubated under the same conditions described above. On the next day the reaction plates were blocked and samples were transferred from the inhibition plate to the respective wells in the reaction plate, and after further incubation at 37°C, the plates were washed and incubated with goat anti-mouse immunoglobulin G peroxidase conjugate. The reaction was developed with o-phenylenediamine as described previously (6). The optical densities (ODs) were read at 490 nm; the values obtained with the inhibition standards were plotted to produce a standard inhibition curve. A regression model constructed with the reciprocal values of fixed concentrations of H. capsulatum var. capsulatum CYA and the ODs were used to calculate the antigen concentration in the samples tested. The cutoff point was established as the upper limit of the 90% least significant difference (LSD) confidence interval of the OD values obtained from the negative controls (NHS). All the standards, samples, and controls were tested in duplicate.
Antibody detection by CF and ID tests.
The 50% hemolysis microtest described by the Centers for Disease Control and Prevention, Atlanta, Ga. (14), was used to determine the CF titers at the time of diagnosis and during follow-up for each patient. The CF test used the histoplasmin and the whole-yeast-cell antigens. The macro-ID test was performed at the time of diagnosis by a standard methodology and with the histoplasmin antigen (13).
Statistical analysis.
The studies in which the inhibition standard curves were generated were performed in duplicate and by at least four independent assays. A regression model was constructed by using the reciprocal values of the antigen concentrations and the OD values that were obtained. The data were analyzed by analysis of variance type III of the square sum by incorporating factors such as the time of follow-up (in weeks) and the clinical presentation. Interactions above the second level were excluded. Comparisons of the values for each factor were based on the LSD determined by the multiple-range test. Statistical analysis was performed with Statgraphics plus release 2 (1996; Statgraphics Corp., Rockville, Md.).
RESULTS
The regression coefficient of the inhibition standard curve was 0.97; it accounted for 97.4% of the variations in the antigen concentrations in the samples. The inhibition standard curve was used to determine the concentration of H. capsulatum var. capsulatum antigen in each sample tested. The cutoff was defined as the upper limit of the 90% confidence interval (CI) for the negative controls (2.2 μg/ml). Thus, samples with an antigen concentration greater than this value were considered positive.
Patients were classified into four groups according to their clinical presentations (Table 1). The mean antigen concentration at the time of diagnosis is expressed as the means ± 90% CIs as follows: acute form (n = 5), 7.3 ± 3.1 μg/ml; disseminated form (n = 4), 10.37 ± 3.45 μg/ml; disseminated form with AIDS (n = 6), 11.57 ± 2.83 μg/ml; chronic form (n = 1), 6.97 μg/ml; and healthy controls (n = 50), 1.23 ± 0.75 μg/ml. Patients with the acute form, the disseminated form, and the disseminated form with AIDS had significantly higher levels of antigenemia compared to those for uninfected controls (P < 0.0001). It is noteworthy that patients with disseminated histoplasmosis (both patients with AIDS and patients without AIDS) had higher concentrations of circulating antigen compared to those in patients with other clinical forms (P < 0.01, based on the LSD by the multiple-range test).
Four of the five patients with the acute form of histoplasmosis had falls in antigen levels and became negative by week 16 (Fig. 1a). Two of them (patients 2 and 4) subsequently became briefly antigen positive before becoming negative again. The remaining patient (patient 5) had a more variable antigenemia with a peak at week 24, followed by a subsequent decline toward negativity. Overall, in this group of patients there was a significant reduction (P < 0.01) in the level of circulating antigen by week 13 after the initiation of therapy. This reduction was maintained until the end of the follow-up period. All patients in this group improved clinically and mycologically with itraconazole therapy, but certain residual abnormalities persisted. Generally, the antibody titers determined at the time of diagnosis by the CF test were low (Fig. 1b). In most individuals the titers subsequently rose and remained persistent.
FIG. 1.
Follow-up of patients with acute form of histoplasmosis. (a) Measurement of circulating 70-kDa antigen (Ag) levels as determined by inhibition ELISA. ■, patient 1; ○, patient 2; ▴, patient 3; ✻, patient 4; ●, patient 5. The dashed line represents the cutoff point (2.2 μg/ml) of the inhibition ELISA. (b) Measurement of antibody titers as determined by CF test. Symbols are the same as those in panel a. The patient numbers are listed in Table 1. NR, nonreactive.
The non-AIDS patients with disseminated histoplasmosis had decreasing titers of circulating antigen, and three of them became antigen negative by week 32 (Fig. 2a). The fourth patient (patient 8) was lost to follow-up at week 30, when a low antigen concentration (4.8 μg/ml) was detected; this individual returned for follow-up at week 68, at which point the antigen levels were undetectable (Fig. 2a). By taking these patients as a group, a significant (P < 0.01) fall in the circulating antigen level was observed by week 2 after the initiation of treatment. Clinical evaluation revealed that these patients improved markedly shortly after the initiation of therapy; this improvement was maintained over the period of observation. In this group underlying illness was not apparent, although the two youngest patients (patients 7 and 8) were severely undernourished. Antibody levels in this group at the time of diagnosis were variable, and they remained so and did not predict the clinical outcome.
FIG. 2.
Follow-up of patients with the disseminated form of histoplasmosis. (a) Measurement of circulating 70-kDa antigen (Ag) levels as determined by inhibition ELISA. ■, patient 6; ✻, patient 7; ▴, patient 8; ○, patient 9. The dashed line represents the cutoff point (2.2 μg/ml) of the inhibition ELISA. (b) Measurement of antibody titers as determined by CF test. Symbols are the same as those in panel a. The patients numbers are listed in Table 1. NR, nonreactive.
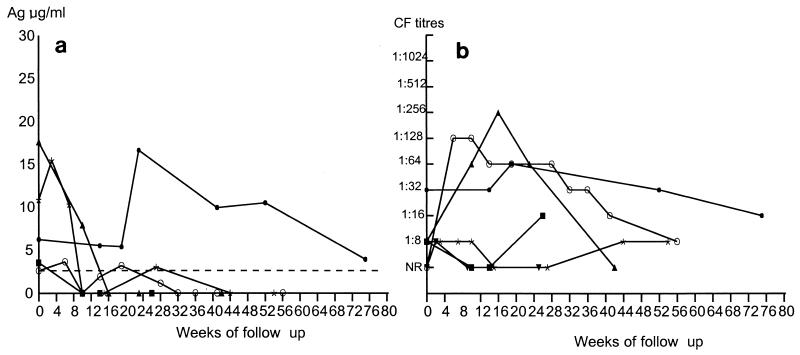
Patients with AIDS and disseminated histoplasmosis typically showed initial increases in circulating antigen levels (Fig. 3a), and only two patients (patients 10 and 15) became antigen negative during the course of follow-up. The remaining four patients maintained high levels of antigen during the follow-up period, with values that were, in some cases, greater than those detected at the time of diagnosis. Over the whole follow-up period there was a decrease in the concentration of circulating antigen which was significant (P < 0.01) only several weeks after the initiation of treatment. The clinical responses of this group were complex. The two patients (patients 10 and 13), in whom major clinical improvements were observed, had been treated not only with itraconazole but also with a combination of three antiretroviral drugs. In contrast, patient 14 did not respond to treatment, despite combined antimycotic and antiretroviral therapies. Finally, the three patients (patients 11, 12, and 15) who received only antimycotic treatment had poor clinical responses. The antibody responses in these patients were generally low (Fig. 3b), and only one patient had detectable antibody levels at the time of diagnosis, with two other patients developing antibody responses by the first follow-up appointment.
FIG. 3.
Follow-up of patients with disseminated form of histoplasmosis and AIDS. (a) Measurement of circulating 70-kDa antigen (Ag) levels as determined by inhibition ELISA. The dashed line represents the cutoff point (2.2 μg/ml) of the inhibition ELISA. ■, patient 10; ○, patient 11; ▴, patient 12; ⧫, patient 13, ✻, patient 14; ▾, patient 15. (b) Measurement of antibody titers as determined by CF test. Symbols are the same patients those in panel a. The patient numbers are listed in Table 1. NR, nonreactive.
In this study we were able to monitor only one patient (patient 16) with the chronic pulmonary form of histoplasmosis. This individual demonstrated an increase in detectable antigen concentration from 6.97 to 8.73 μg/ml by the first follow-up appointment (data not shown). Subsequently, by week 10 of follow-up, this patient became antigen negative. Clinically, the patient was reported as being cured. The antibody response in this patient increased after the first follow-up visit (1:64) and remained positive (1:16) during the whole follow-up period.
DISCUSSION
The inhibition ELISA described previously has now been applied to the follow-up of patients undergoing treatment and has proved to be useful in this context. The assay methodology required no modification from that published originally (6), and the mean antigen concentrations at the time of diagnosis in patients with different clinical forms of histoplasmosis were highly reproducible. The correlation coefficient and the antigen cutoff point reported in this study were also similar to those described previously (6). In addition, samples which tested negative in the former study were also negative in this study (data not shown).
The inhibition ELISA appeared to be very useful for monitoring of the response to therapy in patients presenting with acute pulmonary histoplasmosis. Overall, these patients showed decreasing levels of circulating antigen, a fall which became statistically significant by week 13. The reductions in antigen levels closely mirrored the clinical improvements. In contrast, the detection of antibody levels was less useful since, in some patients, titers were low and remained so during the follow-up period, while in other patients they remained elevated even after an apparent clinical cure. The progress of patients with the acute pulmonary form of the disease has historically been difficult to monitor by antibody detection techniques (3, 7).
All of the non-AIDS patients with disseminated histoplasmosis demonstrated a statistically significant fall in antigen levels 2 weeks after the commencement of treatment, a fall which was more rapid than that seen in patients with the acute form of histoplasmosis. Circulating antigen was completely cleared from all patients with the disseminated form of the disease. This correlated well with complete clinical cure. In contrast, the antibody levels in these patients were variable and did not appear to predict the clinical outcome. Similar variabilities in antibody levels in patients with this form of histoplasmosis have been documented previously (8, 10).
In general, there was no clear pattern in terms of antigenemia during the follow-up of AIDS patients presenting with the disseminated form of histoplasmosis. However, all six patients exhibited some increase in the concentration of circulating antigen at the first follow-up visit after the induction of therapy. Similar observations have previously been reported by Wheat et al. (22, 23), who used an RIA which detects a polysaccharide antigen. It is unclear why this should be so, and this question awaits further investigation. Following the rise in antigenemia, there was then a trend toward reductions in antigen levels; such reductions attained statistical significance by week 8 after the initiation of treatment. However, in the majority of the patients (four of six), high levels of circulating antigen persisted up to the last follow-up visit. The persistence of H. capsulatum var. capsulatum polysaccharide antigen during follow-up has also been observed by RIA (18, 22, 23), although apparently not to the same extent seen here. All of the patients included in this study received itraconazole therapy at various dosages (100 to 400 mg/day), and Wheat et al. (18) reported slower rates of clearance of antigenemia after treatment with itraconazole than after treatment with amphotericin B. The persistence of antigenemia is also seen in AIDS patients with cryptococcosis (9). Antigen levels may remain detectable in AIDS patients due to the persistence of low-level infection that is partially controlled by maintenance therapy but which is not completely eradicated due to the impairment of the cellular immune response. Five of six of these patients had CD4 counts of less than 150 cells/mm3 at the time of diagnosis; unfortunately, CD4 counts were not determined at subsequent time points, making it impossible to correlate cellular immune status with a fall in antigenemia.
Three patients in this group were also receiving antiretroviral treatment (patients 10, 13 and 14); only one of them (patient 10) became antigen negative and at the same time showed a marked clinical improvement. Overall, there would appear to be no clear correlation between the use of combined antifungal and antiretroviral treatment and a fall in antigenemia in our study. However, the situation is made more complex by the fact that some of the patients included in this group were severely immunocompromised and presented with up to four concomitant opportunistic infections (patients 11 and 12). As has been reported elsewhere (3), antibody levels in these patients were low and were not generally useful for follow-up.
A fall in antigen titers was also seen in the patient with the chronic pulmonary form of disease. This correlated well with clinical cure, but because the group had only one patient, it would be unwise to draw conclusions as to the general effectiveness of the inhibition ELISA for such patients.
In conclusion, the inhibition ELISA previously described as a diagnostic tool has also proved to be useful for the monitoring of the progress of patients under treatment for acute pulmonary and disseminated forms of histoplasmosis. This is of particular significance given that there are no previous reports about the follow-up of these groups of patients by antigen detection techniques. Its effectiveness in AIDS patients with the disseminated form of histoplasmosis is less clear, as is the case with regard to patients with the chronic pulmonary form of the disease; there is a need for an expanded study to encompass a larger number of such individuals. The results of our work indicate that regular assessment of antigen titers by the inhibition ELISA is useful in the determination of the response to therapy. In general, on the basis of the results of this study, we suggest that human immunodeficiency virus-positive patients be tested for antigenemia at 2-week intervals during the first 2 months of therapy, followed by more sporadic testing according to clinical judgment. Patients not infected with the human immunodeficiency virus but with the disseminated form of histoplasmosis should be tested at least twice during the first month of therapy and monthly thereafter; patients with the chronic form should be tested monthly for a period of up to 6 months after diagnosis.
ACKNOWLEDGMENTS
This work was supported by COLCIENCIAS, Bogotá, Colombia (grant no. 2213- 05-158-97), and by the Special Trustees of Guy’s Hospital, London, England.
REFERENCES
- 1.Bava A J, Robles A M, Negroni R, Bianchi M. Study of 102 cases of histoplasmosis not associated to AIDS, diagnosed in Muñiz Hospital of Buenos Aires, during 1975-1994. Rev Inst Med Trop Sao Paulo. 1995;37:531–535. doi: 10.1590/s0036-46651995000600010. [DOI] [PubMed] [Google Scholar]
- 2.Bradsher R W. Histoplasmosis and blastomycosis. Clin Infect Dis. 1996;22:S102–S111. doi: 10.1093/clinids/22.supplement_2.s102. [DOI] [PubMed] [Google Scholar]
- 3.Bullock W E. Histoplasma capsulatum. In: Mandell G L, Bennett J, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 2340–2353. [Google Scholar]
- 4.Fotjasek M F, Kleiman M B, Connolly-Stringfield P, Blair R, Wheat J. The Histoplasma capsulatum antigen assay in disseminated histoplasmosis in children. J Clin Microbiol. 1994;30:381–385. doi: 10.1097/00006454-199409000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Garner J A, Kerdnodle D. False-positive Histoplasma antigen test in a patient with pulmonary blastomycosis. Clin Infect Dis. 1995;21:1054. doi: 10.1093/clinids/21.4.1054. [DOI] [PubMed] [Google Scholar]
- 6.Gómez B L, Figueroa J I, Hamilton A J, Ortiz B, Robledo M A, Restrepo A, Hay R J. Histoplasmosis: development of a novel antigen detection test. J Clin Microbiol. 1997;35:2618–2622. doi: 10.1128/jcm.35.10.2618-2622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin R A, Loyd J E, Des Prez R M. Histoplasmosis in normal hosts. Medicine. 1981;60:231–266. doi: 10.1097/00005792-198107000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin R A, Shapiro J L, Thurman G H, Thurman S S, Des Prez R M. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine. 1980;59:1–33. [PubMed] [Google Scholar]
- 9.Hay R J. Clinical manifestations and management of cryptococcosis in the compromised patient. In: Warnock D W, Richardson M D, editors. Fungal infection in the compromised patient. Chichester, United Kingdom: John Wiley & Sons Ltd.; 1991. pp. 85–115. [Google Scholar]
- 10.Kwon-Chung K J, Bennett J E. Histoplasmosis. In: Kwon-Chung K J, Bennett J E, editors. Medical mycology. Philadelphia, Pa: Lea & Febiger; 1992. pp. 464–513. [Google Scholar]
- 11.McKinsey D S, Spiegel R A, Hutwagner L, Stanford J, Driks M R, Brewer J, Gupta M R, Smith D L, O’Connor M C, Dall L. Prospective study of histoplasmosis in patients infected with human immunodeficiency virus: incidence, risk factors and pathophysiology. Clin Infect Dis. 1997;24:1195–1203. doi: 10.1086/513653. [DOI] [PubMed] [Google Scholar]
- 12.Negroni R, Robles A M, Arechavala A, Taborda A. Histoplasmosis diseminada en pacientes con SIDA, su evolución y tratamiento. Rev Arg Micol. 1991;14:5–12. [Google Scholar]
- 13.Palmer D F, Kaufman L, Kaplan W, Cavallaro J. Macroimmunodiffusion test for histoplasmosis. In: Balows A, editor. Serodiagnosis of mycotic diseases. Springfield, Ill: Charles C Thomas, Publisher; 1977. pp. 19–27. [Google Scholar]
- 14.Palmer D F, Kaufman L, Kaplan W, Cavallaro J. The complement fixation test. In: Balows A, editor. Serodiagnosis of mycotic diseases. Springfield, Ill: Charles C Thomas, Publisher; 1977. pp. 155–178. [Google Scholar]
- 15.Rios Fabra A, Restrepo A, Isturiz R. Fungal infection in Latin American countries. Infect Dis Clin N Am. 1994;8:129–154. [PubMed] [Google Scholar]
- 16.Tobón A M, Franco L, Espinal D, Gomez I, Arango M, Trujillo H, Restrepo A. Disseminated histoplasmosis in children: the role of itraconazole therapy. Pediatr Infect Dis J. 1996;15:1002–1008. doi: 10.1097/00006454-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Tobón A M, Franco L, Correa A L, Bedoya F, Ortega J, Soto M, Arango M, Valencia O, Restrepo A. Histoplasmosis en el adulto: bases para su diagnóstico. Acta Med Colomb. 1997;22:277–284. [Google Scholar]
- 18.Wheat J, Hafner R, Korzun A, Limjoco M T, Spencer P, Larsen R, Hecht F, Powderly W. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Med. 1995;98:336–342. doi: 10.1016/s0002-9343(99)80311-8. [DOI] [PubMed] [Google Scholar]
- 19.Wheat L J. Diagnosis and management of histoplasmosis. Eur J Clin Microbiol Infect Dis. 1989;8:480–490. doi: 10.1007/BF01964063. [DOI] [PubMed] [Google Scholar]
- 20.Wheat L J. Histoplasmosis: recognition and treatment. Clin Infect Dis. 1994;19:19–27. doi: 10.1093/clinids/19.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 21.Wheat L J. Histoplasmosis in the acquired immunodeficiency syndrome. Curr Top Med Mycol. 1996;7:7–18. [PubMed] [Google Scholar]
- 22.Wheat L J, Connolly-Stringfield P, Blair R, Connolly K, Garringer T, Katz B P. Histoplasmosis relapse in patients with AIDS: detection using Histoplasma capsulatum variety capsulatum antigen levels. Ann Intern Med. 1991;115:936–941. doi: 10.7326/0003-4819-115-12-936. [DOI] [PubMed] [Google Scholar]
- 23.Wheat L J, Connolly-Stringfield P, Blair R, Connolly K, Garringer T, Katz B, Gupta M. Effect of successful treatment with amphotericin B on Histoplasma capsulatum variety capsulatum polysaccharide antigen levels in patients with AIDS and histoplasmosis. Am J Med. 1992;92:153–160. doi: 10.1016/0002-9343(92)90106-l. [DOI] [PubMed] [Google Scholar]
- 24.Wheat L J, Connolly-Stringfield P, Williams B, Connolly K, Blair R, Bartlett M. Diagnosis of histoplasmosis in patients with the acquired immunodeficiency syndrome by detection of Histoplasma capsulatum polysaccharide antigen in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1992;145:1421–1424. doi: 10.1164/ajrccm/145.6.1421. [DOI] [PubMed] [Google Scholar]
- 25.Wheat L J, Kohler R, Tewari R. Diagnosis of disseminated histoplasmosis by detection of Histoplasma capsulatum antigen in serum and urine specimens. N Engl J Med. 1986;314:83–88. doi: 10.1056/NEJM198601093140205. [DOI] [PubMed] [Google Scholar]
- 26.Wheat L J, Wheat H, Conolly-Stringfield P, Kleiman M, Supparantpinyo K, Nelson K, Bradsher R, Restrepo A. Cross-reactivity in the Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycosis. Clin Infect Dis. 1997;24:1169–1171. doi: 10.1086/513647. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerman S E, Connolly-Stringfield P, Wheat L J, Morris L, French M, Kohler R B. Comparison of sandwich solid-phase radioimmunoassay and two enzyme-linked immunosorbent assays for detection of Histoplasma capsulatum polysaccharide antigen. J Infect Dis. 1989;160:678–685. doi: 10.1093/infdis/160.4.678. [DOI] [PubMed] [Google Scholar]