ABSTRACT
African swine fever (ASF) is a highly contagious viral disease of domestic pigs and wild boars. For disease surveillance and control, we developed a rapid and easy luciferase immunoprecipitation assay (MB-LIPS) to detect ASF virus (ASFV) antibody. The MB-LIPS is based on magnetic beads modified with protein A/G and the recombinant fusion protein of ASFV p30 and luciferase, where p30 functioned as the recognition element and luciferase as the signal component. Incubation and washing could be finished automatically on a machine with magnetic rods. Under optimal conditions, the MB-LIPS showed 96.3% agreement to a commercial enzyme-linked immunosorbent assay (ELISA) kit for detecting ASFV antibody in swine sera. Analyzing serial dilutions of a swine serum sample showed that the MP-LIPS assay was 4 times more sensitive than the ELISA kit. The whole run of the MB-LIPS could be completed within 30 min. With its high sensitivity and simple operation, the MB-LIPS platform has great potential to be used for the detection of ASFV antibody and ASF control in small labs and farms.
KEYWORDS: African swine fever virus, luciferase immunoprecipitation system, magnetic beads, rapid, sensitivity
INTRODUCTION
African swine fever (ASF), which is a highly contagious viral disease affecting domestic and wild pigs with a high mortality rate and rapid spread, causes tremendous socioeconomic loss (1–3). Its etiological agent is a large and enveloped DNA virus, ASF virus (ASFV). ASFV can also infect ticks from the Ornithodoros genus (4) and is the sole member of the Asfarviridae family, with a double-stranded DNA genome of 170 to 190 kbp. Currently, there are no approved vaccines or effective treatments against ASFV. Therefore, disease control mainly depends on early diagnosis, culling the infected pigs in time, and improving the biosecurity control of the pig industry (5). After infection with ASFV, especially subacute infection, surviving pigs may have detectable levels of ASFV antibodies. Therefore, it is a good marker for ASFV antibody detection in enzootic areas affected by ASF. Antibody assays are economical, compatible with automated devices, and suitable for high-throughput screening (6). These advantages make antibody testing convenient in areas where ASFV is endemic and for incursions involving low-virulence ASFV isolates (7). The epidemic genotype of ASFV in China has been classified as a highly virulent genotype II strain that has evolved in the field since August 2018 in China (8). Sun et al. (9) isolated some nonhemadsorbing natural mutants with low virulence (10). Since there are no commercial vaccines available yet, antibodies are still a definitive indication of ASFV infection for this situation. Therefore, it is meaningful to develop a rapid antibody detection method for surveillance of ASFV in the field.
Currently, a few antibody tests, such as enzyme-linked immunosorbent assay (ELISA) and indirect immunoperoxidase test (IPT), have been approved by the World Organization for Animal Health (OIE) (11). IPT is a sensitive test for detecting ASFV antibodies and can detect many different types of samples from pigs, such as blood, tissue exudates, or body fluids (12). However, due to its complex procedure and time consumption, IPT is only suitable for use as a confirmatory test in the laboratory. ELISA is widely used for ASFV antibody screening (13), but it is also labor-intensive and time-consuming, needing more than 2 h for incubation and wash steps. Thus, there are unmet needs for developing fast and cost-effective methods for ASFV surveillance.
The luciferase immunoprecipitation system (LIPS) is a method to detect target molecules based on specific immune recognition and binding with antigen fused to the luciferase reporter, which is widely used for the identification of biomarkers (14) and infectious diseases (15–17) and characterization of human immune response (18). Compared to regular immunoassays, such as ELISA, LIPS has certain advantages in small sample volumes, high speed, and sensitivity (19). Traditional agarose resin bead-based LIPS needs a membrane filter plate to rinse out the unbound luciferase antigen with wash buffer, which involves multiple steps needing manual operation and is error-prone. Here, we replaced the agarose resin beads with protein A/G-modified magnetic beads to develop a magnetic bead-based luciferase immunoprecipitation system (MB-LIPS), which can be separated from liquid phase within 30 s by a magnet so that antibody capture and washing steps could be automatically performed and the detection time could be shortened from around 90 min to 30 min. The newly developed MB-LIPS for the rapid and easy detection of ASFV antibody with high sensitivity is promising for ASF diagnosis and ASFV antibody monitoring in small labs and farms.
MATERIALS AND METHODS
Experimental materials.
Swine serum samples (n = 304) were collected from pig farms in Wuhan and stored at −80°C until use. CSF virus (CSFV) antibody-positive and Porcine reproductive and respiratory syndrome virus (PRRSV) antibody-positive swine sera (n = 4) were kindly gifted from the Laboratory of Microbiology, Huazhong Agriculture University, Wuhan, China. A plasmid with luciferase gene, pET28a (+)-Luc, was preserved by our lab. PrimerSTAR Max DNA polymerase, T4 DNA ligase, and the competent cell preparation kit were purchased from TaKaRa (Dalian, China). The restriction endonucleases were obtained from Thermo Scientific Fermentas. Isopropyl β-d-thiogalactopyranoside (IPTG) and kanamycin sulfate were purchased from Genview. Ni sepharose 6 fast flow was obtained from GE Healthcare (10286564). A bicinchoninic acid (BCA) protein assay kit and protein A (S1425S) and protein G (S1430S) modified magnetic beads were purchased from Thermo Fisher Scientific and New England BioLabs (Ipswich, MA, USA), respectively. ASFV VP72 antibody enzyme-linked immunosorbent assay (ELISA) kit was obtained from Ingenasa (INGEZIM 11.PPA.K3; Spain). Luciferin (L6882) and ATP (S1985) were bought from Sigma and Selleckchem, respectively; 96-deep-well plates and 96-well luminescence test plates (LTP021296) were purchased from Jet Bio-Filtration Co. (Guangzhou, China). All other chemical reagents used in the experiments were bought from Sinopharm Chemical Reagent Co. (Shanghai, China) unless otherwise indicated. Double-distilled water was used for all experiments.
Prokaryotic expression and purification of recombinant fusion protein of p30 and firefly luciferase.
The ASFV-p30 full-length gene (CP204L; GenBank accession no. MN393476.1) (20) was amplified by PCR using DNA extracted from an ASFV-positive blood sample as the template. To facilitate the construction of expression plasmids, primers (CP204L-forward, 5′-GGCCCATATGAAAATGGAGGTCATC-3′; CP204L-reverse, 5′-CCGGCTCGAGTTTTTTTTTTAAAAG-3′; the forward primer of CP204L with GGGGS linker, 5′-GGCGCATATGAAAATGGAGGTCATCTTC-3′; the reverse primer of CP204L with GGGGS linker, 5′-TATAGGATCCGCCTCCACCTTTTTTTTTTAAAAGTTTAATAACC-3′; the underlined bases indicate the restriction endonuclease sites) were used in the PCR. The p30 gene was cloned into plasmid pET28a (+)-Luc by following the general procedure used in molecular biology (21). After confirming the recombinant expression plasmids, named pET28a-p30-Luc, were constructed correctly by sequencing, the plasmids were transformed into Escherichia coli BL21 cells for protein expression. Protein p30-Luc was expressed for 18 h at 16°C using 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) as the inducer and then purified by nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography. SDS-PAGE was used to check the expression and the purity of p30-Luc. Protein concentrations were determined using a BCA protein assay kit by following the manufacturer's instructions. The specificity of the p30-Luc protein with anti-p30 mouse serum (a kind gift from J. Yan, Wuhan Institute of Virology) diluted 1/200 was confirmed by Western blotting.
The procedure for MB-LIPS.
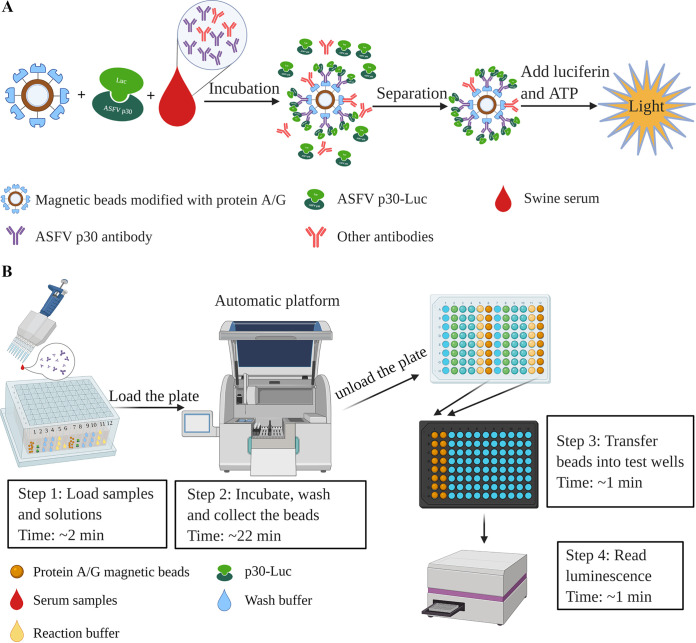
The principle of the MB-LIPS assay is illustrated in Fig. 1A. Briefly, high-capacity magnetic protein A and protein G beads were used as a solid-phase medium to capture antibodies directly from the serum sample and coincubated with recombined p30-Luc, which can recognize and bind with ASFV p30 antibody specifically. After wash and separation of magnetic beads, the light signal intensity of p30-Luc was measured to detect the ASFV p30 antibody level.
FIG 1.
Schemes of the principle (A) and the process (B) of the MB-LIPS assay for ASFV antibody detection.
Figure 1B shows the procedure of the assay with an automated system for incubation, washing, and high-throughput luminescence reading. First, the detection-related reagents are loaded into the wells of 96-deep-well plates, and then the samples to be tested are added into the wells containing the p30-Luc. Afterwards, the plates are loaded into an automated machine with 4 rows of 8-channel magnet rods (Jifan Biotechnologies Inc., China) for a series of steps of incubation, washing, and collection of the magnetic beads automatically. Up to 32 samples could be handled and tested simultaneously. For the detailed procedure, serum samples diluted severalfold with buffer A (20 mM Tris, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100) were added into the wells containing p30-Luc in buffer A. The protein A/G-modified magnetic beads then were moved into the wells by an automated machine, and the mixtures were incubated at 37°C under shaking for 15 min to form the complex of protein A/G-IgG-P30-Luc. After that, the magnetic beads with the complex were washed two times with buffer A and one time with d-luciferase buffer (5 mM Tris-HCl, 10 mM MgCl2·6H2O, pH 7.5) by the machine. Finally, the beads were released into the wells containing 180 μl d-luciferase buffer by the automated machine, and the plates were taken out manually for luminescence measurements. The magnetic beads in the 96-deep-well plates were transferred into wells of a black 96-well luminous microplate containing 20 μl/well 5 mM luciferin and 1 μM ATP for measurement by a microplate reader (Bio-Tek).
Statistical analysis.
Statistical analysis was done by using GraphPad Prism version 5.0 software (San Diego, CA, USA). The optimal signal-to-noise (S/N) cutoff value was established by the receiver operating characteristic (ROC) curve analysis of a panel of ASFV antibody-positive and -negative sera using IBM SPSS statistics 25.0 software. ASFV antibody results of the sera were tested with a commercial ASFV antibody ELISA kit. All data are shown as means ± standard deviations (SD). Student's t test was used for assessing the significant difference between different samples. A P value of <0.05 was considered statistically significant. Repeatability was evaluated by the coefficient of variation (CV).
RESULTS
SDS-PAGE and luciferase activity of recombinant p30-Luc protein.
As shown in Fig. 2A, the recombinant p30-Luc protein was successfully expressed, with a molecular weight of about 85 kDa on the SDS-PAGE gel. The result of Western blotting (Fig. 2B) showed that there was an obvious band between 75 and 100 kDa on the polyvinylidene difluoride (PVDF) membrane reacted with anti-p30 mouse serum, which indicated that p30-Luc expressed by E. coli could recognize the p30 antibody. Further testing of the luminescence generated by a serial dilution of p30-Luc showed that the light intensity had a wide linear range with the concentration of p30-Luc (Fig. 2C), demonstrating that the purified p30-Luc protein had good luciferase activity. The yield of the purified p30-Luc was about 17 mg from 1 liter of the fermentation broth after analyzing the protein concentration by BCA assay.
FIG 2.
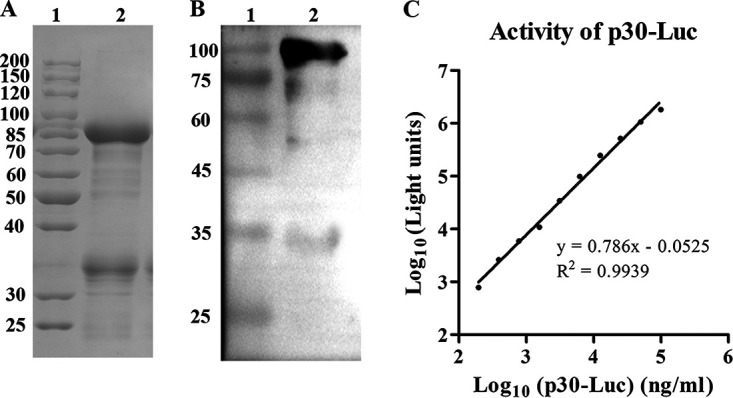
SDS-PAGE analysis (A), Western blot analysis (B), and luciferase activity (C) of the purified p30-Luc. (A) Lane 1, protein marker; lane 2, purified p30-Luc with a molecular weight of 85 kDa. (B) Lane 1, protein marker; lane 2, recombinant p30-Luc hybridized with anti-p30 mouse serum. (C) Luminescence intensity versus the concentration of p30-Luc. Data are shown as the mean from three repeats. Error bars were small enough not to be shown in the plot.
Optimization of the conditions for the MB-LIPS assay.
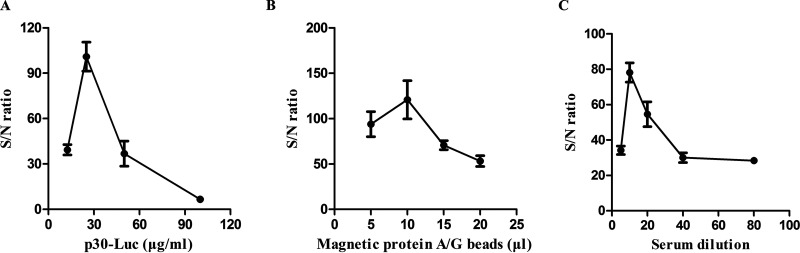
There were three conditions that were supposed to be important for detection performance, the concentration of p30-Luc, the amount of the protein A/G magnetic beads, and the dilution of the serum samples in the assay. Analysis of the signal-to-noise (S/N) ratios of an ASFV-positive serum and a negative serum by the MB-LIPS assay was used to optimize the test conditions.
If the concentration of p30-Luc is too low, the light signal of this system would be so weak that the sensitivity of this assay would become low, while if the concentration is too high, the nonspecific adsorption on beads would cause the low S/N ratio. As shown in Fig. 3A, the group of 25 μg/ml p30-Luc showed the highest S/N ratio.
FIG 3.
Optimization of the MB-LIPS assay for ASFV p30 antibody detection. (A) S/N ratios of an ASFV-positive serum and a negative serum versus p30-Luc concentrations. (B) S/N ratios of an ASFV-positive serum and a negative serum versus the amount of protein A/G-modified magnetic beads. (C) S/N ratios of an ASFV positive serum and a negative serum versus the dilution folds of sera. Data are shown with means ± SD. Error bars represent the standard deviations from triplicates.
The amount of protein A/G-modified magnetic beads is also a vital factor for the sensitivity of the MB-LIPS assay. Small amounts of magnetic beads would not be enough to capture all antibodies in the sample, but excessive beads would lead to a high background with the nonspecific adsorption of p30-Luc on the surface of beads, which would decrease the S/N ratio. From the results shown in Fig. 3B, it was obvious that the optimal amount of the magnetic beads (1 mg/ml) was 10 μl.
Likewise, if the amount of swine serum samples for testing was too much, the signal of luciferase may be affected by some inhibitors from the serum sample, but if the dilutions of the serum samples were too high, the low levels of the antibodies would reduce the sensitivity of the MB-LIPS assay. Thus, the MB-LIPS assay also needs an optimal dilution of serum sample. As displayed in Fig. 3C, 10 times dilution of the serum samples showed the highest S/N ratio among the groups. Therefore, these optimized conditions were used in the following experiments.
Determination of the cutoff value of the MB-LIPS assay.
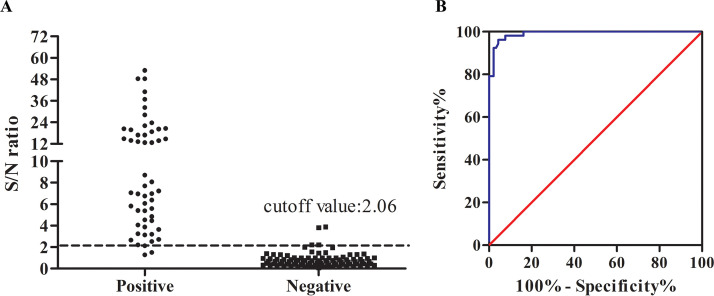
One hundred forty-two swine sera, including four CSFV and PRRSV antibody-positive sera, were used to determine the cutoff value of S/N for the ASFV antibody detection system based on the ROC curve. Among them, 52 samples were classified to be positive and the others were negative, determined by the Ingezim ELISA ASFV antibody kit. As shown in Fig. 4A, ASFV antibody levels in the swine serum samples measured by the MB-LIPS assay ranged from 0.08 to 53.01, with good clusters between the positive and negative samples (P < 0.0001). From the corresponding ROC curve (Fig. 4B), a diagnostic cutoff value of 2.06 was assigned, which could give 95.7% specificity and 96.2% sensitivity for the detection of ASFV antibody compared with the ELISA kit (Table 1). There were two false-negative samples, which were found positive by the Ingezim ELISA kit, and two false-positive samples, which were indicated negative by the Ingezim ELISA kit. The difference may be caused by different antibody levels of different antigens obtained at different days postinfection. The indirect immunoperoxidase test (see Fig. S1A in the supplemental material) was applied to further confirm the results of the four samples, which were consistent with ELISA analysis.
FIG 4.
Determination of the cutoff value of the MB-LIPS for ASFV antibody detection through ROC. (A) S/N ratios obtained by the MB-LIPS assay from a panel of the sera that are classified as positive or negative by the ELISA kit. (B) ROC curve based on the data obtained.
TABLE 1.
ROC analysis of MB-LIPS to detect ASFV antibody
| Characteristic | Value for p30-Luc |
|---|---|
| Optimized cutoff value (S/N) | 2.06 |
| Sensitivity (%) | 96.2 |
| Specificity (%) | 95.7 |
| Area under the concentration-time curve | 0.991 |
| 95% Confidence interval | 0.981–1.001 |
Detection sensitivity and reproducibility of the MB-LIPS assay.
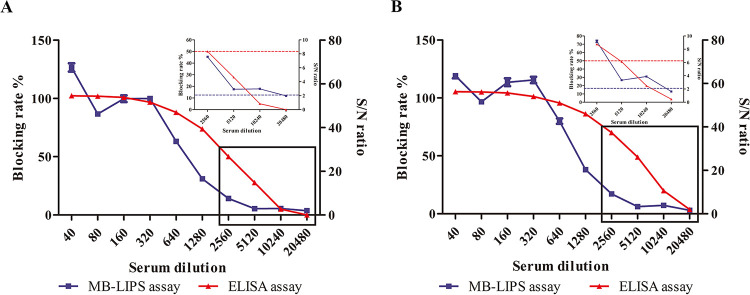
Determining the detection sensitivity of a method should be based on a sample or standard with a known antibody concentration, which was not available in our lab. Instead, serial dilutions of two ASFV antibody-positive swine sera (28 and 29) and a negative swine serum (NC) were used to compare the sensitivity of the MB-LIPS with that of the ELISA kit. As shown in Fig. 5, the MB-LIPS could give positive results after the sera were diluted up to 10,240 times, while the ELISA kit could detect them up to dilutions of 2,560 times. Therefore, the MB-LIPS assay is about 4 times more sensitive than the ELISA kit. The results of testing the same samples 20 times showed that the repeatability (in percentage coefficient of variation [CV]) of the MB-LIPS assay was 5.68% for intraplate assay and 12.20% for interplate assay. A CV value less than 15% for the repeatability of LIPS assay was considered an acceptable level (22).
FIG 5.
Sensitivity comparison between the MB-LIPS (blue line with squares) and the ELISA kit (red line with triangles) for ASFV antibody detection. Two ASFV (28 and 29)-positive sera are diluted to different folds. (A and B) The plots of the inset line charts show the signals under high dilutions, with dotted lines as the cutoff value for positive results. Data are shown with means ± SD. Error bars represent the standard deviations from triplicates.
Detection of swine sera collected from farms by the MB-LIPS assay.
One hundred sixty-two more swine sera were used to test the performance of the MB-LIPS assay under the optimal conditions and with the cutoff S/N ratio of 2.06. As shown in Table 2, the MB-LIPS assay showed 96.3% agreement with the commercial ELISA kit, with a sensitivity of 96.4% (106/110) and a specificity of 96.2% (50/52).
TABLE 2.
Sensitivity and specificity of the MB-LIPS assay for detecting swine seraa
| No. of serum samples with ELISA | MB-LIPS assay |
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 106 | 4 | 110 |
| Negative | 2 | 50 | 52 |
| Total | 108 | 54 | 162 |
| Sensitivity (%) (95% CI) | 96.4 (91.0–99.0) | ||
| Specificity (%) (95% CI) | 96.2 (86.8–99.5) | ||
| PLR (95% CI) | 25.05 (6.43–97.57) | ||
| NLR (95% CI) | 0.04 (0.01–0.10) | ||
| Accuracy (%) (95% CI) | 96.3 (92.1–98.6) | ||
PLR, positive likelihood ratio; NLR, negative likelihood ratio.
DISCUSSION
In this study, a magnetic bead-based LIPS assay was successfully established for rapid and easy detection of ASFV antibody in swine sera. Compared with other antibody detection methods, the MB-LIPS assay has a few advantages.
First, magnetic beads were used instead of agarose beads commonly used in LIPS (23), which made the LIPS assay easier to carry out. Using an automated machine with magnetic rods, some laborious processes, such as incubation and washing, could be done automatically, and many samples could be analyzed in parallel. This feature will overcome the labor-intensive requirements of other assays, such as ELISA and normal LIPS, and make the assay easier to adapt to detect large numbers of samples.
Second, all the reagents in the MB-LIPS assay could be preloaded in 96-well plates (as shown in Fig. 1B), which will reduce the procedure of on-site preparation of the detection reagents, making the antibody assay easier and faster to be carried out in small laboratories. It is well known that transferring liquids is labor-intensive and easily prone to contamination, especially when there are large numbers of samples to be tested.
Third, because of the high activity of luciferase, the MB-LIPS assay is quite sensitive. As shown in Fig. 5, the MB-LIPS assay is more sensitive than the ELISA kit, which means that ASF sera with lower antibody titers could be detected (24). Therefore, the MB-LIPS assay may be usable for finding early ASFV infections.
Lastly, the good agreement (96.3%) between the MB-LIPS assay and the commercial ELISA kit for testing 162 field serum samples demonstrated that recombinant ASFV p30, the antibody of which was reported to be produced earlier than VP72 (25), is a good antigen for detecting ASFV antibody. There were four false-negative and two false-positive results in the MB-LIPS assay compared with the commercial competition ELISA kit. When we analyzed the signals of these false results of MB-LIPS compared with ELISAs, it was easy to find all the blocking rates of these four false-negative samples were slightly higher than 50% (the cutoff value of the positive was set at 50%). In addition, the S/N ratios of the two false-positive samples were 8.44 and 18.76, respectively, which were significantly higher than the cutoff value of 2.06. The results of the six samples that gave the inconsistent results when tested by the two assays described above were confirmed by the indirect immunoperoxidase test (see Fig. S1A in the supplemental material), which indicated that all six samples were considered positive. This difference may be due to different ASFV antigens being used in the kits. According to the ELISA kit instruction, ASFV VP72 is used as the antigen, while p30 is used in this current study. ASFV p30 has been found highly immunogenic and evokes rapid immune responses for ASFV early infection in pigs (25–27). Furthermore, p30-Luc could be expressed by E. coli with high yields and stability at 4°C for several months in this study (data not shown). Besides, recombinant p30-Luc could be used as an antigen to specifically recognize the p30 antibody from clinical swine serum (Fig. S1B). It is expected that p30-Luc, combined with another ASFV antigen, such as VP72, may further improve the sensitivity of the MP-LIPS assay.
There are still some limitations to the MB-LIPS system. The system needs to be applied to multiple types of samples, such as oral fluids, for ASFV antibody detection. There are reports that swine oral fluid would be better than serum for ASFV antibody detection because it is easier to collect and reduces the transmission of diseases (12, 25, 28). In principle, the highly robust MB-LIPS assay could also be used for noninvasive oral fluid testing instead of a serum as the clinical samples. Ching et al. (29) used LIPS for autoantibody detection in Sjögren's syndrome (SjS), a chronic autoimmune disease affecting the salivary and lacrimal glands. Using saliva, Ro60 autoantibodies showed 75% sensitivity and 96% specificity for the diagnosis of SS and correlated with serum levels of Ro60 autoantibody. Furthermore, transferring the magnetic beads manually is required for the following luminescence measurement, so it is applicable to developing a fully automated platform integrated with the luminescent detection, which would simplify the operations for the surveillance of ASFV in small labs or farms.
In summary, an LIPS assay based on magnetic beads modified with protein A/G has been successfully established for the rapid and easy detection of ASFV antibody. With its high sensitivity and simple operation, the MB-LIPS assay has great potential to be used in small labs and farms for ASF control. As far as we know, this is the first time protein A/G magnetic beads were combined with an automated platform that has 32-channel magnetic rods for LIPS assay. Compared with the commercial ELISA kit for ASFV antibody detection, the MB-LIPS assay could save time and labor greatly. If the MB-LIPS system could be developed into a fully automated system in the near future, it would be more applicable than the current system.
ACKNOWLEDGMENTS
We thank M. L. Jin from Huazhong Agriculture University, who kindly gave us four PRRSV- and CSFV-positive swine sera for our testing. We thank J. Yan from the Wuhan Institute of Virology, who kindly gave us anti-p30 mice serum for our Western blot testing.
H.W., conceptualization, methodology, writing the manuscript, visualization, supervision, project administration, and funding acquisition. H.L., performing the experiments and writing the original draft. J.Y., data curation, validation, and editing the original draft. M.J. and J.Y., collecting swine samples from pig farms. J.X. and F.M., ASFV detection of these clinical samples by qPCR assay. P.H. and J.L., formal analysis and technical assistance.
We declare no conflict of interest.
This work was supported by Key Technologies for ASFV Control from Department of Science and Technology of Hubei province, China (grant no. 2019ABA08) and the National Key Research and Development Program of China from the Ministry of Science and Technology of China (no. 2018YFC0840402).
Footnotes
Supplemental material is available online only.
Contributor Information
Junping Yu, Email: yujp@wh.iov.cn.
Hongping Wei, Email: hpwei@wh.iov.cn.
Randall Hayden, St. Jude Children's Research Hospital.
REFERENCES
- 1.Dixon LK, Sun H, Roberts H. 2019. African swine fever. Antiviral Res 165:34–41. 10.1016/j.antiviral.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Zhao D, Liu R, Zhang X, Li F, Wang J, Zhang J, Liu X, Wang L, Zhang J, Wu X, Guan Y, Chen W, Wang X, He X, Bu Z. 2019. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg Microbes Infect 8:438–447. 10.1080/22221751.2019.1590128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Donnell VK, Grau FR, Mayr GA, Sturgill Samayoa TL, Dodd KA, Barrette RW. 2019. Rapid sequence-based characterization of African swine fever virus by use of the Oxford Nanopore MinION sequence sensing device and a companion analysis software tool. J Clin Microbiol 58:e01104-19. 10.1128/JCM.01104-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Luo Y, Wang Y, Li S, Zhao Z, Bi Y, Sun J, Peng R, Song H, Zhu D, Sun Y, Li S, Zhang L, Wang W, Sun Y, Qi J, Yan J, Shi Y, Zhang X, Wang P, Qiu HJ, Gao GF. 2019. Cryo-EM structure of the African swine fever virus. Cell Host Microbe 26:836–843. 10.1016/j.chom.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Teklue T, Sun Y, Abid M, Luo Y, Qiu HJ. 2020. Current status and evolving approaches to African swine fever vaccine development. Transbound Emerg Dis 67:529–542. 10.1111/tbed.13364. [DOI] [PubMed] [Google Scholar]
- 6.Mur L, Igolkin A, Varentsova A, Pershin A, Remyga S, Shevchenko I, Zhukov I, Sanchez-Vizcaino JM. 2016. Detection of African Swine fever antibodies in experimental and field samples from the Russian Federation: implications for control. Transbound Emerg Dis 63:e436–e440. 10.1111/tbed.12304. [DOI] [PubMed] [Google Scholar]
- 7.Gallardo C, Fernandez-Pinero J, Arias M. 2019. African swine fever (ASF) diagnosis, an essential tool in the epidemiological investigation. Virus Res 271:197676. 10.1016/j.virusres.2019.197676. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Z, Xiao CT, Fan Y, Cai Z, Lu C, Zhang G, Jiang T, Tan Y, Peng Y. 2019. Homologous recombination shapes the genetic diversity of African swine fever viruses. Vet Microbiol 236:108380. 10.1016/j.vetmic.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun E, Zhang Z, Wang Z, He X, Zhang X, Wang L, Wang W, Huang L, Xi F, Huangfu H, Tsegay G, Huo H, Sun J, Tian Z, Xia W, Yu X, Li F, Liu R, Guan Y, Zhao D, Bu Z. 2021. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci China Life Sci 64:752–765. 10.1007/s11427-021-1904-4. [DOI] [PubMed] [Google Scholar]
- 10.Gallardo C, Soler A, Rodze I, Nieto R, Cano-Gomez C, Fernandez-Pinero J, Arias M. 2019. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound Emerg Dis 66:1399–1404. 10.1111/tbed.13132. [DOI] [PubMed] [Google Scholar]
- 11.Bergeron HC, Glas PS, Schumann KR. 2017. Diagnostic specificity of the African swine fever virus antibody detection enzyme-linked immunosorbent assay in feral and domestic pigs in the United States. Transbound Emerg Dis 64:1665–1668. 10.1111/tbed.12717. [DOI] [PubMed] [Google Scholar]
- 12.Mur L, Gallardo C, Soler A, Zimmermman J, Pelayo V, Nieto R, Sanchez-Vizcaino JM, Arias M. 2013. Potential use of oral fluid samples for serological diagnosis of African swine fever. Vet Microbiol 165:135–139. 10.1016/j.vetmic.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Wu P, Lowe AD, Rodriguez YY, Murgia MV, Dodd KA, Rowland RR, Jia W. 2020. Antigenic regions of African swine fever virus phosphoprotein P30. Transbound Emerg Dis 10.1111/tbed.13533. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Yu Y, Li H, Hu Q, Chen X, He Y, Xue C, Ren F, Ren Z, Li J, Liu L, Duan Z, Cui G, Sun R. 2019. Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol Cancer 18:33. 10.1186/s12943-019-0947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aira C, Ruiz T, Dixon L, Blome S, Rueda P, Sastre P. 2019. Bead-based multiplex assay for the simultaneous detection of antibodies to African swine fever virus and classical swine fever virus. Front Vet Sci 6:306. 10.3389/fvets.2019.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luong HQ, Lai HTL, Vu HLX. 2020. Evaluation of antibody response directed against porcine reproductive and respiratory syndrome virus structural proteins. Vaccines 8:533. 10.3390/vaccines8030533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zubair A, Burbelo PD, Vincent LG, Iadarola MJ, Smith PD, Morgan NY. 2011. Microfluidic LIPS for serum antibody detection: demonstration of a rapid test for HSV-2 infection. Biomed Microdevices 13:1053–1062. 10.1007/s10544-011-9575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shabani Azim F, Zare Bavani M, Nikmanesh B. 2016. Luciferase immunoprecipitation system assay, a rapid, simple, quantitative, and highly sensitive antibody detection for parasitic diseases. Iran J Parasitol 11:426–428. [PMC free article] [PubMed] [Google Scholar]
- 19.Aye KM, Nagayasu E, Baba M, Yoshida A, Takashima Y, Maruyama H. 2018. Evaluation of LIPS (luciferase immunoprecipitation system) for serodiagnosis of toxoplasmosis. J Immunol Methods 462:91–100. 10.1016/j.jim.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Xiong D, Zhang X, Xiong J, Yu J, Wei H. 2021. Rapid genome-wide sequence typing of African swine fever virus based on alleles. Virus Res 297:198357. 10.1016/j.virusres.2021.198357. [DOI] [PubMed] [Google Scholar]
- 21.Mirkalantari S, Amirmozafari N, Kazemi B, Irajian G. 2012. Molecular cloning of virB12 gene of Brucella melitensis 16M strain in pET28a vector. Asian Pac J Trop Med 5:511–513. 10.1016/S1995-7645(12)60089-3. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Wang G, Yang D, Zhao B, Zhao Y, Liu Y, Cai X, Nan Y, Zhou EM, Wu C. 2018. Development of luciferase-linked antibody capture assay based on luciferase immunoprecipitation systems for antibody detection of porcine reproductive and respiratory syndrome virus. BMC Biotechnol 18:73. 10.1186/s12896-018-0483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burbelo PD, Riedo FX, Morishima C, Rawlings S, Smith D, Das S, Strich JR, Chertow DS, DaveyRT, Jr, Cohen JI. 2020. Detection of nucleocapsid antibody to SARS-CoV-2 is more sensitive than antibody to spike protein in COVID-19 patients. medRxiv 10.1101/2020.04.20.20071423. [DOI] [Google Scholar]
- 24.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. 2008. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis 198:444–451. 10.1086/589718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gimenez-Lirola LG, Mur L, Rivera B, Mogler M, Sun Y, Lizano S, Goodell C, Harris DL, Rowland RR, Gallardo C, Sanchez-Vizcaino JM, Zimmerman J. 2016. Detection of African swine fever virus antibodies in serum and oral fluid specimens using a recombinant protein 30 (p30) dual matrix indirect ELISA. PLoS One 11:e0161230. 10.1371/journal.pone.0161230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrovan V, Yuan F, Li Y, Shang P, Murgia MV, Misra S, Rowland RRR, Fang Y. 2019. Development and characterization of monoclonal antibodies against p30 protein of African swine fever virus. Virus Res 269:197632. 10.1016/j.virusres.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Filgueira DM, Gonzalez-Camacho F, Gallardo C, Resino-Talavan P, Blanco E, Gomez-Casado E, Alonso C, Escribano JM. 2006. Optimization and validation of recombinant serological tests for African swine fever diagnosis based on detection of the p30 protein produced in Trichoplusia ni larvae. J Clin Microbiol 44:3114–3121. 10.1128/JCM.00406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guinat C, Reis AL, Netherton CL, Goatley L, Pfeiffer DU, Dixon L. 2014. Dynamics of African swine fever virus shedding and excretion in domestic pigs infected by intramuscular inoculation and contact transmission. Vet Res 45:93. 10.1186/s13567-014-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ching KH, Burbelo PD, Gonzalez-Begne M, Roberts ME, Coca A, Sanz I, Iadarola MJ. 2011. Salivary anti-Ro60 and anti-Ro52 antibody profiles to diagnose Sjogren's syndrome. J Dent Res 90:445–449. 10.1177/0022034510390811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental methods and Fig. S1. Download JCM.00990-21-s0001.pdf, PDF file, 0.2 MB (178.8KB, pdf)