Abstract
Substances of abuse (SoA), as well as smoking and alcohol consumption, are well known for their impact on male fertility status, erectile function, and ejaculation. We assessed SoA consumption habits in a cohort of men seeking medical attention for uro-andrological purposes. Data from 7447 men seeking medical attention for the first time for uro-andrological purposes were analyzed. A complete medical and sexual history was collected for each patient. Smoking, alcohol, and SoA consumption were investigated. Descriptive statistics was used to describe the whole cohort. The primary motivations for their evaluation were lower urinary tract symptoms (LUTS), erectile dysfunction (ED), and infertility in 1912 (25.7%), 2944 (39.5%), and 2591 (34.8%) men, respectively. Previous use of SoA was reported by 378 (5.1%) men, and 190 (2.6%) individuals were current users. Patients seeking medical attention for infertility were more frequently current SoA users (107; 4.1%) than men with ED (66; 2.2%) and LUTS (17; 0.9%) (both P < 0.001). Current users of SoA were younger than those with past or no SoA history (P < 0.001). Current SoA users were more frequently smokers (P < 0.001) and alcohol consumers (P < 0.001) than those with a previous history or those who had never tried SoA. In conclusion, approximately 3% of men seeking medical attention for uro-andrological purposes were current SoA consumers. Infertile men reported a higher use of SoA than those with ED or LUTS. Current SoA users were younger and more frequently concomitant smokers and alcohol consumers compared to those who did or had never used SoA.
Keywords: cannabis, cocaine, erectile dysfunction, male infertility, risk factors, substances of abuse
INTRODUCTION
The World Drug Report 2019 of the United Nations Office on Drugs and Crime (UNODC) estimated that, in 2017, approximately 271 million people reported using substances of abuse (SoA) in the previous year, with 35 million people suffering from drug use disorders.1 SoA consumption was more frequently found in younger individuals, with men aged 26–34 years, 35–49 years, and 50 years and older reporting use of illicit drugs in 24.6%, 14.5%, and 7.8% of cases, respectively.2
SoA are well known for their potential impact on overall health status.3 For example, cocaine and cannabis have a detrimental effect on the cardiovascular system4 and on the short-term memory.5 Moreover, increasing evidence has shown that SoA can impair fertility status, erectile function, and ejaculation.
Cannabis is the most used drug worldwide,1 and various studies, using both animal and human models, have shown that its utilization is associated with impaired fertility status.6,7,8,9 Conversely, other investigators have failed to find any association between cannabis use and semen parameters or testosterone levels.10,11,12 Cocaine was originally used as an anesthetic agent in the 1880s, but since the 1970s, it has been considered a recreational drug. Cocaine users were found to have increased risk of impaired sperm concentration13 and low testosterone levels14 as compared to nonusers. Similarly, prolonged cocaine use has been consistently associated with low sexual desire and erection difficulties in middle-age men.15 Opioids are commonly prescribed in pain management, but their recreational use and abuse is well known worldwide. As for their uro-andrological side effects, men reporting heroin addiction have a lower weekly sexual intercourse rate and show decreased masturbatory activity compared to nonusers.16 Furthermore, previous reports have also highlighted the negative impact of opioid use in terms of semen parameters.2,17 A recent Iranian study showed that opium-addicted men presented with a higher rate of lower sperm concentration, lower antioxidant activity, and a higher sperm DNA fragmentation index compared to nonopium users.17 Furthermore, erectile dysfunction (ED) was also found to be highly prevalent among male patients treated with methadone maintenance therapy.18
Overall, a vast literature has shown detrimental effects of SoA on male sexual and reproductive health; however, there is a lack of epidemiological studies investigating the real-life prevalence of SoA consumption in this specific cohort of individuals.
Therefore, the driving hypothesis of this analysis was that infertile men show higher SoA consumption in a real-life setting compared to individuals seeking medical attention for other uro-andrological disorders. In this context, we sought to assess SoA consumption in a large cohort of white-Caucasian European men seeking medical attention for uro-andrological purposes at a single academic center over the course of a decade.
PARTICIPANTS AND METHODS
The analyses of this cross-sectional study were based on a cohort of 7447 white-Caucasian-European men (age range: 18–85 years). These patients were evaluated at a single academic center (IRCCS San Raffaele Hospital, Milan, Italy) for uro-andrological purposes between September 2008 and September 2019. The motivation for the medical evaluation was categorized as follows: lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH), ED, or couple's infertility. According to the World Health Organization (WHO) criteria, infertility was defined as not conceiving a pregnancy after at least 12 months of unprotected intercourse regardless of whether or not a pregnancy ultimately occurs.19
The baseline assessment included a detailed medical history and physical examination. Comorbidities were scored using the Charlson Comorbidity Index (CCI).20 The CCI was categorized as 0 or ≥1. Body mass index (BMI) (kg m−2) was calculated for every patient. Patients assessed for LUTS/BPH and ED completed the International Prostate Symptom Score (IPSS) and the International Index of Erectile Function-Erectile Function domain (IIEF-EF) forms.
On presentation at the clinic for the first appointment following consent, the subject was interviewed by the same uro-andrologist to obtain information regarding his personal recreational habits. Smoking habits were assessed as pack-year history (i.e., a man with a 2-pack-year history smokes 2 packs of cigarettes per day) and then categorized into three groups, as follows: non-smokers, moderate smokers (0–1-pack-year history), and heavy smokers (>1-pack-year history), as previously reported.21 Patients were considered active smokers if they reported smoking for at least 1 year or if their quit date was within three months of the clinical evaluation. Similarly, alcohol consumption was categorized as follows: abstainer (no alcohol consumption), moderate drinkers (up to 2 drinks per day), and heavy drinker (>2 drinks per day).21,22 Consumption of SoA was queried in terms of the type of recreational drug and the frequency of current or former consumption.
Data collection followed the principles outlined in the Declaration of Helsinki. All patients signed informed consent agreeing to share their own anonymous information for future studies. The study was approved by the IRCCS San Raffaele Hospital Ethical Committee, Milan, Italy (Prot. 2014 – Pazienti Ambulatoriali).
Statistical analyses
Normality of data distribution was assessed with the Shapiro–Wilk test and the value of the skewness and kurtosis test. Data were presented as median (interquartile range [IQR]) or frequency (proportion). The Kruskal–Wallis and the Fisher's exact tests were used to examine the association between clinical characteristics and recreational habits among men presenting for LUTS, ED, or infertility. Similarly, descriptive statistics were used to test potential differences in clinical characteristics among never, former, and current SoA users. Finally, univariable (UVA) and multivariable (MVA) logistic regression analyses tested the associations between clinical variables (e.g., age, smoking status, alcohol consumption, and reason for office evaluation) and current SoA consumption status. Statistical analyses were performed using SPSS version 26 (IBM Corp., Armonk, NY, USA). All tests were two sided, and statistical significance was determined at P < 0.05.
RESULTS
Table 1 illustrates the descriptive and clinical characteristics of the study population. Overall, the primary motivation for the office evaluation was LUTS/BPH, ED, and couple's infertility in 1912 (25.7%), 2944 (39.5%), and 2591 (34.8%) men, respectively. Median (range) patient's age was 42 (19–80) years. Patients seeking medical attention for LUTS/BPH and ED were older and had a higher CCI score than infertile men (all P < 0.01). Patients assessed for LUTS/BPH had a median (IQR) IPSS and IIEF-EF score of 18 (7–20) and 19 (8–28), respectively. Similarly, patients presenting for ED had a median IIEF-EF and IPSS score of 15 (8–22) and 8 (3–11), respectively.
Table 1.
Sociodemographic characteristics and descriptive statistics of the whole cohort according to the primary motivation for office evaluation (n=7447)
| Parameters | LUTS | ED | Infertility | P |
|---|---|---|---|---|
| Patients, n (%) | 1912 (25.7) | 2944 (39.5) | 2591 (34.8) | |
| Age (year) | <0.001 | |||
| Median (IQR) | 57 (45–66)* | 46 (33–58)* | 36 (33–40) | |
| Range | 18–85 | 18–70 | 18–60 | |
| BMI (kg m−2) | <0.001 | |||
| Median (IQR) | 25 (23–27) | 25 (23–27)* | 25 (23–27) | |
| Range | 15–43 | 15.6–51 | 18–55 | |
| CCI score | <0.001 | |||
| Median (IQR) | 0 (0–1)* | 0 (0–1)* | 0 (0–2) | |
| Mean (s.d.) | 0.3 (0.3) | 0.3 (0.2) | 0.1 (0.2) | |
| Range | 0–8 | 0–9 | 0–8 | |
| CCI ≥1, n (%) | 364 (19.0) | 542 (18.4) | 193 (7.5) | <0.001 |
| IPSS score | <0.001 | |||
| Median (IQR) | 18 (7–20) | 8 (3–11) | ||
| Range | 0–35 | 0–32 | ||
| IIEF-EF score | <0.01 | |||
| Median (IQR) | 19 (8–28) | 15 (8–22) | ||
| Range | 0–30 | 0–30 | ||
| Smoking status, n (%) | <0.001 | |||
| Nonsmokers/former smokers | 1542 (80.7)* | 2172 (73.8) | 1780 (68.7) | |
| Active smokers | 370 (19.3) | 772 (26.2) | 811 (31.3) | |
| Smoking quantity, n (%) | 0.87 | |||
| Moderate smokers | 236 (12.4) | 494 (16.7) | 511 (19.7) | |
| Heavy smokers | 134 (7.0) | 278 (9.4) | 300 (11.6) | |
| Alcohol consumption, n (%) | <0.001 | |||
| Never | 378 (19.8)* | 491 (16.7) | 391 (15.1) | |
| Active consumption | 1534 (80.2) | 2453 (83.3) | 2200 (84.9) | |
| Alcohol quantity, n (%) | <0.001 | |||
| Moderate drinkers | 1021 (53.4) | 1720 (58.4) | 1683 (64.9) | |
| Heavy drinkers | 513 (26.8) | 733 (24.8) | 517 (19.9) | |
| SoA, n (%) | <0.001 | |||
| Never | 1821 (95.2)* | 2744 (93.2) | 2314 (89.3) | |
| Former users | 74 (3.9) | 134 (4.6) | 170 (6.6) | |
| Active users | 17 (0.9) | 66 (2.2) | 107 (4.1) | |
| Type of current SoA use, n (%) | ||||
| Cannabis/marijuana/hashish | 12 (0.6) | 53 (1.8) | 82 (3.2) | 0.66 |
| Cocaine | 5 (0.3) | 23 (0.8) | 25 (0.9) | 0.26 |
| Heroin (different forms) | 1 (0.05) | 4 (0.1) | 5 (0.2) | 0.38 |
| Other illicit drugs | 2 (0.1) | 5 (0.2) | 16 (0.6) | 0.35 |
| Multiple SoA use, n (%) | 3 (0.2) | 18 (0.6) | 20 (0.7) | 0.37 |
P value according to the Kruskal–Wallis test and the Fisher’s exact test, as indicated. *P<0.01 for selected group versus infertility group. BMI: body mass index; CCI: Charlson Comorbidity Index; SoA: substances of abuse; IPSS: International Prostate Symptom Score; IIEF-EF: International Index of Erectile Function-Erectile Function domain; LUTS: lower urinary tract symptom; ED: erectile dysfunction; s.d.: standard deviation; IQR: interquartile range
Overall, 1953 (26.2%) individuals were current smokers. Infertile men (31.1%) were more frequently current smokers than those with ED (26.2%) and LUTS/BPH (19.3%)(both P < 0.001). Alcohol consumption was reported by 6187 (83.1%) men, with infertile men more frequently being regular alcohol consumers than men in other groups (P < 0.001).
Previous use of SoA was reported by 378 (5.1%) men, and 190 (2.6%) individuals were current users. Current use of cocaine, marijuana, and heroin was reported by 25 (0.9%), 82 (3.2%), and 5 (0.2%) infertile men, respectively. Patients seeking medical attention for infertility were more frequently current SoA users (107; 4.1%) than those evaluated for ED (66; 2.2%) and LUTS (17; 0.9%), respectively (both P < 0.001).
Table 2 depicts the clinical characteristics of patients with never, former, or current SoA consumption profiles. Current users of SoA were younger than those with past or no use of SoA (34 years vs 35 years, and 34 years vs 43 years, respectively; both P < 0.001). Current users of SoA were more frequently smokers (69.4% vs 51.5%, and 69.4% vs 23.6%, respectively; both P < 0.001) and alcohol consumers (93.2% vs 91.8%, and 93.2% vs 82.3%, respectively; both P < 0.001) than those with a previous history or those who had never tried SoA. Individuals with current SoA use were more frequently heavy smokers (P = 0.02) and heavy drinkers (P < 0.001) than those with past or no use of SoA.
Table 2.
Sociodemographic characteristics and descriptive statistics of the whole cohort according to substances of abuse use (n=7447)
| Parameters | Never users | Former users | Current users | P |
|---|---|---|---|---|
| Patients, n (%) | 6879 (92.4) | 378 (5.1) | 190 (2.6) | |
| Age (year) | <0.001 | |||
| Median (IQR) | 43 (34–58) | 35 (30–42)* | 34 (30–41)* | |
| Range | 18–85 | 18–85 | 18–66 | |
| BMI (kg m−2) | <0.01 | |||
| Median (IQR) | 25 (23–27) | 25 (22–26)* | 25 (23–27) | |
| Range | 15–51 | 18–39 | 18–42 | |
| CCI score | <0.001 | |||
| Median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–2) | |
| Mean (s.d.) | 0.2 (0.3) | 0.1 (0.2) | 0.1 (0.2) | |
| Range | 0–9 | 0–6 | 0–8 | |
| CCI ≥1, n (%) | 1049 (15.2) | 36 (9.5) | 14 (7.4) | <0.001 |
| Smoking status, n (%) | <0.001 | |||
| Nonsmokers/former smokers | 5255 (76.4) | 183 (48.5) | 58 (30.6) | |
| Active smokers | 1624 (23.6) | 195 (51.5) | 132 (69.4) | |
| Smoking quantity, n (%) | 0.02 | |||
| Moderate smokers | 1041 (15.1) | 123 (32.5) | 67 (35.2) | |
| Heavy smokers | 583 (8.5) | 72 (19.0) | 65 (34.2) | |
| Alcohol consumption, n (%) | <0.001 | |||
| Never | 1217 (17.7) | 31 (8.2) | 13 (6.8) | |
| Active consumption | 5662 (82.3) | 347 (91.8) | 177 (93.2) | |
| Alcohol quantity, n (%) | <0.001 | |||
| Moderate drinkers | 4088 (59.4) | 222 (58.7) | 109 (57.3) | |
| Heavy drinkers | 1574 (22.8) | 125 (33.1) | 68 (35.7) | |
| Type of SoA, n (%) | ||||
| Cannabis/marijuana/hashish | – | 353 (93.4) | 147 (77.4) | 0.001 |
| Cocaine | – | 84 (22.2) | 53 (27.9) | 0.14 |
| Heroin (different forms) | – | 7 (1.9) | 10 (5.3) | 0.02 |
| Other illicit drugs | – | 2 (0.5) | 23 (12.1) | 0.01 |
P value according to the Kruskal–Wallis test and the Fisher’s exact test, as indicated. *P<0.01 for selected group versus never users. BMI: body mass index; CCI: Charlson Comorbidity Index; SoA: substances of abuse; –: no data; s.d.: standard deviation; IQR: interquartile range
Figure 1 shows the distribution of current smokers, alcohol consumers, and SoA users (any type) throughout the study period stratified by the primary reason for office evaluation. Among infertile men (Figure 1a), active smoking increased (P = 0.02), while active SoA consumption significantly decreased over time (P ≤ 0.001). Smoking and active SoA consumption both decreased over time in men with ED (both P ≤ 0.03; Figure 1b). Conversely, while SoA consumption decreased, smoking and alcohol consumption remained stable between 2008 and 2019 in men presenting for LUTS/BPH (Figure 1c).
Figure 1.
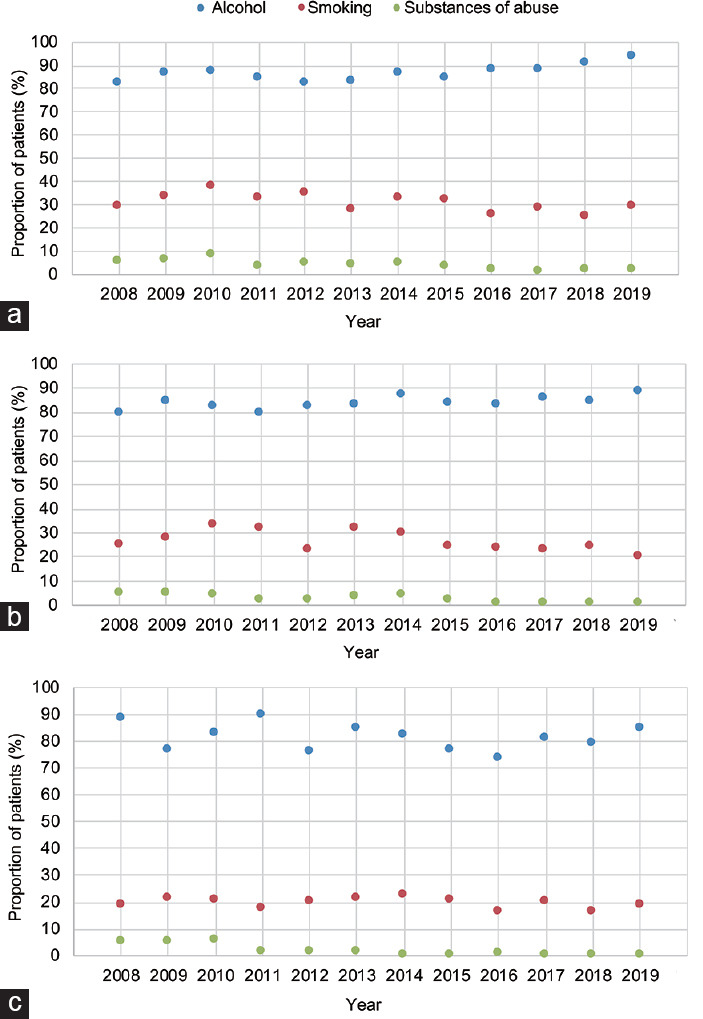
Proportion of patients reporting active cigarette smoking, alcohol consumption and SoA use (any type) between 2008 and 2019 among (a) infertile men, and individuals with (b) ED and (c) LUTS. P value according to the Fisher's exact test. SoA: substances of abuse; LUTS: lower urinary tract symptoms; ED: erectile dysfunction.
The active use of SoA (any type) was consistently higher in infertile men compared to men presenting for either ED or LUTS throughout the decade of investigation (P ≤ 0.01; Figure 2a). Similarly, infertile men reported higher rates of active cigarette smoking and alcohol consumption than men with ED and LUTS over the study period (all P ≤ 0.001; Figure 2b and 2c).
Figure 2.
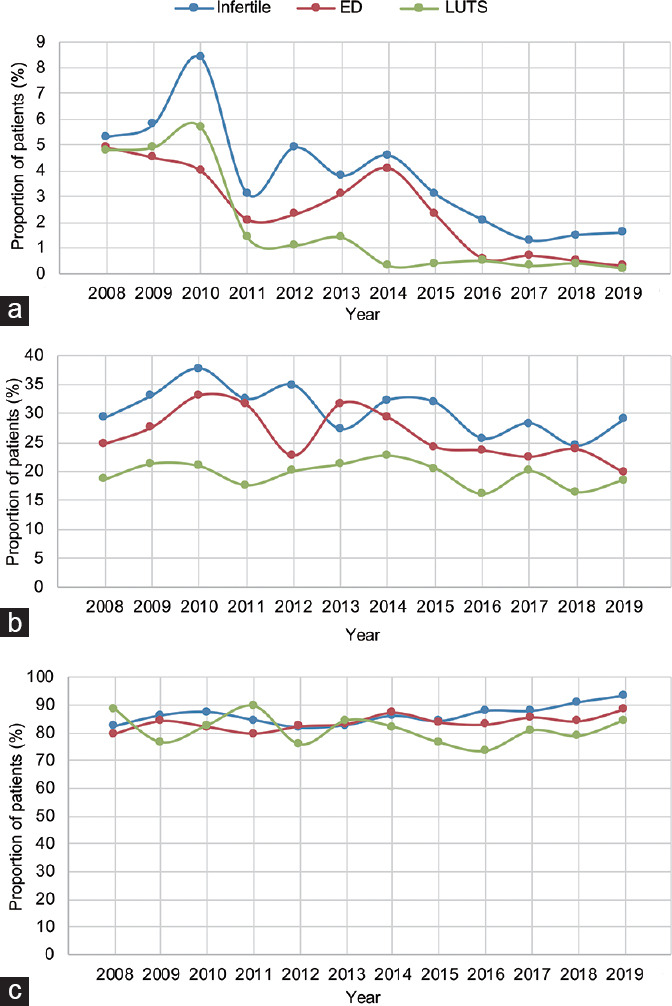
Proportion of patients reporting (a) active SoA use (any type), (b) cigarette smoking, and (c) alcohol consumption between 2008 and 2019 among infertile men, and individuals with ED and LUTS. P value according to the Fisher's exact test. SoA: substances of abuse; LUTS: lower urinary tract symptoms; ED: erectile dysfunction.
Table 3 reports UVA and MVA logistic regression models testing the associations between clinical variables (age, smoking status, alcohol consumption, and motivation for office evaluation) and current SoA consumption status. As revealed by the MVA, younger age (odds ratio [OR]: 0.96; P < 0.001), current smoking status (OR: 5.25; P < 0.001), regular alcohol consumer status (OR: 2.32; P = 0.004), and infertility as the reason for the office evaluation (OR: 2.42; P = 0.002) were independently associated with current SoA status.
Table 3.
Logistic regression models predicting current substances of abuse consumption in the whole cohort (n=190)
| Parameters | UVA model | MVA model | ||
|---|---|---|---|---|
|
|
|
|||
| OR; P | 95% CI | OR; P | 95% CI | |
| Age | 0.95; <0.001 | 0.93–0.96 | 0.96; <0.001 | 0.94–0.97 |
| Current smoker versus nonsmoker | 6.74; <0.001 | 4.92–9.23 | 5.25; <0.001 | 3.82–7.23 |
| Regular alcohol consumer versus abstainer | 2.79; <0.001 | 1.58–4.97 | 2.32; 0.004 | 1.31–4.11 |
| Reason for office evaluation | ||||
| LUTS | Reference | Reference | Reference | Reference |
| ED | 2.55; <0.001 | 1.49–4.37 | 1.63; 0.08 | 0.92–2.88 |
| Infertility | 4.80; <0.001 | 2.86–8.03 | 2.42; 0.002 | 1.41–4.18 |
UVA: univariate model; MVA: multivariate model; LUTS: lower urinary tract symptom; ED: erectile dysfunction; OR: odds ratio; CI: confidence interval
DISCUSSION
This sociobehavioral study shows that approximately 3% of men seeking medical attention for uro-andrological purposes were current SoA consumers. Infertile men reported greater SoA use than those evaluated for ED or LUTS. Marijuana was the most common SoA in our cohort, followed by cocaine and heroin. Current SoA users were younger and more frequently also concomitant smokers and alcohol consumers compared to those who did not currently or had never used SoA. Overall, these results point to the importance of SoA investigation during a patient's evaluation for uro-andrological purposes, particularly in young and infertile men.
Our study was motivated by the extensive amount of literature showing the negative impact of SoA use on men's sexual and reproductive health; however, a detailed characterization of the real-life use of SoA in patients seeking medical attention for uro-andrological purposes was still lacking.
Globally, the use and legalization of cannabis are increasing. Cannabinoid receptors have been found to be expressed in the anterior pituitary, Leydig cells, Sertoli cells, and testicular tissues. Of clinical relevance, cannabis smoking has been found to negatively impact male fertility, affecting the hypothalamus–pituitary–gonadal (HPG) axis, spermatogenesis, and sperm function.6,23 Previous retrospective clinical studies have shown an association between marijuana use and decreased sperm count and poor sperm morphology.7,24 Likewise, Verhaeghe et al.25 reported that cannabis consumption exerts deleterious effects on sperm nuclear quality in infertile men by increasing numerical chromosome abnormalities and DNA fragmentation, thus contributing to poor semen quality and function.
Of note, studies of hormonal changes suggest inconclusive effects of SoA on testosterone levels, lowered luteinizing hormone levels, and unchanged follicle-stimulating hormone levels.6 Moreover, limited emerging evidence points to cannabis use possibly being associated with ED. A recent meta-analysis including 3395 men demonstrated a higher prevalence of ED in cannabis users (up to four times) compared to controls.26 However, the overall low quality of the published studies on this topic may preclude the consideration of cannabis use being a strong risk factor for ED in the general population.27 Our results showed that among current SoA users, 77.3% of them were cannabis consumers. Therefore, infertile men and those with ED should be informed of the negative impact of cannabis on their sexual and reproductive health.
Opioids act on the HPG axis by inhibiting the pulsatility of gonadotropin-releasing hormone secretion with subsequent suppression of follicle-stimulating hormone and luteinizing hormone release, impairing spermatogenesis, and reducing testosterone concentrations.2 Recent data suggest that both sperm concentration and quality are impaired in opioid abusers. Moreover, increased rates of DNA fragmentation and reduced expression of catalase-like and superoxide dismutase-like activity were observed in opioid-addicted men compared to age-matched healthy volunteers.17 Similarly, opioid users were found to have a higher prevalence of ED compared to nonusers.28
Cocaine intake has not been unequivocally associated with impaired semen quality. For instance, Bracken et al.13 reported a greater use of cocaine among subjects with lower sperm counts and motility. However, recent data have suggested that the negative impact of SoA on male fertility might be biased by the concurrent high utilization of tobacco smoking and alcohol in this specific cohort.29 In particular, several studies have shown the detrimental impact of cigarette smoking and alcohol consumption on male sexual and reproductive health. Boeri et al.21 analyzed a cohort of 189 infertile men and found that heavy smokers and heavy drinkers were associated with worse seminal parameters than both moderate smokers/drinkers and nonsmokers/abstainers. Moreover, the detrimental effects on semen parameters were even greater when these two recreational habits were concomitant.21 Similarly, a vast amount of literature has previously reported the negative effects of cigarette smoking and alcohol consumption on ED.30,31
In this study, we showed that current SoA users were more frequently active smokers and alcohol consumers than former or never SoA users. Moreover, men who currently use SoA were more commonly heavy smokers and drinkers than those in the other groups, thus putting these individuals at an even higher risk for sexual dysfunction and impaired semen parameters. Of clinical importance, we showed that infertile men had a 2-fold higher status of current SoA consumption than those with LUTS or ED, even after accounting for smoking and alcohol consumption. Therefore, our real-life, epidemiological investigation suggests that every man seeking medical attention for uro-andrological purposes should be carefully screened for the most common recreational habits including SoA, alcohol consumption, and smoking, because of their negative effects in terms of general health, erectile function, and sperm parameters.
This study is innovative because it shows, for the first time, the real-life utilization of SoA in a very specific and homogeneous cohort of men, those with sexual dysfunction and male infertility, in which the use of these substances has a deleterious impact on several aspects of the individual's well-being, namely erectile function and semen parameters. A second strength of this study is that the comprehensive investigation of recreational habits of this relatively-large cohort of men was conducted by the same uro-andrologist, which ensures consistency in the data collection.
Our study is not devoid of limitations. First, despite the fact that we analyzed a relatively-large cohort of men seeking medical attention for uro-andrological purposes, this was a single center-based study, raising the possibility of selection biases. Particularly, our cohort was based on white-Caucasian European men and our results might not be generalizable to different ethnicities. Therefore, larger studies across different centers and cohorts are needed to externally validate these findings. Second, it was not possible to investigate the route of SoA consumption (e.g., smokable vs edible cannabis), which would have been of relevant clinical interest considering the growing rate of cannabis legalization worldwide. Third, our analysis does not include data on various aspects of sexual dysfunction such as libido, orgasm, and satisfaction. However, the main goal of the study was not to investigate the impact of SoA on ED, which has previously been extensively addressed, but to provide a real-life picture of SoA utilization over the last decade in men with uro-andrological disorders. Fourth, we did not address demographic, education, or socioeconomic information that may be associated with SoA use.
CONCLUSIONS
This sociobehavioral analysis revealed that almost 3% of men seeking medical attention for uro-andrological purposes were current SoA consumers. Cannabis (76.6%) was the most used SoA, followed by cocaine (23.4%) and heroin (4.7%). Infertile men reported the highest rate of SoA use compared to those presenting for ED or LUTS. Current SoA users were younger and more frequently also concomitant smokers and alcohol consumers compared to those who had only previously or had never used SoA. SoA consumption should always be investigated particularly in young and infertile men.
AUTHOR CONTRIBUTIONS
FB and LB designed the study, collected data, performed statistical analyses, and drafted the manuscript. AS designed the study, collected data, and coordinated all the steps of the study. PC, EV, WC, LC, EP, AB, CA, MA, and FM collected data, participated in coordination, and revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
REFERENCES
- 1.United Nations Office on Drugs and Crime (UNODC). World Drug Report. 2019. [Last accessed on 2020 Jun 10]. Available from: https://www.unodc.org/wdr2019/
- 2.Fronczak CM, Kim ED, Barqawi AB. The insults of illicit drug use on male fertility. J Androl. 2012;33:515–28. doi: 10.2164/jandrol.110.011874. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2016 Alcohol and Drug Use Collaborators. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5:987–1012. doi: 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim ST, Park T. Acute and chronic effects of cocaine on cardiovascular health. Int J Mol Sci. 2019;20:584. doi: 10.3390/ijms20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh Z, Gonzalez R, Crosby K, Thiessen MS, Carroll C, et al. Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev. 2017;51:15–29. doi: 10.1016/j.cpr.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Payne KS, Mazur DJ, Hotaling JM, Pastuszak AW. Cannabis and male fertility: a systematic review. J Urol. 2019;202:674–81. doi: 10.1097/JU.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pacey AA, Povey AC, Clyma JA, McNamee R, Moore HD, et al. Modifiable and non-modifiable risk factors for poor sperm morphology. Hum Reprod. 2014;29:1629–36. doi: 10.1093/humrep/deu116. [DOI] [PubMed] [Google Scholar]
- 8.Gundersen TD, Jørgensen N, Andersson AM, Bang AK, Nordkap L, et al. Association between use of marijuana and male reproductive hormones and semen quality: a study among 1,215 healthy young men. Am J Epidemiol. 2015;182:473–81. doi: 10.1093/aje/kwv135. [DOI] [PubMed] [Google Scholar]
- 9.Carroll K, Pottinger AM, Wynter S, DaCosta V. Marijuana use and its influence on sperm morphology and motility: identified risk for fertility among Jamaican men. Andrology. 2020;8:136–42. doi: 10.1111/andr.12670. [DOI] [PubMed] [Google Scholar]
- 10.Lisano JK, Smith JD, Mathias AB, Christensen M, Smoak P, et al. Performance and health-related characteristics of physically active males using marijuana. J Strength Cond Res. 2019;33:1658–68. doi: 10.1519/JSC.0000000000002238. [DOI] [PubMed] [Google Scholar]
- 11.Nassan FL, Arvizu M, Mínguez-Alarcón L, Williams PL, Attaman J, et al. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum Reprod. 2019;34:715–23. doi: 10.1093/humrep/dez002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan T, Ngo B, Jones CA. The use of cannabis and perceptions of its effect on fertility among infertility patients. Hum Reprod Open. 2020;2020:hoz041. doi: 10.1093/hropen/hoz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bracken MB, Eskenazi B, Sachse K, McSharry JE, Hellenbrand K, et al. Association of cocaine use with sperm concentration, motility, and morphology. Fertil Steril. 1990;53:315–22. doi: 10.1016/s0015-0282(16)53288-9. [DOI] [PubMed] [Google Scholar]
- 14.Wisniewski AB, Brown TT, John M, Frankowicz JK, Cofranceso J, et al. Hypothalamic-pituitary-gonadal function in men and women using heroin and cocaine, stratified by HIV status. Gend Med. 2007;4:35–44. doi: 10.1016/s1550-8579(07)80007-6. [DOI] [PubMed] [Google Scholar]
- 15.Rawson RA, Washton A, Domier CP, Reiber C. Drugs and sexual effects: role of drug type and gender. J Subst Abuse Treat. 2002;22:103–8. doi: 10.1016/s0740-5472(01)00215-x. [DOI] [PubMed] [Google Scholar]
- 16.Palha AP, Esteves M. A study of the sexuality of opiate addicts. J Sex Marital Ther. 2002;28:427–37. doi: 10.1080/00926230290001547. [DOI] [PubMed] [Google Scholar]
- 17.Safarinejad MR, Asgari SA, Farshi A, Ghaedi G, Kolahi AA, et al. The effectsof opiate consumption on serum reproductive hormone levels, sperm parameters, seminal plasma antioxidant capacity and sperm DNA integrity. Reprod Toxicol. 2013;36:18–23. doi: 10.1016/j.reprotox.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Nik Jaafar NR, Mislan N, Abdul Aziz S, Baharudin A, Ibrahim N, et al. Risk factors of erectile dysfunction in patients receiving methadone maintenance therapy. J Sex Med. 2013;10:2069–76. doi: 10.1111/jsm.12105. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Infertility Definitions and Terminology. [Last accessed on 2020 Apr 02]. Available from: http://www.who.int/reproductivehealth/topics/infertility/definitions .
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Boeri L, Capogrosso P, Ventimiglia E, Pederzoli F, Cazzaniga W, et al. Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J Androl. 2019;21:478–85. doi: 10.4103/aja.aja_110_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute of Alcohol Abuse and Alcoholism. The Physicians’ Guide to Helping Patients With Alcohol Problems. Washington: U.S. Department of Health and Human Services, National Institutes of Health. 1995:30. [Google Scholar]
- 23.du Plessis SS, Agarwal A, Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet. 2015;32:1575–88. doi: 10.1007/s10815-015-0553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hembree WC, 3rd, Nahas GG, Zeidenberg P, Huang HF. Changes in human spermatozoa associated with high dose marihuana smoking. Adv Biosci. 1978;22:429–39. doi: 10.1016/b978-0-08-023759-6.50038-x. [DOI] [PubMed] [Google Scholar]
- 25.Verhaeghe F, Di Pizio P, Bichara C, Berby B, Rives A, et al. Cannabis consumption might exert deleterious effects on sperm nuclear quality in infertile men. Reprod Biomed Online. 2020;40:270–80. doi: 10.1016/j.rbmo.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Pizzol D, Demurtas J, Stubbs B, Soysal P, Mason C, et al. Relationship between cannabis use and erectile dysfunction: a systematic review and meta-analysis. Am J Mens Health. 2019;13:1557988319892464. doi: 10.1177/1557988319892464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen MS, Walter EE. Health-related lifestyle factors and sexual dysfunction: a meta-analysis of population-based research. J Sex Med. 2018;15:458–75. doi: 10.1016/j.jsxm.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Zhao S, Deng T, Luo L, Wang G, Li E, et al. Association between opioid use and risk of erectile dysfunction: a systematic review and meta-analysis. J Sex Med. 2017;14:1209–19. doi: 10.1016/j.jsxm.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Samplaski MK, Bachir BG, Lo KC, Grober ED, Lau S, et al. Cocaine use in the infertile male population: a marker for conditions resulting in subfertility. Curr Urol. 2015;8:38–42. doi: 10.1159/000365687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verze P, Margreiter M, Esposito K, Montorsi P, Mulhall J. The link between cigarette smoking and erectile dysfunction: a systematic review. Eur Urol Focus. 2015;1:39–46. doi: 10.1016/j.euf.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Wang XM, Bai YJ, Yang YB, Li JH, Tang Y, et al. Alcohol intake and risk of erectile dysfunction: a dose-response meta-analysis of observational studies. Int J Impot Res. 2018;30:342–51. doi: 10.1038/s41443-018-0022-x. [DOI] [PubMed] [Google Scholar]


