The treatment of men with infertility from azoospermia is challenging. As some men with severe oligozoospermia improve sperm output during treatment with isotretinoin, we treated nine men with azoospermia with isotretinoin to determine if azoospermic men would also respond to isotretinoin treatment. One of these men who had a previous negative testicular sperm extraction (TESE) was found to have an area of sperm production on a postisotretinoin testicular extraction. This man achieved a pregnancy and a live birth of a healthy, term daughter after in vitro fertilization coupled with intracytoplasmic sperm injection (IVF/ICSI). A second subject had a positive TESE after treatment as well. Additional study of isotretinoin therapy in men with azoospermia undergoing testicular sperm harvesting procedures is warranted.
Infertility affects 10%–15% of couples.1 Approximately 10% of infertile men have no sperm in their ejaculate, considered as azoospermia. In men with nonobstructive azoospermia who are not candidates for gonadotropin therapy, the current standard of care is an attempt at surgical sperm extraction from the testes using a microscopic testicular sperm extraction (micro-TESE), often coupled with intracytoplasmic sperm injection (ICSI).2 Unfortunately, micro-TESE does not yield sperm in around 40% of cases.2,3 Therefore, new approaches to the treatment of male infertility due to azoospermia are needed to effectively treat these men.
An essential role of retinoids in spermatogenesis has been long appreciated.4 Retinoic acid plays essential roles in spermatogonial differentiation, spermatid adhesion to Sertoli cells, and spermiation.5 Given the importance of retinoic acid in spermatogenesis, it seems possible that some men with infertility might have intratesticular concentrations of retinoic acid below those necessary for spermatogenesis. Indeed, infertile men have significantly lower concentrations of aldehyde dehydrogenase-1A2, the enzyme responsible for retinoic acid biosynthesis in germ cells.6 Recently, we performed a pilot study of isotretinoin in 20 men with infertility due to oligoasthenoteratozoospermia.7 In this study, roughly half of the men had significant improvements in their sperm production during treatment and several pregnancies occurred.
In this study, we sought to examine isotretinoin therapy in men with infertility due to azoospermia in a single-arm pilot study using baseline sperm values as controls. We hypothesized that some men treated with isotretinoin would manifest detectable sperm in their ejaculates, suitable for use in ICSI. Alternatively, we hypothesized that isotretinoin therapy might allow men with a previously negative TESE to undergo successful repeat TESE, allowing for fertility.
PATIENTS AND TECHNIQUE
As isotretinoin had not previously been studied for the treatment of azoospermia, we opted for a single-arm pilot study design. The subjects were infertile men aged between 21 years and 50 years with azoospermia and more than 12 months of an inability to conceive. This study was approved by the Institutional Review Board of the University of Washington (Seattle, WA, USA) and conducted under IND#120703 from the US Food and Drug Administration (FDA). The study was registered on ClinicalTrials.gov with trial No. NCT02061384. Subjects signed the Institutional Review Board-approved informed consent document before participation and were enrolled between July 2017 and September 2018, providing two baseline semen samples to confirm the diagnosis of azoospermia. They also provided a third semen sample before starting therapy and every 4 weeks during the 32-week treatment phase. During the treatment phase, subjects were prescribed isotretinoin at a dose of 20 mg twice daily for 32 weeks (two full cycles of spermatogenesis) for self-administration. Semen samples were allowed by liquefy for 30 min at 37°C and then analyzed within 60 min of liquefaction using the World Health Organization (WHO) protocol.8 All samples were assessed in the same laboratory by the same technicians every 4 weeks during treatment. Samples were initially assessed in “wet drop” preparations. If no sperm were seen, the samples were mixed thoroughly and centrifuged (Model Cerna/CL2, International Equipment Company, Needham Heights, MA, USA) at 3000g for 15 min. The resulting semen pellet was resuspended in approximately 50 ml of seminal plasma and 10 ml aliquots of this mixture were then placed on the slides for microscopic examination (Model Axiostar Plus, Zeiss, White Plains, NY, USA) of up to 400 fields (unless sperm were identified earlier) as per the WHO guidelines.9 Blood counts, serum chemistry, and hormone tests were performed by the clinical laboratory at the University of Washington. The proportion of men with sperm in their ejaculates during treatment was compared with baseline using a Fisher's exact test. For analysis of laboratory assessments, a paired t-test was used with a Bonferroni correction to adjust for multiple comparisons. All analyses were performed using STATA version 10.0 (StataCorp, College Park, TX, USA). For all comparisons, P < 0.05 was considered statistically significant.
Nine men were treated. The median age was 36 (interquartile range: 31–42) years. Seven of the men were White (five non-Hispanic and two Hispanic), and two were Asian. Most men had idiopathic nonobstructive azoospermia; one man had nonmosaic Klinefelter syndrome. Histologically, seven men had complete germ cell aplasia (Sertoli-cell only) pathology on pretreatment testicular biopsy, while one man had hypospermatogenesis (subject #6). Testicular histology was unavailable for one man. Baseline testicular volume, other physical examination, and laboratory parameters are shown in Table 1.
Table 1.
Baseline values and changes during treatment in examination parameters and laboratory measurements (n=9)
| Variable | Baseline | Treatment | |||
|---|---|---|---|---|---|
|
| |||||
| Week 8 | Week 16 | Week 24 | Week 32 | ||
| Physical examination and history | |||||
| Blood pressure (systolic, mmHg) | 130 (125–144) | 124 (114–146) | 132 (115–140) | 125 (116–137) | 122 (117–129) |
| Pulse (beats per min) | 70 (65–78) | 66 (62–77) | 72 (65–87) | 70 (67–83) | 69 (64–84) |
| BMI (kg m−2) | 29 (25–35) | 29 (26–34) | 30 (24–31) | 30 (25–39) | 29 (25–36) |
| Testes volume (right+left; ml) | 21 (17–24) | 20 (12–24) | 22 (18–24) | 22 (18–24) | 20 (18–24) |
| Laboratory assessments | |||||
| Total cholesterol (mg dl−1) | 215 (174–227) | 219 (194–239) | 208 (193–217) | 223 (194–243) | 208 (193–213) |
| HDL cholesterol (mg dl−1) | 54 (37–65) | 49 (42–63) | 46 (37–60) | 54 (42–55) | 46 (38–55) |
| LDL cholesterol (mg dl−1) | 135 (112–152) | 142 (110–171) | 127 (107–158) | 132 (121–164) | 135 (111–151) |
| Triglycerides (mg dl−1) | 72 (55–135) | 129 (73–214)* | 134 (75–215)* | 131 (74–253)* | 116 (76–193) |
| Testosterone (ng ml−1) | 3.0 (2.1–3.8) | 2.6 (2.1–3.5) | 2.6 (2.1–3.5) | 2.8 (2.2–3.3) | 3.3 (2.5–4.3) |
| LH (IU l−1) | 8 (7–14) | 10 (8–13) | 10 (8–16) | 9 (7–12) | 9 (8–13) |
| FSH (IU l−1) | 23 (17–43) | 25 (12–37) | 27 (13–40) | 24 (12–41) | 25 (13–36) |
| Hematocrit (%) | 46 (44–47) | 44 (44–45) | 44 (42–46) | 45 (42–47) | 44 (42–46) |
Data are presented as median and interquartile range (25th–75th percentiles). *P<0.05, the indicated group versus baseline. LH: luteinizing hormone; FSH: follicle-stimulating hormone; HDL: high-density lipoprotein; LDL: low-density lipoprotein; BMI: body mass index
There was one serious adverse event during treatment, a man who developed nephrotic syndrome. Fortunately, this subject fully recovered normal kidney function after treatment with prednisone and cyclosporine by a nephrologist. There were 12 nonserious adverse effects reported by subjects during treatment, including upper respiratory infections (n = 7), muscle cramps (n = 2), worsening of preexisting hypertension (n = 1), rash (n = 1), and headache (n = 1). All adverse effects resolved spontaneously without specific treatment, except for the worsening hypertension, which required additional of a second blood pressure medication. As expected, all men experienced the expected dry facial skin and chapped lips to varying degrees, which was managed with moisturizers and the use of lip balms. Physical examinations, including testicular volume, vital signs, and routine laboratory assessment, and hormone values from subjects did not change significantly during treatment, excepting a small, transient increase in serum fasting triglycerides (Table 1). These increases returned to baseline with isotretinoin discontinuation (data not shown).
The results of the semen samples are presented in Table 2. During treatment, only one man (subject #6) had sperm apparent in his uncentrifuged semen sample at one time point. This sample had a total sperm count of 32 500 sperm, with approximately 20% motility. After centrifugation, four of nine men (44.4%) had detectable sperm in the pellet at any time point, compared to none at baseline (P = 0.04). In 7 out of the 8 samples with sperm, the sperm were nonmotile. There were insufficient sperm present for morphology assessment. One man, who had a previous micro-TESE without finding sperm performed by another surgeon, had several tubules with obvious spermatogenesis on a repeat, postisotretinoin micro-TESE (Figure 1). Four tubule extractions were performed and all were positive (1–8 sperm per 20 high-powered fields) for spermatozoa, and sperm were successfully retrieved for ICSI. His wife had no prior history of pregnancy but was otherwise healthy and free of medical issues or known causes of infertility. Twenty-one previously frozen eggs were injected, resulting in 13 viable embryos. Two embryos were transferred, resulting in a singleton pregnancy and the birth of a healthy girl at term. The baby is doing well and appears to free from obvious birth defects at 6 months of age. Of the remaining subjects, one man (subject #6) had a successful posttreatment micro-TESE with cryopreservation of sperm but has not proceeded with ICSI due to expense. Three additional couples achieved pregnancy using donor sperm, and one couple achieved a pregnancy using donated embryos.
Table 2.
Analyses of semen pellets in nine azoospermic men receiving isotretinoin for 32 weeks
| Subject | Screening | Treatment week | Posttreatment TESE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Screen visit 1 | Screen visit 2 | Week 0 | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 | Week 28 | Week 32 | ||
| 1 | 0 | 0 | 0 | 0 | 0 | 2 sperm | 0 | 0 | 0 | 1 sperm | 5 sperm | + |
| 2 | 0 | 0 | 0 | 1 sperm | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ND |
| 3a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 sperm | 0 | 0 | 0 | ND |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ND |
| 5b | 0 | 0 | 0 | 0 | 0 | NS | NS | NS | NS | NS | NS | ND |
| 6 | 0 | 0 | 0 | 2 sperm | NP | 0 | 0 | 0 | 2 sperm | 0 | 0 | + |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ND |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ND |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ND |
aSubject has Klinefelter syndrome; bsubject withdrew due to adverse event. ND: not done; TESE: testicular sperm extraction; +: posttreatment TESE with sperm recovered; NP: no pellet (sperm were seen on wet drop, so sample was not centrifuged); NS: no sample (due to early drop-out)
Figure 1.
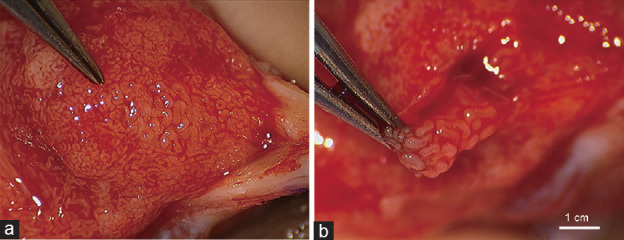
Findings from micro-TESE for subject with the live birth. (a) Note plump seminiferous tubules with active spermatogenesis immediately distal to the forceps. In contrast, most of the remaining tubules are small and likely inactive. (b) This area was excised for sperm retrieval. micro-TESE: microscopic testicular sperm extraction.
COMMENTS
In this report, we present pilot data from the first study to test the hypothesis that men with infertility from azoospermia might benefit from treatment with isotretinoin (13-cis retinoic acid). Unfortunately, isotretinoin was not able to stimulate the appearance of sperm in the uncentrifuged semen sample except in one case; however, it did result in sperm being observed in the centrifuged pellets of four of the nine treated men during treatment. It is theoretically possible that the sperm observed in the pellets were simply a function of repeated assessments. To this point, Jaffe and colleagues have shown that 12% (2/17) of pelleted samples in men with nonobstructive azoospermia can have sperm in a second pelleted sample.10 However, all of the men in our study had three negative pelleted samples analyzed before treatment, suggesting that these men had “true” azoospermia at baseline, and the presence of sperm was not simply due to repeated assessments alone. It may be possible that the longer durations of treatment might have resulted in the appearance of sperm in all subjects; this will need to be examined in future studies.
It is also possible that the successful repeat micro-TESE and live birth described in our report was unrelated to isotretinoin treatment. While the success of repeat micro-TESE in men with a prior successful micro-TESE is known to be above 80%,11,12 the success of repeat micro-TESE after prior unsuccessful procedure appears to be very low, with one series reporting a single live birth in thirty-nine attempts (3%),12 although this success rate may vary with the skill of the surgeon, and the first surgeon may have been unable to find sperm that were present. However, it must be noted that spermatogenesis was readily apparent on the second (postisotretinoin) TESE (Figure 1). Therefore, while we cannot definitively conclude that the isotretinoin treatment was the reason that the man in our study had a positive repeat micro-TESE after a previously negative procedure, it seems more likely than not to be the case. However, study of isotretinoin treatment prior to micro-TESE in a randomized, placebo-controlled trial is needed before the impact of this approach can be determined. Our study was particularly limited in its ability to generalize regarding patients with Klinefelter syndrome, a common cause of azoospermia as we had only one such patient in our pilot study. While there is no reason to believe that men with Klinefelter syndrome would be any more or less likely to respond to isotretinoin therapy, additional study of such men will be needed to determine their relative responsiveness compared to men with azoospermia from causes other than Klinefelter syndrome.
Isotretinoin has been widely prescribed for the treatment of acne since 1982 and has a well-described safety profile.13 Of concern, we had a subject that experienced a serious adverse event, nephrotic syndrome, during our study. To our knowledge, only a single case of contemporaneous nephrotic syndrome and isotretinoin treatment has been reported in the medical literature.14 Nevertheless, isotretinoin use should be monitored closely to preclude adverse events. Isotretinoin is teratogenic (FDA category X) and should not be administered to women of reproductive age without the concomitant use of adequate contraception. From a male infertility standpoint, there is no evidence in the literature to suggest an increased risk of birth defects in the children of women who conceive while their husbands are taking isotretinoin for acne.15
In conclusion, our pilot study suggests that isotretinoin may result in the production of sperm evident in the centrifuged pellet in roughly half of men with azoospermia. This finding suggests that stimulation of spermatogenesis is occurring with isotretinoin therapy, which may benefit some men undergoing testicular sperm harvesting procedures. Additional research of isotretinoin for the treatment of men with infertility from azoospermia before testicular sperm extraction in larger, randomized, placebo-controlled studies is warranted.
AUTHOR CONTRIBUTIONS
All authors conceived of and implemented the protocol. JKA drafted the manuscript, and all authors were involved in editing. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported this work through grant K24HD082231 to John K Amory. The authors would like to thank Ms. Iris Nielsen for coordinating the study, Ms. Connie Peete and Ms. Dorothy McGuinness for performing the seminal fluid assays, and Dr. Kevin Ostrowski, Dr. Laura Shahine, and Dr. Bettina Paek for clinical contributions. In addition, the authors would like to thank Dr. David W Amory and Dr. William J Bremner for critical review of the manuscript.
REFERENCES
- 1.Chandra A, Copen CE, Stephen ED. Infertility and impaired fecundity in the united states, 1982–2010: data from the National Survey of Family Growth. Nat Health Stat Rep. 2013;67:1–17. [PubMed] [Google Scholar]
- 2.Ishikawa T. Surgical recovery of sperm in non-obstructive azoospermia. Asian J Androl. 2012;14:109–15. doi: 10.1038/aja.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramasamy R, Lin K, VeeckGosden L, Rosenwaks Z, Palermo GD, et al. High serum FSH levels in men with nonosbructive azoospermia does not affect success of microdissection testicular sperm extraction. Fert Steril. 2009;92:590–3. doi: 10.1016/j.fertnstert.2008.07.1703. [DOI] [PubMed] [Google Scholar]
- 4.Wolbach SB, Howe PR. Tissue changes following deprivation of fat soluble A vitamin. J Exp Med. 1925;42:753–77. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gewiss R, Topping T, Griswold MD. Cycles, waves, and pulses: retinoic acid and the organization of spermatogenesis. Andrology. 2020;8:892–7. doi: 10.1111/andr.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amory JK, Arnold S, Walsh T, Lardone MC, Piottante A, et al. Levels of the retinoic acid synthesizing enzyme ALDH1A2 are lower in testicular tissue from men with infertility. Fert Steril. 2014;101:960–6. doi: 10.1016/j.fertnstert.2013.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amory JK, Ostrowski KA, Gannon JR, Berkseth K, Stevison F, et al. Isotretinoin administration improves sperm production in some men with infertility from oligoasthenozoospermia: a pilot study. Andrology. 2017;5:1115–23. doi: 10.1111/andr.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 9.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe TM, Kim ED, Hoekstra TH, Lipshultz LI. Sperm pellet analysis: a technique to detect the presence of sperm in men considered to have azoospermia by routine semen analysis. J Urol. 1998;159:1548–60. doi: 10.1097/00005392-199805000-00038. [DOI] [PubMed] [Google Scholar]
- 11.Ramasamy R, Ricci JA, Leung RA, Schlegel PN. Successful repeat microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol. 2011;185:1027–31. doi: 10.1016/j.juro.2010.10.066. [DOI] [PubMed] [Google Scholar]
- 12.Karacan M, Ulug M, Arvas A, Cebi Z, Erkan S, et al. Live birth rate with repeat microdissection TESE and intracytoplasmic sperm injection after a conventional testicular biopsy in men with nonobstructive azoospermia. Eur J Obstet Gynecol Reprod Biol. 2014;182:174–7. doi: 10.1016/j.ejogrb.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Bauer LB, Ornelas JN, Elston DM, Alikhan A. Isotretinoin: controversies, facts, and recommendations. Exp Rev Clin Pharm. 2016;9:1425–42. doi: 10.1080/17512433.2016.1213629. [DOI] [PubMed] [Google Scholar]
- 14.van Oers JA, de Leeuw JD, van Bommel EF. Nephrotic syndrome associated with isotretinoin. Nephrol Dial Transplant. 2000;15:923–4. doi: 10.1093/ndt/15.6.923. [DOI] [PubMed] [Google Scholar]
- 15.Millsop JW, Heller MM, Eliason MJ, Murase JE. Dermatological medication effects on male fertility. Dermatol Ther. 2013;26:337–46. doi: 10.1111/dth.12069. [DOI] [PubMed] [Google Scholar]


