Abstract
Background
The pathogenesis of exercise induced bronchoconstriction is likely multifactorial and is not completely understood. Inflammation plays an important role in the pathogenesis of exercise induced bronchoconstriction in asthmatic subjects but the evidence seems less strong in non‐asthmatic subjects. The management of exercise induced bronchoconstriction focuses on prevention, through both pharmacologic and non‐pharmacologic interventions.
Objectives
The objectives of this review were to evaluate the use of inhaled corticosteroids in the treatment of exercise induced bronchoconstriction in a systematic way. Specifically, the review was designed to:
determine whether inhaled corticosteroids (compared to placebo) has an attenuating effect on exercise induced bronchoconstriction in adult and pediatric asthmatic patients; estimate the magnitude of the attenuating effect.
Search methods
We searched the Cochrane Airways Review Group Specialised Register of trials, the Cochrane Central Register of Controlled Trials, review articles, textbooks and reference list of articles.
Selection criteria
Randomised trials in adults or children comparing inhaled corticosteroids with placebo to prevent bronchoconstriction in patients with exercise induced bronchoconstriction.
Data collection and analysis
Trial quality assessment and data extraction were conducted independently by two reviewers.
Main results
The results from eight randomised controlled trials involving 162 participants were analyzed (two trials involving adults and six involving children). Combining results from the three parallel studies with at least 4 weeks duration of inhaled corticosteroids, the use of inhaled corticosteroids significantly attenuated the percent fall index in forced expiratory volume in 1 second (WMD (fixed): 11.74%; 95% CI: 10.06% to 13.42%). The result from one crossover study with duration of inhaled corticosteroids of 4 weeks revealed significant attenuation of percent fall in forced expiratory volume in 1 second ( WMD 11.70%; 95% CI: 7.51% to 15.90%) and the percent fall in peak expiratory flow ( WMD 11.50%; 95% CI: 6.31% to 16.69%). The small amount of data from placebo‐controlled trials using a single treatment do not currently allow conclusions to be drawn.
Authors' conclusions
Inhaled corticosteroids used for 4 weeks or more before exercise testing significantly attenuated exercise‐induced bronchoconstriction. The relative benefits of inhaled corticosteroids compared to other forms of exercise induced bronchoconstriction treatment (sodium cromoglycate, nedocromil sodium, salbutamol, and other anti‐inflammatory agents) remains unclear.
Plain language summary
Inhaled corticosteroids compared to placebo for prevention of exercise induced bronchoconstriction
Exercise‐induced asthma (bronchoconstriction) can limit a person's exercise endurance and lead to people avoiding exercise. This systematic review found that inhaled corticosteroids taken regularly can reduce exercise induced asthma in both children and adults.
Background
Exercise is the most common trigger of bronchospasm and up to 90% of asthmatics have airways which are hyperactive to exercise (Rundell 2002). Exercise‐induced bronchoconstriction (EIB) also occurs in up to 10% of subjects who are not known to be atopic or asthmatic (Gotshall 2002). The pathogenesis of EIB is likely multifactorial and is not completely understood. Inflammation plays an important role in the pathogenesis of EIB in asthmatic subjects (Koh 2002; Otani 2004) but the evidence seems less strong in non‐asthmatic subjects.
The management of EIB focuses on prevention, through both pharmacologic and non‐pharmacologic interventions. A variety of drugs have been found useful; however, there is considerable debate regarding the merits of each agent, the optimal dose and the optimal delivery method. Traditionally, inhaled bronchodilators have been the drugs of choice. Nedocromil sodium (Spooner 2002), sodium cromoglycate (Kelly 2000) and mast cell stabilizers (Spooner 2003) have been shown to have a statistically and clinically significant effect in attenuating EIB in three Cochrane Reviews. It is our aim to evaluate the use of inhaled corticosteroids (ICS) in the treatment of EIB in a systematic way.
Objectives
The objectives of this review were to evaluate the use of ICS in the treatment of EIB in a systematic way. Specifically, the review was designed to:
Determine whether ICS has an attenuating effect on EIB in adult and pediatric asthmatic patients;
Estimate the magnitude of the attenuating effect.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised, controlled trial comparing ICS with placebo. Studies with an additional treatment arm were included if the effect of ICS could be separately identified. Trials using combination inhalers ( long‐acting beta agonist with inhaled corticosteroids) were not studied here.
Types of participants
All studies including children (>/= 6 years) and/or adults (>/= 17 years) with a history of EIB, or who demonstrate EIB in a standardised exercise challenge prior to entry into the trial, were considered for inclusion. EIB was defined as a fall in forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) following exercise of 10% or greater. Studies that did not clearly state their criteria for EIB or that included people with impairment < 10% were excluded.
Types of interventions
All patients must have been treated with an ICS or placebo as a single prophylactic treatment prior to undergoing a standardised exercise challenge test. If studies reported more than one drug arm, only the comparison of ICS drugs were included. Studies that involved delivery via nasal spray were also excluded.
Types of outcome measures
Primary outcomes
The primary outcome was the maximum percent fall in pulmonary function, which is the conventional approach to quantify EIB (Anderson 1985). The percent (%) fall index expresses the reduction in lung function after exercise as a percent of the pre‐exercise baseline. The formula used to calculate the % fall index is: % fall FEV1 or PEF = 100 x (pre‐exercise value ‐ lowest post‐exercise value) / pre‐exercise value.
Secondary outcomes
The proportion of participants who receive complete protection from EIB. A drug is considered to offer complete protection if the maximum % fall index is < 15% (Spooner 2003; Kelly 2000);
The number of participants who received clinical protection from EIB. As in other EIB reviews (Spooner 2002; Spooner 2003; Kelly 2000), a drug is considered to offer clinical protection if pulmonary function test (PFT) values improve by 50% or more over the placebo effect (ERS 1997). The formula to calculate clinical protection is: (maximum % fall placebo‐ maximum % fall drug)/ maximum % fall placebo x 100] (Anderson 1979);
The number and nature of adverse effects experienced;
Subjective outcomes involving symptom score or performance scores;
Measurement of airway inflammation.
Search methods for identification of studies
Electronic searches
The trials were identified using Cochrane Airways Review Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and hand searching of leading respiratory journals and meeting abstracts (please see the Airways Group Module for further details). All records in the Specialised Register coded as 'asthma' were searched using the following terms:
(exerc* OR train* OR fitness OR physical OR EIB OR EIA ) AND (corticosteroid* OR ciclesonide OR dexa* OR deca* OR fluticasone OR Flovent OR beclo* OR budesonide OR Pulmicort OR flunisolide OR Aerobid OR Bronalide OR triamcinolone OR Azmacort OR Vanceril OR Becotide OR Flixotide OR Aerobec OR Mometasone OR Qvar)
The most recent search was done in October 2008.
Searching other resources
Reference lists of all available primary studies and review articles were reviewed to identify potential relevant citations. Inquiries regarding other published or unpublished studies known and/or supported by the authors of the primary studies were made so that these results could be considered for inclusion in this review. Scientific advisors of the various pharmaceutical industries that manufacture ICS were contacted (AstraZeneca, GlaxoSmithKline, Altana) for any unpublished or interim results on ICS research involving patients with EIB. Finally, personal contact with colleagues, collaborators and other trialists working in the field of asthma were made to identify potentially relevant studies. No language or publication restrictions were applied to these searches.
Data collection and analysis
Selection of studies
Phase 1: Abstracts of articles identified using the above search strategy were reviewed independently by two reviewers (MK, AT) . Phase 2: Articles that appeared to fulfill the inclusion criteria were retrieved. Using the full text of the study, two reviewers independently selected trials for inclusion in the review (MK, AT). Appropriate members of the Cochrane Airways Group screened foreign language articles and abstract data when an article qualified. All articles for which there was agreement were included; third party adjudication ( LI) was used to resolve disagreements when necessary.
Data extraction and management
Data were extracted by two reviewers (MK, AT) and entered into the Cochrane Collaboration software program (RevMan 5.0) . All data, numeric calculations and graphic extrapolations were independently confirmed. Reviewers attempted to contact authors to identify additional papers, confirm data extraction/estimation for correctness and completeness, and to obtain missing data.
Data extraction included the following items:
Methods: study design, method of randomization, definition of EIB, exercise‐testing procedure, withdrawals;
Population: recruitment, sample size, age, gender, inclusion and exclusion criteria;
Intervention: delivery device,agent, dose, timing and duration of therapy, co‐intervention;
Control: agent and dose;
Outcome: PFT, protection,adverse drug reactions
Assessment of risk of bias in included studies
Two reviewers ( AT, LI) assessed the methodological quality of the included trials with particular emphasis on the concealment of allocation, which was ranked using the Cochrane approach and the method of Jadad (Jadad 1996). One point is allocated for randomisation, blinding, and description of withdrawals and dropouts; an extra point can be added for methods of randomisation and blinding that are well‐described and adequate. Studies that use a clearly inadequate method of randomisation or blinding (such as alternating patients) lose the point allocated. The maximum score is 5 points and studies scoring below 3 points are usually regarded as being of low methodological quality. Discussion or a third party adjudication will be used to resolve disagreements when necessary.
Unit of analysis issues
For crossover studies mean differences were obtained for each of the included studies, and 95% CI were obtained for the difference in order to back‐calculate a standard error. The P values from paired statistical tests were used to estimate a standard error, or correspondence with trialists occurred in order to obtain one.
Assessment of heterogeneity
For pooled effects, heterogeneity was tested using the Breslow‐Day test; P < 0.05 was considered statistically significant. Heterogeneity was also quantified using the I2 statistic (Higgins 2003). Where significant heterogeneity exists, the reviewers performed sensitivity analyses based on methodological quality (high Jadad score 3‐5 versus low <3), and fixed vs random effect modelling.
Assessment of reporting biases
Funnel plots were used to investigate the possibility of publication bias. Data from crossover studies were pooled using a random effects model of mean difference, calculated with generic inverse variance.
Data synthesis
Trials were combined using RevMan (Version 5.0). For continuous variables, the individual statistics were reported as mean difference (MD) of treatment effect with 95% confidence interval (CI) and similar studies were pooled using weighted mean differences (WMD) using fixed effect model. For dichotomous outcome measures the individual and pooled statistics were reported as odds ratio (OR) and relative risks (RR) with 95% confidence intervals (95% CI) using fixed effect model. All similar studies were pooled using random effects OR and 95% CIs. Random effects modelling was used as sensitivity analysis.
The data were also evaluated for the presence of publication bias using graphical and statistical methods.
Subgroup analysis and investigation of heterogeneity
A‐priori, heterogeneity was examined using the following subgroups:
Age of patients (less than or greater than 16 years);
Duration of treatment with ICS (less than or more than 2 weeks)
Dose of ICS used in the study (steroid naive, less than or greater than 400 mcg equivalent of beclomethasone dipropionate);
Severity of asthma (degree of EIB before entry into the study, mean Maximum % fall in FEV1 or PEF <30% is mild and more than or greater than 30% is moderate‐severe) Spooner 2003, Kelly 2000);
Atopic asthma versus non‐atopic asthma;
Different delivery devices used.
Results
Description of studies
Results of the search
Using the search strategy described above, 193 titles and abstracts were selected from the computerised databases. From the text in the title, abstract and keywords, two authors independently selected 30 studies for full text review. The two independent reviewers subsequently determined that eight trials met inclusion criteria (100% agreement ). Review of reference lists did not yield any additional trials for inclusion.
Summary details are given in Characteristics of included studies, and described in general terms below.
Included studies
Design issues
Four studies used a parallel group design and four used crossover designs. All eight trials except for one (Jonasson 1998) were double‐blind studies. In the cross‐over studies, wash‐out periods ranged between 24 hours (Yazigi 1978) to 2 weeks (Thio 2001). One study did not mention duration of washout (Hartley 1977).
Population
Studies included children and adults (age range: 4‐45 years). While eight trials were identified for inclusion, results from one study (Hofstra 2000) were presented in geometric mean and attempts in contacting the authors for raw data were unsuccessful. Consequently, the meta‐analysis was performed using results from seven trials with 172 participants (94 males, 39 females, 39 not stated). Two trials involved adults (Hartley 1977; Vathenen 1991) and the other five trials involved children. There were 75 adults between the ages of 17‐45 years and 97 children between the ages of 6‐16 years. There were 10 drop‐outs altogether and analysis was performed using results from 162 patients: 113 patients were in the ICS arm and 110 patients in the placebo arm. Three studies included only stable asthmatics, otherwise stability of asthma was not mentioned. Recruitment procedures were not well described in most studies. Concomitant therapy included a variety of medications and most commonly included short acting beta agonists, which were discontinued for at least 6 hours before the exercise test.
Interventions
Trials evaluated a range of ICS that included budesonide (N=4) between 50 to 200 mcg/day, fluticasone (N=2) between 200 to 1000 mcg/day, betamethasone (N=1) at 800 mcg/day and triamcinolone (N=1) at 300 mcg per day. There was a wide range of treatment duration between the studies. Patients in two studies were given single dose of ICS 15 minutes and 4 hours prior to exercise testing (Yazigi 1978; Thio 2001). The other six studies had duration of ICS ranging between 4 to 12 weeks before a standardised exercise challenge test. All studies except for one (Hartley 1977) used treadmill as the exercise challenge. Hartley 1977 utilised cycle ergometer as a form of exercise challenge test.
Outcomes
Seven studies measured maximum fall in FEV1 after exercise. One study (Hartley 1977) measured fall in PEF after exercise and another (Yazigi 1978) measured both PEF and FEV1 after exercise. In addition, 1 study ( Hofstra 2000) reported PD 20 methacholine and serum eosinophil cationic protein levels, 3 studies measured symptom score ( Hofstra 2000; Hartley 1977; Jonasson 1998), 1 study ( Petersen 2004) measured exhaled nitric oxide levels and 1 study (Vathenen 1991) measured FEV1 response to histamine (PD 20 histamine) and Eucapnic Voluntary Hyperventilation (EVH) response. One study reported area under curve for FEV1values from exercise over 20‐minute period (Stelmach 2008). Two studies monitored adverse events (Petersen 2004; Hofstra 2000).
Excluded studies
Risk of bias in included studies
Using the Cochrane criteria to assess allocation concealment, all the studies were given "unclear" status. Using Jadad's 5 point validity scale, 1 study was rated 4 (very good), 6 studies were rated 3 (good) and one study was rated 2 (poor). All studies except for one lacked sufficient description of the method of randomisation and blinding used.
Effects of interventions
Pulmonary function
Although all eight trials fulfilled the criteria for inclusion, results from one study could not be used for meta‐analysis (Hofstra 2000). As such, the meta‐analysis was conducted using data from 7 studies with 162 participants completing the studies.
Combining results from three parallel studies (Jonasson 1998; Vathenen 1991 ; Stelmach 2008), the use of ICS given for a duration of 4 to 12 weeks significantly attenuated the % fall index in FEV1 (WMD (fixed): 11.74%; 95% CI: 10.06% to 13.42%; Figure 1); the results demonstrated a large degree of heterogeneity (I2 81%). Sensitivity analysis based on a random effects model yielded slightly wider confidence intervals (11.70%; 95% CI: 7.51% to 15.90%). There are several differences in the three study designs. Jonasson 1998 included steroid‐naive children with EIB (aged between 7‐16 years) who received low dose budesonide of 100 mcg bd (via turbohaler inhaler) or placebo inhaler for 12 weeks. Vathenen 1991 included adult patients (aged 18‐45 years) who were positive on bronchoprovocation test (histamine) and EVH test and most of the patients were atopic (37 out of 40) based on positive skin prick to at least one allergen. The treatment arm patients were given higher doses of budesonide (800 mcg bd) via metered dose inhaler (MDI) with spacer and the effects were studied after 6 weeks. Stelmach 2008 included children (aged 6 to 18 years) who were on inhaled budesonide (average of 400 mcg/day) and montelukast or long‐acting bronchodilator for at least 6 months prior to the study. In addition, they were all atopic, as defined by positive skin prick tests to house dust mites. The treatment arm received Inhaled budesonide (100 mcg bid) for 4 weeks. Therefore, the study designs differed in the population studied, type, duration and dose of corticosteroid used. Possibility of publication bias could not be assessed using funnel plot with such a low number of studies.
1.
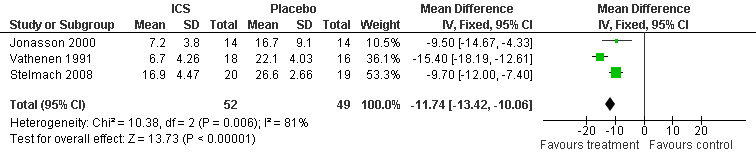
Forest plot of comparison: 1 % fall index, outcome: 1.1 % fall index in FEV1 ( parallel studies, fixed)(>/=4/52).
Hofstra 2000 was in children aged six to fourteen years who had used no inhaled steroids in the previous 4 months. As the data for lung function were not normally distributed natural log‐transformation was applied and geometric means were reported. Compared to placebo both 100 mcg and 250 mcg Fluticasone twice daily significantly attenuated the % fall in FEV1 with exercise when measured at three and six weeks (P< 0.05). The magnitude of this difference (around 17%) was in keeping with the results of Jonasson 2000 and Vathenen 1991.
Data from crossover studies were pooled using a Random effects MD calculated with generic inverse variance. The result from one crossover study with duration of inhaled corticosteroids of 4 weeks revealed significant attenuation of % fall in FEV1 for both doses of budesonide (100 mcg/day and 50 mcg/day), (MD 6.90%; 95% CI: 1.40% to 12.40% and WMD 7.00%; 95% CI: 0.15% to 13.85% respectively, Petersen 2004). Similarly the result from one study with duration of inhaled corticosteroids of 4 weeks significantly attenuated the % fall in PEF (MD 11.50%; 95% CI: 6.31% to 16.69%, Hartley 1977).
Combining results from 2 crossover studies with single dose of ICS use between 15 minutes to 4 hours prior to exercise (Yazigi 1978; Thio 2001), the use of ICS did not significantly reduce the % fall index in FEV1 (MD 5.04%; 95% CI: ‐6.48% to 16.55%); a moderate level of heterogeneity was present, (I 2 = 41.9%). The result from one study (Yazigi 1978) with inhaled corticosteroids given 15 minutes before exercise did not significantly attenuate the % fall in PEF ( WMD= ‐7.50; 95% CI: ‐27.65 to 12.65 ). Yazigi 1978 included asthmatic children (aged 8‐15 years) who had already been receiving treatment with triamcinolone acetonide aerosol (100 mcg od ‐300 mcg qid) for control of severe asthma. Medications were only withheld for 12 hours before a single dose of either triamcinolone (300 mcg) or placebo inhalers were administered and this was separated by a washout period of 24 hours for the crossover study. In contrast, Thio 2001 included patients that were steroid‐naive for 3 months prior to the study and had wash‐out periods of 7 ‐14 days, and the study patients were given fluticasone 1 mg 4 hours prior to exercise. Regular previous use of inhaled corticosteroids may have attenuated the results of treatment in Yazigi 1978. Due to small number of studies and sample sizes, firm conclusions about the effect of single dose treatments cannot be made.
Side effects
There were no significant differences in adverse event reported between the group with beclomethasone 100 mcg/day and placebo in Petersen's group (OR 1.40; 95% CI: 0.28 to 7.00). There were also no significant differences in adverse event reported between the group with beclomethasone 50 mcg/day and placebo (OR 0.31; 95% CI: 0.03 to 3.16). In another study (Hofstra 2000), the authors stated that no clinically significant abnormalities in heart rate and blood pressure were observed and most adverse events were related to respiratory infections, or contacts with allergen; however, no data were presented.
Symptom score
One study (Hofstra 2000) failed to present any raw data on symptom score except to mention that there appeared significantly more days and nights without wheeze when using active treatment (fluticasone) compared to placebo (P<0.05). No effect of fluticasone was found on symptom scores of cough, shortness of breath and exercise‐induced symptoms. In another study (Jonasson 1998), a significant reduction in symptom scores during daytime was found in the treatment group compared with placebo (MD = 0.43; 95% CI 0.2 to 0.7). Finally, one study (Hartley 1977) reported mean symptom score did not differ significantly between ICS and placebo (paired t‐test , P>0.05). Due to the lack of information regarding the symptom score measurement and method of measurement, pooled analyses were not possible.
Airway inflammation
One study (Vathenen 1991) reported the dose of histamine causing 20% fall in FEV1 (PD 20) in the group between those who received ICS and those who received placebo. There were no significant differences between the PD20 in both groups (P=0.28). Another study (Petersen 2004) reported the mean exhaled nitric oxide (eNO) between budesonide and placebo. They found significantly lower mean eNO levels in the ICS groups (both budesonide 50 mcg/day and budesonide 100 mcg/day) compared to placebo (WMD = 5.39; 95% CI: 5.00 to 5.78). There were no differences in mean eNO levels between the 2 doses of budesonide. Although serum levels of eosinophilic cationic protein (sECP) were reported in one study (Hofstra 2000) for active treatment and placebo, results were presented in geometric mean and raw data was unavailable. The authors mentioned in their paper that sECP levels did not change significantly during treatment.
Subgroup analysis
A priori sub‐group comparisons based on age, dose of study medication, severity of asthma, atopic status and delivery devices would be tested for heterogeneity, but due to the small number of trials, we could not perform any sub‐group analysis.
Discussion
Exercise‐induced bronchoconstriction (EIB) is a significant clinical problem for which effective and safe prophylactic treatment is necessary. This systematic review and meta‐analysis of seven randomised controlled trials in 172 patients seems to suggest there are protective effects of ICS against EIB when ICS were given for 4 weeks or more prior to the exercise testing. The small amount of data from 2 studies using single doses of ICS, given 15 minutes and 4 hours prior to exercise testing, do not allow any firm conclusions to be drawn.
The speed of onset of protection and the dose response are currently uncertain. Hofstra 2000 found benefit in children at three weeks and six weeks on both 100 mcg and 250 mcg twice daily. A recent study (Subbarao 2006) compared 4 doses of Ciclesonide to prevent EIB over a four week period, and an attenuation in EIB was found starting after one week of treatment in all but the lowest dose treatment arm (40 micrograms per day) when compared to baseline. The effect seemed to increase over 4 weeks in the higher dose arm (320 micrograms per day). There was no placebo arm in this study and there were no significant differences between the arms of the study, nor between the results at different time points, but the numbers were small. Nevertheless this study gives some useful pointers to the speed of onset of inhaled corticosteroids in the prevention of EIB, which may show benefit as early as one week, but may not reach a plateau until much later. Both the speed of onset and dose response need further research on larger numbers of adults and children.
The protective effects of inhaled corticosteroids ranged between 33% to 56% and clinical protection was demonstrated from 3 studies to be between 33% to 61%. The rate of adverse events between ICS and placebo was not significant from one study which reported this outcome. Due to the lack of information regarding symptom measurements, pooling the data for analysis was not possible. Data also could not be combined for analysis due to differences in measurement of airway inflammation.
Methodological limitations
The authors have identified several potential methodological limitations in this review.
Publication and selection biases. A comprehensive search of published literature for potentially relevant studies was conducted, and attempts were made to contact corresponding and first authors to identify unpublished data due to the possibility of missed unpublished negative trials. Abstracts from meetings were also reviewed for potential inclusion and none were found to fulfill the inclusion criteria. Though it is possible that selection bias occurred, we employed two independent reviewers for the selection process and we are confident that the studies excluded were done so for appropriate reasons and in a consistent manner.
The overall small number of studies and sample sizes introduces a note of caution. Ideally, more RCTs should be conducted to verify the results in the future.
Four studies in this review used a crossover design. The concern regarding inclusion of crossover studies in a meta‐analysis are three‐fold: carry‐over effects, period effects and statistical issues. Data were not presented in a manner that allowed us to confirm the presence or absence of carry‐over effects. Although EIB is a short, transient condition that usually returns to baseline in one hour, and ICS is short‐acting with rapid clearance from the body (Winkler 2004), one of the studies included patients with severe asthmatics on long‐term inhaled corticosteroids for which the inhaled corticosteroid was withheld for only 12 hours before the exercise testing (Yazigi 1978) . Therefore carry‐over effects could be present which may account for the lack of protection of ICS against EIB in this study.
Period effect comes into play since EIB is a variable condition and it is possible that baseline PFT values could vary prior to each exercise challenge. Individuals could randomly experience a change in baseline airflow values depending on many of the factors (humidity, temperature). Had there been a period effect in every study, there would be no reason to believe any systematic bias towards any one period. By averaging the estimates, the period effect would disappear leaving an unbiased estimate of the treatment contrast (Senn 1991).
Details regarding acceptable randomisation, allocation concealment and blinded outcomes assessment were not adequately reported in all the studies.
Authors' conclusions
Implications for practice.
Inhaled corticosteroids given for 4 weeks or more are effective in preventing deterioration in lung function post‐exercise in both adults and children. There is currently insufficient evidence to draw conclusions about shorter durations of treatment.
Implications for research.
Further research should focus on:
Comparing ICS in patients with EIB with different doses of inhaled corticosteroids over a range of durations, more severe EIB ( % fall FEV1 or PEFR > 30%), atopic and non‐atopic asthmatics, and different types of delivery devices.
The effects of ICS should be assessed in eucapnic voluntary hyperventilation (EVH) test ‐proven EIB. EVH is the current challenge test recommended by the International Olympic Committee as the optimal laboratory based challenge test for the identification of EIB.
Improving reporting of recruitment procedures, methodology and effect estimates accompanied by variance estimates for all outcomes.
Correlating physiological benefits with other outcomes such as symptoms scores, athletic or exercise performance and patient preference.
Comparing ICS to other drugs or in combination with other drugs.
What's new
| Date | Event | Description |
|---|---|---|
| 30 October 2008 | New search has been performed | Literature search re‐run; one new study added. Conclusions remain unchanged by the addition of new evidence. |
| 28 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 11 April 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
This protocol has been withdrawn as it is out of date and will be re‐allocated to a new review author team.
Acknowledgements
The authors would like to thank the support staff of the Cochrane Airways Group especially Mr Toby Lasserson and Ms Elizabeth Arnold who have provided invaluable assistance in terms of statistical support and assistance in the electronic search and retrieval of papers. Professor Brian Rowe was the assigned editor for this review.
Data and analyses
Comparison 1. % fall index.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 % fall index in FEV1 ( parallel studies, fixed)(>/=4/52) | 3 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐11.74 [‐13.42, ‐10.06] |
| 2 % fall index in FEV1 ( crossover studies) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 % fall index in FEV1 (crossover studies)(>/=4/52) (BDP100mcg ) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐14.99, 0.99] |
| 2.2 % fall index in FEV1 (crossover studies) (>/=4/52)(BDP 50mcg ) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐14.63, 0.43] |
| 2.3 % fall index in FEV1 ( crossover studies) ( single dose ) | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐5.59 [‐16.14, 4.96] |
| 3 % fall index in PEF ( crossover studies)( >/= 4/52) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐11.5 [‐20.26, ‐2.74] |
| 4 % fall index in FEV1 (crossover studies, GIV, fixed) | 3 | % (Random, 95% CI) | Subtotals only | |
| 4.1 % fall index FEV1 ( crossover studies, GIV, fixed)( >/=4/52)( BDP 50 mcg) | 1 | % (Random, 95% CI) | ‐7.00 [‐13.85, ‐0.15] | |
| 4.2 % fall index FEV1 (crossover studies, GIV, fixed) ( single dose) | 2 | % (Random, 95% CI) | ‐5.04 [‐16.55, 6.48] | |
| 4.3 % fall index FEV1 (crossover studies,GIV, fixed) (>/=4/52)( BDP 100mcg) | 1 | % (Random, 95% CI) | ‐6.9 [‐12.40, ‐1.40] | |
| 5 % fall index PEF (crossover studies, GIV, fixed)( >/= 4/52) | 1 | % (Fixed, 95% CI) | ‐11.5 [‐16.69, ‐6.31] | |
| 6 % fall index FEV1 ( parallel studies , random)(>/=4/52) | 3 | 101 | Mean Difference (IV, Random, 95% CI) | ‐11.70 [‐15.90, ‐7.51] |
| 7 % fall index FEV1 (crossover, GIV, random) | 3 | % (Random, 95% CI) | Subtotals only | |
| 7.1 % fall index FEV1 (crossover, GIV, random) ( BDP50 mcg)(>/=4/52) | 1 | % (Random, 95% CI) | ‐7.00 [‐13.85, ‐0.15] | |
| 7.2 % fall index FEV1 ( crossover, GIV, random) (BDP 100mcg)(>/=4/52) | 1 | % (Random, 95% CI) | ‐6.9 [‐12.40, ‐1.40] | |
| 7.3 % fall FEV1( crossover, GIV, random) (single dose) | 2 | % (Random, 95% CI) | ‐5.04 [‐16.55, 6.48] | |
| 8 % fall index PEF ( crossover, GIV, random)(>/=4/52) | 1 | % (Random, 95% CI) | ‐11.5 [‐16.69, ‐6.31] | |
| 9 % fall index PEF (crossover studies)(single dose) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 7.5 [‐12.65, 27.65] |
| 10 % fall index in FEV1 ( parallel studies, fixed)(>/=4/52) | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
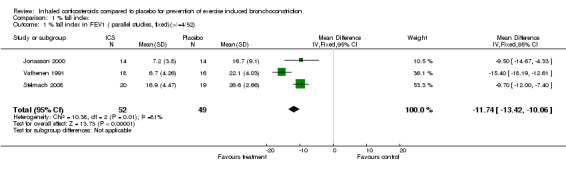
Comparison 1 % fall index, Outcome 1 % fall index in FEV1 ( parallel studies, fixed)(>/=4/52).
1.2. Analysis.
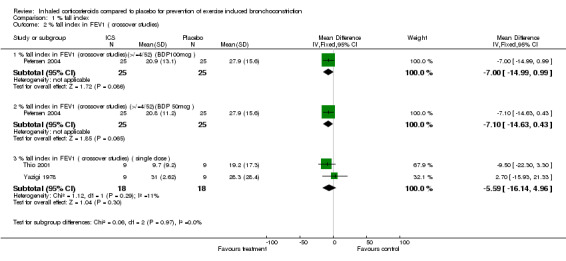
Comparison 1 % fall index, Outcome 2 % fall index in FEV1 ( crossover studies).
1.3. Analysis.
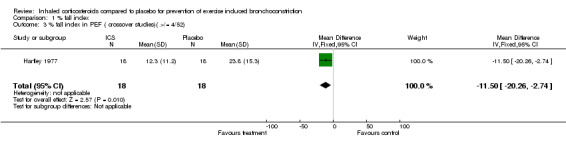
Comparison 1 % fall index, Outcome 3 % fall index in PEF ( crossover studies)( >/= 4/52).
1.4. Analysis.
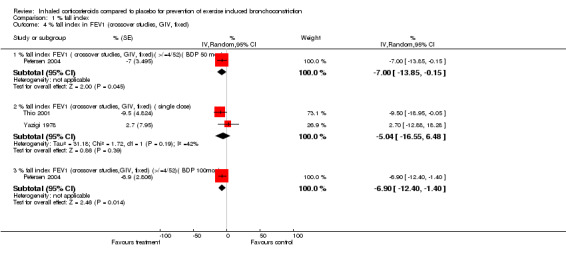
Comparison 1 % fall index, Outcome 4 % fall index in FEV1 (crossover studies, GIV, fixed).
1.5. Analysis.
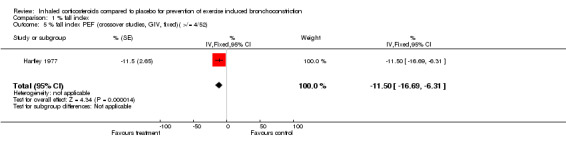
Comparison 1 % fall index, Outcome 5 % fall index PEF (crossover studies, GIV, fixed)( >/= 4/52).
1.6. Analysis.
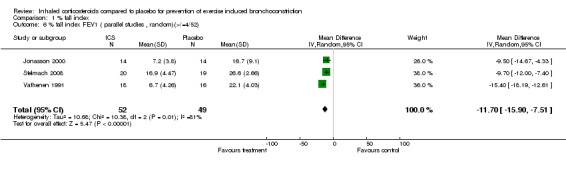
Comparison 1 % fall index, Outcome 6 % fall index FEV1 ( parallel studies , random)(>/=4/52).
1.7. Analysis.
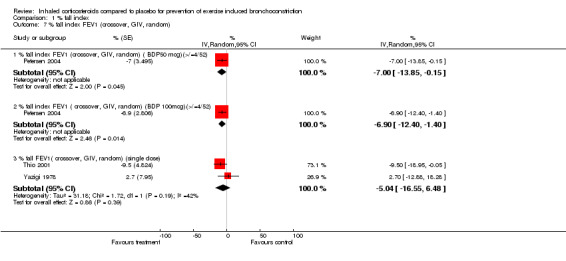
Comparison 1 % fall index, Outcome 7 % fall index FEV1 (crossover, GIV, random).
1.8. Analysis.
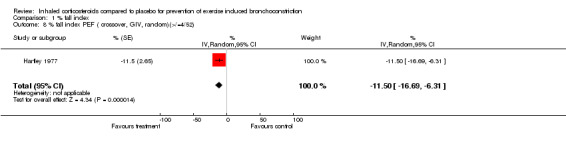
Comparison 1 % fall index, Outcome 8 % fall index PEF ( crossover, GIV, random)(>/=4/52).
1.9. Analysis.
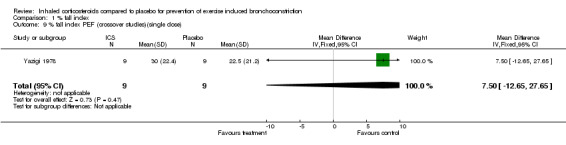
Comparison 1 % fall index, Outcome 9 % fall index PEF (crossover studies)(single dose).
Comparison 2. Adverse outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.28, 7.00] |
| 2 Adverse events | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 3.16] |
2.1. Analysis.
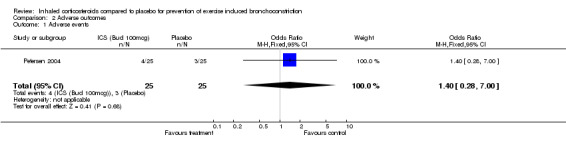
Comparison 2 Adverse outcomes, Outcome 1 Adverse events.
2.2. Analysis.
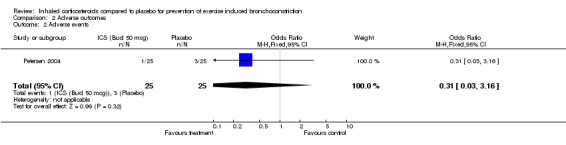
Comparison 2 Adverse outcomes, Outcome 2 Adverse events.
Comparison 3. Measures of airway inflammation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dose of histamine causing 20% fall in FEV1 (PD20) | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.07, 0.23] |
| 2 Exhaled Nitric Oxide ( crossover) | 1 | WMD (Fixed, 95% CI) | ‐5.39 [‐5.78, ‐5.00] | |
| 2.1 Exhaled Nitric Oxide ( crossover) BDP 50mcg vs placebo | 1 | WMD (Fixed, 95% CI) | ‐5.0 [‐7.90, ‐2.10] | |
| 2.2 Exhaled Nitric Oxide ( crossover) BDP 100mcg vs placebo | 1 | WMD (Fixed, 95% CI) | ‐5.4 [‐5.79, ‐5.01] |
3.1. Analysis.
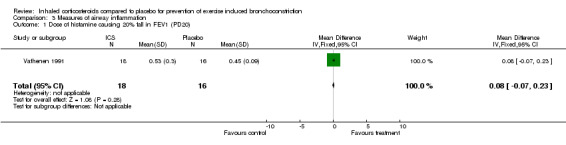
Comparison 3 Measures of airway inflammation, Outcome 1 Dose of histamine causing 20% fall in FEV1 (PD20).
3.2. Analysis.
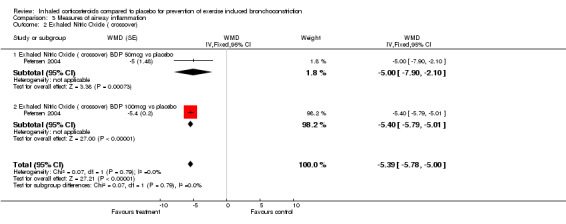
Comparison 3 Measures of airway inflammation, Outcome 2 Exhaled Nitric Oxide ( crossover).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hartley 1977.
| Methods | RCT, double‐blind, crossover study No withdrawal or drop‐out Concomitant treatment: bronchodilators allowed( except < 12 hours of exercise test) Exclusion: no prior corticosteroids or cromoglycate Exercise test: cycle ergometer, 9 min to oxygen uptake of 65‐90mmol/min | |
| Participants | N=18. 12m, 6f. Age 17‐40 years, mean 29 years. 14 had positive skin prick test to common allergens. All had EIB as defined by fall in peak expiratory flow rate (PEFR) of at least 15% after exercise. | |
| Interventions | In random order: betamethasone valerate inhaler 200 mcg QDS for 4 weeks or placebo inhaler in identical container | |
| Outcomes | PEF immediately, and at 5 min interval for 20 min. The lowest value was taken as indicating PEFR after exercise(a) and this figure compared with PEFR before exercise(b)> Percentage fall index was then calculated. Ratio of a/b and its logarithmic form were also analysed. The difference in PEFR fall after bethamethasone and placebo were compared by a paired sample t‐test on log of a/b. Adverse events were not mentioned. | |
| Notes | Jada score =3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Hofstra 2000.
| Methods | RCT, double‐blind, parallel study 1 drop‐out due to non‐compliance Concomitant treatment: intranasal and topical steroids, eye cromones, inhaled cromoglycate ( stopped at screening visit) exercise test: treadmill to achieve heart rate of >90% predicted. | |
| Participants | N= 37. 23m,14f. mean age 10.3 years. 32 patients were atopic to at least one inhalant allergen on RAST test. All had fall in FEV1 of at least 20% post exercise. | |
| Interventions | In random order: fluticasone propionate 100 mcg bid or fluticasone propionate 250mcg bid or placebo using MDI attached to volumetric spacer for 6 weeks. | |
| Outcomes | FEV1, % fall after exercise, PD20 methacholine, serum eosinophil cationic protein and symptom scores were measured. Adverse events were monitored. | |
| Notes | Jadad score =3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Jonasson 2000.
| Methods | RCT, parallel Concomitant treatment: terbutaline turbohaler as needed Exercise test: treadmill to achieve heart rate of 170‐180/min. | |
| Participants | N= 28.22m,6 f. Mean age 9.7 years. All had EIB as defined by reduction in FEV1 of at least 10 % after exercise. | |
| Interventions | In random order: budesonide turbohaler 100 mcg bid or placebo for 12 weeks. | |
| Outcomes | Mean maximum fall in FEV1 after exercise , symptom score and use of rescue medications were measured. | |
| Notes | Jadad score=2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Petersen 2004.
| Methods | RCT, crossover, double blind study 2 ‐week run‐in period, followed by three 4‐week study periods , each separated by a 1‐week washout. The following treatments were given during the double‐blind periods: budesonide 50 mcg, budesonide 100 mcg and placebo. 2 drop‐outs due to asthma exacerbation and toe fracture Concomitant treatment: short acting beta agonist as needed Exercise test: treadmill to achieve heart rate of > 180/min | |
| Participants | N=27. 20m, 7 f. Age 6‐14 years, mean age 10.6. All had FEV1 > 70% predicted and post exercise reduction in FEV1 of at least 15%. | |
| Interventions | In random order: Budesonide autohaler 50 mcg or 100 mcg or placebo for 4 weeks. | |
| Outcomes | Maximal percentage fall in FEV1 after exercise was measured. The percentage fall from pre‐exercise at each time point was also calculated. The mean percentage fall in FEV1 was plotted against time for each treatment. The area under the curve (AUC) for the percentage fall in FEV1 from exercise over the 20 min period was calculated using trapezoidal rule. In addition, exhaled nitric oxide measurements were made from the start of the run‐in and at the end of study period. Adverse events were recorded. | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Stelmach 2008.
| Methods | RCT, double‐blind, parallel study. Regular medications stopped for 4 weeks. Concomitant treatment: bronchodilators allowed ( except < 6 hours of exercise test). Exercise test: treadmill to achieve heart rate of 95% maximum predicted for age. |
|
| Participants | N= 39. Age 6‐18 years. Stable atopic asthmatics who were on inhaled budesonide ( average 400 mcg/day) and montelukast or long‐acting bronchodilator. Inlcusion criteria: Clinical diagnosis of asthma of at least 6 months, resting FEV1 or at least 70% or more and reduction in FEV1 of 20% or more after exercise. Exclusion criteria: recent upper respiratory tract infection (including sinusitis), previous intubation, or asthma hospitalization during 3 months before pre‐study visit. Patients with significant co‐morbidities and patients using beta‐blocker, oral corticosteroids or immunotherapy were also excluded. |
|
| Interventions | In random order: Budesonide turbuhaler 100mcg bid or placebo inhalers for 4 weeks. Other treatment arms studied were: budesonide + formoterol ( 4.5 mcg bid) inhaler; Budesonide + montelukast ( 5 mg/day or 10mg /day depending on age ), montelukast ( 5mg/day or 10 mg/day depending on age ) |
|
| Outcomes | Maximal percentage fall in FEV1 after exercise was measured. The area under the curve (AUC) for the percentage fall in FEV1 from exercise over the 20 min period was calculated. | |
| Notes | Jadad score 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | information not available |
Thio 2001.
| Methods | RCT, Double‐blind, crossover Concomitant treatment: inhaled short‐acting beta agonist as required ( stop12 hours before test) Wash‐out period of 7‐14 days. Exercise test: treadmill, 6 min, to achieve 90% maximum heart rate | |
| Participants | N= 9. 6m,3f. Age: 9‐16 year ( mean 11.2) Inclusion criteria: fall in FEV1 > 15% post‐exercise, age between 8‐16, ability to do lung function test, FEV1 >70%, clinically stable for 3 weeks, no ICS, nasal or systemic steroids for 3 months, no cromoglycates in 2 weeks. Exclusion criteria: theophylline, anticholinergics, and long‐acting beta agonist. | |
| Interventions | In random order: Fluticasone propionate inhaler 1 mg ( 250 mcg x 4) or placebo inhaler ( with MDI and spacer) . Exercise challenge test was performed 4 hours after the administration of inhalers. | |
| Outcomes | FEV1 in the first 4 hours after administration of inhalers, maximum % fall in FEV1 from baseline FEV1 after exercise and AUC of time‐response curve were measured. Protection index was calculated. | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Vathenen 1991.
| Methods | RCT, double‐blind, parallel study Drop‐out: 1 due to change in employment, 5 due to exacerbations of asthma Concomitant treatment: short‐acting beta agonist( stopped 8 hours before test) Exercise: treadmill; 6 min; to achieve 90% maximum heart rate | |
| Participants | N=40,28m,12f. Age: 18‐45 (median 28). Inclusion criteria: Drop in FEV1 post exercise >20%, asthma for 2 years or more, FEV1% predicted > 50%, PD20 of 4micromol or less ,PV20 of 640L or less, not current smoker and had never smoked more than 10 cigarettes a day for 2 years, be having no treatment other than an inhaled beta2 agonist, stable asthma, no chest infection for at least 6 weeks before entry into the study. skin prick tests were performed in and 37 of the 40 subjects had a positive response( weal of more than 2 mm) to at least one allergen. | |
| Interventions | In random order: Budesonide inhaler 800 mcg bid or placebo inhaler ( with MDI and spacer) for 6 weeks | |
| Outcomes | FEV1 response to histamine, exercise and Eucapnic Voluntary Hyperventilation | |
| Notes | Jadad score 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Yazigi 1978.
| Methods | RCT, double‐blind, crossover 2 dropouts due to negative result on exercise testing with placebo. Concomitant treatment: triamcinolone acetonide which was withheld for 12 hours before the exercise test. Exercise test: treadmill ( 6 minutes, 3 mph, 15% grade) | |
| Participants | N=11. 6 m, 5 f. Age: 8‐15 years. All had EIB as defined by fall in FEV1 of at least 15% after exercise in the last one week. All participants were already receiving triamcinolone acetonide aerosol ( 100mcg od ‐ 300 mcg qid) for control of severe asthma. | |
| Interventions | In random order: A single dose of triamcinolone acetonide inhaler 300 mcg or placebo inhaler 15 minutes before exercise test. 24 hour washout period in‐between. | |
| Outcomes | PEF, FEV1, FEF25‐75% vital capacity were measures immediately before exercise, and at 5 min interval for 15 min. | |
| Notes | Jadad score =3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Information not available |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beck‐Ripp 2002 | No placebo and does not fulfill definition of EIB |
| Duong 2008 | No placebo |
| Freezer 1995 | Does not fulfill criteria of EIB |
| Henriksen 1983 | Compares ICS and placebo vs terbutaline and ICS |
| Henriksen 1985 | No placebo data |
| Hodges 2005 | Does not fulfill criteria of EIB |
| Jonasson 1998 | Does not fulfill definition of EIB |
| Jonasson 2000a | Does not fulfill definition of EIB |
| Konig 1974 | Does not fulfill definition of EIB |
| Molema 1989 | No placebo |
| Molema 1989a | Not RCT |
| Morice 2008 | No placebo |
| Nankani 1990 | Not EIB and not RCT |
| Nathan 2003 | Not EIB |
| Patakas 1978 | Not RCT |
| Pedersen 1995 | No placebo |
| Per Venge 1991 | No placebo |
| Pichaipat 1995 | Not RCT |
| Subbarao 2006 | No placebo |
| Vidal 2001 | No placebo |
| Waalkens 1993 | Does not fulfill definition for EIB |
| Wilson 2005 | Not EIB |
Contributions of authors
Koh MS: reviewed abstracts for inclusion; data extraction; discussion. Tee A: reviewed abstracts for inclusion; data extraction; assessed methodological quality of trials. Irving L: assessed methodological quality of trials. Lasserson T: provided advice on data extraction, entry and analysis for GIV outcomes.
Sources of support
Internal sources
No sources of support supplied
External sources
Cochrane Airways Group, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Hartley 1977 {published data only}
- Hartley JPR, Charles TJ, Seaton A. Bethamethasone valerate inhalation and exercise ‐induced asthma in adults. British Journal of Diseases of the Chest 1977;71(4):253‐8. [PubMed] [Google Scholar]
Hofstra 2000 {published data only}
- Hofstra WB, Neijens HJ, Duiverman EJ, Kouwenberg JM, Mulder PGH, Kuethe MC, et al. Dose‐responses over time to inhaled fluticasone propionate treatment of exercise‐ and methacholine ‐induced bronchoconstriction in children with asthma. Pediatric Pulmonology 2000;29(6):415‐23. [DOI] [PubMed] [Google Scholar]
Jonasson 2000 {published data only}
- Jonasson G, Carlsen KH, Hultquist C. Low‐dose budesonide improves exercise‐induced bronchospasm in schoolchildren. Pediatric Allergy & Immunology 2000;11(2):120‐5. [DOI] [PubMed] [Google Scholar]
Petersen 2004 {published data only}
- Petersen R, Agertoft L, Pedersen S. Treatment of exercise‐induced asthma with beclomethasone dipropionate in children with asthma. European Respiratory Journal 2004;24(6):932‐7. [DOI] [PubMed] [Google Scholar]
Stelmach 2008 {published data only}
- Stelmach I, Grzelewski T, Majak P, Jerzynska J, Stelmach W, Kuna P. Effect of different antiasthmatic treatments on exercise‐induced bronchoconstriction in children with asthma. Journal of Allergy and Clinical Immunology 2008;121:383‐9. [DOI] [PubMed] [Google Scholar]
Thio 2001 {published data only}
- Thio BJ, Slingerland GLM, Nagelkerke AF, Roord JJ, Mulder PGH, Dankert‐Roelse JE. Effects of single‐dose fluticasone on exercise‐induced asthma in asthmatic children: A pilot study. Pediatric Pulmonology 2001;32(2):115‐21. [DOI] [PubMed] [Google Scholar]
Vathenen 1991 {published data only}
- Vathenen AS, Knox AJ, Wisniewski A, Tattersfield AE. Effect of inhaled budesonide on bronchial reactivity to histamine, exercise , and eucapnic dry air hyperventilation in patients with asthma. Thorax 1991;46(11):811‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yazigi 1978 {published data only}
- Yazigi R, Sly RM, Frazer M. Effect of traimcinolone acetonide aerosol upon exercise‐induced asthma. Annals of Allergy 1978;40(5):322‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Beck‐Ripp 2002 {published data only}
- Beck‐Ripp J, Griese M, Arenz S, Koring C, Pasqualoni B, Bufler P. Changes of exhaled nitric oxide during steroid treatment of childhood asthma. European Respiratory Journal 2002;19(6):1015‐9. [DOI] [PubMed] [Google Scholar]
Duong 2008 {published data only}
- Duong M, Subbarao P, Adelroth E, Obminski G, Strinich T, Inman M, Pedersen S, O'Byrne PM. Sputum eosinophils and the response of exercise‐induced bronchoconstriction to corticosteroid in asthma . Chest 2008 ; 133 :404‐1. [DOI] [PubMed] [Google Scholar]
Freezer 1995 {published data only}
- Freezer NJ, Croasdell H, Doull IJM, Holgate ST. Effect of regular inhaled beclomethasone on exercise and methacholine airway responses in schoolchildren with recurrent wheeze. European Respiratory Journal 1995;8(9):1488‐93. [PubMed] [Google Scholar]
Henriksen 1983 {published data only}
- Henriksen JM, Dahl R. Effects of inhaled budesonide alone and comination with low‐dose terbutaline in children with exercise‐induced asthma. American Review of Respiratory Disease 1983;128(6):993‐7. [DOI] [PubMed] [Google Scholar]
Henriksen 1985 {published data only}
- Henriksen JM. Effect of inhalation of corticosteroids on exercise induced asthma: randomised double blind crossover study of budesonide in asthmatic children. BMJ 1985;291(6490):248‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodges 2005 {published data only}
- Hodges ANH, Lynn BM, Koehle MS, McKenzie DC. Effects of inhaled bronchodilators and corticosteroids on exercise induced arterial hypoxaemia in trained male athletes. British Journal of Sports Medicine 2005;39(12):917‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jonasson 1998 {published data only}
- Jonasson G, Carlsen KH, Blomqvist P. Clinical efficacy of low‐dose inhaled budesonide once or twice daily in children with mild asthma not previously treated with steroids. European Respiratory Journal 1998;12(5):1099‐1104. [DOI] [PubMed] [Google Scholar]
Jonasson 2000a {published data only}
- Jonasson G, Carlsen KH, Jonasson C, Mowinckel P. Low‐dose inhaled budesonide once or twice daily for 27 months in children with mild asthma. Allergy 2000;55(8):740‐8. [DOI] [PubMed] [Google Scholar]
Konig 1974 {published data only}
- Konig P, Jaffe P, Godfrey S. Effect of corticosteroids on exercise‐induced asthma. Journal of Allergy & Clinical Immunology 1974;54(1):14‐9. [DOI] [PubMed] [Google Scholar]
Molema 1989 {published data only}
- Molema J, Herwaarden CLA, Folgering HTM. Effects of inhaled budesonide on the relationships between symptoms, lung function indices and airway hyperresponsiveness in patients with allergic asthma. Pulmonary Pharmacology 1989;1(4):179‐85. [DOI] [PubMed] [Google Scholar]
Molema 1989a {published data only}
- Molema J, Herwaarden CLA, Folgering HTM. Effects of long‐term teatment with inhaled cromoglycate and budesonide on bronchial hyperresponsiveness in patients with allergic asthma. European Respiratory Journal 1989;2(4):308‐16. [PubMed] [Google Scholar]
Morice 2008 {published data only}
- Morice AH, Peterson S, Beckman O, Ku kova Z. Efficacy and safety of a new pressurised metered‐dose inhaler formulation of budesonide/formoterol in children with asthma: A superiority and therape u tic equivalence study . Pulmonary Pharmacology and Therapeutics 2008 ; 21 :152‐9. [DOI] [PubMed] [Google Scholar]
Nankani 1990 {published data only}
- Nankani JN, Northfield M, Beran YM, Richardson PD. Changes in asthmatic patients' symptoms and lifestyles on institution of inhaled budesonide therapy. Current Medical Research and Opinion 1990;12(3):198‐206. [DOI] [PubMed] [Google Scholar]
Nathan 2003 {published data only}
- Nathan RA, Dorinsky P, Rosenzweig JRC, Shah T, Edin H, Prillaman B. Improved ability to perform strenuous activities after treatment with fluticasone Priopionate/Salmeterol combination in patients with persistent asthma. Journal of Asthma 2003;40(7):815‐22. [DOI] [PubMed] [Google Scholar]
Patakas 1978 {published data only}
- Patakas D, Louridas G, Sehletidis L. Effect of beclomethasone dipropionate inhalation on exercise induced bronchospasm. IRCS Medical Science. Cardiovascular System 1978;6(11):448. [Google Scholar]
Pedersen 1995 {published data only}
- Pedersen S, Hansen OR. Budesonide treatment of moderate and severe asthma in children: A dose‐response study. Journal of Allergy & Clinical Immunology 1995;95(1 Pt 1):29‐33. [DOI] [PubMed] [Google Scholar]
Per Venge 1991 {published data only}
- Venge P, Henriksen J, Dahl R. Eosinophils in exercise‐induced asthma. Journal of Allergy & Clinical Immunology 1991;88(5):699‐704. [DOI] [PubMed] [Google Scholar]
Pichaipat 1995 {published data only}
- Pichaipat V, Tongpenyai Y, Nerntong T, Sriprapachiranont C. The protective effect of inhaled terbutaline, sodium cromogylcate and budesonide on exercise‐induced asthma in children. Journal of the Medical Association of Thailand 1995;78(10):505‐8. [PubMed] [Google Scholar]
Subbarao 2006 {published data only}
- Subbarao P, Duong M, Adelroth E, Otis J, Obminski G, Inman M, et al. Effect of ciclesonide dose and duration of therapy on exercise‐induced bronchoconstriction in patients with asthma. Journal of Allergy & Clinical Immunology 2006;117(5):1008‐13. [DOI] [PubMed] [Google Scholar]
Vidal 2001 {published data only}
- Vidal C, Fernandez‐Ovide E, PineiroJ, Nunez R, Gonzalez‐Quintela A. Comparison of montelukast versus budesonide in the treatment of exercise‐induced bronchoconstriction. Annals of Allergy, Asthma, & Immunology 2001;86(6):655‐8. [DOI] [PubMed] [Google Scholar]
Waalkens 1993 {published data only}
- Waalkens HJ, Essen‐Zandvliet EEM, Gerritsen J, Duiverman EJ, Kerrebjin KF, Knol K. The effect of an inhaled corticosteroid ( budesonide) on exercise‐induced asthma in children. European Respiratory Journal 1993;6(5):652‐6. [PubMed] [Google Scholar]
Wilson 2005 {published data only}
- Wilson AM, Duong M, Pratt B, Dolovich M, O'Byrne PM. Anti‐inflammatory effects of once daily low dose inhaled ciclesonide in mild to moderate asthmatic patients. Allergy 2006;61(5):537‐42. [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 1979
- Anderson S, Seale JP, Ferris L, Schoeffel R, Lindsay DA. An evaluation of pharmacotherapy for exercise‐induced asthma. Journal of Allergy and Clinical Immunology 1979;64(6 pt 2):612‐24. [DOI] [PubMed] [Google Scholar]
Anderson 1985
- Anderson SD. Issues in exercise‐induced asthma. Journal of Allergy and Clinical Immunology 1985;76(6):763‐72. [DOI] [PubMed] [Google Scholar]
ERS 1997
- European Respiratory Society Task Force. Clinical exercise testing with reference to lung diseases: indications, standardization and interpretation strategies. ERS Task Force on Standardization of Clinical Exercise Testing. European Respiratory Journal 1997;10(11):2662‐8. [DOI] [PubMed] [Google Scholar]
Gotshall 2002
- Gotshall RW. Exercise‐induced bronchoconstriction. Drugs 2002;62(12):1725‐39. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Kelly 2000
- Kelly K, Spooner CH, Rowe BH. Nedocromil sodium versus sodium cromoglycate for preventing exercise‐induced bronchoconstriction in asthmatics. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD002731] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koh 2002
- Koh YI, Choi S. Blood eosinophil counts for the prediction of the severity of exercise‐induced bronchospasm in asthma. Respiratory Medicine 2002;96(2):120‐5. [DOI] [PubMed] [Google Scholar]
Otani 2004
- Otani K, Kanazawa H, Fujiwara H, Hirata K, Fujimoto S, Yoshikawa J. Determinants of the severity of exercise‐induced bronchoconstriction in patients with asthma. Journal of Asthma 2004;41(3):271‐8. [DOI] [PubMed] [Google Scholar]
Rundell 2002
- Rundell KW, Jenkinson DM. Exercise‐induced bronchospasm in the elite athlete. Sports Medicine 2002;32(9):583‐600. [DOI] [PubMed] [Google Scholar]
Senn 1991
- Senn SJ, Hildebrand H. Crossover trials, degress of freedom, the carryover problem and its dual. Statistics in Medicine 1991;10(9):1361‐74. [DOI] [PubMed] [Google Scholar]
Spooner 2002
- Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise‐induced bronchoconstriction. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001183] [DOI] [PubMed] [Google Scholar]
Spooner 2003
- Spooner CH, Spooner GR, Rowe BH. Mast‐cell stabilising agents to prevent exercise‐induced bronchoconstriction. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD002307] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winkler 2004
- Winkler J, Hocchaus G, Derendorf H. How the lung handles drugs. Pharmacokinetics and pharmacodynamics of inhaled corticosteroids. Proceedings of the American Thoracic Society 2004;1(4):356‐363. [DOI] [PubMed] [Google Scholar]


