Key Words: 4-hydroxy-trans-2-nonenal, Alda-1, ALDH2, Basso Mouse Scale score, functional recovery, mitochondrial function, neuroinflammation; neuroprotection, pain, spinal cord injury
Abstract
Spinal cord injury (SCI) is associated with high production and excessive accumulation of pathological 4-hydroxy-trans-2-nonenal (4-HNE), a reactive aldehyde, formed by SCI-induced metabolic dysregulation of membrane lipids. Reactive aldehyde load causes redox alteration, neuroinflammation, neurodegeneration, pain-like behaviors, and locomotion deficits. Pharmacological scavenging of reactive aldehydes results in limited improved motor and sensory functions. In this study, we targeted the activity of mitochondrial enzyme aldehyde dehydrogenase 2 (ALDH2) to detoxify 4-HNE for accelerated functional recovery and improved pain-like behavior in a male mouse model of contusion SCI. N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide (Alda-1), a selective activator of ALDH2, was used as a therapeutic tool to suppress the 4-HNE load. SCI was induced by an impactor at the T9–10 vertebral level. Injured animals were initially treated with Alda-1 at 2 hours after injury, followed by once-daily treatment with Alda-1 for 30 consecutive days. Locomotor function was evaluated by the Basso Mouse Scale, and pain-like behaviors were assessed by mechanical allodynia and thermal algesia. ALDH2 activity was measured by enzymatic assay. 4-HNE protein adducts and enzyme/protein expression levels were determined by western blot analysis and histology/immunohistochemistry. SCI resulted in a sustained and prolonged overload of 4-HNE, which parallels with the decreased activity of ALDH2 and low functional recovery. Alda-1 treatment of SCI decreased 4-HNE load and enhanced the activity of ALDH2 in both the acute and the chronic phases of SCI. Furthermore, the treatment with Alda-1 reduced neuroinflammation, oxidative stress, and neuronal loss and increased adenosine 5′-triphosphate levels stimulated the neurorepair process and improved locomotor and sensory functions. Conclusively, the results provide evidence that enhancing the ALDH2 activity by Alda-1 treatment of SCI mice suppresses the 4-HNE load that attenuates neuroinflammation and neurodegeneration, promotes the neurorepair process, and improves functional outcomes. Consequently, we suggest that Alda-1 may have therapeutic potential for the treatment of human SCI. Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of MUSC (IACUC-2019-00864) on December 21, 2019.
Chinese Library Classification No. R453; R364; R741
Introduction
Spinal cord injury (SCI) causes profound secondary injury-mediated neurodegeneration that compromises locomotor, sensory and autonomic functions (Varma et al., 2013; Siddiqui et al., 2015). In spite of high incidence, particularly among young adults, no FDA-approved drug therapy is available for SCI due to limited understanding of druggable targets and cellular injury mechanisms (Ahuja et al., 2017). Although several experimental drugs are in clinical studies, it is critical to identify new and effective drugs for SCI treatment (Wang et al., 2019). Recent advances in medicine indicate that drugs targeting mitochondria-dependent dysfunction are effective in neurodegenerative diseases. Although mitochondrial dysfunction is one of the significant SCI mechanisms (Scholpa and Schnellmann, 2017), the mitochondrial druggable targets’ identity is less clear. Recently mitochondrial enzyme aldehyde dehydrogenase-2 (ALDH2) activation is reported to down regulate inflammation, restore mitochondrial function and improve pain threshold in many animal models (Chen et al., 2014, 2016a; Zhang et al., 2020; McAllister et al., 2021). ALDH2 is the primary enzyme that detoxifies reactive aldehydes, including 4-hydroxy-trans-2-nonenal (4-HNE) (Chen et al., 2014). If not detoxified, 4-HNE forms pathological protein adducts in excess (Gegotek and Skrzydlewska, 2019).
ALDH2 is a NAD(p)+-dependent mitochondrial matrix enzyme. Major substrates (high affinity) of ALDH2 are short-chain aliphatic lipid aldehydes (e.g., 4-HNE, acetaldehyde, malondialdehyde and acrolein). ALDH2 dysfunction has been associated with neurodegeneration, apoptotic cell death, pain and the process of aging (Chen et al., 2014). ALDH2 KO mice had a significantly high 4-HNE load, increased cytokine levels, and persistent behavioral deficits after closed head traumatic brain injury, supporting the anti-inflammatory and antioxidant role of ALDH2 activity in neurotrauma (Knopp et al., 2020). ALDH2 is an established druggable target, and it can be selectively and robustly activated by Alda-1 and inhibited by daidzin (Chen et al., 2014).
4-HNE is a stable and highly reactive lipid aldehyde, and thus it plays a pathological role in neurotraumatic and neurodegenerative disease conditions (Chen et al., 2016a). They can quickly diffuse across the membrane from the site of their origin and accumulate in their adduct form. Therefore, this study is focused on the role of 4-HNE load and its clearance by Alda-1-induced enhanced activity of ALDH2 for the treatment of SCI.
Alda-1 is a potent activator of ALDH2 (Perez-Miller et al., 2010). Pharmacologically, Alda-1 decreases the Km value (Michaelis constant) and increases the V-max (maximum velocity) by binding to ALDH2 near the catalytic site (Perez-Miller et al., 2010; Chen et al., 2014). Alda-1’s half-life is reported 1.67 ± 0.54 hours in rat plasma when 10 mg/kg dose of Alda-1 was administered intravenously (Taneja et al., 2015). The dose of 10 mg/kg body weight has been reported to be effective with significant efficacy in animal studies (Lu et al., 2017; He et al., 2018; Liu et al., 2020).
Based on Alda-1’s efficacy in neurological disorders and nociception, and high safety profile in animal models of CNS diseases, we investigated, for the first time, the therapeutic efficacy of ALDH2 activation using Alda-1 in a mouse model of contusion SCI.
Materials and Methods
Study design
This study’s principal objective was to evaluate the therapeutic potential of Alda-1, an ALDH2 activator, to confer neurovascular protection and aid in functional recovery in a mouse model of contusion SCI. We determined that SCI-induced inhibition of ALDH2 and the sustained overload of reactive aldehydes are reversed by Alda-1, leading to improved pain-like behavior and the accelerated functional recovery. Mice were randomly divided into the sham, control (SCI) and Alda-1 treatment (Alda-1) groups and the number of mice per group was based on our previous studies (Khan et al., 2018) and Basso Mouse Scale (BMS) score-based power analysis. Only male mice were used in this study because traumatic SCI occurs more commonly in males (~80%) than females (~20%) (Chen et al., 2016b). Furthermore, young adult females recover better and faster than young adult males (Swartz et al., 2007). The experimental protocol is described in Figure 1. Experimental groups were blinded, and the biochemical analysis was performed by personnel blinded to groups and samples’ identity.
Figure 1.
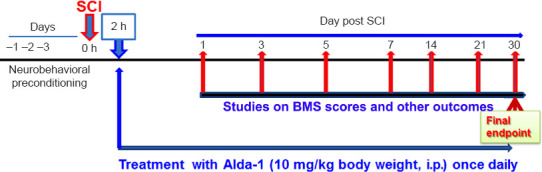
Experimental protocol.
Spinal cord injury (SCI) was induced at T9-10 vertebral levels. Alda-1 (a selective activator of aldehyde dehydrogenase 2) was administered to animals initially at 2 hours post-SCI and the same dose was repeated once daily until the endpoint (30 days). Studies on Basso Mouse Scale (BMS) scores were performed 3 days before SCI, followed by day 3 after SCI and days indicated. Age, sex and environment-matched control (sham) mice were be used in all studies.
Reagents
Alda-1 [N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide] (Item# 21555) was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Ketamine hydrochloride (ketamine) was obtained from Packager Mylan Institutional LLC, Rockford, IL, USA and xylazine was from Patterson Veterinary, Geeley, CO, USA. All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise.
Animals
Young adult male C57BL/6J mice, aged 10–12 weeks, were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Animals (4/cage) were kept in a helicobacter negative room at ~18–22°C (12-hour dark/light cycle). All animals received humane care in compliance with the Medical University of South Carolina’s (MUSC) guidance and the National Research Council’s humane care criteria. The Institutional Animal Care and Use Committee (IACUC) of MUSC approved animal procedures (IACUC-2019-00864 originally approved on December 21, 2019). Animal number in each group is described in all relevant figure legends.
Mouse model of contusion SCI
A combination of ketamine (90 mg/kg, intraperitoneally [i.p.]) from packager and xylazine (10 mg/kg, i.p.) was used to anesthetize experimental mice. Buprenorphine (0.05–0.1 mg/kg, subcutaneously, once 12 hour) was used as an analgesic, and it was administered pre-emptively and after the surgery to alleviate the pain. The toe was pinched to elicit the withdrawal response. When there is no longer response of pinching, the animal was then placed in a stereotaxic frame (Stoelting Co., Wood Dale, IL, USA). SCI at the T9–10 level was produced on the exposed spinal cord following laminectomy between T9–10. SCI was induced by a computer-controlled impactor device described by Dr. Bilgen (Bilgen, 2005) and used in our laboratory (Chou et al., 2011; Khan et al., 2018). SCI was performed with 1–2 mm tissue deformation and an impact velocity of 1.5 m/s and contusion time of 85 ms. Sham animals had similar procedures, with the exception of the impact. After the completion of the surgical procedures and removal from anesthesia, post-surgical reflexes were monitored. During impact, body temperature was maintained at 37°C. The muscle and skin were closed using nylon suture immediately after injury, and 2% lidocaine jelly was applied to the lesion site. The bladders of all animals were expressed as needed. The body weight and humane endpoints were regularly monitored. The animals were euthanized by decapitation under deep anesthesia to harvest the spinal cord for biochemical and immunohistochemical studies. The spinal cords were stored at –70°C for assays later on if needed.
Alda-1 treatment
Alda-1 was slowly administered at 2 hours after spinal cord contusion. Sham and SCI animals were administered vehicle (20% dimethyl sulfoxide (DMSO)/saline, 100 µL, i.p.). This is the first study on the efficacy of Alda-1 in SCI; therefore, we opted for an early therapeutic time window to establish the efficacy of Alda-1. Delayed treatment time window studies will be determined in our next level studies. In our studies, 10 mg/kg body weight dose of Alda-1 had no effects on physiological parameters (heart rate and body/rectal temperature) when measured at 1 hour after the administration of Alda-1’s preparation (10.0 mg/kg body weight in 20% DMSO/saline, 100 µL, i.p.) in mice. Animals in the sham and SCI groups were administered vehicle (20% DMSO/saline, 100 µL, i.p.)
BMS score
The BMS was developed to assess open field locomotion deficits in spinal cord injured mice (Basso et al., 2006). This scoring system is widely used as an indicator of recovery in mouse models of SCI. Later, modifications were added to improve the scale as described (Pajoohesh-Ganji et al., 2010). BMS scoring was performed at the indicated time points, as shown in Figure 1. Two observers, blinded to the identity of groups, independently evaluated the BMS scores.
Video clip of animals’ walking behavior on day 30 after SCI
Animals’ walking behaviors were videotaped using an automated video camera (Canon, VIXIA HF R80, Melville, NY, USA). The entire experiment was performed in an isolated room free from any noise or disturbance.
Evaluation of sensory functions (pain sensitivity)
Pain-like behaviors were assessed by the mechanical allodynia (Khan et al., 2015a) and hyperalgesia (Oghbaei et al., 2020) methods. Improvement in mechanical threshold and thermal withdrawal latency by Alda-1 will indicate improved sensory functions leading to improved pain-like behaviors. The sensory-motor functions were evaluated once a week.
i) Mechanical allodynia: Animals were first acclimatized with a dynamic plantar aesthesiometer (DPA) for about 15 minutes. Dynamic plantar aesthesiometer is an automated version of von Frey hair analysis (Ugo Basile, Italy), and it is used to determine changes in sensation or development of mechanical hyperalgesia (Obata et al., 2004). The animal was placed in an enclosed testing area with a wire mesh on the floor. The dynamic plantar aesthesiometer device was positioned beneath the animal so that the filament directly touched the foot’s surface. The force is increased slowly until it reached 20 g (maximum). The force is recorded at which the foot is withdrawn (paw withdrawal threshold).
ii) Thermal algesia: Mice are gently restrained by placing them in a restrainer, and then the distal 1.0 cm of their tail is dipped into the hot water bath maintained at 49 ± 1.0°C. The latency (time in seconds) between exposure to the hot water and the sudden tail withdrawal is recorded. A 30-second cut-off time was established to minimize the possibility of tissue damage from prolonged heat exposure.
Assay for intraspinal subarachnoid hemorrhage
Mice were perfused with heparinized saline to remove intravascular blood, and a 5 mm segment of cord having the lesion site was homogenized and centrifuged at 16,000 × g for 30 minutes. The supernatant containing hemoglobin was left at room temperature for 15 minutes. The absorbance was measured at 540 nm using CLARIOstar microplate reader (BMG LABTECH, Cary, NC, USA). The procedure was validated, and hemoglobin content was determined using known quantities of bovine erythrocyte hemoglobin (Sigma) as described (Lee et al., 2018).
Evans blue extravasation analyses
Blood-spinal cord barrier (BSCB) leakage was determined as described (Cabrera-Aldana et al., 2017) with slight modification. The mice were administered (i.v.) 100 μL of a 5% solution of Evans blue in saline at 4 hours before the 72 hours endpoint. At 72 hours, cardiac perfusion was performed under deep anesthesia induced byketamine/xylazine as described under mouse model of SCI with 200 mL of saline to remove the circulating Evans blue. The spinal cord was isolated and photographed. The spinal cord was homogenized in 750 μL of N,N-dimethylformamide. The homogenized spinal cord was kept at room temperature in the dark for 72 hours and centrifuged at 10,000 × g for 25 minutes. The supernatant was analyzed spectrofluorometrically (lex 620 nm, lem 680 nm) by CLARIOstar microplate reader to determine the content of Evans blue.
Measurement of edema (spinal cord water content)
At 72 hours following SCI, mice were euthanized using ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) to assess spinal cord water content (edema) described earlier (Cabrera-Aldana et al., 2017). Fresh spinal cord from was collected, and a segment of 0.5 cm of the spinal cord containing the lesion was weighed. Each sample was dried at 60°C for 72 hours, and the dry weight was recorded. Tissue water content was calculated as: water content (%) = (wet weight – dry weight)/wet weight × 100.
Adenosine 5’-triphosphate assay
Cellular adenosine 5’-triphosphate (ATP) contents in the spinal cords’ injured regions were measured using a firefly luciferase-based ATP assay kit (Bioassay Systems, Hayward, CA, USAl; Cat# EATP-100,) as described (Zhang et al., 2020). Briefly, 20 mg of the tissues were homogenized in 200 µL of cold phosphate-buffered saline, centrifuged at 12,000 × g for 5 minutes at 4°C, and the supernatants were collected. The supernatants were equalized based on their protein concentration. A luminescence plate reader measured the ATP level according to the manufacturer’s instructions (CLARIOstar microplate reader).
Western blot analysis
Under deep anesthesia using ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), the animals at the endpoints were euthanized by decapitation and the spinal cord was collected for biochemical studies. The spinal cords were stored at -80°C for subsequent studies.
Using 5 mm of spinal tissue (~1 mm epicenter; 2 mm caudal and 2 mm rostral from the epicenter) from the injured cord, western analysis was performed as described earlier (Khan et al., 2018). For western blot studies, the following antibodies were used for overnight incubation at 4°C. Intercellular adhesion molecule 1 (ICAM-1; Thermo Fisher Scientific, Waltham, CA, USA, Cat# MA5407, RRID:AB_223596, 1:1000 dilution), 4 hydroxynonenal (4-HNE; Abcam, Cambidge, UK, Cat# ab46545, RRID:AB_722490, 1:1000 dilution), aldehyde dehydrogenase 2 (ALDH2; Abcam, Cat # Ab227021, RRID:AB_2868491, 1:2000 dilution), caspase-3 (pro and cleaved) (Cell Signaling, Danvers, MA, USA, Cat# 9664S, RRID:AB_2070042, 1:1000 dilution), neuronal nuclear protein (NeuN; Abcam, Cat# ab104224, RRID:AB_10711040, 1:5000 dilution), ionized calcium-binding adaptor molecule 1 (Iba-1; Abcam, Cat# Ab5076, RRID:AB_2224402, 1:500 dilution), manganese superoxide dismutase (MnSOD; Abcam, Cat# 13533, RRID:AB_300434, 1:2000 dilution), brain-derived neurotrophic factor (BDNF; Abcam, Cat# Ab203573, RRID:AB_2631315, 1:200 dilution), neurotrophin-3 (NT-3 Abcam, Cat# Ab263864, RRID:AB_2884942, 1:1000 dilution), cardiolipin synthase 1 (CLS1; Thermo Protein Tech, Waltham, CA, USA, Cat# 14845-1-AP, RRID:AB_2085336, 1:1000 dilution), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Cell Signaling, Cat# 5174, RRID:AB_10622025, 1:5000 dilution), β-actin (Abcam, Cat# Ab8226, RRID:AB_306371, 1:10,000 dilution). Horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Lab, West Grove, PA, USA, Cat# 111-035-045, RRID:AB_2337938, 1:10,000 dilution) was used for 1.5-hour incubation at room temperature. TBS-T (1X) with 2% non-fat dry milk was used to dilute all antibodies. Protein assay kit from Bio-Rad Laboratories (Hercules, CA, USA) was used to determine protein concentration. Tissue homogenates with 20 µg protein were used for western blot analysis and ImageJ software (version 1.47; NIH, Bethesda, MD, USA) was used for the densitometry of protein expression.
Immunohistochemistry and terminal deoxynucleotidyl transferase dUTP nick end labeling assay
At the 30-day endpoint, mice were anesthetized, sacrificed, and perfused first with saline and 4% paraformaldehyde. The spinal cord was decalcified for 48 hours using decalcification buffer, soaked in cryoprotective solution (30% sucrose) for 48 hours, embedded in optimal cutting temperature (O.C.T) compound, and then stored frozen at –80°C. Frozen tissue samples were sectioned transversely (15-µm thick). The sections were stained with an antibody specific to glial fibrillary acidic protein (GFAP; Abcam, Cat# Ab7260, RRID: AB_305808, 1:500 dilution), neuronal nuclear protein (NeuN; Abcam, Cat# Ab177487, RRID:AB_2532109, 1:500 dilution), or myelin basic protein (MBP; Abcam, Cat# Ab62631, RRID:AB_956157, 1:500 dilution) for 1 hour at room temperature. The expression was detected with a secondary antibody conjugated with a fluorescent molecule for 1 hour at room temperature, as described in the manufacturers’ instructions and our laboratory (Invitrogen, Carlsbad, CA, USA, Cat# A21206/A32794, 1:1000 dilution) (Choi et al., 2020).
To detect apoptotic cell death, spinal cord sections, prepared as described above, were used for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. According to the manufacturer’s instructions, in situ cell death was detected by a kit (Thermo Fisher Scientific, Cat# C10618). Red fluorescent apoptotic cells were manually counted using a BX-60 microscope equipped with a DP70 camera unit (Olympus, Tokyo, Japan).
All digital images were taken using a BX-60 microscope equipped with a DP70 camera unit (Olympus). The acquired images were analyzed by ImageJ software (version 1.47).
Nissl staining
Loss of viable neurons was determined by Nissl (cresyl violet) staining, and thus it reveals the structural features of neurons. The spinal cord sections described above were used to stain with Nissl as previously described (Sakakima et al., 2012). Cells that contained Nissl substance were considered to be viable neurons. Condensed fragmented staining indicates neuronal degeneration. The viable neurons with visible nucleoli were manually counted using a BX-60 microscope equipped with a DP70 camera unit (Olympus).
ALDH2 activity
ALDH2 activity was measured in tissue lysates using an ALDH2 enzymatic activity assay kit from Abcam (Cat# ab115348) according to the manufacturer’s protocol and described by Hua et al. (2018). As described in the western blot analysis section, the frozen spinal cord (5mm segment) was homogenized and centrifuged at 10,000 × g for 30 minutes at 4°C. The activity was measured at 25°C. The nicotinamide adenine dinucleotide hydrogen (NADH) production level was determined spectrophotometrically by monitoring the alterations in absorbance intensity at 340 nm every 30 seconds for 5 minutes using CLARIOstar microplate reader. The reaction rates of ALDH2 were expressed as µmol NADH/min/mg protein.
Statistical evaluation
Statistical analysis was calculated as previously (Khan et al., 2015a, b) using software Graph Pad Prism 5.01 (GraphPad, San Diego, CA, USA). All values are expressed as mean ± standard deviation (SD) of n determinations. Group comparisons were performed by one-way analysis of variances (ANOVA) with Tukey’s post hoc test. A P-value less than 0.05 was considered statistically significant.
Results
SCI induces sustained high levels of 4-HNE
Reactive aldehyde load, more prominently of 4-HNE, is recognized as a pathological event in SCI. Western blot (Figure 2A) and its densitometric analysis (Figure 2B) shows a sustained accumulation of significantly high (P < 0.001) levels of 4-HNE adducts at different time points (24, 48, 72 hours, and 30 days) in SCI groups compared with sham-operated (at 30 days) group.
Figure 2.
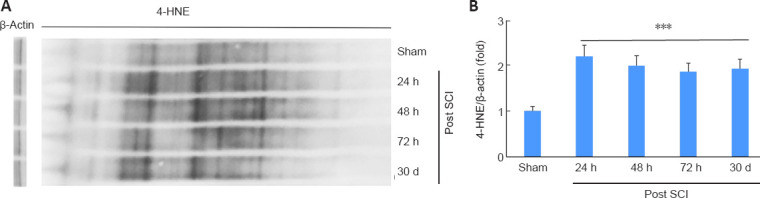
Sustained and prolonged 4-HNE load in a mouse model of SCI.
Western blot (A) and densitometry (B) from the spinal cord of traumatic penumbra (caudal-rostral areas) showing sustained increased levels of 4-HNE in SCI groups compared with Sham (30 days) at different time points (24 hours, 48 hours, 72 hours, 30 days) in a mouse model of SCI. Data are presented as the mean ± SD (n = 5). ***P < 0.001, vs. Sham (one-way analysis of variance with Tukey’s post hoc test). 4-HNE: 4-Hydroxy-trans-2-nonenal; SCI: spinal cord injury.
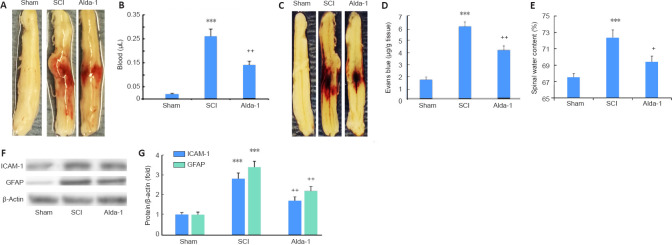
Alda-1 treatment of SCI mice protects against hemorrhage, BSCB leakage, and edema formation by downregulation of pro-inflammatory mediators
BSCB disruption, edema and neurovascular inflammation are critical components of the acute phase of spinal cord contusion injury (Lee et al., 2018). Alda-1 treatment of SCI reduced the severity of contusion injury measured as the blood content in the injured cord (P < 0.01, Figure 3A and B), BSCB disruption (Evans’ blue extravasation, P < 0.01, Figure 3C and D), decreased edema (water content, P < 0.05, Figure 3E) and reduced expression (western blot analysis) of neuroinflammatory mediators ICAM-1 and GFAP (P < 0.01, Figure 3F and G).
Figure 3.
ALDH2 agonist Alda-1 reduces neurovascular dysfunction and inflammation in the acute phase (72 hours) of SCI in wild type mice.
Representative whole spinal cords (A) and spectrophotometric quantification (B) of the amount of blood in homogenates, representative whole spinal cords (C) showing Evans blue dye extravasation into spinal cord and quantification of the Evans blue extravasation (D), levels of edema (spinal water content) (E), Western blot of the expression of ICAM-1 and GFAP (F) and densitometry of ICAM-1 and GFAP (G). Data are presented as the mean ± SD (n = 7). ***P < 0.001, vs. Sham; +P < 0.05, ++P < 0.01, vs. SCI (one-way analysis of variance with Tukey’s post hoc test). Alda-1: N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide; ALDH2: aldehyde dehydrogenase 2; GFAP: glial fibrillary acidic protein; ICAM-1: intercellular adhesion molecule 1; SCI: spinal cord injury.
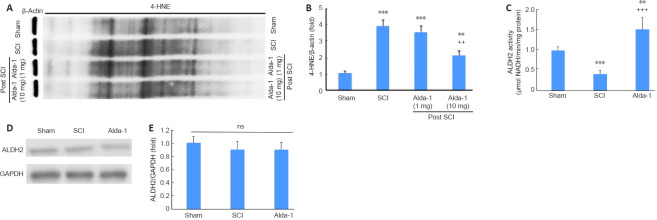
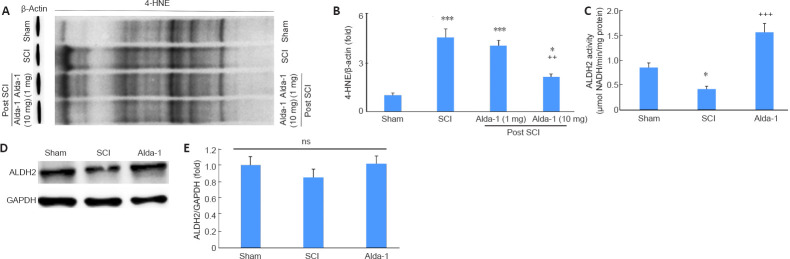
Alda-1 treatment of SCI mice enhances the activity of ALDH2 and decreases 4-HNE load in the acute phase (72 hours study) of SCI
4-HNE is one of the most reactive aldehydes, and it is structurally suitable for the formation of protein adducts due to its strong electrophilic nature. Using the two different doses (1 mg/kg vs. 10 mg/kg) of Alda-1, we determined that the effective dose was 10 mg/kg to reduce SCI-induced 4-HNE load (Figure 4A and B). The dose of 10 mg/kg body weight has been effective with significant efficacy in other animal disease models (Lu et al., 2017; He et al., 2018). Therefore, we used a 10 mg/kg dose of Alda-1 in all other experiments reported in this study. The activity of ALDH2 was significantly inhibited in the SCI group compared with the sham group (Figure 4C). Alda-1 treatment of the SCI group significantly increased the ALDH2 activity (Figure 4C). Interestingly, the expression of ALDH2 remained unchanged among the groups (Figure 4D and E).
Figure 4.
Alda-1 treatment decreases 4-HNE load and enhances the activity of ALDH2 in the acute phase (72 hours) of a mouse model of SCI.
Two different doses of Alda-1 were used to show that only a 10 mg/kg dose effectively reduced 4-HNE load. Western blot (A) and densitometry (B) from the spinal cord of traumatic penumbra (caudal-rostral areas). Measurement of ALDH2 activity (C). Western blot of ALDH2 (D) and densitometry (E). Data are presented as the mean ± SD (n = 7). **P < 0.01, ***P < 0.001, vs. Sham; ++P < 0.01, +++P < 0.001, vs. Alda-1 (one-way analysis of variance with Tukey’s post hoc test). 4-HNE: 4-Hydroxy-trans-2-nonenal; Alda-1: N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide; ALDH2: aldehyde dehydrogenase 2; ns: not significant; SCI: spinal cord injury.
Alda-1 treatment of SCI mice improves locomotor functions in a 30-day mouse model of SCI
Improvement in the BMS score is the gold standard endpoint determining a therapeutic agent’s efficacy in mice following SCI. On the BMS scores, SCI animals exhibited greater functional loss than the Alda-1-treated group (Figure 5). Statistical analysis showed significantly improved scores in the Alda-1-treated compared with the SCI group from day 3 onward (Figure 5).
Figure 5.
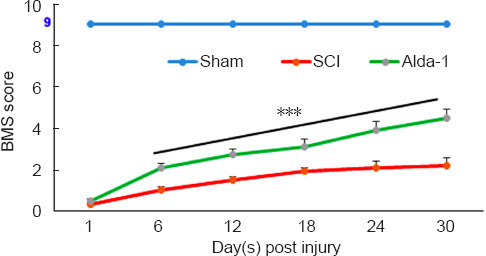
Effect of Alda-1 on locomotor function. Locomotor function was assessed using the BMS scale at indicated days.
An observer, blinded to groups, evaluated the BMS. A score of 9 on the BMS scale was assigned to sham animals displaying coordinated gait, consistent toe clearance, lifted tail and steady trunk. Data are presented as the mean ± SD (n = 7).***P < 0.001, vs. SCI (one-way analysis of variance with Tukey’s post hoc test). Alda-1: N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide; BMS: Basso Mouse Scale; SCI: spinal cord injury.
Functional recovery was also assessed by the walking behavior on day 30 of SCI and SCI + Alda-1 mice (two mice/group) using video photography. The Alda-1-treated mice had significantly improved walking compared with the SCI animals (Additional Video 1).
Alda-1 treatment of SCI mice improves pain-associated behaviors in a 30-day mouse model of SCI
Both inflammatory and neuropathic pain (caused by a lesion or disease of somatosensory function) are present in most SCI patients (Finnerup, 2013). The ALDH2/Alda-1-dependent mechanism has been reported to mitigate pain by reducing the load of reactive aldehyde in animal models of pain (Zambelli et al., 2014; Li et al., 2018). Using mechanical (paw withdrawal threshold, Figure 6A) and thermal withdrawal latency (Figure 6B), we observed that the pain threshold/latency remained almost unchanged for seven days in both the SCI and the Alda-1-treated groups. From day seven onward, the Alda-1 group had a significantly improved pain threshold that further improved with time (Figure 6). Sham animals had no change in pain sensitivity.
Figure 6.
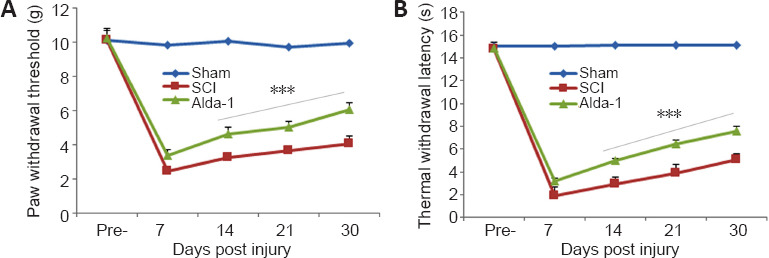
Effect of Alda-1 on pain-like behavior in a mouse model of SCI.
The pain threshold was measured by mechanical allodynia (A) and thermal algesia (B) at the indicated days. Data are presented as the mean ± SD (n = 8). ***P < 0.001, vs. SCI (one-way analysis of variance with Tukey’s post hoc test). Alda-1: N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide; SCI: spinal cord injury.
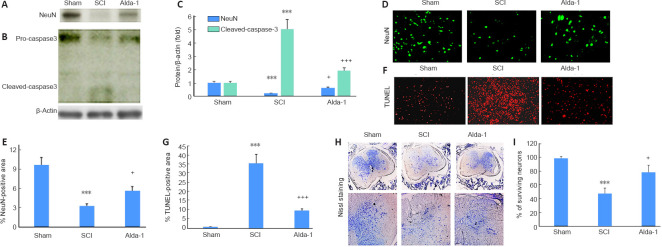
Alda-1 treatment of SCI mice protects against neuronal apoptotic cell death in the chronic phase (30 days study) of SCI
Neuronal cell death is the critical component of a spinal cord contusion injury, and the protection against neuronal cell death is the major objective of SCI therapy. Expectedly, the expression of NeuN was drastically reduced in the SCI group (Figure 7A), which was significantly increased by Alda- 1 treatment (Figure 7A and C). In parallel to NeuN loss in the SCI group, the activity of caspase-3 was increased, as shown by western blot (Figure 7B). In contrast, the Alda-1- treated group had reduced caspase-3 activity (Figure 7B and C). Alda-1 treatment also increased number of neurons measured as the expression of NeuN (Figure 7D and E) and reduced the SCI-induced increased number of TUNEL positive cells (Figure 7F and G), indicating that Alda-1 protects against apoptotic neuronal cell death. The neuroprotective effect of Alda-1 was also supported by Nissl staining showing increased survival of neurons in the Alda-1-treated group (Figure 7H and I). These results suggest a significant neuronal loss in the chronic phase of SCI, and upregulation of ALDH2 activity by Alda-1 may reduce the loss of neuronal cells.
Figure 7.
ALDH2 agonist Alda-1 treatment reduces caspase-3 activity, apoptotic cell death, and neuronal loss in the chronic phase (30 days) of a mouse model of SCI.
Western blot of NeuN (A) and caspase-3 (B) showing the expression of cleaved/uncleaved fragments and their densitometry (C). Immunohistochemistry of NeuN (D; green) and quantified (E), and TUNEL (F; red) and quantified TUNEL positive area (G). Nissl staining (H) and quantified Nissl positive area (I). Data are presented as the mean ± SD (n = 7). ***P < 0.001 vs. Sham; +P < 0.05, +++P < 0.001 vs. SCI. (one-way analysis of variance with Tukey’s post hoc test). Alda-1: N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide; ALDH2: aldehyde dehydrogenase 2; NeuN: neuronal nuclear protein; SCI: spinal cord injury; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
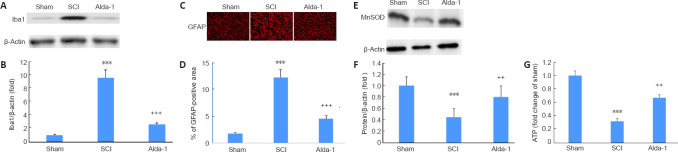
Alda-1 treatment of SCI mice reduces the expression of Iba-1 and GFAP and improves the expression of MnSOD and the level of cellular ATP in the chronic phase (30 days study) of SCI
Inflammation and redox alteration are essential components of SCI, which contributes to neuronal cell death and pain sensitivity, leading to a hindrance to sensory and locomotor functions’ recovery. The expression of activated microglia (Iba-1) (Figure 8A and B) and reactive astrocytes (GFAP) (Figure 8C and D) was significantly increased in the SCI compared to the sham group. Mitochondrial redox, determined as the expression of mitochondria-specific MnSOD, was also compromised considerably in the SCI compared with the sham group. Treatment of the injured animals with Ald-1 decreased the SCI-induced increased expression of Iba-1 and GFAP. The treatment with Alda-1 also improved the SCI-mediated decreased expression of MnSOD (Figure 8E and F) and the reduced levels of ATP (Figure 8G). These results indicate that Alda-1, likely by upregulating ALDH2 activity, is a potent anti-inflammatory and mitochondrial function restoring agent.
Figure 8.
ALDH2 agonist Alda-1 treatment of SCI decreases the activation of Iba-1 and GFAP and normalized mitochondrial oxidative stress measured as the expression of MnSOD in the chronic phase (30 days) of a mouse model of SCI.
Western blot of Iba-1 showing microglial activation (A) and densitometry (B). Immunohistochemistry of GFAP showing the expression of reactive astrocytes (C; red) and densitometry (D). Western blot of MnSOD and its densitometry (F) showing the change in mitochondrial redox. ATP content was measured by a luciferase-based assay showing that Alda-1 improved the ATP level (G). Data are presented as the mean ± SD (n = 7). ***P < 0.001, vs. Sham; ++P < 0.01, +++P < 0.001, vs. SCI (one-way analysis of variance with Tukey’s post hoc test). Alda-1: N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide; ALDH2: aldehyde dehydrogenase 2; GFAP: glial fibrillary acidic protein; Iba-1: ionized calcium binding adaptor molecule 1; SCI: spinal cord injury.
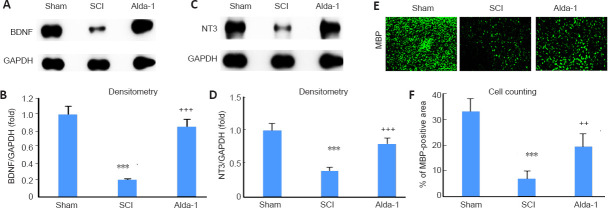
Alda-1 treatment of SCI mice increases the expression of neurotrophic factor BDNF, neurotrophin NT3 and myelin structural protein MBP in the chronic phase (30 days study) of SCI
Stimulation of spinal cord repair is essential for optimum functional recovery following SCI (Cheng et al., 1996). Under the oxidative stress environment, as observed in SCI, neurotrophic factors including BDNF and NT3 are reduced, leading to a hindrance to the reparative process. In accordance, western blot analysis showed the reduced expression levels of both BDNF (Figure 9A and B) and NT3 (Figure 9C and D). The expression levels of MBP was also decreased in the SCI group, as indicated by immunohistochemistry (Figure 9E and F). MBP is a critical structural protein that maintains the structure of myelin via interacting with myelin lipids. Reduced MBP levels are associated with demyelination and inhibition of remyelination (Zhou et al., 2019). The treatment with Alda-1 increased the SCI-induced decreased expression levels of BDNF, NT3, and MBP (Figure 9). Taken together, these data indicate that the enhanced activity of ALDH2 and reduced levels of 4-HNE are linked to the stimulation of the expression of neurorepair mediators.
Figure 9.
ALDH2 agonist Alda-1 treatment of SCI increases the expression of BDNF, NT3, and MBP in the chronic phase (30 days) of a mouse model of SCI.
Western blot studies showing the increased expression levels of BDNF (A) and densitometry (B), NT3 (C), and densitometry (D), and IHC studies showing the increased expression levels of MBP (E; green) and cell counting (F) indicate that Alda-1 stimulates neurorepair process. Data are presented as the mean ± SD (n = 7). ***P < 0.001, vs. Sham; ++P < 0.01, +++P < 0.001, vs. SCI (one-way analysis of variance with Tukey’s post hoc test). Alda-1: N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide; ALDH2: aldehyde dehydrogenase 2; BDNF: brain-derived neurotrophic factor; MBP: myelin basic protein; NT3: neurotrophin-3; SCI: spinal cord injury.
Alda-1 treatment of SCI mice enhances the activity of ALDH2 and decreases 4-HNE load in the chronic phase (30 days study) of SCI
Like in the acute phase, Alda-1 treatment (10 mg/kg but not 1 mg/kg) significantly reduced SCI-induced 4-HNE load in the chronic phase of SCI (Figure 10A and B). While the activity of ALDH2 remained inhibited even at 30-day after the injury, the treatment with Alda-1 enhanced the activity of ALDH2 (Figure 10C). However, like in the acute phase (Figure 4D and E), the expression of ALDH2 had no change among the groups (Figure 10D and E).
Figure 10.
Alda-1 treatment of SCI decreases 4-HNE load and enhances the activity of ALDH2 in the chronic phase (30 days) of a mouse model of SCI.
Like in Figure 4, two different doses of Alda-1 were used to show that daily treatment of 10 mg/kg dose was remarkably effective to reduce 4-HNE load. Western blot (A) and densitometry (B). Measurement of ALDH2 activity (C), western blot of ALDH2 (D), and densitometry (E). Data are presented as the mean ± SD (n = 7). *P < 0.05, ***P < 0.001, vs. Sham; ++P < 0.01, +++P < 0.001, vs. SCI (one-way analysis of variance with Tukey’s post hoc test). 4-HNE: 4-Hydroxy-trans-2-nonenal; Alda-1: N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide; ALDH2: aldehyde dehydrogenase 2; ns: not significant; SCI: spinal cord injury.
Discussion
Enzymatic or non-enzymatic lipid peroxidation (a reaction between polyunsaturated fatty acids and hydrogen peroxide in the presence of free iron) end-product 4-HNE (Ayala et al., 2014) is implicated in several neuroinflammatory diseases, including SCI (Carrico et al., 2009). It is formed in excess and accumulates in CNS traumatic injury conditions such as SCI and traumatic brain injury. Due to an α, β-unsaturated structure-based strong electrophilic nature of 4-HNE, it is highly reactive and forms stable protein adducts (Gegotek and Skrzydlewska, 2019). The accumulation of 4-HNE adduct is the consequence of the down-regulated activity of its major metabolizing enzyme ALDH2 in neurodegenerative diseases (Chen et al., 2014). Although 4-HNE is also metabolized by glutathione and aldo keto reductase superfamily enzymes, mitochondrial ALDH2 is the most prominent among them (Ayala et al., 2014). The role of ALDH2 in various neurodegenerative diseases has been recently reviewed by Chen et al. (2016a). Our data in Figure 2 provides evidence that 4-HNE load is sustained and prolonged, indicating that 4-HNE adducts accumulation is a pathological event during both the acute and chronic SCI phases. Using a mouse contusive SCI model, we show, for the first time, that suppressing 4-HNE load by Alda-1 via enhancing the ALDH2 activity protects against neurovascular inflammatory injury, mitigates pain-like behaviors, and improves locomotor function.
ALDH2 activity is inhibited by reactive aldehydes. 4-HNE is prominent among such reactive aldehydes. To suppress the 4-HNE load, ALDH2 specific agonist/activator Alda-1 has been used in a number of heart and brain disease conditions (Luo et al., 2014). In this study, Alda-1 treatment of SCI enhanced the ALDH2 activity and reduced the levels of 4-HNE. Unchanged protein expression of ALDH2 among the groups indicates that Alda-1-induced enhanced activity is independent of the ALDH2 expression. No change in the ALDH2 expression levels in a liver ischemia-reperfusion model-treated with Alda-1 (Liu et al., 2020) supports our observation that neither SCI nor Alda-1 regulates ALDH2 protein expression. Alda-1 is reported to increase substrate-enzyme interaction and, thus, the activity (Perez-Miller et al., 2010). Alda-1, via the activation of ALDH2, accelerates the detoxification of only smaller straight-chain aliphatic aldehydes such as 4-HNE (Perez-Miller et al., 2010). Interestingly, 4-HNE can diffuse across the biological membranes, and thus it can form adducts with proteins in all cellular compartments (Gegotek and Skrzydlewska, 2019). These observations indicate that Alda-1 directly interacts with the ALDH2 in mitochondria for the up-regulation of ALDH2’s activity, leading to detoxification of 4-HNE and, thus aiding in the amelioration of SCI.
In SCI, the primary injury is physical and structural. The initial physical insult resulting from mechanistic crosstalk between several deleterious pathways is followed by secondary injury of oxidative/nitroxidative exacerbations and neuroinflammation (Xiong et al., 2007; Siddiqui et al., 2015; Wang et al., 2019). Therefore, we first investigated whether Alda-1 treatment had neurovascular protective effects on the initial injury mechanisms. Alda-1-induced protection against BSCB leakage and edema and their correlation with the decreased expression of pro-inflammatory mediator ICAM-1 and reactive astrocytes (GFAP). These results indicate an antioxidant/anti-inflammatory property is associated with Alda-1/ALDH2 in SCI. Previous Alda-1 studies, showing protection against endothelial dysfunction and barrier leakage (Lu et al., 2017), corroborate our findings. Our studies and evidence from other studies using Alda-1 (Chen et al., 2008; Perez-Miller et al., 2010; Lu et al., 2017; Zhang et al., 2018) support increasing the ALDH2 activity will ameliorate the acute phase SCI. The alda-1-mediated decrease in 4-HNE load correlates well with the increased activity of ALDH2 in the Alda-1-treated SCI group. These results indicate that neurovascular protection in the Alda-1-treated SCI groups correlates with the increased activity of ALDH2 and decreased load of 4-HNE.
In the chronic phase of SCI, locomotor function deficits, originating from the combined effect of lesion, neurodegeneration, neuroinflammation, and oxidative exacerbations, are the significant consequence of the overall injury. Therefore, the restoration of such functions is always the primary goal of preclinical studies. Evaluation of locomotor functions using the BMS score in mice is the standard gold method (Basso et al., 2006; Qian et al., 2017) to determine the efficacy of an SCI drug. A slow but steady and significant functional recovery, with time, without reaching a plateau, supports Alda-1 therapy’s potential with time. In contrast to the Alda-1 group, the SCI group’s recovery is reaching a plateau. The more significant functional improvement in the Alda-1 group and its correlation with reduced 4-HNE load and the enhanced ALDH2 activity support the beneficial role of Alda-1-induced ALDH2 activation-mediated 4-HNE load suppression strategy. The greater functional recovery in the Alda-1-treated SCI than the SCI group is further supported by a video clip.
In addition to locomotor and sensory function deficits, the pain has a significant impact on SCI patients’ quality of life. The majority of SCI patients suffer from a combination of inflammatory and neuropathic pain (Finnerup, 2013; Hagen and Rekand, 2015). The mechanisms underlying neuropathic pain are multifactorial and complex. Among them, reactive aldehydes, including 4-HNE-mediated mechanisms, are prominent inducing pain-like behaviors (Chen et al., 2014). Our data shows that SCI-induced load of 4-HNE and reduced activity of ALDH2 correlate well with compromised pain behaviors. In contrast, Alda-1 treatment of SCI improved pain-like behaviors, indicating that Alda-1-induced ALDH2 activation and 4-HNE load suppression regulate nociception and improve the pain threshold.
After SCI, microglial and astrocyte cell activation correlates with neuroinflammation and indicates that the inhibition of their activation-based drugs may contribute to functional improvements (Pannu et al., 2004; Wang et al., 2019). Increased expression/activation of both microglia (Iba1) and astrocytes (GFAP) after 30-day of SCI indicates SCI-induced inflammation in the chronic SCI. Furthermore, the increased expression of Iba1/GFAP correlated with the decreased expression of mitochondria-specific antioxidant enzyme MnSOD, indicating that neuroinflammation and mitochondrial functions are linked with each other. Because Alda-1-induced neurovascular protective activity is associated with the activation and correction of mitochondrial enzyme ALDH2, increased ATP levels, and 4-HNE load suppression, 4-HNE seems to be involved in SCI-induced mitochondrial dysfunction, redox imbalance, and neuroinflammatory events.
The consequence of secondary injury is the accelerated neurodegeneration leading to functional deficits following SCI. 4-HNE-induced neurodegeneration and neuronal loss are well known in a number of neurodegenerative diseases, including animal models of SCI (Xiong et al., 2007; Carrico et al., 2009). Although scavenging 4-HNE is reported to ameliorate CNS trauma, enzymatic detoxification of 4-HNE by enhancing the activity of ALDH2 has not been investigated in SCI. Therefore, ALDH2 activator Alda-1 was a logical choice for 4-HNE load suppression and the neuroprotection following SCI. Remarkable neuronal loss in the SCI group, indicated by significantly reduced NeuN expression as shown by western blot and immunohistochemistry studies, was observed after the four weeks of SCI. In this chronic phase, SCI groups also had increased caspase-3 activity, TUNEL positive, and decreased Nissl positive cells. Furthermore, the expression levels of BDNF, NT3, and MBP were remarkably low in the SCI group. Taken together, these results support that SCI induces apoptotic neuronal loss and neurodegeneration and hinders the stimulation of the neurorepair process. On the other hand, Alda-1 treatment of SCI mitigated SCI-induced neurotoxicity, providing neuroprotection and stimulating the neurorepair process, which correlated well with enhanced activity of ALDH2 and suppressed load of 4-HNE. Taken together, our studies support that enhancing the ALDH2 activity for suppressing 4-HNE load provides neuroprotection, promotes neurorepair process, and aids in functional recovery
Several limitations of this study are recognized. First, the data is based on animal experiments in vivo using wild-type mice, and the cause-and-effect relationship was tested neither in vitro nor in genetically manipulated animals. Second, the study is limited to single-sex (male), and thus, the data may not represent a complete role of ALDH2 activation and the efficacy of Alda-1. We will extend this study to include female animals in next level studies. Third, we investigated only two doses of Alda-1 to determine its effective dose (10 mg/kg). The study is also limited to only one treatment time of Alda-1. We will extend the study to include a delayed treatment time window ranging from 6 hours to 24 hours. Last, the contribution to the SCI of other major reactive aldehydes such as acrolein, formaldehyde, and malondialdehyde, has not been evaluated. However, ALDH2 detoxifies these other aldehydes too. In contrast to beneficial effects, treatment with Alda-1 is also associated with deterioration of renal functions in an ischemia-reperfusion injury animal model due to crystalline nephropathy (Hammad et al., 2018). However, we have not evaluated adverse effects on renal function in this SCI study.
Conclusion
Our data show that Alda-1, a selective agonist of ALDH2, invokes its therapeutic efficacy by targeting the suppression of 4-HNE load in a mouse model of contusion SCI. The study suggests that Alda-1 is a potent antioxidant activity-based drug, and ALDH2 is a druggable target to provide neurovascular protection and aid in functional recovery. Based on our preclinical SCI study’s efficacy and the safety profile in other preclinical studies, Alda-1 seems to be a promising drug to be evaluated in human SCI studies.
Additional file:
Additional Video 1: Effect of Alda-1 on functional improvement on day 30 after spinal cord injury. Animals’ locomotor/walking behaviors were videotaped using an automated video camera. Two animals from spinal cord injury (white label) and another two from the Alda-1 group (green label) were allowed to move freely in a 2.0 × 2.0 feet box. Alda-1: N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide.
Acknowledgments:
We thank Ms. Deborah Davis (Department of Pediatrics, MUSC) for her technical help and secretarial assistance. We also acknowledge Dr. Tom Smith from the MUSC Writing Center for his valuable editing of the manuscript.
Footnotes
C-Editors: Zhao M, Zhao LJ, Li CH; T-Editor: Jia Y
Conflicts of interest:The authors declare that they have no conflict of interests.
Financial support:This study was supported by a grant from the State of South Carolina Spinal Cord Injury Research Fund Board, grant No. SCIRF #2017 (to MK) and the NIH grant No. R21 NS114433 (to JW and MK). This work was also supported by grants from the U.S. Department of Veterans Affairs, grant Nos. RX002090 (IS) and BX003401 (to AKS). The NIH Grants C06 RR018823 and No C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources also supported the animal work.
Institutional review board statement:All animals received humane care in compliance with the Medical University of South Carolina’s (MUSC) guidance and the National Research Council’s criteria for humane care. Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of MUSC (IACUC-2019-00864) on December 21, 2019. Human data or human tissue has not been used in this study.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Funding:This study was supported by a grant from the State of South Carolina Spinal Cord Injury Research Fund Board, grant No. SCIRF #2017 (to MK) and the NIH grant No. R21 NS114433 (to JW and MK). This work was also supported by grants from the U.S. Department of Veterans Affairs, grant Nos. RX002090 (IS) and BX003401 (to AKS). The NIH Grants C06 RR018823 and No C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources also supported the animal work.
References
- 1.Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 2.Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 4.Bilgen M. A new device for experimental modeling of central nervous system injuries. Neurorehabil Neural Repair. 2005;19:219–226. doi: 10.1177/1545968305278635. [DOI] [PubMed] [Google Scholar]
- 5.Cabrera-Aldana EE, Ruelas F, Aranda C, Rincon-Heredia R, Martínez-Cruz A, Reyes-Sánchez A, Guizar-Sahagún G, Tovar-Y-Romo LB. Methylprednisolone administration following spinal cord injury reduces aquaporin 4 expression and exacerbates edema. Mediators Inflamm. 2017;2017:4792932. doi: 10.1155/2017/4792932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrico KM, Vaishnav R, Hall ED. Temporal and spatial dynamics of peroxynitrite-induced oxidative damage after spinal cord contusion injury. J Neurotrauma. 2009;26:1369–1378. doi: 10.1089/neu.2008-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CH, Joshi AU, Mochly-Rosen D. The role of mitochondrial aldehyde dehydrogenase 2 (ALDH2) in neuropathology and neurodegeneration. Acta Neurol Taiwan. 2016a;25:111–123. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94:1–34. doi: 10.1152/physrev.00017.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, He Y, DeVivo MJ. Changing demographics and injury profile of new traumatic spinal cord injuries in the United States, 1972-2014. Arch Phys Med Rehabil. 2016b;97:1610–1619. doi: 10.1016/j.apmr.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- 12.Choi S, Singh I, Singh AK, Khan M, Won J. Asymmetric dimethylarginine exacerbates cognitive dysfunction associated with cerebrovascular pathology. FASEB J. 2020;34:6808–6823. doi: 10.1096/fj.201901318R. [DOI] [PubMed] [Google Scholar]
- 13.Chou PC, Shunmugavel A, Sayed HE, Desouki MM, Nguyen SA, Khan M, Singh I, Bilgen M. Preclinical use of longitudinal MRI for screening the efficacy of s-nitrosoglutathione in treating spinal cord injury. J Magn Reson Imaging. 2011;33:1301–1311. doi: 10.1002/jmri.22574. [DOI] [PubMed] [Google Scholar]
- 14.Finnerup NB. Pain in patients with spinal cord injury. Pain. 2013;154(Suppl 1):S71–76. doi: 10.1016/j.pain.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Gegotek A, Skrzydlewska E. Biological effect of protein modifications by lipid peroxidation products. Chem Phys Lipids. 2019;221:46–52. doi: 10.1016/j.chemphyslip.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Hagen EM, Rekand T. Management of neuropathic pain associated with spinal cord injury. Pain Ther. 2015;4:51–65. doi: 10.1007/s40122-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammad FT, Al-Salam S, Yuvaraju P, Lubbad L. Alda-1, an aldehyde dehydrogenase-2 agonist, causes deterioration in renal functions following ischemia-reperfusion injury due to crystalline nephropathy. Drug Dev Res. 2018;79:315–323. doi: 10.1002/ddr.21454. [DOI] [PubMed] [Google Scholar]
- 18.He M, Long P, Yan W, Chen T, Guo L, Zhang Z, Wang S. ALDH2 attenuates early-stage STZ-induced aged diabetic rats retinas damage via Sirt1/Nrf2 pathway. Life Sci. 2018;215:227–235. doi: 10.1016/j.lfs.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Hua Y, Chen H, Zhao X, Liu M, Jin W, Yan W, Wu Y, Tan Z, Fan H, Wu Y, Xie L, Zhang W, Liu B, Zhou Y. Alda1, an aldehyde dehydrogenase2 agonist, improves longterm survival in rats with chronic heart failure following myocardial infarction. Mol Med Rep. 2018;18:3159–3166. doi: 10.3892/mmr.2018.9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan M, Dhammu TS, Singh I, Singh AK. Amelioration of spinal cord injury in rats by blocking peroxynitrite/calpain activity. BMC Neurosci. 2018;19:50. doi: 10.1186/s12868-018-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan M, Shunmugavel A, Dhammu TS, Matsuda F, Singh AK, Singh I. Oral administration of cytosolic PLA2 inhibitor arachidonyl trifluoromethyl ketone ameliorates cauda equina compression injury in rats. J Neuroinflammation. 2015a;12:94. doi: 10.1186/s12974-015-0311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan M, Dhammu TS, Matsuda F, Baarine M, Dhindsa TS, Singh I, Singh AK. Promoting endothelial function by S-nitrosoglutathione through the HIF-1alpha/VEGF pathway stimulates neurorepair and functional recovery following experimental stroke in rats. Drug Des Devel Ther. 2015b;9:2233–2247. doi: 10.2147/DDDT.S77115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knopp RC, Lee SH, Hollas M, Nepomuceno E, Gonzalez D, Tam K, Aamir D, Wang Y, Pierce E, BenAissa M, Thatcher GRJ. Interaction of oxidative stress and neurotrauma in ALDH2(-/-) mice causes significant and persistent behavioral and pro-inflammatory effects in a tractable model of mild traumatic brain injury. Redox Biol. 2020;32:101486. doi: 10.1016/j.redox.2020.101486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JY, Choi HY, Park CS, Ju BG, Yune TY. Mithramycin A improves functional recovery by inhibiting BSCB disruption and hemorrhage after spinal cord injury. J Neurotrauma. 2018;35:508–520. doi: 10.1089/neu.2017.5235. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Sun W, Gu C, Yang Z, Quan N, Yang J, Shi Z, Yu L, Ma H. Targeting ALDH2 for therapeutic interventions in chronic pain-related myocardial ischemic susceptibility. Theranostics. 2018;8:1027–1041. doi: 10.7150/thno.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Ye S, Zhong X, Wang W, Lai CH, Yang W, Yue P, Luo J, Huang X, Zhong Z, Xiong Y, Fan X, Li L, Wang Y, Ye Q. Pretreatment with the ALDH2 activator Alda1 protects rat livers from ischemia/reperfusion injury by inducing autophagy. Mol Med Rep. 2020;22:2373–2385. doi: 10.3892/mmr.2020.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Q, Mundy M, Chambers E, Lange T, Newton J, Borgas D, Yao H, Choudhary G, Basak R, Oldham M, Rounds S. Alda-1 protects against acrolein-induced acute lung injury and endothelial barrier dysfunction. Am J Respir Cell Mol Biol. 2017;57:662–673. doi: 10.1165/rcmb.2016-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo XJ, Liu B, Ma QL, Peng J. Mitochondrial aldehyde dehydrogenase, a potential drug target for protection of heart and brain from ischemia/reperfusion injury. Curr Drug Targets. 2014;15:948–955. [PubMed] [Google Scholar]
- 29.McAllister SL, Sinharoy P, Vasu M, Gross ER. Aberrant reactive aldehyde detoxification by aldehyde dehydrogenase-2 influences endometriosis development and pain-associated behaviors. Pain. 2021;162:71–83. doi: 10.1097/j.pain.0000000000001949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oghbaei H, Mohaddes G, Hamidian G, Keyhanmanesh R. Sodium nitrate preconditioning prevents progression of the neuropathic pain in streptozotocin-induced diabetes Wistar rats. J Diabetes Metab Disord. 2020;19:105–113. doi: 10.1007/s40200-019-00481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pajoohesh-Ganji A, Byrnes KR, Fatemi G, Faden AI. A combined scoring method to assess behavioral recovery after mouse spinal cord injury. Neurosci Res. 2010;67:117–125. doi: 10.1016/j.neures.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pannu R, Won JS, Khan M, Singh AK, Singh I. A novel role of lactosylceramide in the regulation of lipopolysaccharide/interferon-gamma-mediated inducible nitric oxide synthase gene expression: implications for neuroinflammatory diseases. J Neurosci. 2004;24:5942–5954. doi: 10.1523/JNEUROSCI.1271-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Miller S, Younus H, Vanam R, Chen CH, Mochly-Rosen D, Hurley TD. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat Struct Mol Biol. 2010;17:159–164. doi: 10.1038/nsmb.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian J, Zhu W, Lu M, Ni B, Yang J. D-beta-hydroxybutyrate promotes functional recovery and relieves pain hypersensitivity in mice with spinal cord injury. Br J Pharmacol. 2017;174:1961–1971. doi: 10.1111/bph.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakakima H, Khan M, Dhammu TS, Shunmugavel A, Yoshida Y, Singh I, Singh AK. Stimulation of functional recovery via the mechanisms of neurorepair by S-nitrosoglutathione and motor exercise in a rat model of transient cerebral ischemia and reperfusion. Restor Neurol Neurosci. 2012;30:383–396. doi: 10.3233/RNN-2012-110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholpa NE, Schnellmann RG. Mitochondrial-Based Therapeutics for the Treatment of Spinal Cord Injury: Mitochondrial Biogenesis as a Potential Pharmacological Target. J Pharmacol Exp Ther. 2017;363:303–313. doi: 10.1124/jpet.117.244806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddiqui AM, Khazaei M, Fehlings MG. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog Brain Res. 2015;218:15–54. doi: 10.1016/bs.pbr.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Swartz KR, Fee DB, Joy KM, Roberts KN, Sun S, Scheff NN, Wilson ME, Scheff SW. Gender differences in spinal cord injury are not estrogen-dependent. J Neurotrauma. 2007;24:473–480. doi: 10.1089/neu.2006.0167. [DOI] [PubMed] [Google Scholar]
- 40.Taneja I, Raju KSR, Mittal M, Dev K, Khan MF, Maurya R, Wahajuddin M. Bioavailability, plasma protein binding and metabolic stability studies of a ALDH2 activator, alda-1, using a validated LC-ESI-MS/MS method in rat plasma. RSC Advances. 2015;5:54395–54402. [Google Scholar]
- 41.Varma AK, Das A, Wallace Gt, Barry J, Vertegel AA, Ray SK, Banik NL. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res. 2013;38:895–905. doi: 10.1007/s11064-013-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S, Smith GM, Selzer ME, Li S. Emerging molecular therapeutic targets for spinal cord injury. Expert Opin Ther Targets. 2019;23:787–803. doi: 10.1080/14728222.2019.1661381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong Y, Rabchevsky AG, Hall ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J Neurochem. 2007;100:639–649. doi: 10.1111/j.1471-4159.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- 44.Zambelli VO, Gross ER, Chen CH, Gutierrez VP, Cury Y, Mochly-Rosen D. Aldehyde dehydrogenase-2 regulates nociception in rodent models of acute inflammatory pain. Sci Transl Med. 2014;6:251ra118. doi: 10.1126/scitranslmed.3009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang R, Liu B, Fan X, Wang W, Xu T, Wei S, Zheng W, Yuan Q, Gao L, Yin X, Zheng B, Zhang C, Zhang S, Yang K, Xue M, Wang S, Xu F, Wang J, Cao Y, Chen Y. Aldehyde dehydrogenase 2 protects against post-cardiac arrest myocardial dysfunction through a novel mechanism of suppressing mitochondrial reactive oxygen species production. Front Pharmacol. 2020;11:373. doi: 10.3389/fphar.2020.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang T, Zhao Q, Ye F, Huang CY, Chen WM, Huang WQ. Alda-1, an ALDH2 activator, protects against hepatic ischemia/reperfusion injury in rats via inhibition of oxidative stress. Free Radic Res. 2018;52:629–638. doi: 10.1080/10715762.2018.1459042. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y, Su P, Pan Z, Liu D, Niu Y, Zhu W, Yao P, Song Y, Sun Y. Combination therapy with hyperbaric oxygen and erythropoietin inhibits neuronal apoptosis and improves recovery in rats with spinal cord injury. Phys Ther. 2019;99:1679–1689. doi: 10.1093/ptj/pzz125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.