Regeneration of long axons after the spinal cord injury (SCI) will benefit patients with extensive traumatic damage to the white matter pathways who experience intolerable, permanent, neurologic deficits even after neuroprotective treatment with anti-inflammatory agents (Kwiecien, 2021a). This short paper attempts to synthetize pathologic mechanisms or barriers involved in inhibition of axonal regeneration in the SCI and provides suggestions of therapeutic interventions enabling this regeneration in animal models.
Recently elucidated pathogenesis of the spinal cord injury (SCI) in the rat model indicates that destruction of axonal pathways by a traumatic event is further augmented by the severity of destructive inflammation over months following the trauma (Kwiecien et al., 2020a). The character of the inflammatory response in the spinal cord depends on the location of the area of necrosis and hemorrhage; if it is located deep, surrounded by the spinal cord, it results in formation of the cavity of injury (COI) where myelin-rich necrotic debris, hemorrhage and from day 3, rapidly increasing numbers of pro-inflammatory, CD68+/CD163– macrophages are sequestered by a central nervous system (CNS) tissue response, particularly astrogliosis, within the first week post-SCI. Excess edema fluid from the surrounding spinal cord appears to be transferred to the COI with apparent participation of reactive astrocytes and aquaporin-4 (Rash et al., 2004; Kwiecien et al., 2021b). The other type of inflammatory response, arachnoiditis, involves large disruption of the surface of the spinal cord (pia limitans externa) and involves infiltration of macrophages, fibroblasts and blood vessels with concurrent obliteration of the spinal cord and exclusion of CNS glia including GFAP+ astrocytes (Kwiecien et al., 2020a). While arachnoiditis is essentially a severe form of granulomatous inflammation that resolves into a scar, the COI resolves into a macrophage-free syrinx (Kwiecien et al., 2020a). Both types of inflammation apparently expand at the cost of destroyed spinal cord and are contained and inhibited by progressively thickening wall of astrogliosis and its anti-inflammatory activity mechanisms of which are unknown (Kwiecien, 2013; Kwiecien et al., 2020a).
Given the above pathogenesis of the SCI we have to consider the following barriers to regrowth of long axons in descending and ascending pathways; (1) the severity of inflammation initiated by trauma to the white matter (in the COI and in arachnoiditis) is not only destructive to the adjacent white matter but it lasts for an extraordinarily long period of time, > 16 weeks (Kwiecien et al., 2020a), (2) the COI, with its aqueous content and the resulting syrinx (Kwiecien et al., 2021b) is not crossed by axons unless they are supported by an implanted bridge (Kwiecien, 2013), (3) arachnoiditis and the resulting scar cease to be part of the spinal cord (Kwiecien et al., 2020a) and the CNS axons may not enter it.
Inhibition of inflammation in the SCI has been achieved recently with prolonged, continuous 1–2 week long subdural administration of dexamethasone (Kwiecien et al., 2015, 2016) and two immunomodulatory proteins derived from Myxoma virus, Serp-1 and M-T7 (Kwiecien et al., 2019). Since infusion of dexamethasone resulted in severe toxicity (Kwiecien et al., 2015, 2019), this powerful synthetic glucocorticoid is not suitable for long term sustained administration. An 8 week long subdural infusion of Serp-1 lowered the numbers of macrophages throughout the administration and essentially eliminated them from the COI thus reducing the duration of inflammation by half considered a neuroprotective effect (Kwiecien et al., 2020b). This is the first preclinical study indicating the required duration of sustained administration of an anti-inflammatory agent, 8 weeks, to eliminate inflammation from the COI. Although subdural infusion offers an effective route of administration of agents that do not readily pass the blood-spinal cord barrier (Kwiecien et al., 2019), it is an invasive administration that needs to be maintained for a long period of time. Anti-inflammatory agents administered orally and intravenously still need to be tested for their neuroprotective effectiveness considering the damage to the blood-spinal cord barrier around inflammation confined to the COI with resulting persistent vasogenic edema (Kwiecien et al., 2020a, b, 2021b). While the COI can be implanted with materials secreting anti-inflammatory agents such as Serp-1 (Kwiecien et al., 2020c) or materials tested for ability to support axonal regeneration (Kwiecien, 2016), arachnoiditis, a solid inflammatory tissue (Kwiecien et al., 2020a) is less amenable to neuroregenerative therapies and probably would require the surgical resection to create a lesion leading to the COI for appropriate implantation enabling axonal regeneration.
Axonal regeneration in ascending pathways in the dorsal column can be conveniently studied in myelin-lacking Long Evans Shaker (LES) rat with the dorsal column crush lesion implanted with the rat choroid plexus (Kwiecien, 2013) and dextran axonal tracer microinjected in both sciatic nerves (Kwiecien, unpublished). Ependymal cells derived from the implanted choroid plexus formed elaborate processes enveloping numerous axons in the COI, thus supporting their regrowth across 1–2 mm wide lesion and supporting regenerated axons for beyond 8 weeks. Some of the regenerated axons received myelin from implanted ependymal cells that transdifferentiated into oligodendrocytes by 8 weeks post-SCI indicating that these axons may have regenerated at full length and re-constituted synapses in the caudal brain stem, 4–5 cm from the site of the crush or 2/3 length of the spinal cord (Kwiecien, 2013). Although ependymal cells are very good at supporting axonal regeneration across the COI of the LES rat or in the crushed filum terminale of the LES and normally myelinated rats (Kwiecien and Avram, 2008), their availability is limited and a variety of candidate synthetic materials have been studied in the SCI for their ability to bridge axonal regeneration.
The implantation of the spinal crush injury in normally myelinated rats resulted in complete destruction of all implants by the severity of inflammation (Kwiecien, 2016) rendering normally myelinated spinal cord not useful for such testing due to the severity of post-SCI inflammation (Kwiecien et al., 2020a). The same materials implanted in the spinal crush of dysmyelinated LES rats could be studied conveniently in more detail following the 2 week survival. Most of the implants induced an inflammatory response from the spinal cord involving infiltration by macrophages and multinucleated giant cells and formation of liquid-filled cystic spaces between the spinal cord and the body of an implant indicating rejection (Kwiecien, 2016). One material however, a methacrylate hydrogel, did not induce inflammatory response and adhered closely to the spinal cord around the lesion but axons failed to enter it (Kwiecien, 2016). Observation from this and subsequent studies on other materials (Kwiecien, unpublished) indicate that in a normally myelinated animal model of human SCI, after the inhibition and elimination of inflammation, a candidate material for bridging activity in the COI should be: (a) inert, non-resorbable, not inducing inflammation in the implanted spinal cord, (b) liquid, with ability to gel within 10–30 seconds after microinjection in the lesion (approximately 50 μL in the rat), (c) supporting axonal migration into its delicate, soft, porous structure and their permanent support thereafter including enabling of re-myelination. The last requirement may need participation of tri-dimentional chains of suitable cells, therefore, pre-seeding of a candidate hydrogel material in vitro prior to its micro-injection into the COI. The inhibition of inflammation as the first and necessary step in neuroregeneration leads to an exciting idea of using hydrogels loaded with an effective anti-inflammatory agent for sustained release in situ (Kwiecien et al., 2020c) that would also serve as the bridge for axonal regeneration across the lesion at the same time, a daunting challenge for a potentially fast and effective treatment of a devastating and currently untreatable disease, the SCI.
Once the axons cross the acute lesion or the COI, they will face myelinated white matter at the opposite side and myelin is not permissive to axonal regeneration, it needs to be removed in a gentle fashion not involving initiation of destructive inflammation, at least for a period of time required for axons to re-grow. Such removal of myelin has been achieved in large areas of the white matter of the spinal cord by 1 week long subdural infusion of a very high concentration (50 million times higher than physiological) of kynurenic acid (Dabrowski et al., 2015) indicating the method for therapeutic removal of myelin in areas of the spinal cord targeted for neuroregeneration. Importantly, oligodendrocytes appeared remarkably affected by the treatment with kynurenic acid with markedly retracted cytoplasmic processes and small amount of organelle-poor cytoplasm (Dabrowski et al., 2015) associated with specific “weakening” of oligodendrocytes in vitro, mechanism of which remains uncertain (Langer et al., 2016). It is also not known whether “weakened” oligodendrocytes would revive and remyelinate naked axons after a period of time following administration of kynurenic acid and if so, what is that period of time?
Given the above considerations summarized in the Figure 1, therapeutic neuroregeneration following the SCI is the matter of properly designed pre-clinical experiments targeting the inflammation, involving hydrogels or other materials acting as the bridge for axonal regeneration across the COI and the removal of myelin sheaths in the white matter areas targeted for axonal regrowth with infusion of kynurenic acid. Considering that axonal regeneration in the filum terminale (an integral part of the CNS in the rat) and in the spinal cord is about 2 mm a day at its fastest (Kwiecien and Avram, 2008; Kwiecien, 2013), it will be a slow process in a much longer human spinal cord. Therapeutic neuroregeneration in clinical trials and beyond will require in vivo imaging to monitor regenerating axons throughout the therapy, another challenge for in vivo pre-clinical studies.
Figure 1.
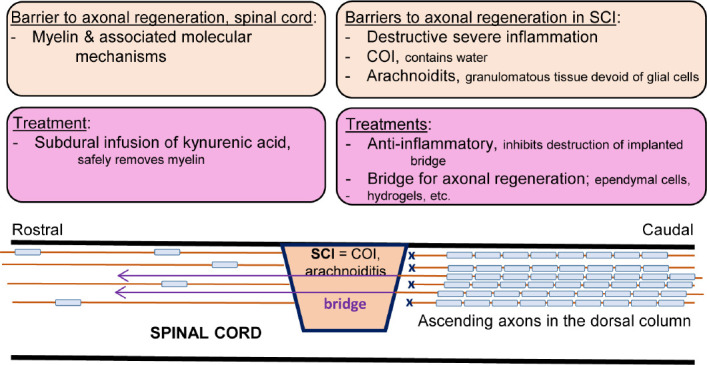
Conceptual presentation of cellular mechanisms involved in inhibition of axonal regeneration in the SCI with putative treatment directions.
A SCI involving the dorsal column cuts ascending axons and results in a locally severe, destructive and extraordinarily protracted inflammation that can destroy any implant to serve as the bridge for axonal regeneration. The site of injury deep in the spinal cord is converted within the first week into a COI filled with water from excess edema fluid and by macrophages or, if it is at the surface of the spinal cord, into arachnoiditis, a type of severe solid, granulomatous inflammation. Both types of inflammation are walled off from the rest of the spinal cord by astrogliosis. Regenerating axons do note enter the COI because it is filled with water and axons do not swim across on their own while arachnoiditis is devoid of glial cells and becomes a solid extra-neural tissue hostile to axons. The administration of anti-inflammatory agents is the first and necessary step in treating the SCI; the inhibition of the severe inflammation that allows for neuroprotection and also for protection of cells and/or synthetic materials implanted into the SCI lesion, or later, into the COI. The implantation of the choroid plexus, rich in ependymal cells, allowed for axons (colored purple and not myelinated) to cross the COI. Myelin sheaths in the spinal cord form an impassable barrier for axonal regeneration beyond the COI. Their widespread and safe removal and creation of large myelin-free areas can be accomplished by subdural infusion for 7 days of a very high concentration of kynurenic acid which allows for axonal plasticity, sprouting and potentially for regeneration of axons (purple, not myelinated) after crossing the COI. COI: Cavity of injury; SCI: spinal cord injury.
The present work was supported in part by VPC NeuroPath CONSULTING, Inc (to JMK).
Footnotes
P-Reviewer: Yildiz-Unal A; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open peer reviewer:Aysegul Yildiz-Unal, Mugla Sitki Kocman University, Turkey.
References
- 1.Dabrowski W, Kwiecien JM, Rola R, Klapec M, Stanisz GJ, Kotlinska-Hasiec E, Oakden W, Janik R, Coote M, Frey BN, Turski WA. Prolonged subdural infusion of kynurenic acid is associated with dose-dependent myelin damage in the rat spinal cord. PLoS One. 2015;10:e0142598. doi: 10.1371/journal.pone.0142598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwiecien JM. Cellular mechanisms of white matter regeneration in adult dysmyelinated rat model. Folia Neuropathol. 2013;51:189–202. doi: 10.5114/fn.2013.37703. [DOI] [PubMed] [Google Scholar]
- 3.Kwiecien JM. Tissue reaction to acellular implants in the acute spinal cord injury in the dysmyelinated rat. In: Berhardt LV, editor. Advances in medicine and biology. Vol. 99. Hauppauge, NY: Nova Science Publishers, Inc; 2016. pp. 111–123. [Google Scholar]
- 4.Kwiecien JM. The pathogenesis of neurotrauma indicates targets for neuroprotective therapies. Curr Neuropharmacol. 2021a doi: 10.2174/1570159X19666210125153308. doi: 102174/1570159X19666210125153308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwiecien JM, Avram R. Long distance axonal regeneration in the filum terminale of adult rats is regulated by ependymal cells. J Neurotrauma. 2008;25:196–204. doi: 10.1089/neu.2007.0454. [DOI] [PubMed] [Google Scholar]
- 6.Kwiecien JM, Dabrowski W, Dąbrowska -Bouta B, Sulkowski G, Oakden W, Kwiecien-Delaney CJ, Yaron JR, Zhang L, Marzec-Kotarska B, Stanisz GJ, Karis JP, Struzynska L, Lucas AR. Protracted inflammation extends damage after spinal cord injury. PLoS One. 2020a;15:e0226584. doi: 10.1371/journal.pone.0226584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwiecien JM, Dabrowski W, Kwiecien-Delaney BJ, Kwiecien-Delaney CJ, Siwicka-Gieroba D, Yaron JR, Zhang L, Delaney KH, Lucas AR. Neuroprotective effect of subdural infusion of Serp-1 in spinal cord trauma. Biomedicines. 2020b;8:372. doi: 10.3390/biomedicines8100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwiecien JM, Dabrowski W, Marzec-Kotarska B, Kwiecien-Delaney CJ, Yaron JR, Zhang J, Schutz L, Lucas AR. Myxoma virus derived immune modulating proteins, M-T7 and Serp-1, reduce early inflammation after spinal cord injury in the rat model. Folia Neuropathol. 2019;57:41–50. doi: 10.5114/fn.2019.83830. [DOI] [PubMed] [Google Scholar]
- 9.Kwiecien JM, Dabrowski W, Yaron RJ, Zhang L, Delaney KH, Lucas AR. The role of astrogliosis in formation of the syrinx in spinal cord injury. Curr Neuropharmacol. 2021b;19:294–303. doi: 10.2174/1570159X18666200720225222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwiecien JM, Jarosz B, Machova-Urdzikova L, Rola R, Dabrowski W. Subdural infusion of dexamethasone inhibits leukomyelitis after acute spinal cord injury in a rat model. Folia Neuropathol. 2015;53:41–51. doi: 10.5114/fn.2015.49973. [DOI] [PubMed] [Google Scholar]
- 11.Kwiecien JM, Jarosz B, Oakden W, Klapec M, Stanisz GJ, Delaney KH, Kotlinska-Hasiec E, Janik R, Rola R, Dabrowski W. An in vivo model of anti-inflammatory activity of subdural dexamethasone following the spinal cord injury. Pol J Neurol Neurosurg. 2016;50:7–15. doi: 10.1016/j.pjnns.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Kwiecien JM, Zhang L, Yaron JR, Schutz LN, Kwiecien-Delaney CJ, Enkidia A, Awo EA, Burgin M, Dabrowski W, Lucas AR. Local chitosan-serpin injection after spinal cord injury reduces inflammatory damage and improves neurologic function. J Clin Med. 2020c;9:1221. doi: 10.3390/jcm9041221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langner E, Lemieszek LM, Kwiecień JM, Rajtar G, Rzeski W, Turski WA. Kynurenate induces impairment of oligodendrocyte viability – on the role of glutamatergic mechanisms. Neurochem Res. 2016;42:838–845. doi: 10.1007/s11064-016-2009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rash JE, Davidson KG, Yasumura T, Furman CS. Freeze-fracture and immunogold analysis of aquaporin-4 (AQP4) square analysis, with models of AQPR lattice assembly. Neurosci. 2004;129:915–934. doi: 10.1016/j.neuroscience.2004.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]


