Abstract
It is well known that coronavirus disease (COVID-19) is associated with a coagulopathy characterized by a prothrombotic state of DIC different from the usual sepsis induced DIC with significant vascular thrombosis and worsening outcomes. This hypercoagulability results in enhanced thrombin generation and fibrin formation. Routine coagulation tests, such as the prothrombin time and the activated partial thromboplastin time, do not always actively reflect this state. Thrombelastography (TEG), appears therefore a potentially reliable method to evaluate COVID-19 related coagulopathy. TEG role in sepsis however has been less defined. Large randomized trials are needed to address whether TEG is able to identify patients likely to benefit from anticoagulation for sepsis induced coagulopathy. By reviewing the available literature on the use of TEG in sepsis, and the current knowledge on COVID-19 induced coagulopathy we provide clinicians with a practical guide on TEG testing in patients with COVID-19.
Introduction:
Thrombelastography (TEG) is viscoelastic, whole-blood test that assesses the coagulation system under low shear rate forces as seen with venous blood flow in real time. As a whole-blood test, TEG evaluates primary and secondary hemostasis and can assess for abnormal, hyperfibrinolysis.1 The viscoelastic properties of blood were first used in 1948 by Dr. Harmut who applied it to monitor hemostasis and guide blood transfusion in patients undergoing liver transplantation.2 The technical developments have improved the reproducibility of the viscoelastic method and allowed its use to monitor hemostasis in patients in various clinical settings such as pre- and post-cardiac surgery, trauma, and detection of anticoagulants or antiplatelet medication when coupled with platelet mapping.
ROTEM that stands for ROtational ElastoMEtry is another viscoelastic test, TEG and ROTEM difference is mainly the bit which rotates (cup vs pin) but they both allow for the evaluation of hemostasis in a similar way.3 The use of TEG in septic patients to identify sepsis induced coagulopathy (SIC), which is a precursor to overt DIC, has been the subject of debate in the literature and lacks any published standard parameters.
As it is becoming increasingly clear that critically ill patients with COVID-19 can develop a prothrombotic form of DIC, which is associated with increased risk of thrombosis and associated mortality, clinicians are now directing their attention towards TEG as a possible tool to identify as early as possible this COVID-19 induced coagulopathy.4
We review here the data on the use of TEGs in sepsis induced coagulopathy, and postulate the use of TEGs in managing COVID-19 patients.
Thrombelastography – real time assessment of patient coagulation:
Major parameters of the TEG tracing are: reaction time (R) which is the period from initiation of the test until the beginning of fibrin clot formation (figure 1), and thus, the (R) time is a direct reflection of the availability and function of clotting factors; a short (R) time indicates a hypercoagulable state and a prolonged (R) time indicates either hypocoagulability or the presence of an anticoagulant. The presence of anticoagulation (e.g. heparins) in a patient sample should prolong the (R) time. The next parameter, the K-time, measures the time from the start of fibrin clot formation until the curve reaches an amplitude of 20 mm. K-time is most reflective of fibrin kinetics, including fibrin formation and cross-linking. The K-time is coupled with the α-angle which is measured between the baseline and the tangent of the TEG curve amplitude. Maximum clot Amplitude (MA) reflects platelets contribution to the clot stability and full platelet potential under maximal stimulation by thrombin. The combination of K-time, α-angle and MA can evaluate both fibrinogen and platelet quantity and function. For example, an increased K-time and a decreased a-angle and MA would indicated reduced clot stability associated with hypofibrinogenemia. The final parameter in the TEG evaluates for fibrinolysis, and is measured by the difference between this maximum and the amplitude measured over 30 min (LY30). In the TEG assay, normal fibrin clots are stable for a few hours due to lack of tissue plasminogen activator. Evidence of clot lysis that starts within 30 minutes of clot formation suggests hyperfibrinolysis.1
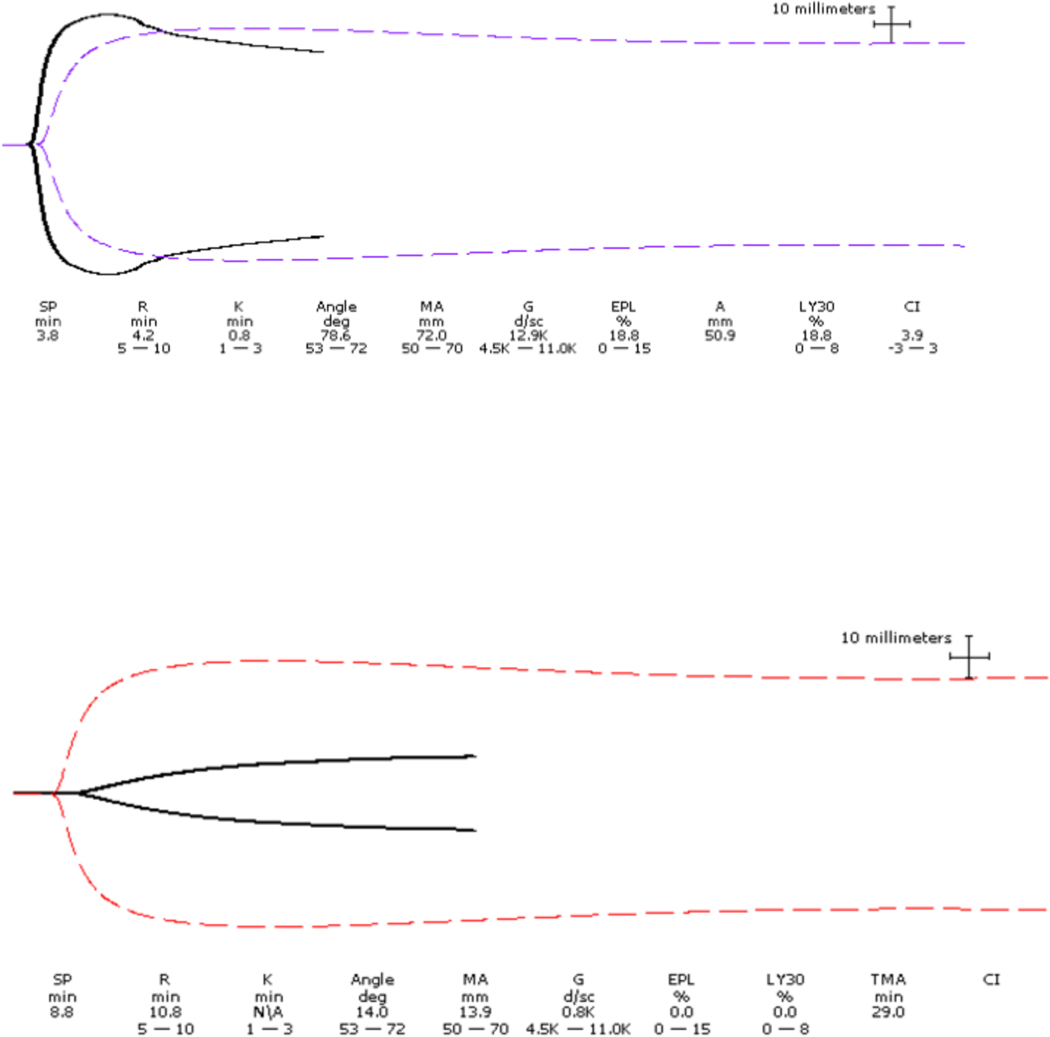
Figure 1.
TEG showed shortened clotting time (R) indicating increased rate of thrombin generation. Clot lysis indices (LY30 = 18.8 % and CI > 1) suggest secondary fibrinolysis, commonly seen in the first phase of sepsis or in disseminated intravascular coagulation (DIC). The excessive rate of clot breakdown clinically may present as bleeding.
TEG showed worsening coagulopathy (overt DIC) which is evidenced by prolonged R (coagulation factors consumption) high K and low angle ( low fibrinogen ) and low MA ( platelets consumption).
Coagulopathy and thrombelastography in sepsis:
Patients with sepsis have systemic activation of the coagulation system. In severe sepsis infections are associated with significant cytokine storms, this amplification of the coagulation system can lead to a significant increase in mortality5. When analyzing TEG tracings in patients with sepsis induced DIC, two states are identified: An early DIC state characterized by a hypercoagulable state and secondary fibrinolysis followed later on by a hypo coagulable state dominated by consumption of the coagulation factors.6
The notion of identifying this early stage of DIC to allow the use of interventions such as heparin, that might slow the process of thrombin generation and therefore prevent a detrimental overt DIC, has led the ISTH to validate a scoring system defining an early stage of sepsis induced coagulopathy (SIC) leading to an overt DIC.7 However, it is based only on regularly measured coagulation parameters and does not include TEG findings; whether TEG is superior or adds value to this scoring system remains subject of debate.
A meta-analysis looking at TEG’s ability to identify SIC and sepsis related DIC showed that the available data is heterogenous at best, and the percentage of patients in whom TEG detected SIC successfully ranged anywhere from 43% to 100%.3 The observed heterogeneity could have been related to the timing when the TEG was performed during an ICU admission. Since coagulopathy in sepsis is a dynamic process the, sensitivity of this test might increase with rigorous, daily TEG profiles during an ICU admission.
Some authors have suggested that TEG might add significant value to conventional, clot-based coagulation parameters in identifying critically ill septic patients with coagulopathy. In a prospective observational study, TEG was able to identify normo-coagulable, hyper-coagulable and hypo-coagulable patients better than conventional coagulation assays.8 In a large cohort of 56 sepsis patients and 52 postoperative controls, using ROTEM, the lysis parameter (lysis index) had a better diagnostic value than did procalcitonin in identifying sepsis,9 and in a different study ROTEM also allowed differentiation between sepsis and post-operative systemic inflammatory response10. Patients who were identified as having hypo-coagulable TEG profile, consistent with overt DIC, had more severe organ failure which translated to higher mortality suggesting that viscoelastic assay can add prognostic value as well3,11 however at this stage of coagulopathy interventions might fail to improve the patient’s outcome.
TEG appear therefore as an attractive tool that could identify SIC in patients with sepsis, however further studies are needed: One to establish uniform definitions of what would be considered as hypo-and hyper-coagulable state as some studies classified patients when measurements were outside the reference values and other studies compared the numbers to those of healthy controls9,11,12. In addition, large randomized trials are also needed to address whether TEG is able to identify patients likely to benefit from anticoagulation for SIC. One case report in a patient with post-operative fibrinolysis treatment with low dose unfractionated heparin showed normalization of LY30 and prevention of progression to DIC. 13 However in another report of 33 patients with severe sepsis, of whom 17 were treated with antithrombin in addition to low dose low molecular weight heparin, hypercoagulability was not reversed by this treatment and did not change the outcome in these patients despite significantly supratherapeutic AT levels.14
The overall quality of evidence on the value of TEG in adults with sepsis remains low, however it remains promising that TEG parameters, especially in the setting of hypo-coagulability might be able to identify patient with sepsis induced DIC and predict more severe disease and higher mortality. There are no large published studies documenting that starting anticoagulation treatment when patients are identified to have a hyper-coagulable process on TEG is any helpful in preventing worsening to DIC or reducing mortality.
Thrombelastography in patients with COVID-19 infection:
Patients with severe COVID-19 infections can develop coagulation activation mimicking a DIC state4, with incidence of macro-thrombotic events in up to 30% of ICU patients15 and multiple autopsy reports showing micro-thrombotic events in the pulmonary vasculature of patients who died from COVI-19 induced lung injury.16
A recent retrospective study describing TEG findings on 24 ICU patients showed findings consistent a phenotype with hyper-coagulability with decreased in (R) and K times and increased angle and MA that was persistent throughout their course. Interestingly, there was no evidence via TEG tracings for hyper-fibrinolysis, suggesting that patients with COVID-19 do not have the usual acute DIC parameters seen in sepsis, which often includes progression to a hypo-coagulable pattern.17
Based on the available data, the TEG assay in patients with COVID-19 might be a potentially useful addition to conventional, clot-based coagulation tests, especially in complicated patients where conventional coagulation assays show inconclusive results. TEG will also allow clinicians (through LY 30 parameter for the TEG 5000® or through Lysis cartridge TEG 6s) to identify a lack of fibrinolysis, which seems to be associated with COVID-19 infections17.This lack of fibrinolysis raises the question as to whether the use of fibrinolytics such as t-PA would help reverse this process and improve these patients condition as has been suggested by multiple case reports17,18. It also can be helpful in monitoring effects of anticoagulation either with unfractionated heparin or LMWH19 and will be very helpful in establishing the full coagulation picture for late-stage COVID-19 patients that have been placed on ECMO.
References:
- 1.Braintree M. TEG® 5000 System user manual for clinical use indications. 2017. [Google Scholar]
- 2.Hartert H. Blutgerinnungsstudien Mit Der Thrombelastographie; Einem Neuen Untersuchungs Verfahren. Klin Wochenschr. 1948;26(37–38):577–583. [DOI] [PubMed] [Google Scholar]
- 3.Müller MC, Meijers JC, Vroom MB et al. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Crit Care. 2014;18(R30 ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iba T, Levi M, Levy JH. Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. Semin Thromb Hemost. 2020;46(1):89–95. [DOI] [PubMed] [Google Scholar]
- 6.MD SJMaKK. Introduction to TEG interpretation. [Google Scholar]
- 7.Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9):e017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muzaffar SN, Baronia AK, Azim A, et al. Thromboelastography for Evaluation of Coagulopathy in Nonbleeding Patients with Sepsis at Intensive Care Unit Admission. Indian J Crit Care Med. 2017;21(5):268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamzik M, Eggmann M, Frey UH, et al. Comparison of thromboelastometry with procalcitonin, interleukin 6, and C-reactive protein as diagnostic tests for severe sepsis in critically ill adults. Crit Care. 2010;14(5):R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner T, Schmidt K, Delang M, et al. Viscoelastic and aggregometric point-of-care testing in patients with septic shock - cross-links between inflammation and haemostasis. Acta Anaesthesiol Scand. 2012;56(10):1277–1290. [DOI] [PubMed] [Google Scholar]
- 11.Daudel F, Kessler U, Folly H, Lienert JS, Takala J, Jakob SM. Thromboelastometry for the assessment of coagulation abnormalities in early and established adult sepsis: a prospective cohort study. Crit Care. 2009;13(2):R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins PW, Macchiavello LI, Lewis SJ, et al. Global tests of haemostasis in critically ill patients with severe sepsis syndrome compared to controls. Br J Haematol. 2006;135(2):220–227. [DOI] [PubMed] [Google Scholar]
- 13.Shafi H, Tcherniantchouk O, Chaffin DJ, Mason H, Klapper E. Transfusion medicine illustrated. Thromboelastography: a more accurate assessment of global hemostasis. Transfusion. 2013;53(11):2605. [DOI] [PubMed] [Google Scholar]
- 14.Gonano C, Sitzwohl C, Meitner E, Weinstabl C, Kettner SC. Four-day antithrombin therapy does not seem to attenuate hypercoagulability in patients suffering from sepsis. Crit Care. 2006;10(6):R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A Report of Thromboelastography Findings and other Parameters of Hemostasis. J Thromb Haemost. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Hajizadeh N, Moore EE, et al. Tissue Plasminogen Activator (tPA) Treatment for COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS): A Case Series. J Thromb Haemost. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias JD, Lopez-Espina CG, Panigada M, Dalton HJ, Hartmann J, Achneck HE. Cartridge-Based Thromboelastography Can Be Used to Monitor and Quantify the Activity of Unfractionated and Low-Molecular-Weight Heparins. TH Open. 2019;3(3):e295–e305. [DOI] [PMC free article] [PubMed] [Google Scholar]