Abstract
Scalp electrode impedance measurements recorded by wired and wireless electroencephalography (EEG) machines in 7 healthy dogs were compared. Eight recordings resulted in 80 impedance readings from subdermal wire electrodes (locations F7/F8, F3/F4, T3/T4, C3/C4, Fz, and Cz). Impedance values were measured first from the wired and then the wireless EEG machine. Wireless impedance measurements were higher than the wired EEG machine in 79/80 readings (P ≤ 0.05), being on average 2.83 kΩ [P ≤ 0.05, 95% confidence interval (CI): 2.51 to 3.14, SD = 1.42] higher. Impedances from the wired machine ranged between < 0.5 and 9 kΩ (mean = 3.09, median = 2.00, SD = 2.15), whereas impedances from the wireless machine ranged between 2.69 and 6.07 kΩ (mean = 5.92, median = 5.05, SD = 2.59). Despite these differences in impedance measurements, both machines measured similar impedance patterns. The wireless EEG machine’s impedance measurements, therefore, should be acceptable for veterinary clinical settings.
Résumé
Les mesures d’impédance des électrodes du cuir chevelu enregistrées par des appareils EEG filaires et sans fil chez sept chiens en bonne santé ont été comparées. Huit enregistrements ont donné 80 lectures d’impédance à partir de fils-électrodes sous-cutanés (emplacements F7/F8, F3/F4, T3/T4, C3/C4, Fz et Cz). Les valeurs d’impédance ont été mesurées d’abord à partir de la machine EEG filaire puis sans fil. Les mesures d’impédance sans fil étaient plus élevées que l’EEG filaire dans 79/80 lectures (P ≤ 0,05), étant en moyenne de 2,83 kΩ [P ≤ 0,05, intervalle de confiance (IC) à 95 % : 2,51 à 3,14, SD = 1,42] plus élevé. Les impédances de la machine filaire étaient comprises entre < 0,5 et 9 kΩ (moyenne = 3,09, médiane = 2,00, SD = 2,15), tandis que les impédances de la machine sans fil étaient comprises entre 2,69 et 6,07 kΩ (moyenne = 5,92, médiane = 5,05, SD = 2,59). Malgré ces différences dans les mesures d’impédance, les deux machines ont mesuré des patrons d’impédance similaires. Les mesures d’impédance de la machine EEG sans fil doivent donc être acceptables pour les paramètres cliniques vétérinaires.
(Traduit par Docteur Serge Messier)
Electroencephalography (EEG) is the recording of cerebrocortical electrical activity using electrodes placed on the head (1,2). The main indication of EEG is the diagnosis of seizures, a common canine neurological disorder (3,4). Electroencephalography is required to attain the highest tier of confidence in the diagnosis of canine epilepsy (4). In EEG recordings, the impedance is a measure of the connection quality between tissue and electrode and is obtained by calculating the opposition to current flowing between electrodes (5). The EEG recording standards for humans accept impedance values up to 10 kΩ, but it is recommended that impedance values be kept to < 5 kΩ to produce accurate EEG recordings (6). In a recent veterinary EEG study, however, higher impedances were observed using novel wireless equipment (7).
The objective of this study was to investigate electrode impedance discrepancies between wired and wireless monitors used for recording canine scalp EEG (7). The hypothesis is that no significant difference exists between electrode impedance values measured by the 2 EEG devices.
Animal procedures were conducted in accordance with the Canadian Council on Animal Care and approved by the University of Guelph Animal Care Committee (Animal Utilization Protocol 3954, 6 April 2018). Seven healthy neutered or spayed adult research beagle dogs were recorded: 3 male and 4 females, weighing between 6.5 kg to 12.2 kg (mean: 9.3 kg), 1 to 2 y of age, with normal physical and neurological examinations. One of the dogs was recorded twice, resulting in 8 recording sessions. Each dog was recorded on a separate day and acted as its own comparison. To keep the dogs calm during the instrumentation process, Propofol (Propofol injection; Novopharm, Toronto, Ontario) was titrated intravenously to effect to obtain sedation and muscle relaxation while maintaining jaw tone and spontaneous breathing. Once sedated, new subdermal wire electrodes (SWEs) (Subdermal wire electrode, model F-SWEL-48; Grass Technologies, an Astro-Med Product Group, West Warwick, Rhode Island, USA) were placed on 10 skull locations: F7/F8, F3/F4, T3/T4, C3/C4, Fz, and Cz, with the ground placed on the dorsal midline of the neck and the reference placed midline between the medial canthi (7). Adhesive tape was placed on top of the SWE insertion points to ensure electrodes did not fall out. During the recording sessions, the SWEs were first plugged into the wired EEG machine (Stellate Harmonie Systems, models HSYS-REC-DUO and HSYS-REC-WP2; Stellate Systems, Montreal, Quebec) for impedance measurement. The SWEs were then disconnected and immediately reconnected to the wireless EEG machine (Trackit MK3 EEG/Polygraphy Recorder with Video; Lifelines Neurodiagnostic Systems, Troy, Illinois, USA) through which a second impedance measurement was taken.
Impedance measurements were compared between the 2 EEG machines for 10 electrode positions in the 8 recording sessions resulting in 80 comparisons. For each comparison, the difference between the 2 impedance measurements was calculated by subtracting 1 value from the other. Statistical analysis was performed blinded to machine identification. The Shapiro-Wilk test was used to check for normality. If the difference between the paired impedance measurements were normally distributed, a paired t-test was used, otherwise a Wilcoxon rank-sum test was done instead. A Bland-Altman plot and calculation of Lin’s concordance correlation coefficient were used to measure agreement between the paired impedance measurements. Significance was set at P ≤ 0.05.
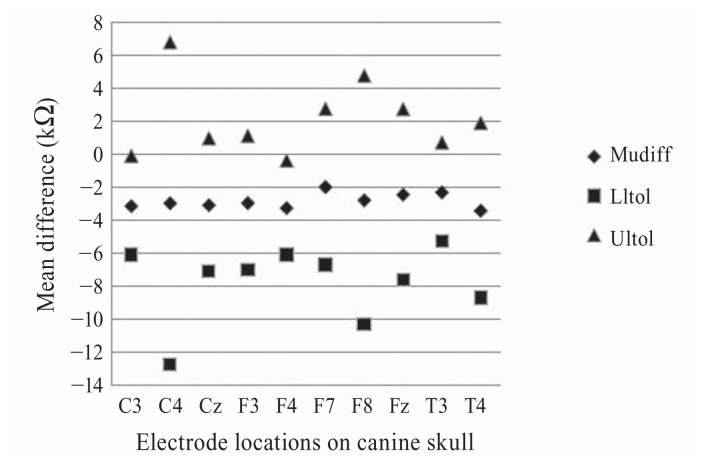
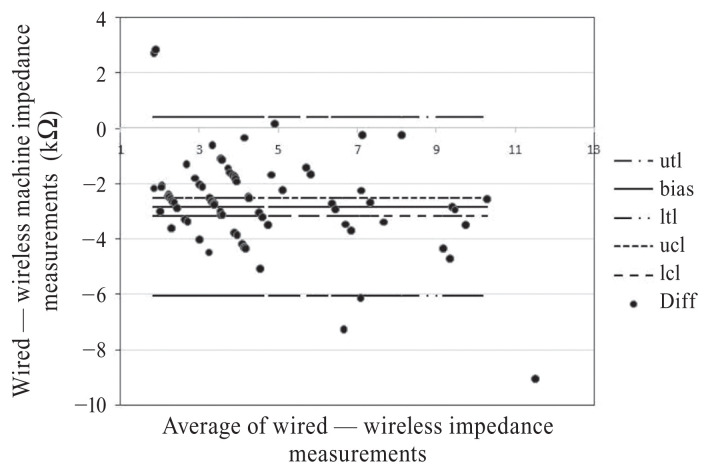
The wired EEG machine’s impedances ranged between < 0.5 and 9.0 kΩ [mean = 3.09, median = 2.00, standard deviation (SD) = 2.15], whereas the wireless machine’s impedances were between 2.69 and 16.07 kΩ (mean = 5.92, median = 5.05, SD = 2.59) (Figure 1). On average the wireless EEG impedance readings were higher than the wired EEG in 79/80 measurements. The wireless EEG machine’s impedance readings were 2.83 kΩ higher than the wired EEG impedance readings [upper tolerance limit (UTL) = 0.40, lower tolerance limit (LTL) = −6.07] (Figure 2). The Lin’s concordance correlation coefficient was 0.48 (P < 0.05, 95% CI). When impedance measurement differences were compared for each electrode skull location, the smallest difference between the 2 machines was at F7 and the largest difference was at T4 (Table I); this likely reflects the variability of the differences.
Figure 1.
Plot of the mean difference (Mudiff) (wired — wireless, diamonds) of each electrode location with respective upper tolerance limits (Ultol) and lower tolerance limits (Lltol) for all dogs.
Figure 2.
Bland-Altman plot showing an average difference (bias) of 2.83 kΩ in which the wireless EEG impedance measurements were greater than the wired EEG impedance measurements, with a 2-sided upper tolerance limit (utl) and lower tolerance limit (ltl) of 0.403 kΩ and −6.065 kΩ, respectively. The confidence intervals on the differences (circles) have an upper control limit (ucl) of −2.514 kΩ and a lower control limit (lcl) of −3.148 kΩ.
Table I.
Comparison of the mean impedance difference for each location on the dog’s skull with a 2-sided lower tolerance limit and upper tolerance limit. The mean difference was obtained by subtracting the impedance measurement of the wireless EEG machine from the wired EEG machine. All mean difference values are statistically significant (P < 0.0001).
| Electrode location on skull | Mean difference (kΩ) | Lower tolerance limit (kΩ) | Upper tolerance limit (kΩ) | P-value |
|---|---|---|---|---|
| C3 | −3.13 | −6.13 | −0.09 | 0.000012304 |
| C4 | −2.96 | −12.72 | 6.80 | 0.015055867 |
| Cz | −3.08 | −7.13 | 0.97 | 0.000088978 |
| F3 | −2.95 | −7.02 | 1.12 | 0.000120298 |
| F4 | −3.25 | −6.12 | −0.39 | 0.000006425 |
| F7 | −1.99 | −6.73 | 2.76 | 0.003066006 |
| F8 | −2.78 | −10.33 | 4.77 | 0.005967053 |
| Fz | −2.45 | −7.62 | 2.73 | 0.001575756 |
| T3 | −2.31 | −5.32 | 0.71 | 0.000086880 |
| T4 | −3.41 | −8.73 | 1.91 | 0.000260980 |
Given the lack of impedance standard in veterinary EEG, it is assumed that most clinicians follow the human impedance standard recommendation of ≤ 5 kΩ (6). Our study showed that impedance values for the wireless EEG machine were on average > 5 kΩ, whereas the wired EEG was recording impedance values < 5 kΩ using the same electrodes on the same dogs in the same recording sessions. In addition, although all the wired EEG machine’s impedance readings stayed below an acceptable range of 10 kΩ, the wireless machine’s impedance readings exceeded 10 kΩ and even reached as high as 16.07 kΩ. The mean impedance of 5.92 kΩ and median impedance of 5.05 kΩ for the wireless EEG machine are also both higher than the recommended 5 kΩ, whereas the wired EEG machine has a mean and median < 5 kΩ (mean = 3.09, median = 2.00). Not only do the 2 machines demonstrate a significant difference in measured impedance values but based on the human impedance standard, the wireless machine measures impedance values are higher than acceptable for veterinary clinical use. However, the machines do seem to be measuring the same impedance patterns as the wireless machine’s impedance values are consistently (79/80) higher than the wired EEG. This almost uniform difference in measured impedances could be due to differences in the calculation algorithms and sensitivity calibrations with which the 2 EEG machines were originally designed or programmed. The Lin’s concordance correlation value of 0.48 shows moderate strength of agreement between impedance values. Despite this moderate agreement, the Bland-Altman plot indicates that the differences between the upper and lower tolerances vary too much for impedances from one EEG machine to be used to clinically predict the other (P < 0.05, 95% CI).
One of the limitations during the data collection process was that, due to additional data being recorded for another study, the wired EEG machine was used first on all dogs, not allowing for randomization in the order of recording with the EEG machines. However, the 2 machines’ impedance measurements on each dog were separated by only the time required to switch the electrode plugs from one machine to the other, which was as close to simultaneous measurement as could be achieved. Another limitation is that not all the possible scalp electrode locations were tested on the dogs. Again, this was due to the electrode protocol designed for the other study. In addition, since each dog was measured on a separate day variability between days could not be statistically confirmed. Future testing would ideally collect all data on 1 day for all dogs and repeat for several days. Lastly, this study examined only 1 type of electrode and compared only 2 EEG machines. Future studies could test various electrode types and other EEG machines to determine the pervasiveness of this impedance variation.
In conclusion, significant impedance measurement differences were detected between these wired and wireless EEG machines using SWEs in dogs, with the wireless EEG machine averaging impedance values 2.83 kΩ higher. Even though the measured impedances between the 2 machines are not the same, they do follow the same patterns with the wireless EEG machine impedance reading being greater than that of the wired machine in 79/80 measurements. Although it is expected that the wireless EEG machine will yield higher impedance measurements in dogs than the human EEG standard, these values should still be acceptable in veterinary clinical canine EEG. Having confirmed this discrepancy between impedance readings, thorough investigation would require calibration with external impedance standards and testing machine-to-machine variability.
Acknowledgments
Thank you to the technicians from the Central Animal Facility at the University of Guelph for their assistance during the data collection process. This study was supported by an equipment grant from the Canada Foundation for Innovation/Ontario Ministry of Research and Innovation (project #30953 grant #460425), and the Ontario Veterinary College Pet Trust Fund (grant #053614).
References
- 1.De Risio L, Platt S. Canine and Feline Epilepsy: Diagnosis and Management. Boston, Massachusetts: CABI; 2014. pp. 325–346. [Google Scholar]
- 2.Packer RMA, Berendt M, Bhatti S, et al. Inter-observer agreement of canine and feline paroxysmal event semiology and classification by veterinary neurology specialists and non-specialists. BMC Vet Res. 2015;11:39. doi: 10.1186/s12917-015-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandler K. Canine epilepsy: What can we learn from human seizure disorders? Vet J. 2006;172:207–217. doi: 10.1016/j.tvjl.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.De Risio L, Bhatti S, Muñana K, et al. International veterinary epilepsy task force consensus proposal: Diagnostic approach to epilepsy in dogs. BMC Vet Res. 2015;11:148. doi: 10.1186/s12917-015-0462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kappenman ES, Luck SJ. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology. 2010;5:888–904. doi: 10.1111/j.1469-8986.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis EK, Frey LC. Electroencephalography: An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants. Chicago, Illinois: American Epilepsy Society; 2016. p. 22. [PubMed] [Google Scholar]
- 7.James FMK, Cortez MA, Monteith G, et al. Diagnostic utility of wireless video-electroencephalography in unsedated dogs. J Vet Intern Med. 2017;5:1469–1476. doi: 10.1111/jvim.14789. [DOI] [PMC free article] [PubMed] [Google Scholar]