Abstract
Dogs with lower airway pathology that present in respiratory distress often receive oxygen therapy as the first line of treatment regardless of the underlying cause. Conventional “low-flow” systems deliver oxygen with a maximum flow rate of 15 L/minute. Traditionally, when an animal’s respiratory status does not improve with conventional oxygen therapy and treatments for underlying disease, options might be limited to either intubation and mechanical ventilation or humane euthanasia. High-flow oxygen therapy (HFOT) has been gaining popularity in veterinary medicine as an alternative route of oxygen supplementation for animals that require support beyond conventional therapy. High-flow oxygen therapy can supply a mixture of air and oxygen via a heated and humidified circuit. It is user friendly and can be used in an environment in which mechanical ventilation is unavailable.
This review article is written for emergency doctors and general practitioners who lack access to mechanical ventilation. This article briefly reviews pertinent respiratory physiology, traditional oxygen supplementation techniques, the physiology of HFOT, and the limited evidence available in veterinary medicine regarding the use of HFOT, its applications, and limitations. Guidelines for the use of HFOT are suggested and HFOT is compared to conventional therapy.
Résumé
Les chiens avec une pathologie des voies respiratoires inférieures qui présentent une détresse respiratoire reçoivent souvent une oxygénothérapie en première intention, quelle que soit la cause sous-jacente. Les systèmes conventionnels à « faible débit » fournissent de l’oxygène avec un débit maximum de 15 L/minute. Traditionnellement, lorsque l’état respiratoire d’un animal ne s’améliore pas avec l’oxygénothérapie conventionnelle et les traitements de la maladie sous-jacente, les options peuvent se limiter à l’intubation et à la ventilation mécanique ou à l’euthanasie. L’oxygénothérapie à haut débit (HFOT) gagne en popularité en médecine vétérinaire en tant que voie alternative de supplémentation en oxygène pour les animaux qui nécessitent un soutien au-delà de la thérapie conventionnelle. L’oxygénothérapie à haut débit peut fournir un mélange d’air et d’oxygène via un circuit chauffé et humidifié. Il est convivial et peut être utilisé dans un environnement où la ventilation mécanique n’est pas disponible.
Cet article de revue est écrit pour les médecins urgentistes et les médecins généralistes qui n’ont pas accès à la ventilation mécanique. L’article passe brièvement en revue la physiologie respiratoire pertinente, les techniques traditionnelles de supplémentation en oxygène, la physiologie de la HFOT et les preuves limitées disponibles en médecine vétérinaire concernant l’utilisation de la HFOT, ses applications et ses limites. Des lignes directrices pour l’utilisation de la HFOT sont suggérées et la HFOT est comparée au traitement conventionnel.
(Traduit par Docteur Serge Messier)
Dogs that present in respiratory distress typically either have difficulty bringing oxygen into the lungs or have reductions in gas exchange between the alveoli and pulmonary capillaries. The most common examples of diseases leading to reduced oxygen intake are either an upper airway obstruction blocking airflow into the trachea or bronchi, or pleural space disease reducing the ability to expand the lungs. In contrast, diseases that cause decreased gas exchange usually involve the lung tissue itself (i.e., lower airway injury) and include pulmonary edema, pulmonary hemorrhage, or pneumonia. Regardless of the exact cause, the end result of upper or lower airway conditions is tissue hypoxia due to inadequate oxygen supply. Specific treatment and diagnosis of upper airway disease and pleural space disease have been well-documented but are beyond the scope of this article (1). This article focuses on oxygen supplementation techniques to improve the clinical status of veterinary patients with lower airway injury with a brief review of traditional techniques available to clinics without access to mechanical ventilation as well as the newer option of high-flow oxygen therapy.
Introduction
Under normal settings, the movement of oxygen and carbon dioxide into and out of the lungs or to and from the tissues occurs via simple diffusion. When an animal breathes, air moves through the conducting airways (trachea, bronchi) into the alveoli where the oxygen in the inhaled air diffuses into the bloodstream (2). The oxygen is then transported in the blood to the tissues either in a chemically bound form associated with hemoglobin or a free unbound dissolved form (2,3).
In a normal setting, 97 to 98% of oxygen in the body is transported in combination with hemoglobin (i.e., the bound form) and only a very small amount is dissolved in plasma. Although numerically small, the dissolved oxygen is the oxygen that diffuses into the tissues. A major role of hemoglobin is to release oxygen to replenish the dissolved pool, thus allowing continual oxygen diffusion from the blood into tissues. It is important to note that severe anemia (typically hematocrit < 12%) will reduce this storage pool of oxygen enough to contribute to hypoxemia.
The overall arterial oxygen content of the blood (CaO2) is described by:
The bound oxygen content of the arterial blood depends on the hemoglobin concentration (Hgb) and what percentage of the oxygen binding sites on hemoglobin are saturated with oxygen (SaO2). The dissolved oxygen content is determined by the partial pressure of oxygen (PaO2) as governed by Henry’s law: for each 1 mmHg of pressure, there are 0.003 mL of oxygen per deciliter (dL) of blood.
Clinically, the aim of oxygen supplementation is to increase the fraction of inspired oxygen (FiO2) above room air (21%). In so doing, oxygen concentrations in the alveoli should improve, increasing oxygen diffusion into the blood, fully saturating the oxygen binding sites on Hgb and increasing the partial pressure of plasma oxygen. The overall effect is improvement in the CaO2.
Hypoxemia
Many types of lower airway pathology prevent these normal physiologic processes, resulting in hypoxemia (low arterial oxygen content) and decreased oxygen delivery to tissues (tissue hypoxia). Hypoxemia is documented by directly measuring dissolved oxygen levels in an arterial blood sample (i.e., measuring the PaO2). A less invasive, readily available option for estimating the PaO2 is measuring the oxygen saturation of hemoglobin (SpO2) using a pulse oximeter. Hypoxemia is defined as a PaO2 < 80 mmHg and/or a SpO2 < 95% (4). The various causes for hypoxemia with examples are listed in Table I. Note that although listed separately, some pathologies can fit into more than one category.
Table I.
Mechanisms of hypoxemia with examples.
| Mechanism of hypoxemia | Example |
|---|---|
| Low FiO2 | High altitude Anesthetic accidents in which oxygen source is reduced, occluded, or otherwise not appropriate for the patient |
| Hypoventilation | Cervical myelopathy |
| Reduced movement of air into and out of the alveoli | Neuromuscular disease Sedation/anesthesia |
| Diffusion impairment | Interstitial lung disease (smoke inhalation, ARDS) |
| Thickening or other abnormality of the alveolar interstitial membrane reducing gas exchange | Pulmonary fibrosis |
| High V:Q mismatch | Pulmonary thromboembolism |
| Ventilation normal (normal oxygen delivery to alveoli), pulmonary blood flow compromised | Atelectasis |
| Low V:Q mismatch | Pulmonary edema |
| Ventilation compromised (low oxygen delivery to alveoli), pulmonary blood flow normal | Pneumonia Pulmonary neoplasia |
| Right to left anatomic shunts | Right to left patent ductus arteriosus Ventricular septal defect |
FiO2 — Fraction of inspired oxygen; ARDS — Acute respiratory distress syndrome; V — Ventilation; Q — Perfusion.
Movement of air
Minute ventilation is the amount of oxygen/carbon dioxide entering or leaving the lungs per minute (5). It is expressed as a product of respiratory rate and tidal volume. Minute ventilation decreases at rest and increases during exercise. The formula for minute ventilation is:
For a 40-kg dog with a normal resting respiratory rate (20 to 30 breaths/min; bpm) will have a minute ventilation of:
When an animal is hypoxemic, the minute ventilation increases as a compensatory mechanism to increase oxygen concentration in the blood (i.e., the animal breathes faster and/or deeper to get more oxygen into its lungs). This same 40-kg dog in respiratory distress could compensate by breathing rapidly at a respiratory rate of 60 bpm, and therefore its minute ventilation could increase as follows:*
Similarly, if the same 40-kg dog demonstrated its respiratory distress by breathing more deeply but with a normal respiratory rate, its minute ventilation could still increase as follows:*
Supplementing oxygen
Low-flow oxygen systems
Traditional oxygen supplementation is through low-flow systems. Common low-flow oxygen techniques (Table II) used in lower airway injury in veterinary medicine include flow-by oxygen, nasal cannula, face mask, oxygen cages, and the oxygen hood. The goal of each is to enrich the inspired gas to increase oxygen concentration in the alveoli and increase oxygen diffusion into the bloodstream.
Table II.
Supplemental oxygen (low-flow versus high-flow systems).
| Device | Flow | Estimated maximal FiO2 | Advantages | Disadvantages |
|---|---|---|---|---|
|
| ||||
| Low-flow systems | ||||
| Flow-by oxygen | 6 to 8 L/min | 25 to 45% | Minimal equipment, Easy patient accessibility for treatments | Oxygen dilution with room air, patient intolerance, personnel required to hold tube |
| Oxygen face mask | 1 to 6 L/min | 35 to 45% | Minimal equipment, easy patient accessibility for treatments | Patient intolerance, risk of CO2 rebreathing, personnel required to hold mask |
| Oxygen cage | N/A | 50 to 60% | Low stress to patient, suitable for small dogs and cats | Difficult to monitor patient, oxygen dilution with room air every time cage door opened, development of hyperthermia due to heat and humidity in cage, cost of commercial cages |
| Nasal cannulas | Flow not to exceed a total of 5 L/min through each nasal cannula | 30 to 60% (depending on flow rate) | Easy patient access for evaluations and treatment, can effectively achieve high oxygen concentrations | Nasal irritation, sneezing, nasal drying, panting leading to air entrainment |
|
| ||||
| High-flow system | ||||
|
| ||||
| High-flow oxygen therapy | Up to 60 L/min | 21 to 100% space, PEEP effects, | Washout of anatomic dead patient mobility, nasal mucosal humidification and warming of inspired air | Noise of machine, reduced irritation, can delay initiation of mechanical ventilation |
FiO2 — Inspired oxygen content; L/min — Liters per minute; N/A — Not applicable; CO2 — Carbon dioxide; PEEP — Positive end expiratory pressure.
Low-flow oxygen systems will provide oxygen at a flow rate up to 15 L/min; as shown, this flow rate is often lower than a dyspneic patient’s inspiratory demands as dictated by its minute ventilation. As a result, when an animal receiving low-flow oxygen is breathing very fast or very deep, the animal will breathe in the delivered oxygen as well as additional room air with each breath. This process is called “air entrainment” (6,7). Air entrainment is detrimental because it causes the animal to receive a lower FiO2 per breath than intended due to the mixing of room air with the oxygen being delivered.
Flow-by-oxygen
Flow-by oxygen is the easiest method to provide oxygen in an emergency. Oxygen is provided via a plastic tube that is held ~2 cm from the patient’s nostrils. The FiO2 with flow-by oxygen can vary between 24 to 45% at a flowmeter setting of 6 to 8 L/min of oxygen (8). Disadvantages of this system include diluting oxygen with room air during each breath even without entrainment and patient intolerance (the dog moving its head away from the oxygen) negating long-term use.
Oxygen face mask
A face mask is another method used to provide oxygen to dogs and can achieve a FiO2 between 35 to 45% at flow rates of 1 to 6 L/min (8). In a recent study in dogs, oxygen supplementation through a face mask was more effective in improving the FiO2 compared with flow-by oxygen (9). There are several inherent reasons why a face mask is not a good long-term option for delivering oxygen to an animal including patient intolerance of the mask, the need for someone to constantly hold the mask to the patient, and the risk of carbon dioxide rebreathing if the mask is too tight and does not allow gases to leave it. Additionally, and paradoxically, when the mask isn’t fitted tightly to the animal’s face, air entrainment often occurs due to dilution of oxygen with room air within the mask.
Oxygen cage
Oxygen can be delivered into a tightly sealed box made of materials such as acrylic, plexiglass, or polyvinyl chloride, a standard cage fitted with a sealed door of similar materials, or through commercial oxygen cages wherein the temperature, humidity, and oxygen concentration of the cage can be controlled. The biggest advantage of an oxygen cage over other low-flow techniques is that oxygen can be provided “hands off” to the animal, thus reducing the stress of handling. Oxygen cages can reach a FiO2 as high as 60% when an airtight seal is created with minimal to no air entrainment.
Disadvantages of oxygen cages include expense (when purchasing commercial cages), the time it takes to fill the cage with oxygen and achieve a desirable oxygen concentration, as well as the risk of hyperthermia if the temperature and humidity in the cage is not adequately maintained. In addition, due to the need to keep the cage closed as much as possible to minimize dilution and loss of oxygen levels, patient monitoring and handling is difficult. Oxygen cages are not suitable for larger patients due to restricted cage size and the rapid development of hyperthermia with large dogs in a relatively tight space even with temperature and humidity control. Furthermore, with tightly sealed oxygen cages or cages not equipped with material to reabsorb carbon dioxide there is a potential for carbon dioxide, gas buildup, and rebreathing which can be harmful to the patient.
Nasal oxygen
Oxygen can be delivered by either nasal cannulas or nasal catheters and can either be unilateral or bilateral. Nasal oxygen cannulas/catheters require minimal equipment and, in many cases, can be placed with minimal or no sedation. Various tubes (red rubber catheters, feeding tubes, urinary catheters) can be used to deliver nasal oxygen making this technique adaptable for different dogs and practices. Nasal cannulas/catheters are placed so that the end of the cannula resides just above the soft palate or within the nasopharynx. Alternatively, nasal prongs can achieve a similar FiO2, but have the disadvantage of easy dislodgement due to short length.
Nasal oxygen efficiently delivers a constant flow of oxygen, thus creating a reservoir of oxygen-rich gas in the upper airway during each breath. As a result, in healthy dogs with closed mouth breathing, flow rates as low as 0.1 L/kg BW per minute can reach a FiO2 of 50 to 60%, whereas higher flow rates of 0.2 L/kg BW per minute can achieve a FiO2 as high as 80% (10). The oxygen supply delivered by nasal cannulas is typically connected to a bubble humidifier filled with sterile water or saline at room temperature to minimize drying of the nasal mucosa. However, despite humidification, nasal oxygen can only be effectively delivered up to a maximum of 4 to 6 L/min using nasal cannulas (11). Flow rates any higher will lead to drying and irritation of nasal mucosa, leading to patient discomfort.
When a dog has a high minute ventilation requirement, it may exceed the flow rate provided by nasal oxygen and air entrainment will likely occur. Similarly, when dogs pant, the room air mixing with the delivered oxygen in the oro- and nasopharynx will lead to entrainment.
Nasal cannulas, as well as nasal catheters, are well-tolerated in dogs but may cause nasal irritation and sneezing. Nasal catheters also need to be used with caution in patients with coagulopathies (due to the possibility of inducing epistaxis during placement) and intracranial disease (nasal cannula placement has been shown to increase intracranial pressure).
High-flow oxygen therapy
High-flow oxygen therapy (see Table II) is a relatively new therapy in veterinary medicine which provides oxygen support via heated humidified air delivered at flow rates up to 60 L/min with a FiO2 ranging between 21 to 100%. (12). High-flow oxygen therapy is indicated in patients that have failed traditional oxygen therapy; failure is defined as a SpO2 < 93% or PaO2 < 70 mmHg despite traditional oxygen therapy (13). Due to their ability to provide higher flow rates of oxygen which meet or exceed a patient’s minute volume, high-flow devices should be able to provide enough oxygen to meet a patient’s ventilation requirements even when the animal is breathing deeply or rapidly, thus avoiding or minimizing entrainment. Several veterinary studies comparing the effects of HFOT to traditional oxygen therapy have shown significant improvement in PaO2 and minimal to no complications (14–16).
Physiologic benefits and favorable effects of high-flow oxygen therapy
-
Washout of physiologic dead space.
Anatomic dead space describes air entering the lungs that is not participating in gas exchange. This includes air in the nasopharynx, trachea, and mainstem bronchi. Since these areas are designed simply to conduct gases (oxygen and carbon dioxide to and from the alveoli), a quantity of carbon dioxide always remains in the upper airways (i.e., nasopharynx) creating a carbon dioxide reservoir. When an animal is in respiratory distress and breathing quickly and/or deeply, a greater amount of carbon dioxide-rich air from this reservoir is rebreathed.
The high-flow rates of oxygen delivered during HFOT continuously flush the carbon dioxide from the dead space in the upper airways, filling the nasopharyngeal dead space with oxygen (11,12). Thus, when an animal on HFOT breathes, it draws from a reservoir of oxygen-rich air (rather than carbon dioxide-rich air) in the upper airways, improving oxygen delivery and thereby oxygenation.
-
Humidification and warming of inspired air.
Ideally, the oxygen delivered to the lungs is both warm and moist, having been shown in humans to increase patient comfort and tolerance of oxygen administration (13). Warming is also important since cold air can lead to bronchoconstriction in patients that are already experiencing respiratory compromise, worsening or creating a V:Q mismatch. High-flow oxygen therapy is designed to warm the inspired air close to body temperature and to humidify the air to add moisture.
Inspiration of warm and moist air improves mucociliary function and prevents damage to epithelial cells lining the respiratory tract, thus maximizing the effectiveness of the mucociliary apparatus to remove particulate matter (6). Inspiration of dry air can cause damage to the ciliated epithelium and result in submucosal inflammation (17). Studies evaluating gas humidification in human airway epithelial cells showed an increase in inflammatory markers within 8 h of receiving oxygen at low humidity (18).
Dehydrated mucosal tissues also prevent effective ciliary clearance of mucus resulting in retention of mucous secretions. Dry air alters the water content of mucus, increasing the viscosity and making it more difficult for a patient to cough and expel mucus and particulate matter. Therefore, the warmth and moisture provided by HFOT should improve clearance of mucous and particulate matter, reducing the likelihood of pneumonia formation in the airways and allowing for clearance of pre-existing lower airway injury.
-
Minimizing air entrainment and delivery of fixed FiO2.
As shown, larger dogs (> 20 kg) in respiratory distress often have high inspiratory flow demands that cannot be met by conventional oxygen systems. High-flow oxygen systems with flow rates as high as 60 L/min can minimize air entrainment in these settings so that the patient is reliably breathing less room air and more oxygen-rich air. This leads to greater confidence that the oxygen received closely matches the oxygen setting on the machine, resulting in improved oxygenation at the level of the lungs (19). Despite these advantages, the exact FiO2 delivered to the patient can still decrease in situations with open mouth breathing or panting due to mixing of room air with oxygen rich air supplied by the machine.
-
Positive end expiratory pressure effects.
Theoretically, HFOT can provide positive end expiratory pressure (PEEP) effects in humans (20,21). Positive end expiratory pressure refers to the pressure remaining in the airways at the end of each respiratory cycle. This pressure should improve oxygenation by reopening any collapsed alveoli, increasing the surface area of the lungs to improve gas exchange. The exact mechanism by which HFOT therapy generates PEEP is not fully understood.
In humans, HFOT has been shown to deliver up to 1 mmHg of pressure for every 10 L/min of oxygen inspired during close mouth breathing. True PEEP effects in humans are usually seen at flow rates of 40 to 50 L/min (22). One veterinary study involving healthy dogs demonstrated that HFOT could generate a positive airway pressure at flow rates of 1 to 2 L/kg BW per min (10 to 72 L/min) (23).
Factors that can affect the amount of PEEP successfully provided to the patient include panting/open mouth breathing and the HFOT flow rate delivered to the patient. Since PEEP effects can be negligible at lower flow rates and many dogs do not require flow rates above 40 L/min due to their size, whether PEEP is truly provided is unclear. Additionally, for maximum PEEP effects, humans must keep their mouths closed since open mouth breathing/panting can lead to escape of pressure (24). One human study showed no PEEP effects with an open mouth even at flow rates as high as 80 L/min (24). Since many dogs tend to pant whether or not they are dyspneic, whether HFOT can truly provide PEEP effects in those dogs is brought into question.
-
Reduced metabolic expenditure.
Dogs in respiratory distress for prolonged periods of time are working so hard to breathe that it can result in depletion of energy stores and respiratory muscle fatigue (25). This is one of the reasons to mechanically ventilate dogs; mechanical ventilation will reduce the “work of breathing” and prevent respiratory fatigue. High-flow oxygen therapy also has the potential to reduce energy expenditures related to breathing by decreasing the energy used by the respiratory muscles.
There are several proposed mechanisms by which HFOT reduces the work of breathing. First, high-flow rates ensure a unidirectional flow of air through the upper airway. This in turn decreases dead space, washes out carbon dioxide, and generates PEEP (26). This decreases the need for elevated minute ventilation by ensuring each breath has a higher FiO2; reduction in respiratory rates and depth of respiration lessen the work of breathing. Additionally, by supplying heated and humidified air, HFOT is able to conserve the energy normally used to raise the temperature of inhaled gases, again reducing the work of breathing (27,28).
Applications of high-flow oxygen therapy in human and veterinary medicine
High-flow oxygen therapy has been gaining popularity in human and veterinary medicine in recent years in situations in which conventional oxygen therapy is determined insufficient. It is often considered an intermediate between traditional oxygen therapy and mechanical ventilation. Common clinical scenarios in which HFOT is used in human medicine include acute respiratory failure (28,29), cardiogenic pulmonary edema (30), and during the post-extubation period (31). The clinical applications of HFOT in veterinary medicine are extrapolated from humans.
Very little data are published in clinical veterinary medicine regarding HFOT. Two prospective (15,32) and one retrospective (16) study in acutely dyspneic dogs presenting to veterinary hospitals showed improvements in oxygen parameters. The first of these studies was a retrospective study involving 6 dogs with primary pulmonary hypoxemia (16). It demonstrated HFOT significantly improved PaO2 with minimal complications (median PaO2 133.75 mmHg on HFOT versus median PaO2 61.85 mmHg with traditional therapy) (16).
In the first of the prospective studies, 11 dogs with acute respiratory distress were initially given standard oxygen therapy and then converted to HFOT when there was no improvement in respiratory signs within 30 min (15). These dogs showed statistically significant increases in PaO2 (171 ± 123 mmHg versus 73 ± 24 mmHg) and SpO2 (97.7 ± 2.3% versus 91.6 ± 7.2%) along with increases in the measured flow rate (18 ± 12 L/min versus 3.2 ± 2.0 L/min) of delivered oxygen after transitioning to HFOT (15). Furthermore, HFOT therapy was well-tolerated in all 11 dogs with minimal complications (15). Limitations of this study included a small sample size, absence of a control group, and lack of exploration of long-term HFOT (beyond 60 min).
In the second prospective sequential trial performed in 22 hypoxemic dogs that failed traditional oxygen therapy, physiologic variables along with sedation, dyspnea, tolerance scores, and arterial blood gases were recorded from the time of initiation of HFOT up to 7 h (32). Improvements in dyspnea scores and SpO2 were recorded at all time points along with improvement in respiratory rates at 1 and 7 h. In addition, 60% of the patients responded to HFOT within 30 min with 45% of the dogs surviving to discharge. No complications were noted from administration of HFOT (32). This is the first prospective veterinary study to demonstrate the use of high-flow therapy for up to 7 h.
Dovetailing with the safety and efficacy demonstrated clinically in dogs with respiratory distress, a single veterinary study in 6 healthy dogs undergoing dental procedures revealed that transpulmonary pressures, which would put a dog at risk of pneumothorax or other barotrauma, were not significantly different in dogs receiving traditional oxygen therapy versus HFOT (14). Specifically, dogs in this study receiving conventional oxygen therapy at 100 mL/kg BW per minute (1.7 to 3.6 L/min) and later HFOT at 20 L/min and 30 L/min did not have significant changes in transpulmonary pressures from baseline (14). Additionally, the median PaO2 was significantly higher with HFOT (> 500 mmHg with HFOT of 20 or 30 L/m in) compared to traditional oxygen therapy (202.9 mmHg) (14).
A more recent study described the use of HFOT in the post-anesthesia recovery period in a small number of brachycephalic dogs undergoing surgery for obstructive airway disease. This study concluded that nasal prongs were well-suited for the brachycephalic facial structure with improvement in dyspnea scores in the postoperative period (33).
Drawbacks of high-flow oxygen therapy
High-flow oxygen therapy has some limitations. One major drawback reported in human medicine is the increased complexity of the system and the training required to initiate care compared to low-flow systems (11). Other shortcomings reported in humans include reduced patient mobility when connected to the high-flow system, nasal irritation, alteration in sense of smell after treatment, and frequent displacement of nasal prongs (11,12). In humans, noise during HFOT is also associated with significant discomfort (34).
In veterinary medicine, the need to acquire HFOT equipment is a disadvantage along with the need for a source of medical air (such as a compressor) as well as large quantities of oxygen needed to use the unit. Furthermore, significant adaptation of the human nasal prongs is required to make them fit the conformation of canine nostrils. Despite several modification techniques) that have been developed to secure them in place (23), it would be ideal to develop nasal cannulas specifically designed for dogs to ensure optimal oxygen delivery.
At this time, HFOT has only been described in dogs and may not be possible in cats for several reasons. First, the nasal cannulas that are currently available are not designed and cannot be adapted to fit cat nostrils. Secondly, to place the cannulas and set up HFOT, patient handling is required which may not be possible in dyspneic cats. Cats in respiratory distress also may not tolerate the noise associated with HFOT machines.
Barotrauma leading to leakage of air into the pleural space (air leak syndrome) has been mentioned in multiple human studies as an uncommon complication induced by HFOT (12,13). In a human case series, 2 cases of pneumothorax and 1 case of pneumomediastinum were reported in 3 children within hours of initiation of HFOT (32). The exact mechanism of barotrauma is unknown, but the authors hypothesized that the positive airway pressure created by HFOT created overdistension and rupture of alveoli (35). Barotrauma has not been reported in veterinary medicine as of yet, but aerophagia secondary to HFOT was reported in 8 research dogs (23). However, no additional clinical signs linked to aerophagia (vomiting, regurgitation or abdominal discomfort) were reported in the dogs (23).
A final potential drawback of HFOT in humans is that it can lead to a delay in intubation and mechanical ventilation which may worsen outcomes (36). In one human study, early intubation was associated with overall better ICU mortality rates compared to those patients that were intubated after receiving 48 h of HFOT (36). Whether this is relevant in veterinary medicine is controversial since HFOT is often used in cases in which mechanical ventilation is not an option for clients and failure of HFOT would lead to humane euthanasia.
There are no specific protocols that have been described in veterinary medicine for set up of HFOT. If you believe HFOT is a good choice for your patient, a suggested protocol for set up and maintenance of dogs on HFOT, adapted from the author’s institution, is included below. A quick set up version of this protocol is shown in Table III; its use is demonstrated in the case example.
Table III.
Quick guide for set up of high-flow oxygen therapy.
| Settings | Recommendations |
|---|---|
| Nasal prongs | Occlude less than 50% of the nostrils, specifically designed for HFOT unit |
| Flow rate | Option 1: Start at 0.5 L/kg BW per minute Titrate up to meet dog’s respiratory demands Option 2: Perform minute ventilation calculations: Minute ventilation = RR × TV Start with RR of 20 to 30 breaths and TV of 10 to 20 mL/kg BW and titrate up until a satisfactory PaO2 and FiO2 has been reached |
| FiO2 | Start at 100% FiO2 Once satisfactory SpO2/PaO2 is reached, titrate down to no less than 40 to 60% |
| Temperature | Set to patient body temperature, do not go above 38°C Temperature (°C) = [Temperature (°F) – 32°F] × 5/9 |
| Monitoring | Continuous monitoring of heart rate, respiratory rate and effort, SpO2 ± PaO2, BP |
| Weaning | Reduce FiO2 by 5 to 10%, assess respiratory parameters within 1 to 2 h If tolerated, reduce flow rate by 5 to 10% and assess respiratory parameters within 1 to 2 h Transition from high-flow to low-flow oxygen systems when flow rate ≤ 25 L/min and FiO2 ≤ 40% |
| Treatment escalation | Lack of clinical response and improvement in respiratory parameters within 1 to 2 hours of initiation of HFOT warrants other treatments (i.e., indicates a failure of HFOT for that patient) |
RR — Respiratory rate; TV — Tidal volume; HFOT — High-flow oxygen therapy; FiO2 — Inspired oxygen content; PaO2 — Partial pressure of oxygen in arterial blood; BP—Blood pressure, SpO2 — Pulse oximetry reading.
Protocol for set up and maintenance of dogs on high-flow oxygen therapy
-
Parts of the high-flow oxygen system.
The high-flow oxygen system (Figures 1, 2) is an open circuit consisting of 4 major parts: flow generator, active heated humidifier, single-limb heated circuit, and nasal cannulas (37). The flow generator creates a high flow of oxygen-rich air that travels to the active humidifier where it picks up moisture. The humidified air then moves to the inspiratory limb that contains a heating wire to warm the air. Finally, the warm and moist oxygen is delivered to the patient via nasal prongs. It is important to note that compressed air (aka, medical air) is necessary for effective oxygen delivery using the high-flow system. Medical air can be used from a medical air outlet if the hospital has central medical air, from a compressor, or from tanks. Be aware that tanks will empty very rapidly if the flow rate on the machine is set to a high level.
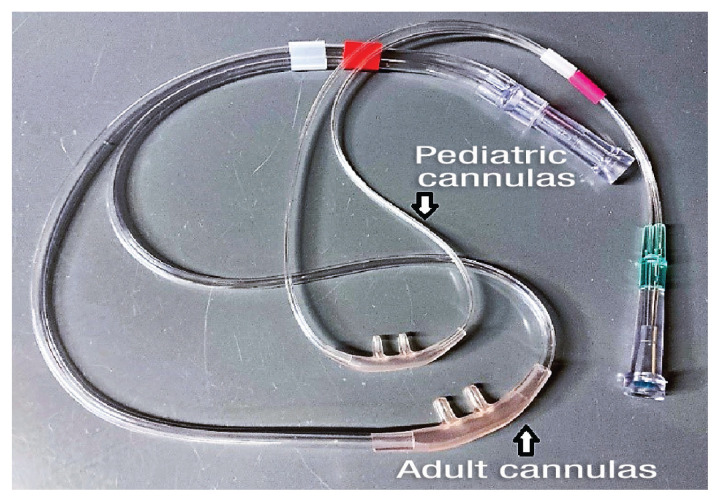
Nasal prongs recommended by the manufacturer should be used rather than red rubber catheters since high-flow rates collapse the lumen of red rubber catheters, impeding oxygen delivery. The nasal prongs used in HFOT should only occlude about 50% of the nostril (12,32). There are several sizes of nasal prongs available depending on the size of the high-flow system: intermediate infant, pediatric/adult small, and adult (Figure 2). As per manufacture guidelines, the infant size is used for the pediatric (1 to 8 L/min) circuit and the adult and adult small sizes are used with the adult circuit (5 to 40 L/min).
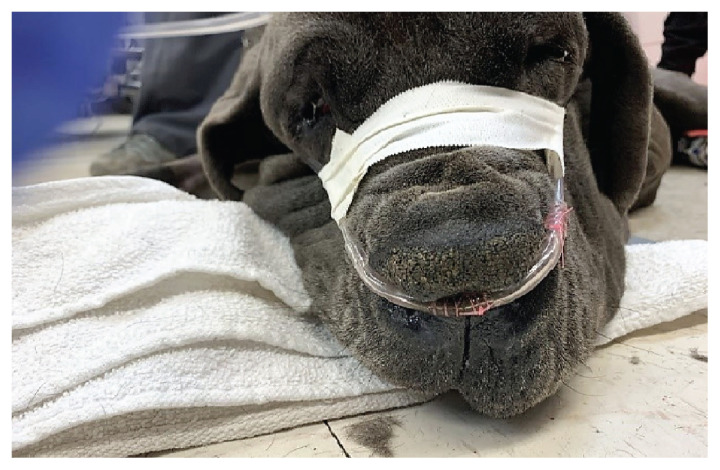
Different techniques have been recommended to secure the nasal prongs in place in dogs including simple interrupted sutures, Chinese finger trap sutures, and adhesive bandages (Figures 3, 4). Additionally, modeling clay can be used to better seat the nasal cannulas in those dogs in which the nasal prongs are not an exact fit (23). In brachycephalic dogs, suturing the nasal prongs lateral to the nostrils on each side of the cheek will better secure the prongs in place (Figure 4). In non-brachycephalic dogs, tape tabs can be used to hold the nasal prongs in place with sutures to the side of the nares (Figure 3). Sedation may be needed for dyspneic dogs receiving HFOT to improve tolerance to the nasal prongs.
-
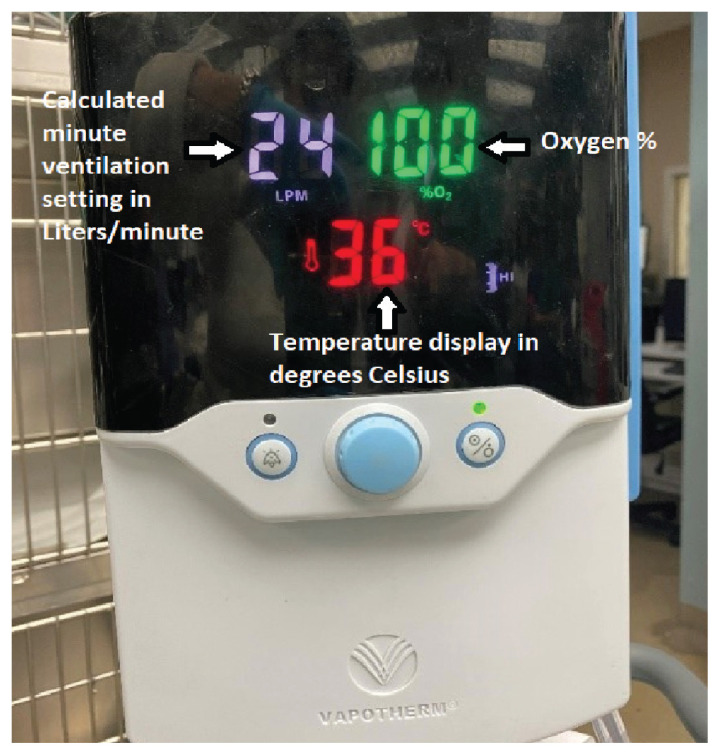
Machine setup (Figure 5)
Calculate the desired flow rate to choose the correct patient circuit.
-
Set the L/min delivered by the machine using 1 of the 2 formulas as:
-
Flow rate: MV = RR × Tv
Normal tidal volume for dogs is 10 to 20 mL/kg BW. Use an initial respiratory rate of 20 to 30 breaths/min and titrate up in 5 to 10 mL/kg BW per minute increments as needed depending on the patient’s work of breathing* and respiratory rate after being placed on HFOT.
OR
-
Flow rate: 0.5 to 2 L/kg BW per minute (23)
Start at the lower flow rate (0.5 L/kg BW per minute) and titrate up as needed depending on the animal’s work of breathing* after being placed on HFOT.
-
Depending on the flow rate calculated, select either the adult/high-flow (5 to 40 L/min) tubing and cannulas versus pediatric/low-flow tubing and cannulas (1 to 8 L/min).
-
Set the delivered FiO2
This parameter determines what fraction of oxygen is supplied to the animal and hopefully will be inspired. Initially maintain the patient on 100% FiO2. Once the animal’s condition has stabilized (respiratory rate < 40 breaths, SpO2 > 95%, or PaO2 > 80 mmHg), the oxygen levels can be slowly titrated down to a FiO2 no lower than 40 to 60%. Typically, the SpO2 is measured every 1 to 2 h and arterial blood gas (ABG) values are measured at least q8h to document improvement along with observation of the clinical signs in the animal. Administration of 100% oxygen for > 12 h theoretically can increase the risk of oxygen toxicity through free radicle production and cell membrane damage. Differentiating oxygen toxicity from hypoxemia can often be challenging due to similar clinical signs. Although, oxygen toxicity from HFOT is uncommon due to the concept of air entrainment and the mixing of gases during panting and open mouth breathing, an attempt should be made to reduce the FiO2 below 100% within the first 12 to 24 h of therapy.
-
Set the machine temperature:
Note that the machine temperature is in Celsius (°C). To convert Fahrenheit (°F) to °C, use the formula:
Initially set the machine temperature to the measured patient body temperature to administer oxygen comfortably. Check the animal’s body temperature every 2 to 4 h after starting HFOT. Alter the temperature as needed on the machine in response to the animal’s body temperature.
Based on the authors’ experience, febrile patients tolerate cooler temperatures better than temperatures matching their elevated body temperatures; therefore, it is recommended to not increase temperature of the high-flow machine above 38.8 to 39.4°C (102 to 103°F). A veterinary study evaluating combined effects of different flow rates and temperature in 14 healthy dogs, showed no significant differences in tolerance scores at 31°C or 37°C (38).
-
Monitoring
Monitoring the respiratory status of patients receiving HFOT is important to detect any critical changes in the patient’s health and institute appropriate measures in a timely fashion. However, it is also important not to stress already fragile dogs collecting monitoring data.
Typically, the respiratory status can be monitored by checking respiratory rate, SpO2, and (if possible) PaO2. However, in a clinical setting in which the patient is stressed or monitoring PaO2 is not practical, assessing baseline respiratory rate/effort and pulse oximetry can give a fair indication of respiratory status. Once a dog’s respiratory status stabilizes (and only if amenable to restraint), other cardiovascular parameters such as heart rate and blood pressure are commonly monitored.
Pulse oximetry is often checked every 1 to 2 h and, if possible, arterial blood gases are usually sampled at least 1 to 2 times a day. In patients in which serial arterial blood sampling will be performed, an arterial catheter can be placed to aid with frequent blood sampling ± to measure direct blood pressure.
-
Weaning.
No specific weaning protocols have been established in veterinary medicine with regard to HFOT. Weaning recommendations are instead extrapolated from human medicine. However, even in human medicine, there is a lack of consensus and standardization as far as weaning protocols (39).
A reasonable target for initiation of weaning is resolution of the underlying disease condition to the point where the animal can perform adequate gas exchange in the absence of the high-flow system. In other words, once the dog is able to maintain its respiratory rate, visible respiratory effort, and either SpO2 or PaO2 parameters on low-flow rates (< 25 L/min) and FiO2 settings < 40%, it can be switched to traditional low-flow oxygen systems (13). Weaning is done by reducing FiO2 first and then the flow rate (12). Typically, the FiO2 is reduced by 5 to 10% and the dog’s oxygenation and respiratory effort is reassessed after 1 to 2 h. If respiratory parameters are holding, the flow rate is then repeatedly reduced by 5 to 10 % with assessment of the animal’s oxygenation and respiratory effort again in 1 to 2 h. The reduction of flow rate to a large extent is dependent on patient size and should be done more gradually in smaller patients to avoid sudden drops. As mentioned previously, an animal is considered eligible for a return to traditional oxygen therapy when the HFOT flow rate is < 25 L/min and the FiO2 setting is ≤ 40% (13).
-
Escalation of treatment.
When initiating HFOT, clinical improvement [i.e., improvement in respiratory rate, respiratory effort, and oxygenation parameters (PaO2/SpO2)] should be noted within 1 to 2 h. If no improvement is seen in these parameters within this timeframe, and all other patient and environmental factors have been assessed, then other treatments should be instituted (e.g., mechanical ventilation (12) or humane euthanasia should be considered. Criteria for mechanical ventilation include severe hypoxemia (PaO2 < 60 mmHg) despite oxygen supplementation, severe hypoventilation (PaCO2 > 60 mmHg) and respiratory fatigue (40).
Figure 1.
High-flow oxygen machine (Precision Flow Plus; Vapotherm, Exeter, New Hampshire, USA). The yellow hose is attached to medical/compressed air, the green hose is attached to the oxygen source and the black cord connects to the power source. The flow generator and humidifier are contained within the unit (black arrow).
Figure 2.
Two sizes of nasal prongs used in high-flow oxygen therapy (Nasal prongs; Vapotherm).
Figure 3.
Combination of tape and suture to secure nasal prongs in a dolichocephalic dog receiving high-flow oxygen therapy. Sutures are present on the lateral aspect of each nare.
Figure 4.
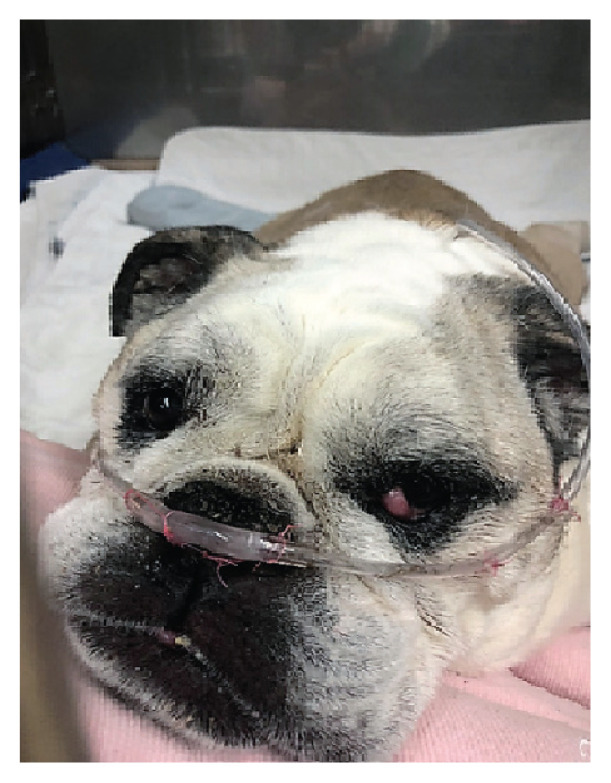
Brachycephalic dog with nasal prongs sutured lateral to the nostrils. Note that a nasogastric feeding tube is also attached to the left nostril.
Figure 5.
High-flow oxygen machine display. Photo courtesy of Dr. Mariana Pardo, MV, BVSc, DACVECC.
Case example
A 4-year-old neutered male mixed breed dog weighing 30 kg and diagnosed with pulmonary blastomycosis presented to the Purdue Emergency Service for oxygen supplementation. On presentation, the dog was tachypneic with increased respiratory effort. Thoracic radiographs revealed a diffuse alveolar to interstitial pattern. The SpO2 on entry was 90% and the dog was placed on low-flow oxygen with bilateral nasal cannulas at 4 L/min while treatment with itraconazole and steroids was initiated. The dog’s SpO2 on low-flow oxygen initially improved to 95%.
On day 2 of treatment, the animal’s respiratory status declined with marked increases in respiratory rate and effort noted on physical examination. A recheck SpO2 showed a decrease to 88% on low-flow oxygen therapy and an arterial blood gas confirmed severe hypoxemia with a PaO2 of 55 mmHg. Treatment escalation was deemed necessary, and the dog was placed on HFOT at a FiO2 of 100% starting at 30 L/min [MV = 60 bpm × (30 kg × 15 mL/kg BW) = 27 L/min, rounded to 30 L/min].
Within 60 min of initiating HFOT, there was marked improvement in the dog’s respiratory rate and effort. A recheck SpO2 within 60 min was 97% and a recheck arterial blood gas revealed a PaO2 of 109 mmHg. The dog was maintained on HFOT for 24 h and then weaned off HFOT by reducing first the FiO2 followed by flow rate. By the end of day 3 of hospitalization, he was switched back to a low-flow oxygen system (normal nasal cannulas) with a flow rate of 4 L/min.
In conclusion, HFOT is an evolving and growing modality which can serve as an intermediate between traditional oxygen therapy and mechanical ventilation. It is cost-effective and offers several physiologic benefits (washout of anatomic dead space, improved FiO2, small amount of PEEP, superior humidification) to assist with respiratory management of patients. Since veterinary medicine differs from human medicine in that mechanical ventilation is often either not available or not considered an option by clients for various reasons including personal ethics and cost, having a 3rd option that is “better than” traditional oxygen therapy but still not mechanical ventilation is helpful. High-flow oxygen therapy cannot and will not replace mechanical ventilation, especially for cases of hypoventilation, but may help in cases of hypoxemia and with increased work of breathing despite traditional oxygen therapy. Further studies are needed to fully determine the impact of HFOT on the veterinary field.
Footnotes
In a clinical setting, depending on the reason for the hypoxemia, there may also be an increase in the dead space in the lung which will reduce the amount of lung tissue available for gas exchange and reduce the overall tidal volume. Thus, increasing respiratory rate alone may not be as effective as predicted in the above equations.
Increased work of breathing is clinically characterized by thoraco-abdominal asynchrony (abdomen contracting while thorax expands or vice versa), contraction of abdominal muscles to breath, and/or orthopnea. A positive response to HFOT involves a gradual improvement in these clinical signs within 15 to 20 min once the appropriate flow rate is established.
References
- 1.Rozanski E, Chan DL. Approach to the patient with respiratory distress. Vet Clin North Am Small Anim Pract. 2005;35:307–317. doi: 10.1016/j.cvsm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE. Transport of oxygen and carbon dioxide in blood and tissue fluids. In: Hall JE, editor. Guyton and Hall Textbook of Medical Physiology. 13th ed. St. Louis, Missouri: Elsevier; 2015. pp. 527–537. [Google Scholar]
- 3.West JB. Gas transport by the blood. In: West JB, editor. Respiratory Physiology: The Essentials. 10th ed. Philadelphia, Pennsylvania: Wolters Kluwer; 2016. pp. 87–107. [Google Scholar]
- 4.Haskins SC. Hypoxemia. In: Silverstein DC, Hopper K, editors. Small Animal Critical Care Medicine. 2nd ed. St. Louis, Missouri: WB Saunders; 2015. pp. 81–86. [Google Scholar]
- 5.Hall JE. Pulmonary ventilation. In: Hall JE, editor. Guyton and Hall Textbook of Medical Physiology. 13th ed. St. Louis, Missouri: Elsevier; 2015. pp. 497–507. [Google Scholar]
- 6.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults. Chest. 2015;148:253–261. doi: 10.1378/chest.14-2871. [DOI] [PubMed] [Google Scholar]
- 7.Tancredi F, Lajoie I, Hoge R. A simple breathing circuit allowing precise control of inspiratory gases for experimental respiratory manipulations. BMC Research Notes. 2014;7:235. doi: 10.1186/1756-0500-7-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mannig AM. Oxygen therapy and toxicity. Vet Clin North Am Small Anim Pract. 2002;32:1005–1020. doi: 10.1016/s0195-5616(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 9.Wong A, Uquillas E, Hall E, Dart CM, Dart AJ. Comparison of the effect of oxygen supplementation using flow-by or a face mask on the partial pressure of arterial oxygen in sedated dogs. N Z Vet J. 2019;67:36–39. doi: 10.1080/00480169.2018.1528903. [DOI] [PubMed] [Google Scholar]
- 10.Dunphy ED, Mann FA, Dodam JR, et al. Comparison of unilateral versus bilateral nasal catheters for oxygen administration in dogs. J Vet Emerg Crit Care. 2002;12:245–251. [Google Scholar]
- 11.Sharma S, Danckers M, Sanghavi D, Chakraborty RK. High flow nasal cannula. StatPearls Publishing; 2020. [accessed August 23, 2020]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526071/Last. [PubMed] [Google Scholar]
- 12.Renda T, Corrado A, Iskandar G, Pelaia G, Abdalla K, Navalesi P. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br J Anaesth. 2018;120:18–27. doi: 10.1016/j.bja.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Tseng LW, Drobatz KJ. Oxygen supplementation and humidification. In: King LG, editor. Textbook of Respiratory Diseases of the Dog and Cat. Philadelphia, Pennsylvania: WB Saunders; 2003. pp. 205–213. [Google Scholar]
- 14.Daly JL, Guenther CL, Haggerty JM, Keier I. Evaluation of oxygen administration with a high-flow nasal cannula to clinically normal dogs. Am J Vet Res. 2017;78:624–630. doi: 10.2460/ajvr.78.5.624. [DOI] [PubMed] [Google Scholar]
- 15.Pouzot-Nevoret C, Hocine L, Nègre J, et al. Prospective pilot study for evaluation of high-flow oxygen therapy in dyspnoeic dogs: The HOT-DOG study. J Small Anim Pract. 2019;60:656–662. doi: 10.1111/jsap.13058. [DOI] [PubMed] [Google Scholar]
- 16.Keir I, Daly J, Haggerty J, Guenther C. Retrospective evaluation of the effect of high flow oxygen therapy delivered by nasal cannula on PaO2 in dogs with moderate-to-severe hypoxemia. J Vet Emerg Crit Care. 2016;26:598–602. doi: 10.1111/vec.12495. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch JA, Tokayer JL, Robinson MJ, Sackner MA. Effects of dry air and subsequent humidification on tracheal mucous velocity in dogs. J Appl Physiol. 1975;39:242–246. doi: 10.1152/jappl.1975.39.2.242. [DOI] [PubMed] [Google Scholar]
- 18.Chidekel A, Zhu Y, Wang J, Mosko JJ, Rodriguez E, Shaffer TH. The effects of gas humidification with high-flow nasal cannula on cultured human airway epithelial cells. Pulm Med. 2012;2012:1–8. doi: 10.1155/2012/380686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chikata Y, Onodera M, Oto J, Nishimura M. FiO2 in an adult model simulating high-flow nasal cannula therapy. Respir Care. 2017;62:193–198. doi: 10.4187/respcare.04963. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura M. High-flow nasal cannula oxygen therapy in adults: Physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61:529–541. doi: 10.4187/respcare.04577. [DOI] [PubMed] [Google Scholar]
- 21.Papazian L, Corley A, Hess D, et al. Use of high-flow nasal cannula oxygenation in ICU adults: A narrative review. Intensive Care Med. 2016;42:1336–1349. doi: 10.1007/s00134-016-4277-8. [DOI] [PubMed] [Google Scholar]
- 22.Parke RL, McGuinness SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care. 2013;58:1621–1624. doi: 10.4187/respcare.02358. [DOI] [PubMed] [Google Scholar]
- 23.Jagodich TA, Bersenas AME, Bateman SW, Kerr CL. Comparison of high flow nasal cannula oxygen administration to traditional nasal cannula oxygen therapy in healthy dogs. J Vet Emerg Crit Care. 2019;29:246–255. doi: 10.1111/vec.12817. [DOI] [PubMed] [Google Scholar]
- 24.Luo J, Lu M, Zhao Z, et al. Positive end-expiratory pressure effect of 3 high-flow nasal cannula devices. Respir Care. 2017;62:888–895. doi: 10.4187/respcare.05337. [DOI] [PubMed] [Google Scholar]
- 25.Barton L. Respiratory muscle fatigue. Vet Clin North Am Small Anim Pract. 2002;32:1059–1071. doi: 10.1016/s0195-5616(02)00036-0. [DOI] [PubMed] [Google Scholar]
- 26.Gotera C, Díaz Lobato S, Pinto T, Winck JC. Clinical evidence on high flow oxygen therapy and active humidification in adults. Rev Port Pneumol. 2013;19:217–227. doi: 10.1016/j.rppneu.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Ricard J-D, Boyer A. Humidification during oxygen therapy and non-invasive ventilation: Do we need some and how much? Intensive Care Med. 2009;35:963–965. doi: 10.1007/s00134-009-1457-9. [DOI] [PubMed] [Google Scholar]
- 28.Sztrymf B, Messika J, Bertrand F, et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: A prospective pilot study. Intensive Care Med. 2011;37:1780–1786. doi: 10.1007/s00134-011-2354-6. [DOI] [PubMed] [Google Scholar]
- 29.Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55:408–413. [PubMed] [Google Scholar]
- 30.Carratalá Perales JM, Llorens P, Brouzet B, et al. High-flow therapy via nasal cannula in acute heart failure. Rev Esp Cardiol (English Edition) 2011;64:723–725. doi: 10.1016/j.recesp.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Nugent K. Postextubation management of patients at high risk for reintubation. J Thorac Dis. 2016;8:E1679–E1682. doi: 10.21037/jtd.2016.12.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jagodich TA, Bersenas AME, Bateman SW, Kerr CL. High-flow nasal cannula oxygen therapy in acute hypoxemic respiratory failure in 22 dogs requiring oxygen support escalation. J Vet Emerg Crit Care. 2020;30:364–375. doi: 10.1111/vec.12970. [DOI] [PubMed] [Google Scholar]
- 33.Jagodich TA, Bersenas AME, Bateman SW, Kerr CL. Preliminary evaluation of the use of high-flow nasal cannula oxygen therapy during recovery from general anesthesia in dogs with obstructive upper airway breathing. J Vet Emerg Crit Care. 2020;30:487–492. doi: 10.1111/vec.12971. [DOI] [PubMed] [Google Scholar]
- 34.Kubo T, Nakajima H, Shimoda R, et al. Noise exposure from high-flow nasal cannula oxygen therapy: A bench study on noise reduction. Respir Care. 2018;63:267–273. doi: 10.4187/respcare.05668. [DOI] [PubMed] [Google Scholar]
- 35.Hegde S, Prodhan P. Serious air leak syndrome complicating high-flow nasal cannula therapy: A report of 3 cases. Pediatrics. 2013;131:e939–e944. doi: 10.1542/peds.2011-3767. [DOI] [PubMed] [Google Scholar]
- 36.Kang BJ, Koh Y, Lim C-M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41:623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura M. High-flow nasal cannula oxygen therapy devices. Respir Care. 2019;64:735–742. doi: 10.4187/respcare.06718. [DOI] [PubMed] [Google Scholar]
- 38.Harduin C, Allaouchiche B, Nègre J, et al. Impact of flow and temperature on non-dyspnoeic dogs’ tolerance undergoing high-flow oxygen therapy. J Small Anim Pract. 2021;62:265–271. doi: 10.1111/jsap.13284. [DOI] [PubMed] [Google Scholar]
- 39.Morris L, Cook N, Ramsey A, et al. Weaning humidified high flow oxygen therapy among paediatric patients: An integrative review of literature. J Pediatr Nurs. 2020;50:37–45. doi: 10.1016/j.pedn.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Pilbeam SP. Establishing the need for mechanical ventilation. In: Cairo JM, editor. Mechanical Ventilation Physiologic and Clinical Applications. 6th ed. St. Louis, Missouri: Elsevier; 2016. pp. 43–55. [Google Scholar]