Abstract
C-reactive protein (CRP) is an acute phase protein, which is used to evaluate and monitor the response of the innate immune system to a variety of inflammatory processes in the dog. The purpose of this study was to analytically validate a point-of-care assay (IDEXX Catalyst CRP Test) and an immunoturbidimetric assay (Gentian Canine CRP Immunoassay) for the measurement of serum CRP concentrations in dogs. These 2 assays (Catalyst, Gentian) were compared to a previously validated enzyme-linked immunosorbent assay (Tridelta Development EIA Canine CRP Assay). Linearity, precision, reproducibility, and accuracy were assessed using leftover serum samples. Agreement between assays was assessed using leftover serum samples and serum from clinically healthy dogs. Observed to expected ratios (O/E) for dilutional parallelism were 83.9 to 163.1% and 108.3 to 160.6% for the Catalyst and the Gentian assays, respectively. Coefficients of variation for intra-assay variability ranged from 6.4 to 9.5% for the Catalyst assay and 1.5 to 2.6% for the Gentian assay. Coefficients of variation for inter-assay variability ranged from 3.8 to 18.2% for the Catalyst assay and 4.5 to 5.8% for the Gentian assay. The mean O/E for recovery were 97.9% and 98.5% for the Catalyst and Gentian assays, respectively. Correlations between assays were as follows: Catalyst and Tridelta (R2 = 0.76), Gentian and Tridelta (R2 = 0.79), and Catalyst and Gentian (R2 = 0.98). The Catalyst and Gentian assays are both acceptable for measuring CRP in dog serum, but their results are not directly comparable with the Tridelta assay.
Résumé
La protéine C réactive (CRP) est une protéine de phase aiguë, qui est utilisée pour évaluer et surveiller la réponse du système immunitaire inné à une variété de processus inflammatoires chez le chien. Le but de cette étude était de valider analytiquement un test au point de service (test IDEXX Catalyst CRP) et un test immunoturbidimétrique (Gentian Canine CRP Immunoassay) pour la mesure des concentrations sériques de CRP chez le chien. Ces deux tests (Catalyst, Gentian) ont été comparés à un test immuno-enzymatique précédemment validé (Tridelta Development EIA Canine CRP Assay). La linéarité, la précision, la reproductibilité et l’exactitude ont été évaluées à l’aide d’échantillons de sérum restants. La concordance entre les tests a été évaluée à l’aide d’échantillons de sérum restants et de sérum provenant de chiens cliniquement sains. Les rapports observés/attendus (O/E) pour le parallélisme de dilution étaient de 83,9 à 163,1 % et de 108,3 à 160,6 % pour les tests Catalyst et Gentian, respectivement. Les coefficients de variation pour la variabilité intra-test variaient de 6,4 à 9,5 % pour le test Catalyst et de 1,5 à 2,6 % pour le test Gentian. Les coefficients de variation pour la variabilité inter-test variaient de 3,8 à 18,2 % pour le test Catalyst et de 4,5 à 5,8 % pour le test Gentian. L’O/E moyen pour la récupération était de 97,9 % et de 98,5 % pour les tests Catalyst et Gentian, respectivement. Les corrélations entre les tests étaient les suivantes : Catalyst et Tridelta (R2 = 0,76), Gentian et Tridelta (R2 = 0,79) et Catalyst et Gentian (R2 = 0,98). Les tests Catalyst et Gentian sont tous deux acceptables pour mesurer la CRP dans le sérum de chien, mais leurs résultats ne sont pas directement comparables avec le test Tridelta.
(Traduit par Docteur Serge Messier)
Introduction
C-reactive protein (CRP) is a hepatically synthesized acute phase protein, which increases in concentration following exposure to pro-inflammatory cytokines, such as interleukin (IL)-6 and IL-1 (1). Clinically, measurement of serum CRP concentration offers a quantitative measurement of the response of the innate immune system to a wide array of traumatic, infectious, immune-mediated, and neoplastic processes, thus serving as a tool to monitor disease progression and treatment efficacy in dogs (2–6). For example, serum CRP concentrations were shown to be significantly increased in dogs with acute pancreatitis (2), lymphoma (2), immune-mediated thrombocytopenia (2), leptospirosis (1), parvovirus infection (1), and surgical trauma (7).
There are currently a number of assays available to measure canine serum CRP concentration, including the Tridelta Development canine CRP (8), Gentian canine CRP (9,10), TECOmedical CRP (11), EUROLyser solo cCRP (11), and LifeAssay canine CRP (11). A previously validated canine-specific enzyme-linked immunosorbent assay (ELISA) (Tridelta Development EIA Canine CRP) was shown to effectively discriminate between healthy and non-healthy dogs, but had a relatively high (23.9 to 28.4%) inter-assay coefficient of variation (%CV), suggesting suboptimal repeatability (8,12). Enzyme-linked immunosorbent assays are time-consuming when performed manually and are relatively expensive.
Previously published work evaluated 3 automated immunoturbidimetric CRP assays marketed for use in humans to determine their utility in dogs. Although there was cross-reactivity in 2 of the 3 assays, they were rendered unsuitable for routine use in veterinary medicine due to a lack of common antigenicity (13,14). More recently, an automated canine-specific immunoturbidimetric assay (Gentian Canine CRP Immunoassay) was shown to rapidly and effectively measure canine CRP on the Abbott Architect c4000 and ABX Pentra 400 clinical chemistry analyzers (9,10). This assay offers a less costly and time-consuming alternative to measuring canine CRP when compared to its ELISA counterpart. However, variations in performance between different clinical chemistry analyzers have been noted previously (15). Therefore, assay revalidation should be performed for different types of instruments.
A commercially available point-of-care canine CRP assay has recently been released (IDEXX Catalyst CRP Test). This point-of-care test (POCT) is a sandwich immunoassay that uses gold nanoparticles to measure canine CRP in serum or lithium heparin plasma from dogs. Point-of-care tests are useful when results are needed immediately, such as with a critically ill animal, or as an efficient method to repeatedly monitor CRP measurements in-house. A validation study of a POCT (Point Strip Canine CRP Assay) to measure CRP in dogs was previously published, but that assay had intra- and inter-assay %CVs that exceeded the desired range of 10 to 15% in the CRP range of 20 to 30 mg/L (16,17). In another validation and comparison study of 3 POCTs (TECOmedical, EUROLyser solo cCRP test, and LifeAssays canine CRP test), it was shown that the assays could discriminate between low and high CRP concentrations in dogs, but lacked agreement with the reference ELISA (TECOmedical AG ELISA) (11). To the authors’ knowledge, validation data comparing the IDEXX Catalyst One against both the Gentian Canine CRP Immunoassay and the previously validated canine CRP ELISA (Tridelta Development EIA Canine CRP) has yet to be published in the peer-reviewed literature.
The aims of our study were to: i) analytically validate the Gentian assay on the Beckman Coulter AU480 Chemistry Analyzer and calculate a reference interval for this assay for this instrument, ii) analytically validate the Catalyst assay, and iii) perform a method comparison with the Tridelta assay as a reference (12).
Materials and methods
Serum samples
Surplus dog serum samples (N = 136) submitted to the Gastrointestinal Laboratory, Department of Small Animal Clinical Sciences, Texas A&M University, were used for assay validation and method comparison. A total of 58 canine surplus serum samples from healthy dogs were evaluated for the determination of a reference interval and method comparison. These dogs were classified as being healthy based on results of a client questionnaire, physical examination, complete blood (cell) count, and serum biochemistry profile. These samples were collected for the purpose of another study. Collection of blood was approved by the Texas A&M Institutional Animal Care and Use Committee and informed client consent was obtained. All serum samples were aliquoted and stored between −20˚C and −80˚C, until CRP measurement. Individual samples were thawed on an as-needed basis to prevent variation due to freeze-thaw cycles.
CRP measurement
C-reactive protein concentrations were determined using 3 assays: i) the Catalyst (IDEXX Catalyst CRP Test; IDEXX Laboratories, Westbrook, Maine, USA), ii) the Gentian (Canine CRP Immunoassay; Gentian, Moss, Norway) on a fully automated clinical chemistry analyzer (AU480 Chemistry Analyzer; Beckman Coulter, Brea, California, USA), and iii) the Tridelta (EIA Canine CRP Assay; Tridelta Development, Kildare, Ireland), which served as the reference assay. For the Catalyst assay, there is no quality control (QC) specific for CRP, though the precision and accuracy of the analyzer was evaluated in accordance with manufacturer guidelines using the provided QC clip and VetTrol control fluid (IDEXX Laboratories). Samples for the Catalyst assay were evaluated once, as per the manufacturer’s instructions, using 1 lot of CRP kits. For the Gentian and Tridelta assays, samples were run in duplicate, per the manufacturer’s instructions, using the provided QC materials.
Method validation
Linearity was assessed by manual stepwise dilution. Five samples were serially diluted (1:2, 1:4, and 1:8) with pools of serum with negligible CRP concentrations, as measured by the Tridelta assay.
Observed to expected (O/E) ratios were then calculated using the formula:
For the determination of intra-assay variation, 10 aliquots of a serum sample were run consecutively (Catalyst, 5 samples; Gentian, 6 samples). Inter-assay %CV was determined from measurements performed over 7 consecutive days (7 samples). Intra- and interassay %CVs were calculated as:
The goal for inter and intra-assay variation was set at < 15% (17).
To determine recovery percentage, 6 serum samples from dogs with a known concentration of CRP were mixed in all possible variations (A + B, A + C, etc.) and O/E ratios were calculated. In accordance with previously published guidelines for immunoassay validation, the goal for recovery was set at 80 to 120% (18).
Establishment of a reference interval for the Gentian assay
C-reactive protein concentrations from 58 serum samples from healthy adult dogs were tested for normality using the Shapiro-Wilk test and JMP Pro software (version 14.1.0; SAS Institute, Cary, North Carolina, USA). In accordance with recent international recommendations for the determination of reference intervals from small sample groups, the Box-Cox transformation was performed to normalize the data sets from healthy dogs and determine the reference interval (19). Reference Value Advisor freeware (Version 2.1 for Microsoft Excel; Biostatistiques, Toulouse, France) was used for this process (19).
Method comparison
The Catalyst and the Gentian CRP concentrations for each sample were both compared to a previously validated reference ELISA (Tridelta) (7,8). In total, 136 samples from healthy and diseased dogs were run on each assay for use in this comparison study. Graphical analysis of the comparison data included Bland-Altman plots and linear as well as Deming regression. R2 was calculated for linear and Deming regression models and bias with a 95% confidence interval (CI) was calculated from Bland-Altman analyses. Statistical evaluation was conducted using GraphPad Prism (Version 5.0 for Windows; GraphPad Software, La Jolla, California, USA). Statistical significance was set as P < 0.05.
Results
Observed to expected ratios for dilutional parallelism ranged from 83.9 to 164.1% for the Catalyst assay (Table I). For the Gentian assay, O/E ratios for dilutional parallelism ranged from 108.3 to 160.6% (Table I).
Table I.
Dilutional parallelism of C-reactive protein (CRP) in 5 canine serum samples. Serum samples were serially diluted 1:2, 1:4, and 1:8 in canine serum with an unmeasurable CRP concentration. Observed to expected (O/E) ratios were then calculated.
| Catalyst assay | Gentian assay | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Sample | Dilution | Observed | Expected | O/E (%) | Sample | Dilution | Observed | Expected | O/E (%) |
| 1 | Neat | 8.7 | — | — | 1 | Neat | 132 | — | — |
| 1 to 2 | 4.8 | 4.35 | 110.3 | 1 to 2 | 78 | 66 | 118.2 | ||
| 1 to 4 | 2.8 | 2.175 | 128.7 | 1 to 4 | 53 | 33 | 160.6 | ||
| 1 to 8 | 1.6 | 1.088 | 147.1 | 1 to 8 | 21 | 16.5 | 127.3 | ||
| 2 | Neat | 9.3 | — | — | 2 | Neat | 113 | — | — |
| 1 to 2 | 4.6 | 4.65 | 98.9 | 1 to 2 | 66 | 56.5 | 116.8 | ||
| 1 to 4 | 2.3 | 2.325 | 98.9 | 1 to 4 | 34 | 28.25 | 120.4 | ||
| 1 to 8 | 1.5 | 1.163 | 129.0 | 1 to 8 | 20 | 14.125 | 141.6 | ||
| 3 | Neat | 10.0 | — | — | 3 | Neat | 94 | — | — |
| 1 to 2 | 5.2 | 5.0 | 104.0 | 1 to 2 | 56 | 47 | 119.1 | ||
| 1 to 4 | 2.5 | 2.5 | 100.0 | 1 to 4 | 29 | 23.5 | 123.4 | ||
| 1 to 8 | 1.7 | 1.250 | 136.0 | 1 to 8 | 17 | 11.75 | 144.7 | ||
| 4 | Neat | 7.8 | — | — | 4 | Neat | 145 | — | — |
| 1 to 2 | 4.0 | 3.9 | 102.6 | 1 to 2 | 82 | 72.5 | 113.1 | ||
| 1 to 4 | 2.3 | 1.95 | 117.9 | 1 to 4 | 43 | 36.25 | 118.6 | ||
| 1 to 8 | 1.6 | 0.975 | 164.1 | 1 to 8 | 23 | 18.125 | 126.9 | ||
| 5 | Neat | 9.3 | — | — | 5 | Neat | 157 | — | — |
| 1 to 2 | 3.9 | 4.65 | 83.9 | 1 to 2 | 85 | 78.5 | 108.3 | ||
| 1 to 4 | 2.2 | 2.325 | 94.6 | 1 to 4 | 44 | 39.25 | 112.1 | ||
| 1 to 8 | 1.5 | 1.163 | 129.0 | 1 to 8 | 25 | 19.625 | 127.4 | ||
| Parallelism O/E (%) | Parallelism O/E (%) | ||||||||
| Minimum | 83.9 | Minimum | 108.3 | ||||||
| Maximum | 164.1 | Maximum | 160.6 | ||||||
| Mean | 115.7 | Mean | 126.4 | ||||||
| SD | 22.2 | SD | 14 | ||||||
SD — standard deviation.
Coefficients of variation for intra-assay variability ranged from 6.4 to 9.5% for the Catalyst assay and from 1.5 to 2.6% for the Gentian assay, with mean CRP values between 8.7 and 49.1 mg/L, and 17.2 and 165.1 mg/L, respectively (Table II). Coefficients of variation for inter-assay variability ranged from 3.8 to 18.2% for the Catalyst assay and 4.5 to 5.8% for the Gentian assay, with mean CRP values between 6.9 and 89.6 mg/L, and 12.6 and 149.9 mg/L, respectively (Table III). The %CV of 18.2% was an outlier (mean concentration of sample = 6.9 mg/L), with the next highest %CV for inter-assay variability of the Catalyst being 10.5% (Table III).
Table II.
Intra-assay variation of C-reactive protein (CRP) in canine serum samples. Coefficients of variation (%CV), standard deviation (SD), and mean CRP concentrations (mg/L) were calculated for the Catalyst and Gentian assays. Five (Catalyst) and 6 (Gentian) serum samples were run 10 times consecutively.
| Catalyst assay | Gentian assay | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Sample | Mean | SD | %CV | Sample | Mean | SD | %CV |
| 1 | 8.7 | 0.8 | 9.5 | 1 | 17.2 | 0.4 | 2.6 |
| 2 | 12.4 | 1.0 | 8.7 | 2 | 17.7 | 0.5 | 2.7 |
| 3 | 19.2 | 1.6 | 8.8 | 3 | 77 | 1.0 | 1.4 |
| 4 | 37.1 | 2.0 | 5.7 | 4 | 107.3 | 1.6 | 1.5 |
| 5 | 49.1 | 3.0 | 6.4 | 5 | 150.4 | 2.6 | 1.8 |
| 6 | 165.1 | 2.4 | 1.5 | ||||
Table III.
Inter-assay variation of C-reactive protein (CRP) in canine serum samples. Coefficients of variation (%CV), standard deviation (SD), and mean CRP concentrations (mg/L) were calculated for the Catalyst and Gentian CRP assays. For each assay, 7 serum samples were run on 7 separate days.
| Catalyst assay | Gentian assay | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Sample | Mean | SD | %CV | Sample | Mean | SD | %CV |
| 1 | 6.9 | 1.2 | 18.2 | 1 | 13 | 0.8 | 5.8 |
| 2 | 8.4 | 0.7 | 8.6 | 2 | 12.6 | 0.5 | 3.9 |
| 3 | 8.6 | 0.9 | 10.5 | 3 | 14.6 | 0.7 | 5.0 |
| 4 | 25.3 | 1.3 | 5.1 | 4 | 84 | 3.8 | 4.5 |
| 5 | 25.1 | 1.7 | 6.9 | 5 | 106.1 | 3.6 | 3.4 |
| 6 | 54.4 | 3.6 | 6.6 | 6 | 111.1 | 3.8 | 3.4 |
| 7 | 89.6 | 3.4 | 3.8 | 7 | 149.9 | 6.8 | 4.5 |
Observed to expected ratios for recovery ranged from 85.6 to 110.7% and 95.7 to 103.4% for the Catalyst assay and Gentian assay, with mean O/E ratios of 97.9% ± 8.2% and 98.5% ± 3.8%, respectively (Table IV).
Table IV.
Recovery of C-reactive protein (CRP) in dog serum samples. Six canine serum samples of known concentrations were mixed in all possible combinations and evaluated using the IDEXX Catalyst One and Gentian Canine CRP assays. Different sets of serum samples were used for each assay. Observed to expected (O/E) ratios were then calculated.
| Catalyst assay | Gentian assay | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Sample | Spiking concentration | Observed (mg/L) | Expected (mg/L) | O/E (%) | Sample | Spiking concentration | Observed (mg/L) | Expected (mg/L) | O/E (%) |
| A | N/A | 1.0 | N/A | N/A | A | N/A | 12.0 | N/A | N/A |
| A + B | 2.0 | 1.6 | 1.5 | 106.9 | A + B | 17.0 | 14.5 | 14.5 | 100.0 |
| A + C | 3.5 | 2.3 | 2.2 | 103.4 | A + C | 40.0 | 25.5 | 26.0 | 98.1 |
| A + D | 9.2 | 5.3 | 5.1 | 104.0 | A + D | 134.0 | 75.0 | 73.0 | 102.7 |
| A + E | 5.4 | 2.9 | 3.2 | 89.8 | A + E | 100.5 | 54.5 | 56.25 | 96.9 |
| A + F | 4.0 | 2.3 | 2.5 | 93.9 | A + F | 118.0 | 59.5 | 65.0 | 91.5 |
| B | N/A | 2.0 | N/A | N/A | B | N/A | 17.0 | N/A | N/A |
| B + C | 3.5 | 2.9 | 2.7 | 106.4 | B + C | 40.0 | 28.5 | 28.5 | 100.0 |
| B + D | 9.2 | 5.1 | 5.6 | 91.9 | B + D | 134.0 | 78.0 | 75.5 | 103.3 |
| B + E | 5.4 | 3.4 | 3.7 | 91.2 | B + E | 100.5 | 58.5 | 58.75 | 99.6 |
| B + F | 4.0 | 2.8 | 3.0 | 93.2 | B + F | 118.0 | 63.0 | 67.5 | 93.3 |
| C | N/A | 3.5 | N/A | N/A | C | N/A | 40.0 | N/A | N/A |
| C + D | 9.2 | 7.0 | 6.3 | 110.7 | C + D | 134.0 | 90.0 | 87.0 | 103.4 |
| C + E | 5.4 | 4.4 | 4.5 | 97.8 | C + E | 100.5 | 69.5 | 70.25 | 98.9 |
| C + F | 4.0 | 3.2 | 3.7 | 85.9 | C + F | 118.0 | 75.0 | 79.0 | 94.9 |
| D | N/A | 9.2 | N/A | N/A | D | N/A | 134.0 | N/A | N/A |
| D + E | 5.4 | 7.6 | 7.3 | 103.8 | D + E | 100.5 | 121.0 | 117.25 | 103.2 |
| D + F | 4.0 | 6.9 | 6.6 | 104.6 | D + F | 118.0 | 120.0 | 126.0 | 95.2 |
| E | N/A | 5.4 | N/A | N/A | E | N/A | 100.5 | N/A | N/A |
| E + F | 4.0 | 4.0 | 4.7 | 85.6 | E + F | 118.00 | 104.5 | 109.25 | 95.7 |
| Recovery O/E (%) | Recovery O/E (%) | ||||||||
| Minimum | 85.6 | Minimum | 91.5 | ||||||
| Maximum | 110.7 | Maximum | 103.4 | ||||||
| Mean | 97.9 | Mean | 98.5 | ||||||
| SD | 8.2 | SD | 3.8 | ||||||
N/A — not applicable; SD — standard deviation.
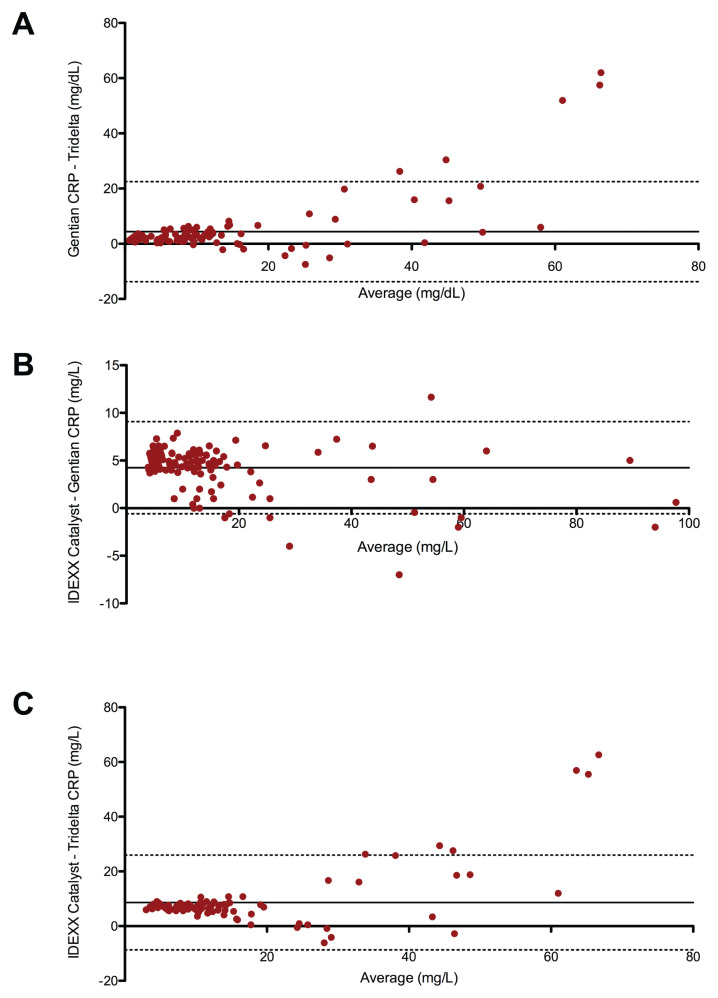
The bias between the Gentian and Tridelta assays was 4.4 with a 95% CI from 22.5 to −13.8 (Figure 1 A) and between the Catalyst and Gentian assays, the bias was 4.2 with a 95% CI from −0.6 to 9.1 (Figure 1 B). Bias between the Catalyst and Tridelta was reported to be 8.6% with a 95% CI from −8.7 to 25.9 (Figure 1 C). Proportional biases were seen between the Catalyst and Tridelta and between the Gentian and Tridelta; no proportional bias was observed between the Catalyst and Gentian assays. The coefficient of determination, or R2 value, between the Catalyst and Tridelta was 0.76, while R2 between the Gentian and Tridelta was 0.79 (Figure 2 A,B). The Catalyst and Gentian assays correlated most strongly with an R2 value of 0.98 (Figure 2 C). P-value for Figure 2 A,C was < 0.0001.
Figure 1.
Bland-Altman plots comparing canine C-reactive protein (CRP) measurements by 2 different assays (Catalyst and Gentian) with a reference enzyme-linked immunosorbent assay (Tridelta). The solid black line represents the bias and the dotted black lines delineate the 95% confidence intervals. A — Comparison of the Gentian with the Tridelta. B — Comparison of the Catalyst with the Gentian. In each comparison, 136 canine serum samples were used. C — Comparison of the Catalyst with the Tridelta.
Figure 2.
Correlation of canine C-reactive protein (CRP) measurements by 2 different assays (Catalyst and Gentian) compared with the reference enzyme-linked immunosorbent assay (Tridelta). The solid black line represents the linear regression line, while the dotted black line represents the Deming regression line. A — Correlation between the Catalyst and Tridelta (R2 = 0.76). B — Correlation between the Gentian and Tridelta (R2 = 0.79). C — Correlation between the Catalyst and Gentian (R2 = 0.98). In each comparison, 136 canine serum samples were used.
The reference interval calculated for the Catalyst and Gentian assays were 6.0 to 15.1 mg/L and 1.3 to 9.1 mg/L, respectively (Figure 3).
Figure 3.
Serum C-reactive protein (CRP) concentrations in 58 healthy dogs measured by the Tridelta, Gentian, and Catalyst assays. The upper limits of these assays’ reference intervals are 7.6 mg/L (Tridelta) and 10 mg/L (Gentian and IDEXX). For the Catalyst assay, CRP concentrations > 30 mg/L represent the cutoff point for clinically relevant inflammation.
Discussion
The aims of our study were i) to analytically validate the Gentian canine CRP assay on the Beckman Coulter AU480 Chemistry Analyzer, ii) to analytically validate the Catalyst CRP Test, and iii) to compare these assays with the Tridelta Development EIA Canine CRP Assay as a reference standard. We found that both assays had linearity, precision, repeatability, and accuracy but their results were not directly comparable with the Tridelta assay.
Linearity of each assay was determined by diluting dog serum samples with dog serum that had an unmeasurable CRP concentration and calculating O/E ratios. Dog serum was chosen as a diluent as this was the matrix that both assays were expected to be compatible with. Observed to expected ratios for dilutional parallelism were 83.9 to 163.1% and 108.3 to 160.6% for Gentian and Catalyst assays, respectively. Thus, linearity appeared to be suboptimal for both assays compared to the target for O/E ratios between 80 to 120%. It was also generally observed that as the dilution factor increased, the O/E ratio increased above 100%. As such, regardless of the diluent used, 1:2 and 1:4 dilutions had more favorable O/E ratios compared to 1:8 dilutions. However, this apparent lack of linearity is not expected to have any clinical implications, as canine serum samples would rarely need to be diluted by a factor of 1:8 for the measurement of CRP, given that the upper limits of the reference intervals for the Gentian and Catalyst assays were 300 mg/L and 100 mg/L, respectively, which were well above the highest CRP concentrations measured in serum samples in this study.
Although there is no generally accepted maximum %CV, intra-and inter-assay %CVs of 10 or less are considered acceptable (17). In the case of both the Catalyst and Gentian assays, nearly all of the intra- and inter-assay %CVs remained below 10%. The 2 exceptions to this, with inter-assay %CVs of 10.5% and 18.2%, were 2 samples measured with the Catalyst assay (Table III). However, these samples reported mean serum CRP concentrations (9 mg/L and 7 mg/L, respectively) that were in the lower limit of the assay’s working range (1.0 to 100.0 mg/L). It is not uncommon for assay repeatability to be lower in the lower limit of the working range. Additionally, all results for these samples were well below 30 mg/L, the cutoff point for clinically important inflammation with this assay; thus, this limited repeatability would not have affected clinical decision-making.
When compared to a previously published evaluation of the Gentian assay, run on the ABX Pentra 400 analyzer, the intra-assay %CV was similar and acceptable when the Beckman Coulter AU480 was used (10). Both instruments demonstrated comparable interassay %CVs of < 7.9% (10).
The mean O/E ratios for recovery of canine CRP in dog serum samples were 97.9% ± 8.2% (minimum = 85.6%; maximum = 110.7%) and 98.5% ± 3.8 % (minimum = 95.7%; maximum = 103.4%) for the Catalyst and Gentian assays, respectively. This falls within the generally accepted criteria for recovery of 80 to 120%, suggesting that both assays are accurate. Recovery %CVs for the Gentian assay could not be directly compared between the ABX Pentra 400 and Beckman Coulter AU480 analyzers, as different dilution factors were used. In the case of the Abbott Architect c4000, a recovery study of 2 spiked samples yielded recoveries of 123% and 116% (9). These recoveries are greater than those seen in the present study, although further evaluation is required to definitively establish this relationship.
Taken together, these findings demonstrate that both the Catalyst and Gentian assays have sufficient precision, reproducibility, and accuracy to be suitable for clinical use, and that the linearity characteristic, although not ideal, is unlikely to detract from this.
The reference interval calculated from 58 healthy dogs for the Catalyst assay was 6.0 to 15.1 mg/L; that of the Gentian assay measured on the Beckman Coulter AU480 was calculated to be 1.3 to 9.1 mg/L. The reference interval for the Catalyst assay fits well within the manufacturer’s cutoff of 30 mg/L for clinically significant inflammation. The reference interval for the Gentian assay is concordant with that suggested by the manufacturer (< 10 mg/L) and suggests that this reference interval can be transferred to this instrument. All 58 healthy dogs had CRP concentrations < 7.6 mg/L when measured using the Tridelta assay, which is concordant with the previous reference interval reported by our laboratory of 2.4 to 7.6 mg/L. Interestingly, 6 of our 58 dogs had a serum CRP concentration above the upper limit of the manufacturer’s specified reference interval (> 10.0 mg/L) when measured with the Catalyst assay. The reason for this apparent discrepancy is unknown, as the 58 healthy dogs were all screened extensively. However, it should be noted that none of these dogs had a CRP concentration > 30 mg/L, the cutoff point of the Catalyst assay for clinically relevant inflammation, and so clinical decision making would not have been affected.
The agreement between the Catalyst, Gentian, and Tridelta assays varied (Figure 1 A–C). Generally, the Bland-Altman diagram demonstrates good agreement between 2 assays when the differences between their data points are centered around a 0-difference line and the data points are distributed equally above and below said line (20). The agreement between the Catalyst and Gentian assays was better than their agreement with the Tridelta assay. This is not surprising as the Gentian assay was used to calibrate the Catalyst assay (in-house study; Graham E. Bilbrough, IDEXX Laboratories 2017). When compared to the Tridelta assay, both the Catalyst and Gentian assays demonstrated a negative proportional bias and heteroscedasticity, with more variation for samples with higher concentrations (Figure 1 A,C). No proportional bias was apparent between the Catalyst and Gentian assays when they themselves were compared using a Bland-Altman plot (Figure 1 B); of the assays evaluated, these 2 had the closest agreement. The results of this study demonstrate that canine serum CRP concentrations are not directly interchangeable between these 3 assays. Thus, the clinical interpretation of a dog’s serum CRP value must be made in conjunction with the appropriate diagnostic reference range/cutoff value for each assay and the same assay should be used each time a patient’s serum CRP concentration is measured longitudinally.
This study is subject to several limitations. Firstly, as mentioned in other method comparison studies, it is probable that there are inherent variations within the reference ELISA that may have contributed to the bias seen between assays (15). The Tridelta assay was chosen as a reference as it has been validated for use in dogs (8). However, it may not be valid to assume that this assay is the gold standard. Although the Tridelta assay was able to detect clinically important changes of serum CRP concentrations and distinguish between diseased and healthy dogs, it had a higher than acceptable inter-assay %CVs (8,12). In addition, although serum samples that appeared grossly lipemic or hemolytic were not included in the study, the authors did not investigate any interference that triglycerides or hemoglobin may have had on the measured canine serum CRP concentrations across these 3 assays. Although the Gentian and Tridelta assays were run in duplicate, as per the manufacturers’ directions, the Catalyst assay was not. However, POCT assays are usually not run in duplicate, and the manufacturer of this assay does not recommend this practice; thus, our study sought to mimic how each assay would be used under field conditions. Lastly, although we evaluated canine serum samples with a wide range of CRP concentrations, we did not evaluate the ability of the assays to differentiate between dogs with and without acute phase inflammation or the utility of the assays for the longitudinal assessment of canine serum CRP concentrations.
In conclusion, both the Catalyst and Gentian demonstrated sufficient precision, reproducibility, and accuracy to be acceptable for measuring CRP in dog serum, but their results are not directly comparable to those of the Tridelta assay. The Catalyst and Gentian assays had the strongest agreement, whereas their agreement with the Tridelta assay was less favorable due to proportional bias and heteroscedasticity. Therefore, assay specific reference intervals/cutoff points should be used when interpreting canine CRP concentration measurements and the same assay should be used each time a patient’s serum CRP concentration is measured longitudinally.
Footnotes
IDEXX Laboratories provided the Catalyst One and related materials used in this study free of charge. Dr. Steiner is a paid consultant for IDEXX Laboratories. Dr. Lidbury has acted as a paid speaker for IDEXX Laboratories. The authors are affiliated with the Gastrointestinal Laboratory, Texas A&M University, which offers measurement of canine C-reactive protein on a fee-for-service basis.
This work was presented in the form of an abstract at the 2018 American College of Veterinary Internal Medicine Forum in Seattle, Washington.
References
- 1.Cerón JJ, Eckersall PD, Martínez-Subiela S. Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Vet Clin Pathol. 2005;34:85–99. doi: 10.1111/j.1939-165x.2005.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura M, Takahashi M, Ohno K, et al. C-reactive protein concentration in dogs with various diseases. J Vet Med Sci. 2008;70:127–131. doi: 10.1292/jvms.70.127. [DOI] [PubMed] [Google Scholar]
- 3.Higgins MA, Berridge BR, Mills BJ, et al. Gene expression analysis of the acute phase response using a canine microarray. Toxicol Sci. 2003;74:470–484. doi: 10.1093/toxsci/kfg142. [DOI] [PubMed] [Google Scholar]
- 4.Cray C, Zaias J, Altman NH. Acute phase response in animals: A review. Comp Med. 2009;59:517–526. [PMC free article] [PubMed] [Google Scholar]
- 5.Eckersall PD, Bell R. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet J. 2010;185:23–27. doi: 10.1016/j.tvjl.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Gebhardt C, Hirschberger J, Rau S, et al. Use of C-reactive protein to predict outcome in dogs with systemic inflammatory response syndrome or sepsis. J Vet Emerg Crit Care (San Antonio) 2009;19:450–458. doi: 10.1111/j.1476-4431.2009.00462.x. [DOI] [PubMed] [Google Scholar]
- 7.Conner JG, Eckersall PD, Ferguson J, Douglas TA. Acute phase response in the dog following surgical trauma. Res Vet Sci. 1988;45:107–110. [PubMed] [Google Scholar]
- 8.Kjelgaard-Hansen M, Kristensen AT, Jensen AL. Evaluation of a commercially available enzyme-linked immunosorbent assay (ELISA) for the determination of C-reactive protein in canine serum. J Vet Med A Physiol Pathol Clin Med. 2003;50:164–168. doi: 10.1046/j.1439-0442.2003.00509.x. [DOI] [PubMed] [Google Scholar]
- 9.Hillström A, Hagman R, Tvedten H, Kjelgaard-Hansen M. Validation of a commercially available automated canine-specific immunoturbidimetric method for measuring canine C-reactive protein. Vet Clin Pathol. 2014;43:235–243. doi: 10.1111/vcp.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindenberg S, Klenner-Gastreich S, Kneier N, et al. Evaluation of a species-specific C-reactive protein assay for the dog on the ABX Pentra 400 clinical chemistry analyzer. BMC Vet Res. 2017;13:146. doi: 10.1186/s12917-017-1065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jasensky AK, Klenner S, Einspanier R, Kohn B. Evaluation of three different point-of-care tests for quantitative measurement of canine C-reactive protein. Vet Clin Pathol. 2015;44:205–214. doi: 10.1111/vcp.12264. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Subiela S, Cerón JJ. Analytical validation of commercial assays for the determination of haptoglobin, C-reactive protein and serum amyloid A in dogs. Arch Med Vet. 2005;37:61–66. [Google Scholar]
- 13.Klenner S, Bauer N, Moritz A. Evaluation of three automated human immunoturbidimetric assays for the detection of C-reactive protein in dogs. J Vet Diagn Invest. 2010;22:544–552. doi: 10.1177/104063871002200408. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto S, Miyaji S, Abe N, Otabe K, Furukawa E, Naiki M. Canine C-reactive protein (CRP) does not share common antigenicity with human CRP. Vet Res Commun. 1993;17:259–266. doi: 10.1007/BF01839216. [DOI] [PubMed] [Google Scholar]
- 15.Farr AJ, Freeman KP. Quality control validation, application of sigma metrics, and performance comparison between two biochemistry analyzers in a commercial veterinary laboratory. J Vet Diagn Invest. 2008;20:536–544. doi: 10.1177/104063870802000502. [DOI] [PubMed] [Google Scholar]
- 16.Hindenberg S, Keβler M, Zielinsky S, Langenstein J, Moritz A, Bauer N. Evaluation of a novel quantitative canine species-specific point-of-care assay for C-reactive protein. BMC Vet Res. 2018;14:99. doi: 10.1186/s12917-018-1415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steiner JM, Teague SR, Williams DA. Development and analytic validation of an enzyme-linked immunosorbent assay for the measurement of canine pancreatic lipase immunoreactivity in serum. Can J Vet Res. 2003;67:175–182. [PMC free article] [PubMed] [Google Scholar]
- 18.Andreasson U, Perret-Liaudet A, van Waalwijk van Doorn LJ, et al. A practical guide to immunoassay method validation. Front Neurol. 2015;6:179. doi: 10.3389/fneur.2015.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geffré A, Concordet D, Braun JP, Trumel C. Reference Value Advisor: A new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet Clin Pathol. 2011;40:107–112. doi: 10.1111/j.1939-165X.2011.00287.x. [DOI] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]