Abstract
Purpose
To assess safety and patency of the Venovo venous stent for the treatment of iliofemoral vein obstruction.
Materials and Methods
Twenty-two international centers enrolled 170 patients in the VERNACULAR study (93 post-thrombotic syndrome; 77 non-thrombotic iliac vein lesions). Primary outcome measures were major adverse events at 30 days and 12-month primary patency (freedom from target vessel revascularization, thrombotic occlusion, or stenosis > 50%). Secondary outcomes included the Venous Clinical Severity Score Pain Assessment and Chronic Venous Quality-of-Life Questionnaire assessments (hypothesis tested). Secondary observations included primary patency, target vessel and lesion revascularization (TVR/TLR), and assessment of stent integrity through 36 months.
Results
Freedom from major adverse events through 30 days was 93.5%, statistically higher than a pre-specified performance goal of 89% (p = 0.032) while primary patency at 12 months was 88.6%, also statistically higher than a performance goal of 74% (p < 0.0001). Mean quality-of-life measures were statistically improved compared to baseline values at 12 months (p < 0.0001). Primary patency at 36 months was 84% (Kaplan–Meier analysis) while freedom from TVR/TLR was 88.1%. There was no stent embolization/migration, and no core laboratory assessed stent fractures reported through 36 months. Six deaths were reported; none adjudicated as device or procedure related.
Conclusion
The Venovo venous stent was successfully deployed in obstructive iliofemoral vein lesions and met the pre-specified primary outcome measures through 12 months. At 3 years, primary patency was 84%, reintervention rates were low, standardized quality-of-life and pain measures improved from baseline, and there was no stent migration or fractures.
Level of Evidence
Level 2—prospective, multicenter, controlled clinical study without a concurrent control or randomization. Pre-specified endpoints were hypothesis-tested to performance goals derived from peer-reviewed clinical literature.
Registration clinicaltrials.gov
Unique Identifier NCT02655887.
Keywords: Iliofemoral venous obstruction, Iliac and femoral vein occlusive disease, Percutaneous endovenous stent, Post-thrombotic syndrome, Non-thrombotic iliac vein lesion
Introduction
Iliofemoral venous obstruction, caused by anatomic vein compression or deep vein thrombosis (DVT), can lead to severe venous insufficiency and a reduction in quality-of-life. Exercise, compression therapy, anticoagulation with thrombolysis, catheter-directed thrombolysis, or pharmaco-mechanical thrombectomy do not address the underlying causes of venous obstruction and have provided mixed therapeutic results [1–3]. Surgery has been used to bypass occlusions, but stent use has largely replaced surgery as the interventional procedure of choice [4]. Bare metal stents designed for arterial use have been used to treat peripheral venous obstructions, yet few have been systematically studied in the venous system [5–7]. Dedicated venous stents for iliofemoral venous outflow obstruction have emerged over the past few years as alternatives to conservative therapy, surgery, and the use of arterial stents. Observational single-center studies using the Venovo venous stent have reported good early patency and low complication rates [8–10]. The current prospective, multicenter study was designed and powered to evaluate major adverse events and primary patency of this dedicated, nitinol self-expanding, open-cell venous stent for iliofemoral obstructions through 12 months with secondary observations through 3 years.
Methods and Materials
Study Design
VERNACULAR was a prospective, multicenter study of the Venovo venous stent used to treat iliac and femoral vein thrombotic obstruction or venous compression. Between June 2016 and May 2017, 170 patients were treated at 22 centers in the USA, Europe, and Australia. Investigators followed a protocol approved by their institutional review board or ethics committee, patients provided written informed consent to participate in the study, and study procedures were conducted in accordance with the Declaration of Helsinki, good clinical practice, and other applicable healthcare regulations and privacy laws. A clinical events committee (CEC) provided independent oversight of patient safety, and a medical monitor reviewed adverse event trends. The Yale Cardiovascular Research Group (New Haven, CT) independently analyzed X-rays and venographic images while VasCore, the Vascular Ultrasound Core Laboratory (Massachusetts General Hospital, Boston, MA), reviewed duplex-ultrasound (DUS) images. VERNACULAR was sponsored by Bard/Becton, Dickinson and registered on clinicaltrials.gov (Unique Identifier: NCT02655887) prior to patient enrollment.
Eligible patients had symptomatic, non-malignant venous outflow obstruction in the iliac or femoral veins (≥ 50% by contrast venography) with a clinical–etiologic–anatomic–pathophysiologic clinical score (CEAP “C” score) ≥ 3 or a venous clinical severity score (VCSS) pain component ≥ 2. Exclusion criteria included venous obstruction that extended into the inferior vena cava or below the level of the lesser trochanter, prior stent placement in the target vessel, or an iliac or femoral vein unsuitable for treatment with available device sizes.
Outcome Measures
The primary outcome measures included freedom from major adverse events (MAEs) through 30 days and 12-month primary patency, both compared to performance goals (PGs) derived from published clinical literature. MAEs included target vessel revascularization (TVR), device- or procedure-related DVT, target-limb major amputation, symptomatic pulmonary embolism, vascular injury requiring intervention, device migration, or death. Primary patency was defined as freedom from TVR, thrombotic occlusion, or stenosis greater than 50% measured by DUS and reviewed by the core laboratory. Secondary outcomes included quality-of-life (QoL) measures evaluated with the pain component of the VCSS and the Chronic Venous Quality-of-Life Questionnaire (CIVIQ-20 Global Index). QoL assessments were hypothesis-tested at 12 months to mean baseline values. Additional secondary observations included technical success, defined as successful deployment of the stent to the intended location with adequate lesion coverage and procedural success, technical success with no major adverse events between index procedure and discharge. Primary patency, TVR and target lesion revascularization (TLR; both core laboratory evaluated), CEAP classification scores, and stent integrity (i.e., migration and fracture) were reported through 3 years.
Baseline Patient and Lesion Characteristics
The treated population consisted of 170 patients who consented to participate in the study and received a stent (Fig. 1) and included 93 patients with post-thrombotic syndrome (PTS) and 77 patients with symptomatic non-thrombotic iliac vein lesions (NIVL). Baseline patient demographics and medical histories are summarized in Table 1. At baseline, 235 lesions were evaluated by the venographic core laboratory. Patients could present with more than one lesion in multiple veins; 94.5% of patients had lesions in the common iliac vein, 40.5% in the external iliac vein, and 9.2% in the common femoral vein. The mean total lesion length per treated limb was 67.8 ± 39.7 mm, and the mean pre-procedure percent diameter stenosis was 75.7 ± 17.0% (Table 2).
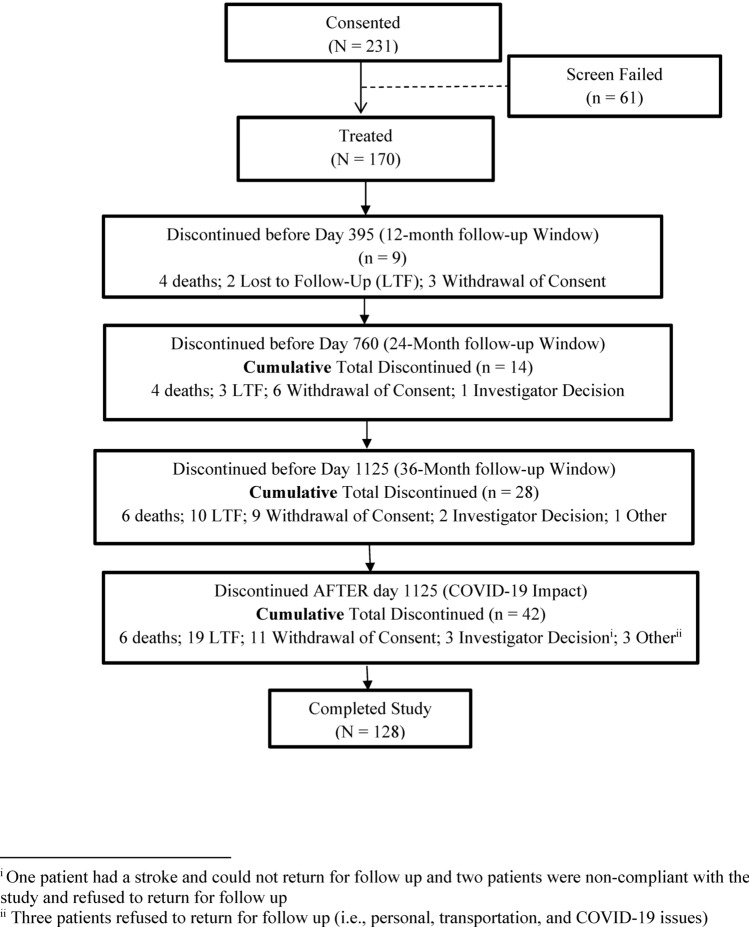
Fig. 1.
Summary of the final disposition of patients in the VERNACULAR Study. Two hundred thirty-one patients were enrolled, and 170 were treated with the Venovo venous stent. One hundred twenty-eight patients completed the study while 42 patients either withdrew consent or were withdrawn by an investigator (14), were lost to follow-up (19), died (6), or discontinued participation for other reasons (3). Due to the COVID-19 pandemic, the final 36-month follow-up window was extended beyond 1125 days to allow patients to complete the study
Table 1.
Baseline patient demographics, medical history, and additional procedures
| Patient demographics | PTS subgroup N = 93 | NIVL subgroup N = 77 | All treated N = 170 |
|---|---|---|---|
| Age (years), X ± SD | 49.8 ± 15.0 | 55.0 ± 15.4 | 52.1 ± 15.3 |
| Male/Female, % / % | 45.2/54.8 | 27.3/72.7 | 37.1/62.9 |
| BMI, kg/m2 ± SD | 28.6 ± 6.4 | 29.1 ± 7.7 | 28.8 ± 7.0 |
| Race, % (n) | |||
| Caucasian | 89.2 (83) | 94.8 (73) | 91.8 (156) |
| African American | 7.7 (7) | 2.6 (2) | 5.3 (9) |
| Asian | 3.2 (3) | 1.3 (1) | 2.4 (4) |
| Medical history, % (n) | |||
| Varicose Veins | 76.3 (71) | 80.5 (2) | 78.2 (133) |
| May–Thurner Syndrome | 37.6 (35) | 87.0 (67) | 60.0 (102) |
| Deep Vein Thrombosis | 98.9 (92) | 0 (0) | 54.1 (92) |
| Cigarette Smoking | 30.1 (28) | 39.0 (30) | 34.1 (58) |
| Hypertension | 29.0 (27) | 36.4 (28) | 32.4 (55) |
| Dyslipidemia | 21.5 (20) | 35.1 (27) | 27.6 (47) |
| Respiratory Disorder | 14.0 (13) | 9.1 (7) | 11.8 (20) |
| Diabetes | 5.4 (5) | 16.9 (13) | 10.6 (18) |
| Peripheral Arterial Disease | 6.5 (6) | 15.6 (12) | 10.6 (18) |
| Gastrointestinal Disease | 11.8 (11) | 9.1 (7) | 10.6 (18) |
| Coronary Artery Disease | 6.5 (6) | 11.7 (9) | 8.8 (15) |
| Coronary Intervention | 6.5 (6) | 10.4 (8) | 8.2 (14) |
| Venous Valve Disease | 7.5 (7) | 6.5 (5) | 7.1 (12) |
| Inferior Vena Cava Filter | 10.8 (10) | 0 (0) | 5.9 (10) |
| Additional procedures1, % (n) | |||
| Previous Procedure2 | 20.4 (19) | 13.0 (10) | 17.1 (29) |
| 30-Day Planned Procedure3 | 9.7 (9) | 2.6 (2) | 6.5 (11) |
1Some patients had multiple procedures
2Procedure within 1 year of the study; most peripheral vascular interventions were for vena cava filter placement (33%), thrombectomy (16%), or venous procedures (e.g., venoplasty, ablation, or lysis)
3Planned peripheral endovascular or surgical intervention 30 days before or after the study procedure
Table 2.
Baseline lesion characteristics and stent details
| Lesion characteristics1 | PTS subgroup N = 89 | NIVL subgroup N = 74 | All treated N = 1632 |
|---|---|---|---|
| Lesion Location3, % (n/N) | |||
| Common Iliac Vein | 92.1 (82/89) | 10.8 (8/74) | 94.5 (154/163) |
| External Iliac Vein | 58.4 (52/89) | 18.9 (14/74) | 40.5 (66/163) |
| Common Femoral Vein | 14.6 (13/89) | 2.7 (2/74) | 9.2 (15/163) |
| Lesion Length, mm, μ ± SD | 80.5 ± 42.8 | 55.2 ± 32.0 | 67.8 ± 39.7 |
| Length Range, mm | 18.1–199.7 | 22.3–183.4 | 18.1–199.7 |
| Vessel Diameter, mm, μ ± SD | 16.0 ± 2.7 | 17.4 ± 3.3 | 16.7 ± 3.0 |
| Diameter Stenosis, % ± SD | |||
| Baseline | 81.0 ± 18.4 | 69.3 ± 12.6 | 75.7 ± 17.0 |
| After Pre-dilation | 49.9 ± 21.1 | 40.6 ± 28.2 | 47.8 ± 22.9 |
| Final | 16.1 ± 7.0 | 12.1 ± 5.3 | 14.3 ± 6.5 |
| Stent Details4 | |||
| Diameter, mm, μ ± SD | 15.4 ± 2.1 | 16.6 + 2.0 | 15.9 ± 2.1 |
| Length, mm, μ + SD | 100.1 ± 33.2 | 83.0 ± 26.3 | 93.5 ± 31.7 |
| Stents Placed, N | 134 | 85 | 219 |
| Single Stent, % (n) | 69.4 (93) | 90.6 (77) | 77.6 (170) |
| Distal Overlap, % (n) | 20.1 (27) | 4.7 (4) | 14.2 (31) |
| Proximal Overlap, % (n) | 10.4 (14) | 4.7 (4) | 8.2 (18) |
| Pre-dilation, % (n) | 87.1 (81) | 66.2 (51) | 77.6 (132) |
| Post-dilation, % (n) | 92.5 (86) | 88.3 (68) | 90.6 (154) |
| Technical Success, % (n/N) | 100 (93/93) | 100 (77/77) | 100 (170/170) |
| Procedural Success, % (n/N) | 97.8 (91/93) | 100 (77/77) | 98.8 (168/170)5 |
1Quantitative Vascular Analysis by the Venographic Core Laboratory
2163 patients in the ITT cohort had images that could be evaluated by the core laboratory
3Some patients had lesions in multiple locations
4219 stents were used in 170 patients
5Two patients in the PTS subgroup experienced a TVR for stent thrombosis prior to discharge
Study Procedures and Follow-Up
A comprehensive clinical history, physical examination, QoL assessment, and diagnostic venogram were performed prior to treatment to confirm study eligibility. Patients received prophylactic low-molecular-weight heparin prior to the procedure, 3000–5000 Units of unfractionated heparin during the procedure, and appropriate antiplatelet or anticoagulation medications post-procedure (e.g., acetylsalicylic acid, clopidogrel, rivaroxaban, apixaban) based on physician and institution standard-of-care guidelines. The Venovo venous stent (Bard/Becton, Dickinson and Company, Tempe, Arizona, USA) was used according to the device instructions for use. Stent diameters ranged from 10 to 20 mm (2 mm increments) and lengths from 40 to 160 mm (20 mm increments), the stent ends were flared approximately 3 mm larger than nominal stent diameter to provide fixation and prevent migration, and three radiopaque tantalum markers on each end of the stent enhanced visibility to aid in placement. Stents were introduced through an 8–10 F sheath depending on device size. Up to two stents per patient were allowed per protocol with a recommended overlap of 10 mm. Venograms were completed pre-deployment, at stent deployment, and at procedure completion and were sent to the venographic core laboratory for analysis. Follow-up visits at 1, 6, 12, 24, and 36 months included physical examination, target-limb assessment, documentation of reintervention or adverse events, assessment of changes in QoL, and DUS imaging. Anterior–posterior (A–P) radiographs were taken at 12, 24, and 36 months to evaluate possible stent fractures (analyzed by the Yale Core Laboratory).
Statistical Analysis
The treated sample size of 170 patients provided an overall power of > 85% for the pre-specified outcome measures. Freedom from MAEs was achieved if the one-sided p value was less than 0.05 or the lower limit of the two-sided 90% confidence limit was greater than a literature-derived PG of 89% (exact binomial method). MAEs reported in 19 peer-reviewed articles (> 2400 patients) were used to derive a mean overall freedom from MAEs of 99% [11–29]. The PG was set at a 10% non-inferiority margin below the literature-derived value at 30 days. Similarly, primary patency was achieved if the one-sided p value was less than 0.05 or the lower limit of the two-sided 90% confidence limit was greater than a PG of 74% (weighted Z-statistics). The patency PG was derived from published data on stent treatment of lower-limb venous obstructions; primary patency data from fifteen NIVL studies (1149 limbs) [11, 13, 15, 23, 25, 27, 28, 30–37] and fourteen PTS studies (908 limbs) [11, 12, 14, 19–22, 24, 26, 28, 29, 38–40] were weighted and used to derive an overall mean primary patency rate (84%); the PG was set at a 10% non-inferiority margin below the weighted literature-derived mean at 12 months. The VCSS pain score and CIVIQ-20 assessment at 12 months were hypothesis-tested to baseline values (Hochberg method for controlling multiple comparisons); the reduction in pain and discomfort was deemed statistically significant if the two-sided p value (paired t test) was less than 0.05.
Additional secondary outcomes were reported using Kaplan–Meier (K–M) estimates and descriptive statistics through 3 years. Descriptive statistics included categorical variables presented as percentages and frequency counts, and continuous variables that included means ± standard deviation (SD) and confidence intervals (CIs). Secondary outcomes beyond the hypothesis-tested measures at 12 months were observational.
Results
Two hundred nineteen (219) stents were used in 170 patients; one device was used in 77.6% of cases (Table 2). The mean stent length was 93.5 ± 31.7 mm (range 40–160 mm), and the mean stent diameter was 15.9 ± 2.1 mm (range 10–20 mm). Technical success was achieved in all cases (100%) while procedural success was 98.8% (168/170); two PTS patients experienced a TVR for stent thrombosis prior to discharge. The final mean percent diameter stenosis after stent placement was 14.3 ± 6.5%.
Post-procedure Follow-up and Outcomes
One hundred twenty-eight patients completed the study at 3 years (Fig. 1). Primary and secondary outcome measures are summarized in Table 3. Freedom from MAEs at 30 days was 93.5% [90% CI: 89.5%, 96.3%], statistically higher than the literature-derived PG of 89% (one-sided exact binomial test; p value = 0.03). Analysis of patient subgroups demonstrated a numerical difference, but not a statistically significant difference in MAEs between the PTS subgroup (88.2%) and the NIVL subgroup (100%; p = 0.94 logistic regression with subgroup as fixed effect). Twelve-month primary patency was 88.6% [90% CI: 82.8%, 94.4%], also statistically higher than the literature-derived PG of 74% (one-sided exact binomial test; p value < 0.0001). This was a weighted mean based on the distribution between the mean primary patency for the PTS and NIVL subgroups. Analysis of patient subgroups demonstrated a statistically higher primary patency rate for the NIVL subgroup (96.9%) compared to the PTS subgroup (81.3%; p = 0.01; type III test by logistic regression with subgroup as fixed effect). The VCSS pain score and the CIVIQ-20 global index were secondary QoL endpoints hypothesis-tested at 12 months to baseline values. The mean improvement in the VCSS pain score was −1.7 [95% CI: −1.8, −1.5] at 12 months (two-sided paired t test; p < 0.0001; Fig. 2A) and the mean improvement in the CIVIQ-20 global index score was −15.7 [95% CI: −18.4, −13.0] (two-sided paired t test: p < 0.0001; Fig. 2B), representing statistically significant improvements in overall pain and patient comfort from baseline.
Table 3.
Primary results, hypothesis-tested secondary outcomes, and secondary observations
| Primary outcome measures | PTS subgroup N = 93 | NIVL subgroup N = 77 | All treated N = 170 | p value [90% CI] |
|---|---|---|---|---|
| Primary Patency1, % (n//N) | 81.7 (67/82) | 97.1 (66/68) | 88.62 |
< 0.00013 [82.8, 94.4] |
| Freedom from MAEs4, % (n/N) | 88.2 (82/93) | 100 (77/77) | 93.5 |
0.0325 [89.5, 96.3] |
| Secondary outcome measures (hypothesis tested) | p value | |||
|---|---|---|---|---|
| VCSS Pain Score6 | ||||
| Baseline |
2.27 [2.1, 2.4] |
2.3 [2.1, 2.4] |
2.3 [2.2, 2.4] |
|
| 12 Months |
0.7 [0.5, 0.9] |
0.5 [0.3, 0.7] |
0.6 [0.5, 0.7] |
|
| Change from Baseline |
−1.5 [−1.8, −1.3] |
−1.8 [−2.0, −1.6] |
−1.7 [−1.8, −1.5] |
< 0.00018 |
| CIVIQ-20 Score9 | ||||
| Baseline |
52.510 [48.6, 56.4] |
45.7 [41.8, 49.5] |
49.3 [46.5, 52.0] |
|
| 12 Months |
34.0 [30.5, 37.6] |
33.1 [29.3, 36.9] |
33.6 [31.0, 36.2] |
|
| Change from Baseline |
−18.5 [−22.2, −14.7] |
−12.6 [−16.5, −8.7] |
−15.7 [−18.4, −13.0] |
< 0.000111 |
| Secondary observations | [95% CI] | |||
|---|---|---|---|---|
| CEAP Score, mean ± SD | ||||
| Baseline | 3.4 ± 1.0 | 3.5 ± 0.8 | 3.5 ± 0.9 | |
| 36 Months | 2.1 ± 1.6 | 2.0 ± 1.5 | 2.1 ± 1.6 | |
| Change from Baseline |
−1.3 [−1.7, −1.0] |
−1.5 [−1.9, −1.2] |
−1.412 [−1.7, −1.2] |
|
| Freedom from TVR/TLR | ||||
| 12 Months, % (n/N) | 87.6 (78/89) | 98.6 (73/74) | 92.6 (151/163) | [87.5, 96.1] |
| 24 Months, % (n/N) | 82.8 (72/87) | 97.3 (72/74) | 89.4 (144/161) | [83.6, 93.7] |
| 36 Months, % (n/N) | 80.5 (66/82) | 97.1 (67/69) | 88.1 (133/151) | [81.8, 92.8] |
| Primary Patency13, % (n/N) | [90% CI] | |||
| 24 Months | 75.6 (62/82) | 95.4 (62/65) | 84.4 (124/147) | [78.6, 89.1] |
| 36 Months | 70.0 (49/70) | 93.6 (44/47) | 79.5 (93/117) | [72.4, 85.4] |
| Stent Migration14, % (n/N) | 0 (0/93) | 0 (0/77) | 0 (0/170) | |
| Stent Fracture15, % (n/N) | ||||
| 12 Months | 0 (0/72) | 0 (0/65) | 0 (0/137)16 | |
| 24 Months | 0 (0/65) | 0 (0/63) | 0 (0/128) | |
| 36 Months | 0 (0/53) | 0 (0/45) | 0 (0/98) | |
1Primary patency was defined as freedom from TVR, thrombotic occlusion, or stenosis greater than 50% measured by duplex ultrasonography (DUS) and reviewed by the DUS core laboratory
2Weighted mean based on the patency rates of PTS and NIVL subgroups
390% CI and one-sided p value is from the weighted Z-statistics and the combined patency rate was tested against the performance goal (PG) (74%)
4MAEs included target vessel revascularization (TVR), device- or procedure-related deep vein thrombosis (DVT), target-limb major amputation, symptomatic pulmonary embolism, vascular injury requiring either surgical or endovascular intervention, device migration, or death
5The p value is computed compared with performance goal of 89%. The 90% confidence interval is calculated using the exact binomial method
6Pain component of the venous clinical severity score (VCSS) hypothesis-tested against baseline values at 12 months
7Mean and 95% CI are presented based on normal assumption
8The p value is calculated from a two-sided paired t test
9The Chronic Venous Quality-of-Life Questionnaire (CIVIQ-20) score hypothesis-tested against baseline values at 12 months
10Mean and 95% CI are presented based on normal assumption
11The p value is calculated from a two-sided paired t test
12Mean and 95% CI are presented based on normal assumption
13Unweighted proportional values with 90% CI estimated by exact binomial method
14CEC adjudicated stent embolism/migration through 3 years
15Based on AP and lateral X-rays for each evaluated stent analyzed by the core laboratory
16Number of stents that had images available and readable for review
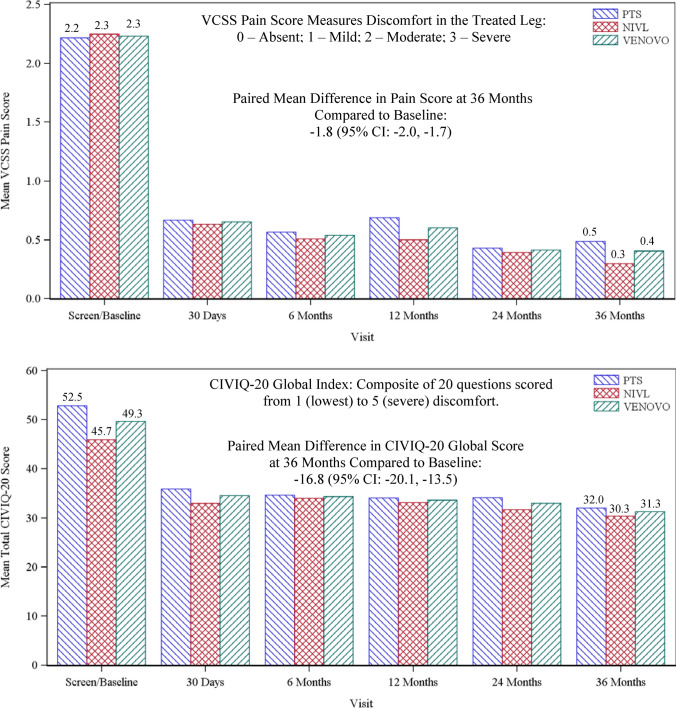
Fig. 2.
Quality-of-life summary bar graphs through 36 months. The paired mean improvement in the VCSS pain score at 36 months was -1.8 [95% CI: −2.0, −1.7] and the paired mean improvement in CIVIQ-20 global index score was −16.8 [95% CI: −20.1, −13.5] both compared to baseline values
Supportive analyses and secondary observations without formal hypothesis testing were conducted through 3 years. The unweighted primary patency rate (binary analysis) was 79.5% [90% CI 72.4%, 85.4%] while the K–M estimate of primary patency, accounting for patients censored (e.g., lost to follow-up) in the binary analysis, was 84.0 ± 3.0% (1125 days: Fig. 3). The mean improvement from baseline in the VCSS pain score was −1.8 [95% CI: −2.0, −1.7], CIVIQ-20 global index was −16.8 [95% CI: −20.1, −13.5], and CEAP “C” score was −1.4 [95% CI: −1.7, −1.2]. Freedom from TVR/TLR (binary) was 88.1%. No stent embolization or migration was reported, and A−P and lateral radiographs at 12, 24, and 36 months, reviewed by the X-ray core laboratory, revealed no stent fractures.
Fig. 3.
Kaplan–Meier curve of primary patency through 3 years. The estimated primary patency rate at 1125 days, the end of the 3-year follow-up window, was 84.0% [95% CI: 78.2%, 88.3%]
Six deaths were reported through 3 years. In three cases, the investigator listed a specific cause of death (i.e., one case of rectal cancer and two cases of myocardial infarction). In the remaining three cases, the specific cause of death was not listed; post-mortem summaries, however, indicated the apparent causes as recurrent cancer, complications of liver cirrhosis, and age-related natural causes. The CEC adjudicated that no deaths were related to the stent.
Discussion
Contemporary clinical practice has established stenting of iliofemoral venous obstruction as a durable therapy with relatively few complications. Stents have been used most often in acute cases with severe symptoms and in chronic DVT patients with symptoms of moderate to severe PTS; most were designed for arterial use, so the guidance was appropriately prudent and meant to discourage overuse of arterial stents in cases without evidence-based justification [41, 42]. Four self-expanding nitinol stents, specifically designed to meet the challenges of venous obstructive disease, have now been FDA-approved and address the long-held realizations that venous lesions are fundamentally different than the pathological disease that typically affects arteries; that vessel compliance, wall composition, and blood flow are distinctly dissimilar between arteries and veins; and that the basic dimensions (e.g., diameter, wall thickness) of iliac arteries and veins are disparate.
One- and two-year data from the current trial and the three other venous-specific stent trials—VIRTUS (Vici Venous stent), VIVO (Zilver Vena), and ABRE (Abre venous stent)—have been presented or published [43–45]. The 3-year results of the VERNACULAR trial, however, are the longest-term follow-up data published on a venous-specific stent. Primary patency was superior to a literature-based performance goal at 1 year (p < 0.0001) and was sustained at 3 years (79.5%) with a relative drop in the patency rate of only 9% from 1 to 3 years. The Kaplan–Meier estimate of primary patency was 84% while freedom from TVR and TLR was 88.1% at 3 years.
It is difficult to compare results between the venous stent trials because of differences in patient demographics, anatomic characteristics as well as specific differences in protocol-defined inclusion criteria and outcome measures. There are also differences in stent designs, open cell (Venovo, Zilver Vena, Abre) versus closed cell (Vici); the former typically providing greater device flexibility while closed-cell stents accentuate resistance to external compression [46]. The Venovo stent has some distinct features such as 3 mm flared stent ends designed for anchoring and the largest venous stent diameter range (10–20 mm). Given the mean diameter of treated veins in multiple trials was > 15 mm, and 13% of patients in the VIRTUS trial were excluded because the vein diameter was < 12 mm or > 16 mm, the large diameter range may prove useful [43].
Although not directly comparable, observations from the four trials can provide insight on the performance of venous stents. First, patient demographics across trials were similar, composed predominantly of women (range 63% to 70%), with a mean age ranging from 51 to 54 years, and with lesions overwhelmingly affecting the left leg (up to 90%). Total occlusions were present in 26% to 32% of cases with a baseline mean diameter vein stenosis of 63–78%. Second, there appeared to be no clear differences in safety endpoints observed through 30 days between the studies based on similar composite metrics. Freedom from MAEs at 30 days in the VERNACULAR trial was 93.5%, statistically higher compared to the safety performance goal. Third, primary patency rates were clustered from 84 to 90% at 1 year. Patency outcomes for the NIVL subgroups ranged from 96 to 99%, and as anticipated, the patency outcomes for the PTS subgroups were uniformly lower (VERNACULAR 81.7%; VIRTUS 79.8%, ABRE 79.8%, and Vici Venous Stent Trial 59%). At 3 years, the primary patency rate for the VERNACULAR trial was 79.5% (93.6% and 70%, respectively, for the NIVL and PTS subgroups). Fourth, paralleling the patency outcomes, TVR-free rates through the first 24 months were consistent across the dedicated venous stent studies (e.g., VERNACULAR 89.4%, VIRTUS 88.7%) while the rate in the VERNACULAR trial remained consistent at 3 years (88.1%). Fifth, there was no stent embolization or migration and no stent fractures observed in the VERNACULAR trial through 3 years; migration (0–1%) and fractures (0–4%) were also rare in the other trials at 1 and 2 years. Finally, lower VCSS scores and improved quality-of-life results (EQ-5D Index, Chronic Venous Quality-of-Life Questionnaire-CIVIQ-20 Global Index) were observed through all follow-up time points compared to pre-intervention assessments. At 3 years in the VERNACULAR trial, the mean improvement from baseline in the VCSS pain score was −1.8 and in the CIVIQ-20 score was −16.8. Similar QoL improvements were observed in the other dedicated venous stent trials at earlier time points, but clinical outcome studies comparing arterial stents and dedicated venous stents for management of iliofemoral venous disease have not been performed.
Limitations of the current trial included the single-arm design that used historical rather than concurrent, randomized controls. The trial was prospective, multicentered, and had pre-specified endpoints powered to detect differences compared to performance goals; there can be selection bias, however, in the choice of historical studies. The case mix, trial design, and study methodology may be different than the studies used to determine the PGs, and the use of other studies could have yielded different results. The study was not designed to assess clinical success compared to non-venous stents. It is also possible that the Venovo stent performed differently than venous stents used in other trials. A comparative, randomized study powered to determine differences between stents would be needed within the same demographic population. Forty-two patients discontinued prior to the end of the study resulting in an overall follow-up rate of 75.3%. The COVID-19 pandemic impacted the final follow-up at 3 years, and an attrition of approximately 25% could have impacted the results. The primary outcome measures and secondary quality-of-life parameters at 12 months were statistically powered and hypothesis-tested, but additional secondary analyses out to 3 years were observational only.
Recent data from dedicated venous stent trials for iliac and femoral vein occlusive disease provide interventionalists with clear outcomes that can be anticipated through 1 year following stent placement. Three-year results from the VERNACULAR trial using the Venovo venous stent are the longest-term follow-up data from a controlled multicenter trial. Primary patency and stent integrity were sustained at 2 and 3 years, reintervention rates were low, and patient quality-of-life improved, providing physicians and patients with important predictability and confidence in the durability of the earlier outcomes.
Acknowledgements
The authors thank Katie Retherford, Senior Manager, Clinical Sciences, Becton Dickinson Peripheral Interventions for her oversight and management of the VERNACULAR Study. The authors also wish to thank Alexandra Lansky, MD and Ecaterina Cristea, MD of the Yale Cardiovascular Research Group and Ido Weinberg, MD and Gail Hadley, RN of the Vascular Ultrasound Core Laboratory. Finally, the authors thank the following investigators who participated in the VERNACULAR trial: Professor Thomas Zeller, MD, Steven Black, MD, Michiel de Haan, MD, Rick de Graaf, MD, Professor Luis Miguel Izquierdo Lamoca, MD, Houman Jalaie, MD, Steven Dubenec, MD, Jeffrey Apple, MD, Robert Lookstein, MD, John Mullins, MD, David Dexter, MD, Shadi Abu-Halimah, MD, Brian Ferris, MD, Barbara Karenko, MD, Fadi Saab, MD, Robert Mendes, MD, Khanjan Nagarsheth, MD, Robert Attaran, MD, Ronald Morford, MD, and Paul Gagne, MD.
Funding
VERNACULAR was a U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) Study supported by C. R. Bard, a wholly owned subsidiary of Becton, Dickinson and Company.
Declarations
Conflict of interest
MDD is a member of the Scientific Advisory Board of W.L. Gore and consultant to Cook Medical. GO is a consultant and received speaker honoraria from Cook Medical, C. R. Bard (Bard)/Becton, Dickinson and Company (BD), Boston Scientific, Philips, Medtronic, WhiteSwell, VeinWay, and Vivasure as well as a shareholder in Marvao Medical. NWS has received research and educational grants from Bard/BD, Boston Scientific, Phillips, AngioDynamics, and VentureMed Group, is on the advisory board of VentureMed group and CSI, and has received speaker honoraria from Janssen, Boehringer Ingelheim, Lilly, Kiniksa, Amgen, and Esperion. ML has received research grants and speaker honoraria from Bard/BD. BPM has received research and educational grants from Boston Scientific, Getinge, and Medtronic Australia as well as speaker honoraria from Bard/BD, Boston Scientific, Getinge, Gore, and Medtronic Australia. RAS is an employee and shareholder of Becton, Dickinson and Company.
Consent for publication
Consent for publication was obtained as part of the written informed consent. All patients consented to publication of the study results if their identity remained confidential.
Ethical Approval
All procedures performed on human participants were done in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
This article was updated to correct the formatting of Table 3 which occurred after authors reviewed proofs.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/19/2021
A Correction to this paper has been published: 10.1007/s00270-021-02982-3
Contributor Information
Michael D. Dake, Email: mddake@arizona.edu
Gerard O’Sullivan, Email: gerard.osullivan2@hse.ie.
Nicolas W. Shammas, Email: shammas@mchsi.com
Michael Lichtenberg, Email: klichte@gmx.net.
Bibombe P. Mwipatayi, Email: patrice@bibombe.com
Richard A. Settlage, Email: rasettlage@msn.com
References
- 1.Enden T, Haig Y, Klow NE, Slagsvold CE, Sandvik L, Ghanima W, Hafsahl G, Holme PA, Holmen LO, Njaastad AM, Sandbaek G, Sandset PM, CaVenT Study Group Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomized controlled trial. Lancet. 2012;379:31–38. doi: 10.1016/S0140-6736(11)61753-4. [DOI] [PubMed] [Google Scholar]
- 2.Haig Y, Enden T, Grotta O, Klow NE, Slagsvold CE, Ghanima W, Sandvik L, Hafsahl G, Holme PA, Holmen LO, Njaastad AM, Sandbaek G, Sandset PM, CaVenT Study Group Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label randomized controlled trial. Lancet Haematol. 2016;3:e64–e71. doi: 10.1016/S2352-3026(15)00248-3. [DOI] [PubMed] [Google Scholar]
- 3.Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, Magnuson E, Razavi MK, Comerota AJ, Gornik HL, Murphy TP, Lewis L, Duncan JR, Nieters P, Derfler MC, Filion M, Gu CS, Kee S, Schnieder J, Saad N, Blander M, Moll S, Sacks D, Lin J, Rundback J, Garcia M, Razdan R, Vander-Woude E, Marques V, Kearon C, ATTRACT Trial Investigators Pharmacomechanical catheter-directed thrombolysis for deep vein thrombosis. N Eng J Med. 2017;377:2240–2252. doi: 10.1056/NEJMoa1615066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jost CJ, Gloviczki P, Cherry KJ, Jr, McKusick MA, Harmsen WS, Jenkins GD, Bower TC. Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg. 2001;33:320–328. doi: 10.1067/mva.2001.112805. [DOI] [PubMed] [Google Scholar]
- 5.Gutzeit A, Zollikofer CHL, Dettling-Pizzolato M, Graf N, Largiade`r J, Binkert CA. Endovascular stent treatment for symptomatic benign iliofemoral venous occlusive disease: Long-term results 1987–2009. Cardiovasc Intervent Radiol. 2011;34:542–549. doi: 10.1007/s00270-010-9927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ignatyev IM, Pokrovsky A, Gradusov E. Long-term results of endovascular treatment of chronic iliofemoral venous obstructive lesions. Vasc Endovascular Surg. 2019;53(5):373–378. doi: 10.1177/1538574419839256. [DOI] [PubMed] [Google Scholar]
- 7.Qiu P, Zha B, Xu A, Wang W, Zhan Y, Zhu X, Yuan X. Systematic review and meta-analysis of iliofemoral stenting for post-thrombotic syndrome. Eur J Vasc Endovasc Surg. 2019;57:407–416. doi: 10.1016/j.ejvs.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Lichtenberg MKW, Stahlhoff WF, Stahlhoff S, Özkapi A, Rassaf T, Breuckmann F, de Graff R. Venovo venous stent in the treatment of non-thrombotic or post-thrombotic iliac vein lesions—long-term results from the Arnsberg venous registry. Vasa. 2020;50:52–58. doi: 10.1024/0301-1526/a000893. [DOI] [PubMed] [Google Scholar]
- 9.Shammas NW. Worsening back and lower leg pain post stenting of the common iliac vein: is there evidence it is related to stent size? J Invasive Cardiol. 2020;32(10):E250–E253. doi: 10.25270/jic/20.00245. [DOI] [PubMed] [Google Scholar]
- 10.Tang TY, Lim MHH, Damodharan K, Yap CJQ, Lee SQW, Yap HY, Chong TT, Tan JWH. Use of the VENOVOTM and Sinus ObliquusTM venous stents in the treatment of non-thrombotic or post-thrombotic iliac vein lesions – Short-term results from a multi-centre Asian cohort. Phlebology. 2021;36(1):70–78. doi: 10.1177/0268355520946219. [DOI] [PubMed] [Google Scholar]
- 11.Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990. doi: 10.1016/j.jvs.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 12.Kurlinsky AK, Bjarnason H, Friese JL, Wysokinski WE, McBane RD, Misselt A, Moller SM, Gloviczki P. Outcomes of venoplasty with stent placement for chronic thrombosis of the iliac and femoral veins: single-center experience. J Vasc Interv Radiol. 2012;23:1009–1015. doi: 10.1016/j.jvir.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng Q-y, Li X-q, Qian A-m, Sang H-f, Rong J-j, Zhu L-w. Endovascular treatment of iliac vein compression syndrome. Chin Med J. 2011;124(20):3281–3284. doi: 10.3760/cma.j.issn.0366-6999.2011.20.013. [DOI] [PubMed] [Google Scholar]
- 14.AbuRahma AF, Perkins SE, Wulu JT, Ng HK. Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001;233:752–760. doi: 10.1097/00000658-200106000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye K, Lu X, Li W, Huang Y, Huang X, Lu M, Jiang M. Long-term outcomes of stent placement for symptomatic nonthrombotic iliac vein compression lesions in chronic venous disease. J Vasc Interv Radiol. 2012;23:497–502. doi: 10.1016/j.jvir.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 16.O’Sullivan GJ, Sheehan J, Lohan D, McCann-Brown JA. Iliofemoral venous stenting extending into the femoral region: initial clinical experience with the purpose-designed Zilver Vena stent. J Cardiovasc Surg. 2013;54:255–261. [PubMed] [Google Scholar]
- 17.George R, Verma H, Ram B, Tripathi R. The effect of deep venous stenting on healing of lower limb venous ulcers. Eur J Vasc Endovasc Surg. 2014;48(3):330–336. doi: 10.1016/j.ejvs.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 18.Raju S, Tackett P, Jr, Neglen P. Reinterventions for nonocclusive iliofemoral venous stent malfunctions. J Vasc Surg. 2009;49:511–518. doi: 10.1016/j.jvs.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Köbel T, Lindh M, Åkesson M, Wassèlius J, Gottsäter A, Ivancev K. Chronic iliac vein occlusion: midterm results of endovascular recanalization. J Endovasc Ther. 2009;16:483–491. doi: 10.1583/09-2719.1. [DOI] [PubMed] [Google Scholar]
- 20.Rosales A, Sandbæck G, Jørgensen JJ. Stenting for chronic post-thrombotic vena cava and iliofemoral venous occlusions: Mid-term patency and clinical outcome. Eur J Vasc Endovasc Surg. 2010;40(2):234–240. doi: 10.1016/j.ejvs.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Wahlgren C-M, Wahlberg E, Olofsson P. Endovascular treatment in postthrombotic syndrome. Vasc Endovascular Surg. 2010;44(5):356–360. doi: 10.1177/1538574410369710. [DOI] [PubMed] [Google Scholar]
- 22.Alerany MB, Lamoca LMI, Ortega MR, Rivas IL, Desboeufs RZ, Kiuri SS. Endovascular treatment of iliofemoral chronic post-thrombotic venous flow obstruction. J Vasc Surg Venous and Lym Dis. 2014;2:2–7. doi: 10.1016/j.jvsv.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda A, Yamada N, Ogihara Y, Tsuji A, Ota S, Ishikura K, Nakamura M, Ito M. Early and long-term outcomes of venous stent implantation for iliac venous stenosis after catheter-directed thrombolysis for acute deep vein thrombosis. Circ J. 2014;78:1234–1239. doi: 10.1253/circj.CJ-13-1247. [DOI] [PubMed] [Google Scholar]
- 24.Hokimoto S, Saito T, Oshima S, Ogawa H. Initial and mid-term outcomes of pulse infusion thrombolysis using a unique pump system and stent placement for deep vein thrombosis. Inter Med. 2008;47:1663–1667. doi: 10.2169/internalmedicine.47.1024. [DOI] [PubMed] [Google Scholar]
- 25.Hurst DR, Forauer AR, Bloom JR, Greenfield LJ, Wakefield TW, Williams DM. Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106–113. doi: 10.1067/mva.2001.114213. [DOI] [PubMed] [Google Scholar]
- 26.Nazarian GK, Bjarnason H, Dietz CA, Jr, Bernadas CA, Hunter DW. Iliofemoral venous stenoses: effectiveness of treatment with metallic endovascular stents. Radiology. 1996;200(1):193–199. doi: 10.1148/radiology.200.1.8657909. [DOI] [PubMed] [Google Scholar]
- 27.Park JY, Ahn JH, Jeon YS, Cho SG, Kim JY, Hong KC. Iliac vein stenting as a durable option for residual stenosis after catheter-directed thrombolysis and angioplasty of iliofemoral deep vein thrombosis secondary to May-Thurner syndrome. Phlebology. 2013;29(7):461–470. doi: 10.1177/0268355513491724. [DOI] [PubMed] [Google Scholar]
- 28.Titus JM, Moise MA, Bena J, Lyden SP, Clair DG. Iliofemoral stenting for venous occlusive disease. J Vasc Surg. 2011;53:706–712. doi: 10.1016/j.jvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Ye K, Lu X, Jiang M, Yang X, Li W, Huang Y, Huang X, Lu M. Technical details and clinical outcomes of transpopliteal venous stent placement for postthrombotic chronic total occlusion of the iliofemoral vein. J Vasc Interv Radiol. 2014;25:925–932. doi: 10.1016/j.jvir.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Jeon UB, Chung JW, Jae HJ, Kim H-C, Kim SJ, Ha J, Park JH. May-thurner syndrome complicated by acute iliofemoral vein thrombosis: helical CT venography for evaluation of long-term stent patency and changes in the iliac vein. AJR. 2010;195:751–757. doi: 10.2214/AJR.09.2793. [DOI] [PubMed] [Google Scholar]
- 31.Oguzkert L, Tercan F, Ozkan U, Gulcan O. Iliac vein compression syndrome: Outcome of endovascular treatment with long-term follow-up. Eur J Radiol. 2008;68:487–492. doi: 10.1016/j.ejrad.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Binkert CA, Schoch E, Stuckmann G, Largiader J, Wigger P, Schoepke W, Zollikofer CL. Treatment of pelvic venous spur (may–thurner syndrome) with self-expanding metallic endoprostheses. Cardiovasc Intervent Radiol. 1998;21:22–26. doi: 10.1007/s002709900205. [DOI] [PubMed] [Google Scholar]
- 33.Hartung O, Otero A, Boufi M, Decaridi G, Barthelemy P, Juhan C, Alimi YS. Mid-term results of endovascular treatment for symptomatic chronic nonmalignant iliocaval venous occlusive disease. J Vasc Surg. 2005;42:1138–1144. doi: 10.1016/j.jvs.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Knipp BS, Ferguson E, Williams DM, Dasika NJ, Cwikiel W, Henke PK, Wakefield TW. Factors associated with outcome after interventional treatment of symptomatic iliac vein compression syndrome. J Vasc Surg. 2007;46:743–749. doi: 10.1016/j.jvs.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 35.Kwak H-S, Han Y-M, Lee Y-S, Jin G-Y, Chung G-H. Stents in Common iliac vein obstruction with acute ipsilateral deep venous thrombosis: early and late results. J Vasc Interv Radiol. 2005;16:815–822. doi: 10.1097/01.RVI.0000157690.91690.38. [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan GJ, Semba CP, Bittner CA, Kee ST, Razavi MK, Sze DY, Dake MD. Endovascular management of iliac vein compression (may-thurner) syndrome. J Vasc Interv Radiol. 2000;11:823–836. doi: 10.1016/s1051-0443(07)61796-5. [DOI] [PubMed] [Google Scholar]
- 37.Zhu QH, Zhou CY, Chen Y, Wang J, Mo HY, Luo MH, Huang W, Yu XF. Percutaneous manual aspiration thrombectomy followed by stenting for iliac vein compression syndrome with secondary acute isolated iliofemoral deep vein thrombosis: a prospective study of single-session endovascular protocol. Eur J Vasc Endovasc Surg. 2014;47:68–74. doi: 10.1016/j.ejvs.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 38.Sang H, Li X, Qian A, Meng Q. Outcome of endovascular treatment in postthrombotic syndrome. Ann Vasc Surg. 2014;28:1493–1500. doi: 10.1016/j.avsg.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 39.Bjarnason H, Kruse JR, Asinger DA, Nazarian GK, Dietz CA, Jr, Caldwell MD, Key NS, Hirsch AT, Hunter DW. Iliofemoral deep venous thrombosis safety and efficacy outcome during 5 years of catheter-directed thrombolytic therapy. J Vasc Interv Radiol. 1997;8:405–418. doi: 10.1016/s1051-0443(97)70581-5. [DOI] [PubMed] [Google Scholar]
- 40.Warner CJ, Goodney PP, Wallaert JB, Nolan BW, Rzucidlo EM, Powell RJ, Walsh DB, Stone DH. Functional outcomes following catheter-based iliac vein stent placement. Vasc Endovascular Surg. 2013;47(5):331–334. doi: 10.1177/1538574413487443. [DOI] [PubMed] [Google Scholar]
- 41.Wittens C, Davies AH, Bækgaard N, Broholm R, Cavezzi A, Chastanet S, de Wolf M, Eggen C, Giannoukas A, Gohel M, Kakkos S, Lawson J, Noppeney T, Onida S, Pittaluga P, Thomis S, Toonder I, Vuylsteke M. Management of chronic venous disease: clinical practice guidelines of the European Society for Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2015;49:678–737. doi: 10.1016/j.ejvs.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Kahn SR, Comerota AJ, Cushman M, Evans NS, Ginsberg JS, Goldenberg NA, Gupta DK, Prandoni P, Vedantham S, Walsh E, Weitz JI, on behalf of the American Heart Association Council on Peripheral Vascular Disease, Council on Clinical Cardiology, and Council on Cardiovascular and Stroke Nursing The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies. Circulation. 2014;130:1636–1661. doi: 10.1161/CIR.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 43.Razavi MK, Black S, Gagne P, Chiacchierini R, Nicolini P, Marston W, VIRTUS Trial Investigators Pivotal study of endovenous stent placement for symptomatic iliofemoral venous obstruction. Circ Cardiovasc Interv. 2019;12:e008268. doi: 10.1161/CIRCINTERVENTIONS.119.008268. [DOI] [PubMed] [Google Scholar]
- 44.Razavi M, Marston W, Black S, Bentley D, Neglen P. The initial report on 1-year outcomes of the feasibility study of the VENITI VICI VENOUS Stent in symptomatic iliofemoral venous obstruction. J Vasc Surg Venous Lym Dis. 2018;6:192–200. doi: 10.1016/j.jvsv.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Black S, Gwozdz A, Karunanithy N, Silickas J, Breen K, Hunt B, Smith A, Cohen A, Saha P. Two year outcome after chronic iliac vein occlusion recanalization using the Vici Venous Stent. Eur J Vasc Endovasc Surg. 2018;56:710–718. doi: 10.1016/j.ejvs.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Dabir D, Feisst A, Thomas D, Luetkens JA, Meyer C, Kardulovic A, Menne M, Steinseifer U, Schild HH, Kuetting DLR. Physical properties of venous stents: an experimental comparison. Cardiovasc Intervent Radiol. 2018;41(6):942–950. doi: 10.1007/s00270-018-1916-1. [DOI] [PubMed] [Google Scholar]