Abstract
Purpose
Several studies found reduced retinal thickness on optical coherence tomography (OCT) in Alzheimer’s disease (AD), even in preclinical stages, labelling this technique of interest as biomarker. In this study, we examine retinal thickness changes in preclinical AD, as defined by cognitively normal individuals with amyloid‐beta (Aβ) on positron emission tomography (PET).
Methods
For this monocentre study, 145 cognitively healthy monozygotic twins aged ≥ 60 were included from the Netherlands Twin Register taking part in the EMIF‐AD PreclinAD study. At baseline, participants underwent [18F] flutemetamol PET that was visually rated for cortical Aβ. Binding potential was calculated as continuous measure for Aβ. Optical coherence tomography (OCT) was performed at baseline and after 22 months to assess changes in total and individual inner retinal layer thickness in the macular region (ETDRS circles) and peripapillary retinal nerve fibre layer thickness. Differences in rate of change between amyloid‐beta positive and negative individuals and associations between binding potential and change in retinal thickness were evaluated.
Results
Sixteen participants (11%) were positive for Aβ. Change in retinal thickness did not differ in any region between Aβ+ and Aβ− individuals. A positive association between binding potential and change in inner plexiform layer thickness was observed in the inner macular ring (beta = 1.708, CI = 0.575 to 2.841, p = 0.003).
Conclusion
Aβ+ individuals did not differ in rate of change of any retinal layer compared to controls, but higher binding potential at baseline was associated with less IPL thinning over time. Optical coherence tomography (OCT) as a longitudinal screening tool for preclinical AD seems limited, but IPL changes offer leads for further research.
Keywords: OCT, ocular biomarkers, preclinical Alzheimer’s disease, retina, twins
Introduction
Alzheimer’s disease (AD) is the most common form of dementia and a major cause of disability and death in the western world (Murray et al. 2013). With our increasing life expectancy, incidence of AD is expected to only increase in the oncoming years, up to an expected prevalence of 115 million in the year 2050 (Prince et al. 2013). So far, no curative treatment for AD has been found, although many trials are being performed to find one (Kumar et al. 2015; Sun et al. 2018; Vina & Sanz‐Ros 2018). Due to disappointing results, attention is shifting from AD patients with dementia to individuals with preclinical AD, who have cerebral amyloid pathology but still normal cognition and limited neurodegeneration, reasoning that at this stage therapy could be more effective (Vlassenko et al. 2012; Kumar et al. 2015; Khan 2018; Sun et al. 2018).
Accumulation of cortical amyloid‐beta (Aβ) can now be visualized in vivo using positron emission tomography (PET) or by detecting their levels in cerebrospinal fluid obtained with a lumbar puncture (Vlassenko et al. 2012; Blennow & Zetterberg 2018; Khan 2018). Unfortunately, these biomarkers are not suitable for large‐scale screening, due to costs, limited availability, the need for injection of a radioactive tracer and/or their invasiveness (Scheinin et al. 2007; Mitka 2013; Leuzy et al. 2014; Hornberger et al. 2017; Nishii et al. 2018).
The retina is receiving more and more attention as a source for possible non‐invasive biomarkers for diseases such as AD. The retina has a similar embryological origin as the central nervous system and may therefore reflect brain diseases (London et al. 2013). Indeed, various groups have found changes in vascular retinal parameters and in retinal thinning as measured by optical coherence tomography (OCT) in individuals suffering from AD, including those with preclinical AD (Frost et al. 2010; Lim et al. 2016; Snyder et al. 2016; den Haan et al. 2017; McGrory et al. 2017; Santos et al. 2018). However, the diagnostic accuracy of OCT in screening for (preclinical) AD is as yet limited. This may be explained by the high variation in retinal thickness due to for example genetic differences or aging effects (Hougaard et al. 2003; Chamberlain et al. 2006; Hinrichs et al. 2010; Kwun et al. 2011; Demirkaya et al. 2013; Wei et al. 2017; Van de Kreeke et al. 2019a; van de Kreeke et al. 2019b). A way to eliminate this natural variation is to use longitudinal imaging, which may be more sensitive in detecting subtle changes due to neurodegeneration.
Retinal thickness is suggested to have a highly genetic origin, as demonstrated by several earlier studies including our own (Hougaard et al. 2003; Chamberlain et al. 2006; Hinrichs et al. 2010; Kwun et al. 2011; van de Kreeke et al. 2019e Kreeke et al. 2019). No groups, however, have looked at the influence of genes on potential changes in retinal thickness. As even elderly twins are still very similar in their retinal thickness, one would expect age‐related changes to retinal thickness to have a genetic component as well.
In our earlier cross‐sectional study with monozygotic twins, we could not find differences in retinal layer thickness between individuals with preclinical AD (i.e. amyloid‐beta (Aβ) positive on PET with normal cognition) and healthy controls (van de Kreeke et al. 2019e Kreeke et al. 2019). The aims of this study in the same population were to (1) test whether Aβ+ individuals showed more prominent retinal (layer) thinning over a 2‐year period compared to Aβ− individuals, (2) test if the binding potential of the Aβ sensitive PET‐tracer at baseline (as a continuous measure for Aβ load) is associated with increased retinal (layer) changes over this 2‐year period, and (3) investigate the maximum contribution of genetic factors to retinal thinning.
Methods
Participants
This longitudinal study was performed within a sub‐sample of the European Medical Information Framework for AD (EMIF‐AD) PreclinAD cohort from the Amsterdam UMC, location VUmc (Konijnenberg et al. 2018). For this cohort, 194 participants aged ≥60 years were recruited from the Netherlands Twin Register (Boomsma et al. 2006; Konijnenberg et al. 2018; Ten Kate et al. 2018ate et al. 2018; van de Kreeke et al. 2018) and followed for 2 years. The study followed the Tenets of the Declaration of Helsinki, and written informed consent was obtained from all participants. The study was approved by the Medical Ethics Committee of the VU University Medical Center in Amsterdam.
Inclusion criteria were as follows: age ≥ 60 years, monozygosity, cognitively healthy as defined by: Telephone Interview for Cognitive Status modified (TICS‐m) score > 22 (de Jager et al. 2003), Geriatric Depression Scale (GDS) score < 11 (Yesavage et al. 1982), Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) 10 word list immediate and delayed recall >−1.5 SD of age adjusted normative data (Morris et al. 1989) and Clinical Dementia Rating (CDR) scale of 0 with a score on the memory sub domain of 0 (Morris 1993).
Exclusion criteria were as follows: stroke resulting in physical impairment, neurodegenerative disorders, cancer with terminal life expectancy, uncontrolled diabetes mellitus, and alcohol consumption > 35 units (1 unit = 10 ml or 8 g of pure alcohol) per week.
Apart from these criteria, additional ophthalmological exclusion criteria for this study were defined (see below).
Ophthalmological examination
Participants underwent the following ophthalmological examinations at baseline and follow‐up: best corrected visual acuity, intra‐ocular pressure, refraction measurement, slit lamp examination, indirect fundoscopy, fundus photography and OCT. To enable these examinations, tropicamide 0.5% was used. All fundus images and OCT scans were assessed by an experienced ophthalmologist (HTN or FDV) for unexpected pathology. Participants suffering from ophthalmological conditions interfering with the retina or severely inhibiting image quality were excluded from analyses (cataract, macular degeneration, glaucoma, diabetic retinopathy, vascular occlusions).
Optical coherence tomography
Dense macular scans (49 B‐scans, 20° × 20° equalling 5.7 mm × 5.7 mm) and axonal ring scans (Automatic Real‐time Tracking averaged over at least 100 scans) around the optic nerve head (ONH) were acquired using spectral domain OCT (Spectralis, Heidelberg). Total retinal thickness and individual layer thicknesses were obtained in the macular region using build‐in Heidelberg segmentation software (version 1.9.14.0). The following retinal layers were analysed besides total retinal thickness: retinal nerve fibre layer (RNFL), ganglion cell layer (GCL) and inner plexiform layer (IPL). Both the inner and outer macular ring according to the standard ETDRS macular grid (1–3 mm around the fovea for inner ring and 3–6 mm around the fovea for outer ring) were analysed separately. Values of the 4 quadrants within a macular ring were averaged to obtain a single value for that entire ring. Peripapillary RNFL (pRNFL) thickness was obtained as an average value over all sectors. Data from scans were averaged over right and left eyes, but if only one suitable scan was available only data from that eye was used. We synchronized this over the 2 visits (i.e. if at baseline only the right or left eye could be used, we also used only the same right or left eye at follow‐up).
Amyloid PET scanning
Participants underwent amyloid PET scanning at baseline. Positron emission tomography (PET) scans were performed using an Ingenuity TF PET‐MRI scanner (Philips Medical Systems, Best, the Netherlands). Participants were scanned using a ‘coffee‐break’ dynamic scan protocol from 0 to 30 min and again from 90 to 110 min after intravenous injection of 185 MBq (±10%) [18F]flutemetamol (Heeman 2018). Before each part of the PET scan, a T1‐weighted gradient echo pulse MRI scan was obtained for attenuation correction. The first dynamic emission scan was reconstructed into 18 frames with progressive increase in frame length (6 × 5, 3 × 10, 4 × 60, 2 × 150, 2 × 300, 1 × 600 s), and the second part of the scan consisted of 4 frames of 5 min. Vinci viewing software 2.56 (Max Planck Institute for neurological research, Cologne, Germany) and in‐house build tools were used to co‐register and combine the two PET scans into a single multi‐frame image sequence. In addition, each individual’s T1 was co‐registered to the dynamic PET image using the generic multimodality setting of Vinci with a linear rigid‐body scheme and normalized mutual information as the similarity measure. Parametric binding potential relative to the non‐displaceable compartment (non‐displaceable binding potential or BP ND) images were generated from the entire image set using the receptor parametric mapping (RPM) (Gunn et al. 1997; Wu & Carson 2002) implementation in PPET (Boellaard 2006). Standard uptake value ratio (SUVr) images were generated based on the 90–110 min data. As reference region for both analyses, cerebellar grey matter, as defined by the Hammers atlas, was used (Hammers et al. 2003). Finally, global values were computed based on the volume‐weighted average of frontal (superior, middle, and inferior frontal gyrus), parietal (posterior cingulate, superior parietal gyrus, postcentral gyrus, and inferolateral remainder of parietal lobe), and temporal (parahippocampal gyrus, hippocampus, medial temporal lobe, superior, middle, and inferior temporal gyrus) regions (Tolboom et al. 2009).
All SUVr images were visually read by an experienced nuclear physician (BvB) and images were classified as positive or negative rating according to criteria defined by the manufacturer (GE Healthcare) (Collij et al. 2019).
Statistical analysis
Change in retinal (layer) thickness over time was measured by subtracting the follow‐up values from the baseline values to create a Δ‐variable for each layer. Differences in the created Δ‐variables between Aβ+ and Aβ− participants were then analysed by Generalized Estimating Equations (GEE) in SPSS (IBM, version 22), to correct for clustering in the data from twin pairs. Covariates included age, gender and a diagnosis of diabetes (as previous studies have shown that diabetes can have an impact on the rate of retinal thinning) (De Clerck et al. 2015). As the time between baseline and follow‐up visits varied between 15 and 32 months, we included follow‐up time as an additional covariate in the statistical models. The association between BP ND at baseline and Δ‐variables for retinal layers was analysed with a similar approach by GEE. p‐Values adjusted for multiple testing were obtained in addition to the raw p‐values using the Holm–Bonferroni method (Holm 1979).
Results
Of the total number of 194 participants, 29 were excluded at baseline due to ophthalmological pathology (N = 26, mostly due to age‐related macular degeneration, glaucoma, epiretinal membranes or vascular occlusions) or insufficient image quality (N = 3), leaving data from 165 participants for analysis. Of these 165 participants, 148 participants also underwent ophthalmological testing at the follow‐up visit. Three of these were excluded due to newly developed ophthalmological pathology (N = 1) or insufficient image quality at follow‐up (N = 2). Based on the baseline data, the group lost to follow‐up (N = 20) was significantly older than the final studied population (N = 145, respectively 75.8 versus 68.6 years, p < 0.001, independent samples t‐test), consisted of significantly more females (85% versus 54%, p = 0.008, chi‐square test) and had a significantly lower MMSE (p = 0.020, Mann–Whitney U test), but did not differ significantly in any of the ophthalmological or PET variables. The difference in MMSE score was not present after correction for age. The reason for the age difference lies in the set‐up of the follow‐up visit: if participants had difficulty coming in to the hospital (e.g. due to age/comorbidity), a house visit was performed instead. These individuals were thus not included for an ophthalmological follow‐up examination, as the diagnostic devices were at the hospital. Table 1 shows the demographic information of all 145 participants included for analyses.
Table 1.
Demographics of the study population
| Baseline | Follow‐up | Raw p‐value | Adjusted p‐value | |
|---|---|---|---|---|
| Number of participants (N) | 145 | ‐ | ‐ | |
| Follow‐up time in months (range) | 22 (15–32) | ‐ | ‐ | |
| Sex, female N (%) | 78 (54%) | ‐ | ‐ | |
| Age (years) | 68.6 (±6.3) | 70.5 (±6.2) | ‐ | ‐ |
| BCVA (LogMAR) | 0.02 (±0.10) | 0.04 (±0.16) | ‐ | ‐ |
| IOP (mmHg) | 14.2 (±2.6) | 15.6 (±2.5) | ‐ | ‐ |
| Spherical Equivalent | 0.39 (±1.82) | ‐ | ‐ | |
| MMSE (median, IQR) | 29 (29–30) | 29 (28–30) | ‐ | ‐ |
| Brain amyloid status, positive N (%) | 16 (11%) | ‐ | ‐ | ‐ |
| Global BP ND of Aβ (median, IQR) | 0.120 (0.088–0.174) | ‐ | ‐ | ‐ |
| Macular layer thickness: | ||||
| Total RT inner ring (µm) | 342.5 (±15.3) | 339.4 (±15.2) | <0.001 | <0.001 |
| Total RT outer ring (µm) | 296.0 (±12.2) | 292.9 (±12.6) | <0.001 | <0.001 |
| RNFL inner ring (µm) | 22.0 (±2.0) | 22.1 (±2.1) | 0.420 | 0.840 |
| RNFL outer ring (µm) | 36.7 (±4.7) | 36.8 (±4.8) | 0.494 | 0.494 |
| GCL inner ring (µm) | 50.4 (±4.7) | 49.8 (±4.7) | <0.001 | <0.001 |
| GCL outer ring (µm) | 34.5 (±3.2) | 34.0 (±3.2) | <0.001 | <0.001 |
| IPL inner ring (µm) | 41.4 (±3.2) | 41.1 (±3.1) | <0.001 | <0.001 |
| IPL outer ring (µm) | 28.7 (±2.4) | 28.0 (±2.4) | <0.001 | <0.001 |
| Peripapillary RNFL thickness | 98.3 (±8.7) | 95.0 (±9.0) | <0.001 | <0.001 |
Data are means unless otherwise specified. p‐Values were obtained using paired samples t‐test.
Aβ = amyloid‐beta, BCVA = Best Corrected Visual Acuity (both eyes averaged), BP ND = non‐displaceable binding potential, GCL = Ganglion Cell Layer, IOP = Intra‐Ocular Pressure (both eyes averaged), IPL = Inner Plexiform Layer, IQR = inter‐quartile range, MMSE = Mini‐Mental State Examination, NA = not applicable, RNFL = Retinal Nerve Fibre Layer, RT = Retinal Thickness.
Values in bold are significant (p < 0.05).
All retinal layers except macular RNFL decreased significantly in thickness over the follow‐up period (Table 1).
There were no significant differences in the change of retinal (layer) thickness over time between participants who were Aβ+ or Aβ− at baseline (Fig. 1 & 2, Tables S1 & S2).
Fig. 1.
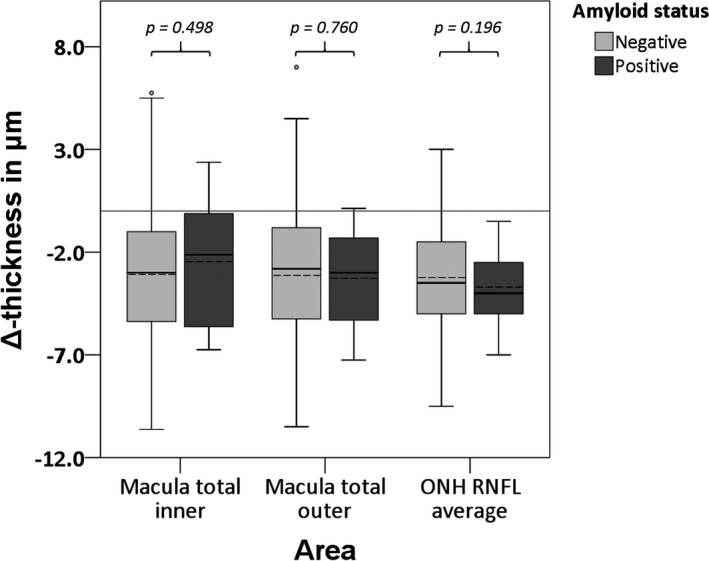
Boxplots for differences in rate of change in retinal thickness between baseline and follow‐up visit for Aβ− and Aβ+ participants. Dotted lines represent the mean. p‐Values were obtained using GEE, corrected for time between measurements, age, sex and a diagnosis of diabetes. Aβ = Amyloid Beta, pRNFL = peripapillary Retinal Nerve Fibre Layer.
Fig. 2.
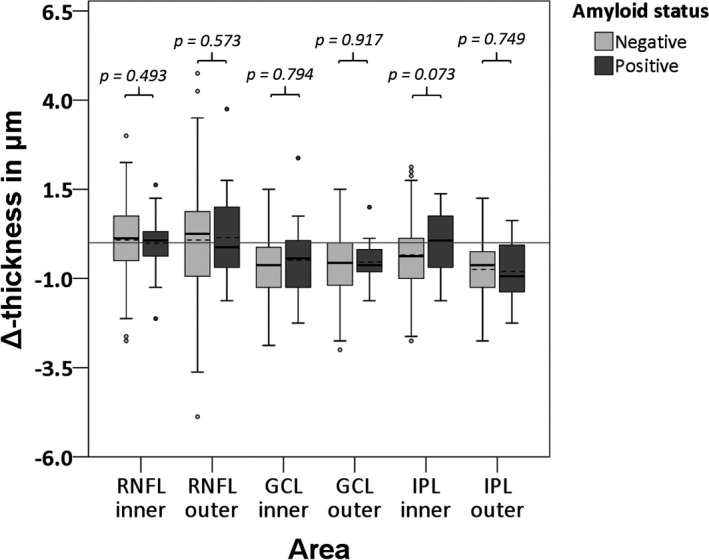
Boxplots for differences in rate of change in macular retinal layer thickness between baseline and follow‐up visit for Aβ− and Aβ+ participants. Dotted lines represent the mean. p‐Values were obtained using GEE, corrected for time between measurements, age, sex and a diagnosis of diabetes. Aβ = Amyloid Beta, RNFL = Retinal Nerve Fibre Layer, GCL = Ganglion Cell Layer, IPL = Inner Plexiform Layer.
Global BP ND of [18F]flutemetamol (as a continuous measure for brain amyloid load) was not associated with the retinal layer change in any of the retinal layers, except for the inner ring of the IPL, where there was a positive association (i.e. a higher BP ND at baseline was associated with less thinning of the IPL, Tables 2, S3).
Table 2.
Associations between rate of change in retinal layers between baseline and follow‐up visit with BP nd
| Beta | 95% CI | Raw p‐value | Adjusted p‐value | |
|---|---|---|---|---|
| Macula | ||||
| Δ total inner ring | 2.548 | −1.882 to 6.979 | 0.260 | 0.999 |
| Δ total outer ring | −0.427 | −3.582 to 2.728 | 0.791 | 0.999 |
| Δ RNFL inner ring | −0.228 | −1.536 to 1.079 | 0.732 | 0.999 |
| Δ RNFL outer ring | 0.346 | −1.947 to 2.639 | 0.768 | 0.999 |
| Δ GCL inner ring | −0.224 | −1.783 to 1.334 | 0.778 | 0.999 |
| Δ GCL outer ring | −0.092 | −0.838 to 0.653 | 0.808 | 0.808 |
| Δ IPL inner ring | 1.708 | 0.575 to 2.841 | 0.003 | 0.027 |
| Δ IPL outer ring | −0.172 | −1.235 to 0.891 | 0.751 | 0.999 |
| Δ pRNFL | −1.586 | −5.352 to 2.179 | 0.409 | 0.999 |
GEE, corrected for time between measurements, age, sex and a diagnosis of diabetes.
CI = Confidence Interval, GCL = Ganglion Cell Layer, IPL = Inner Plexiform Layer, pRNFL = peripapillary RNFL, RNFL = Retinal Nerve Fibre Layer.
Values in bold are significant (p < 0.05).
Twins from a same pair correlated weakly in changes over time of the total macular thickness in the outer ring, IPL thickness in the inner ring and average pRNFL thickness. Table 3 shows all intra‐twin pair correlations for changes over time in all retinal (layer) thicknesses.
Table 3.
Intra‐twin correlations for rate of change in retinal layer thicknesses over time
| Correlation coefficient | p‐Value | |
|---|---|---|
| Macula: | ||
| Δ total inner ring | 0.242 | 0.052 |
| Δ total outer ring | 0.316 | 0.011 |
| Δ RNFL inner ring | 0.092 | 0.466 |
| Δ RNFL outer ring | 0.189 | 0.136 |
| Δ GCL inner ring | 0.182 | 0.148 |
| Δ GCL outer ring | 0.160 | 0.205 |
| Δ IPL inner ring | 0.262 | 0.035 |
| Δ IPL outer ring | 0.165 | 0.191 |
| Δ pRNFL | 0.280 | 0.025 |
GCL = Ganglion Cell Layer, IPL = Inner Plexiform Layer, RNFL = Retinal Nerve Fibre Layer.
Values in bold are significant (p < 0.05).
Discussion
A thinning over time of all retinal layers except macular RNFL was observed, but no differences in the amount of thinning were seen between participants with and without Aβ on PET. There was an unexpected positive association between change of IPL thickness with BP ND of [18F]flutemetamol (i.e. individuals with a higher BP ND showed less thinning of the IPL over time).
Several studies, including our own (manuscript submitted for publication), have shown that retinal layers become thinner with increasing age (Demirkaya et al. 2013; Won et al. 2016; Hoffmann et al. 2018; Van de Kreeke et al. 2019e Kreeke et al. 2019). This is supported by our data in which over a 22‐month period all retinal layers except macular RNFL decreased in thickness (Table 1). As such, neuroretinal degeneration can to some extent be considered a physiological process occurring with age, which should be taken into account when looking at retinal neurodegeneration in the context of pathologies such as dementias.
We did not find a decrease in thickness of the macular RNFL area, despite finding a decrease in the peripapillary RNFL. The absence of this decrease may be due to (subclinical) vitreomacular interface pathology. Whilst obvious epiretinal membranes (ERM) interfering with retinal thickness were excluded, the formation of a slight ERM, also an aging phenomenon, may add to the RNFL thickness layer in the macular area.
We did not find a difference in retinal neurodegeneration between Aβ+ and Aβ− participants. Our previous cross‐sectional analysis was also unable to illustrate differences between these groups (van de Kreeke et al. 2019e Kreeke et al. 2019). The absence of a difference may be due to the relatively short follow‐up time of 22 months. As the preclinical stages of AD can take up to 20 years before dementia starts, the increase in neurodegeneration in this population is likely to be very subtle and may thus remain undetected in this relatively short follow‐up period (Jansen et al. 2015). Another study did demonstrate that Aβ+ individuals had a stronger decrease in macular RNFL, IPL and outer nuclear layer (ONL) volume over a 27‐month time period, but these differences did not reach statistical significance when looking at thickness rather than volume, as we did in our study (Santos et al. 2018). The absence of significant differences suggests that even if increased retinal neurodegeneration is present in Aβ+ individuals, it will be at such a subtle rate that its clinical usefulness in detecting preclinical AD will be minimal.
Individuals with higher Aβ (i.e. BP ND) at baseline have less decrease of IPL thickness over time. Interestingly, Snyder et al showed in their preclinical AD population that IPL volume was increased in Aβ+ individuals, although this lost statistical significance after correcting for multiple testing (Snyder et al. 2016). Their proposed explanation was the presence of retinal inclusion bodies (recognized on blue autofluorescence, postulated to contain fibrillar Aβ) close to or within the IPL, causing an increase in thickness. We were unable to detect such inclusion bodies in our study population. Another explanation of the absence of a decrease in IPL thickness in these preclinical stages of AD may be one of inflammation. Alzheimer’s disease (AD) is known to have an inflammatory component, and this process may well be already present in preclinical stages (Kinney et al. 2018). If this occurs in the retina as well, inflammation may cause microscopic fluid assembly resulting in thickening of the IPL. Other studies already demonstrated an increase in mostly INL, but also IPL thickness due to such ‘subclinical macular edema’ is possible (Bandello et al. 2015). As the IPL is known to become thinner with age, this may be cancelled out by a thickening due to subclinical macular edema, resulting in a stabilization or even an increase of IPL thickness over time (Demirkaya et al. 2013; Won et al. 2016; Van de Kreeke et al. 2019e Kreeke et al. 2019). Santos et al also looked at the relationship between the amount of cortical Aβ using SUVr and subsequent change in IPL volume over time, but found no significant association between the two, not supporting our finding (Santos et al. 2018). Further studies are needed to elucidate this intriguing association between change in IPL thickness and cortical Aβ.
In a previous paper, we demonstrated that the retinal thickness between monozygotic twins from the same pair correlated moderately to highly (r of 0.59 to 0.92), suggesting a high influence of genes (van de Kreeke et al. 2019e Kreeke et al. 2019). This finding is supported by other literature as well (Hougaard et al. 2003; Chamberlain et al. 2006; Hinrichs et al. 2010; Kwun et al. 2011). In this study, twins from the same pair correlated weakly with respect to changes over time in total macular thickness in the outer ring, IPL thickness in the inner ring and average pRNFL thickness, suggesting weakly genetic origins in how the retina evolves with age. It is interesting to see that even changes in this thickness are caused partly by genes, likely contributing to why, even at a higher age, monozygotic twins remain very similar in their retinal layer thickness.
A strength of this study is its extensive characterization of the participants, from both an ophthalmological and a neurological perspective. We performed comprehensive screening for cognitive functioning at baseline, enrolling only cognitively healthy participants, thereby ensuring that only true preclinical AD cases were included. Conditions interfering with retinal layer thickness were either excluded (macular degeneration, glaucoma, vascular occlusions, epiretinal membranes) or controlled for (diabetes mellitus). Due to the longitudinal aspect of this study, we were now also able to show changes over time, thereby eliminating limitations of most cross‐sectional studies, which suffer from the pre‐existing high inter‐person variability in retinal layer thickness. Finally, another strength of this study is the use of a dynamic 18F‐Flutemetamol acquisition, making is possible to generate BP ND parametric images, enabling us to capture the earliest phase of amyloid deposition.
The main limitation of this study lies in its relatively small group of Aβ+ individuals (N = 16), limiting statistical power. This is a problem often encountered in studies looking at preclinical AD. As only around 20% of individuals around ages 70 are positive for Aβ, this would mean that a large number of cognitively normal individuals need to undergo expensive PET scanning in order to identify an Aβ+ group of sufficient size. Another limitation is the relatively short follow‐up period of 22 months, making subtle changes over time hard to detect.
In conclusion, we found no significant differences in changes of retinal layer thickness over time in Aβ+ individuals compared to Aβ− controls, suggesting the use of OCT as a longitudinal screening tool for preclinical AD is currently limited. A positive relationship between the change in IPL thickness over time and BP ND was found, possibly due to a microscopic inflammatory process. This finding may offer a starting point for further research on the topic of OCT as a biomarker for preclinical AD.
Supporting information
Table S1. Differences in rate of change in retinal layers between Aβ− and Aβ+ participants.
Table S2. Differences in rate of change in retinal layers between Aβ− and Aβ+ participants.
Table S3. Associations between rate of change in retinal layers between baseline and follow‐up visit with BP nd.
This work has received support from the EU/EFPIA Innovative Medicines Initiative Joint Undertaking (EMIF grant number 115372).
These funding sources had no involvement in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Jacoba A. van de Kreeke, H. Ton Nguyen, Elles Konijnenberg, Jori Tomassen, Anouk den Braber, Mara ten Kate, Maqsood Yaqub, Bart van Berckel, Adriaan A. Lammertsma, Dorret I. Boomsma and H. Stevie Tan have no financial disclosures. Frank D. Verbraak reports income from consulting and lectures from Bayer, Novartis and IDx. Pieter Jelle Visser reports grants from Innovative Medicine Initiative; grants from ZonMw, during the conduct of the study; non‐financial support from GE Healthcare; and grants from Biogen, outside the submitted work. None of these disclosures cause any conflicts of interest, financial or otherwise, with regard to this paper.
The corresponding author is a member of the Dutch Ophthalmological Societies only
References
- Bandello F, Tejerina AN, Vujosevic S et al. (2015): Retinal layer location of increased retinal thickness in eyes with subclinical and clinical macular edema in diabetes type 2. Ophthalmic Res 54: 112–117. [DOI] [PubMed] [Google Scholar]
- Blennow K & Zetterberg H (2018): Biomarkers for Alzheimer's disease: current status and prospects for the future. J Intern Med 284: 643–663. [DOI] [PubMed] [Google Scholar]
- Boellaard R, Yaqub M, Lubberink M & Lammertsma A (2006): PPET: a software tool for kinetic and parametric analyses of dynamic PET studies. NeuroImage 31: T62. [Google Scholar]
- Boomsma DI, de Geus EJ, Vink JM et al. (2006): Netherlands Twin Register: from twins to twin families. Twin Res Hum Genet 9: 849–857. [DOI] [PubMed] [Google Scholar]
- Chamberlain MD, Guymer RH, Dirani M, Hopper JL & Baird PN (2006): Heritability of macular thickness determined by optical coherence tomography. Invest Ophthalmol Vis Sci 47: 336–340. [DOI] [PubMed] [Google Scholar]
- Collij LE, Konijnenberg E, Reimand J et al. (2019): Assessing amyloid pathology in cognitively normal subjects using (18)F‐flutemetamol PET: comparing visual reads and quantitative methods. J Nucl Med 60: 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clerck EE, Schouten JS, Berendschot TT et al. (2015): New ophthalmologic imaging techniques for detection and monitoring of neurodegenerative changes in diabetes: a systematic review. Lancet Diabetes Endocrinol 3: 653–663. [DOI] [PubMed] [Google Scholar]
- Demirkaya N, van Dijk HW, van Schuppen SM, Abramoff MD, Garvin MK, Sonka M, Schlingemann RO & Verbraak FD (2013): Effect of age on individual retinal layer thickness in normal eyes as measured with spectral‐domain optical coherence tomography. Invest Ophthalmol Vis Sci 54: 4934–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost S, Martins RN & Kanagasingam Y (2010): Ocular biomarkers for early detection of Alzheimer's disease. J Alzheimers Dis 22: 1–16. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP & Cunningham VJ (1997): Parametric imaging of ligand‐receptor binding in PET using a simplified reference region model. NeuroImage 6: 279–287. [DOI] [PubMed] [Google Scholar]
- den Haan J, Verbraak FD, Visser PJ & Bouwman FH (2017): Retinal thickness in Alzheimer's disease: A systematic review and meta‐analysis. Alzheimers Dement (Amst) 6: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp MJ et al. (2003): Three‐dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp 19: 224–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeman FYM, Heurling, K., Lopes Alves, I., Gispert, J.D., Foley, C., Lammertsma, A.A.(2018): P20: Optimized coffee‐break protocol for quantitative [18F]flutemetamol studies. Human Amyloid Imaging Conference. Miami, Florida.
- Hinrichs AL, Mintun MA, Head D, Fagan AM, Holtzman DM, Morris JC & Goate AM (2010): Cortical binding of pittsburgh compound B, an endophenotype for genetic studies of Alzheimer's disease. Biol Psychiatry 67: 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann EM, Schmidtmann I, Siouli A, Schuster AK, Beutel ME, Pfeiffer N & Lamparter J (2018): The distribution of retinal nerve fiber layer thickness and associations with age, refraction, and axial length: the Gutenberg health study. Graefes Arch Clin Exp Ophthalmol 256: 1685–1693. [DOI] [PubMed] [Google Scholar]
- Holm S (1979): A simple sequentially rejective multiple test procedure. Scand J Stat 6: 65–70. [Google Scholar]
- Hornberger J, Bae J, Watson I, Johnston J & Happich M (2017): Clinical and cost implications of amyloid beta detection with amyloid beta positron emission tomography imaging in early Alzheimer's disease ‐ the case of florbetapir. Curr Med Res Opin 33: 675–685. [DOI] [PubMed] [Google Scholar]
- Hougaard JL, Kessel L, Sander B, Kyvik KO, Sorensen TI & Larsen M (2003): Evaluation of heredity as a determinant of retinal nerve fiber layer thickness as measured by optical coherence tomography. Invest Ophthalmol Vis Sci 44: 3011–3016. [DOI] [PubMed] [Google Scholar]
- de Jager CA, Budge MM & Clarke R (2003): Utility of TICS‐M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry 18: 318–324. [DOI] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL et al. (2015): Prevalence of cerebral amyloid pathology in persons without dementia: a meta‐analysis. JAMA 313: 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan TK (2018): An algorithm for preclinical diagnosis of Alzheimer's disease. Front Neurosci 12: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM & Lamb BT (2018): Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y) 4: 575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konijnenberg E, Carter SF, Ten Kate M et al. (2018): The EMIF‐AD PreclinAD study: study design and baseline cohort overview. Alzheimers Res Ther 10: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kreeke JA, Nguyen HT, den Haan J et al. (2019): Retinal layer thickness in preclinical Alzheimer's disease. Acta Ophthalmol 97(8): 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kreeke JA, Nguyen HT, Konijnenberg E et al. (2018): Retinal and cerebral microvasculopathy: relationships and their genetic contributions. Invest Ophthalmol Vis Sci 59: 5025–5031. [DOI] [PubMed] [Google Scholar]
- Kumar A, Singh A & Ekavali, (2015): A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacol Rep 67: 195–203. [DOI] [PubMed] [Google Scholar]
- Kwun Y, Sung J, Yang Y, Yang S, Ham DI & Song YM (2011): Genetic influences on macular thickness in Koreans: the healthy twin study. Invest Ophthalmol Vis Sci 52: 9523–9526. [DOI] [PubMed] [Google Scholar]
- Leuzy A, Zimmer ER, Heurling K, Rosa‐Neto P & Gauthier S (2014): Use of amyloid PET across the spectrum of Alzheimer's disease: clinical utility and associated ethical issues. Amyloid 21: 143–148. [DOI] [PubMed] [Google Scholar]
- Lim JK, Li QX, He Z et al. (2016): The eye as a biomarker for Alzheimer's disease. Front Neurosci 10: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London A, Benhar I & Schwartz M (2013): The retina as a window to the brain‐from eye research to CNS disorders. Nat Rev Neurol 9: 44–53. [DOI] [PubMed] [Google Scholar]
- McGrory S, Cameron JR, Pellegrini E et al. (2017): The application of retinal fundus camera imaging in dementia: A systematic review. Alzheimers Dement (Amst) 6: 91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitka M (2013): PET imaging for Alzheimer disease: are its benefits worth the cost? JAMA 309: 1099–1100. [DOI] [PubMed] [Google Scholar]
- Morris JC (1993): The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED & Clark C (1989): The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 39: 1159–1165. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Atkinson C, Bhalla K et al. (2013): The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA 310: 591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishii R, Higashi T, Kagawa S et al. (2018): (18)F‐FPYBF‐2, a new F‐18 labelled amyloid imaging PET tracer: biodistribution and radiation dosimetry assessment of first‐in‐man (18)F‐FPYBF‐2 PET imaging. Ann Nucl Med 32: 256–263. [DOI] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W & Ferri CP (2013): The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9: 63‐75 e62. [DOI] [PubMed] [Google Scholar]
- Santos CY, Johnson LN, Sinoff SE, Festa EK, Heindel WC & Snyder PJ (2018): Change in retinal structural anatomy during the preclinical stage of Alzheimer's disease. Alzheimers Dement (Amst) 10: 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinin NM, Tolvanen TK, Wilson IA, Arponen EM, Nagren KA & Rinne JO (2007): Biodistribution and radiation dosimetry of the amyloid imaging agent 11C‐PIB in humans. J Nucl Med 48: 128–133. [PubMed] [Google Scholar]
- Snyder PJ, Johnson LN, Lim YY, Santos CY, Alber J, Maruff P & Fernandez B (2016): Nonvascular retinal imaging markers of preclinical Alzheimer's disease. Alzheimers Dement (Amst) 4: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BL, Li WW, Zhu C et al. (2018): Clinical research on alzheimer's disease: progress and perspectives. Neurosci Bull 34: 1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Kate M, Sudre CH, den Braber A et al. (2018): White matter hyperintensities and vascular risk factors in monozygotic twins. Neurobiol Aging 66: 40–48. [DOI] [PubMed] [Google Scholar]
- Tolboom N, Yaqub M, van der Flier WM et al. (2009): Detection of Alzheimer pathology in vivo using both 11C‐PIB and 18F‐FDDNP PET. J Nucl Med 50: 191–197. [DOI] [PubMed] [Google Scholar]
- Van de Kreeke JA, Legdeur N, Badissi M et al. (2019a): Ocular biomarkers for cognitive impairment in nonagenarians; a prospective cross‐sectional study. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed]
- Vina J & Sanz‐Ros J (2018): Alzheimer's disease: only prevention makes sense. Eur J Clin Invest 48: e13005. [DOI] [PubMed] [Google Scholar]
- Vlassenko AG, Benzinger TL & Morris JC (2012): PET amyloid‐beta imaging in preclinical Alzheimer's disease. Biochim Biophys Acta 1822: 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Jiang H, Shi Y, Qu D, Gregori G, Zheng F, Rundek T & Wang J (2017): Age‐related alterations in the retinal microvasculature, microcirculation, and microstructure. Invest Ophthalmol Vis Sci 58: 3804–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JY, Kim SE & Park YH (2016): Effect of age and sex on retinal layer thickness and volume in normal eyes. Medicine (Baltimore) 95: e5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y & Carson RE (2002): Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 22: 1440–1452. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M & Leirer VO (1982): Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17: 37–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Differences in rate of change in retinal layers between Aβ− and Aβ+ participants.
Table S2. Differences in rate of change in retinal layers between Aβ− and Aβ+ participants.
Table S3. Associations between rate of change in retinal layers between baseline and follow‐up visit with BP nd.


