Abstract
Objective
Neuropsychiatric symptoms (NPS) are associated with the risk of incident mild cognitive impairment (MCI) and dementia. We examined associations between NPS and the outcomes of global and domain‐specific cognitive trajectories.
Methods
In this longitudinal study conducted in the setting of the population‐based Mayo Clinic Study of Aging, 5081 community‐dwelling, nondemented individuals aged ≥50 years (51% males) underwent NPS assessment using Neuropsychiatric Inventory Questionnaire (NPI‐Q), and Beck Depression and Anxiety Inventories (BDI‐II, BAI). Global and domain‐specific (memory, language, attention, and visuospatial skills) cognitive performance was assessed through neuropsychological testing every 15 months. Associations between baseline NPS and trajectories for individual yearly change in cognitive z‐scores were calculated using linear mixed‐effect models.
Results
Cognition declined regardless of NPS status over the median follow‐up of 4.5 years. Presence of NPS was associated with increased cognitive decline. Differences in annualized change in global cognition z‐scores for participants with NPS compared to without NPS ranged from −0.018 (95% CI −0.032, −0.004; p = 0.011) for irritability to −0.159 (−0.254, −0.065; p = 0.001) for hallucinations. Associations between NPS and annual decline in global cognition were significant for most NPI‐Q‐assessed NPS and clinical depression (BDI‐II≥13). Participants with NPI‐Q‐assessed depression, apathy, nighttime behavior, and clinical depression had greater decline in all domain‐specific z‐scores; presence of delusions and anxiety was associated with more pronounced decline in language, attention and visuospatial skills.
Conclusion
NPS were associated with a more accelerated cognitive decline. Clinical assessment and potential treatment of NPS is warranted even in a community setting as NPS may impact cognitive decline in nondemented individuals.
Keywords: cognitive trajectory, longitudinal study, neuropsychiatric symptoms, nondemented, older adults
Key points
Presence of neuropsychiatric symptoms was associated with a more accelerated global and domain‐specific cognitive decline
Participants with depression or apathy had greater decline in all domain‐specific z‐scores
Presence of delusions and anxiety was associated with more pronounced decline in language, attention and visuospatial skills
Clinical assessment and potential treatment of neuropsychiatric symptoms is warranted even in a community setting
1. INTRODUCTION
Neuropsychiatric symptoms (NPS) such as depression or apathy are very common in older adults with or without cognitive impairment.1, 2, 3, 4, 5, 6, 7, 8 Furthermore, prospective studies have shown that NPS are risk factors for new onset of categorical outcomes of mild cognitive impairment (MCI)9, 10, 11 or dementia.12, 13, 14, 15, 16
However, little is known about the population‐based associations between various NPS and cognitive trajectories in older adults free of dementia. For example, a recent analysis derived from the Cache County study showed that overall NPS burden among clinically normal older adults was associated with a more rapid cognitive decline over 5.7 years of follow‐up, but results for individual NPS (e.g., depression or anxiety) and their respective associations with cognitive decline were conflicting.17
Therefore, the aim of this study was to examine the longitudinal associations between self‐as well as informant‐reported NPS and longitudinal trajectories of cognitive performance in a sample of nondemented community‐dwelling older individuals aged ≥50 years. We hypothesized that baseline NPS, be it self‐reported or informant‐observed, would be associated with a more accelerated decline in global and domain‐specific cognitive performances over time.
2. METHODS
2.1. Setting and study sample
The study was conducted in the setting of the population‐based Mayo Clinic Study of Aging (MCSA) in Olmsted County, MN, USA. We included persons aged ≥50 years who were either cognitively unimpaired (CU) or had MCI, and on whom self‐ and informant‐based information on NPS at baseline as well as cognitive performance measurements were available. Neuropsychological testing was conducted approximately every 15 months. MCSA study protocols have been approved by the IRB of the Mayo Clinic and Olmsted Medical Center in Rochester, MN. All participants provided written informed consent.
2.2. Cognitive evaluation
The MCSA study protocol is described in detail elsewhere.18 Briefly, all participants underwent a face‐to‐face evaluation including a neurological examination performed by a physician, a risk factor ascertainment conducted by a study coordinator, and standardized neuropsychological testing which was administered by a psychometrist supervised by a board‐certified neuropsychologist, in order to assess performance in four cognitive domains: (1) memory (delayed recall trials from Auditory Verbal Learning Test,19 Wechsler Memory Scale‐Revised,20 Logical Memory and Visual Reproduction subtests); (2) attention/executive function (Trail‐Making Test Part B,21 Wechsler Adult Intelligent Scale‐Revised,22 Digit Symbol Substitution subtest); (3) language (Boston Naming Test,23 category fluency);24 and (4) visuospatial skills (Wechsler Adult Intelligence Scale‐Revised,22 Picture Completion and Block Design subtests). An expert consensus panel consisting of physicians, study coordinators, and neuropsychologists reviewed the results for each participant and determined whether a participant was CU or had MCI. Individuals were classified as CU based on normative data developed on a different sample in this community.25, 26, 27, 28 For MCI, the Mayo Clinic criteria for MCI29, 30 were used: (1) cognitive concern expressed by a physician, informant, participant, or study coordinator; (2) impairment in one or more cognitive domains (memory, attention/executive function, language, or visuospatial skills); (3) essentially normal functional activities; and (4) absence of dementia. Participants with MCI had a Clinical Dementia Rating (CDR) score of 0 or 0.5; however, the final diagnosis of MCI was based on all available data.
2.3. Measurement of neuropsychiatric symptoms
NPS were assessed using the Neuropsychiatric Inventory Questionnaire (NPI‐Q;31), the Beck Depression Inventory (BDI‐II;32), and the Beck Anxiety Inventory (BAI;33). The NPI‐Q was administered as a structured interview to an informant by a study coordinator and assessed the presence/absence of twelve emotional behaviors (i.e., depression, anxiety, apathy, agitation, delusions, hallucinations, euphoria, disinhibition, irritability, aberrant motor behavior, nighttime behavior/sleep, and eating/appetite). The BDI‐II and BAI are validated, self‐administered inventories. The BDI‐II is a 21‐item questionnaire that measures symptoms of depression over the last two weeks, such as feeling guilty and loss of interest. Similarly, the BAI is a 21‐item questionnaire that assesses symptoms of anxiety such as nervousness and fear of losing control over the last seven days. The severity of each symptom in both BDI‐II and BAI is rated on a scale ranging from 0 (low severity) to 3 (high severity). The total score for both inventories thus ranges from 0 to 63, with a higher score indicating a higher severity of depressive and anxiety symptoms, respectively.
2.4. Measurement of neuropsychological test scores
From all neuropsychological tests described under “cognitive evaluation,” we used continuous measures that were not age normed. Longitudinal z‐scores were calculated relative to the baseline scores by converting individual test scores to z‐scores. We then created domain‐specific z‐scores by z‐scoring the averages of the test‐specific z‐scores and also created a global z‐score by z‐scoring the averages of the domain‐specific z‐scores. The outcome of interest for the linear mixed‐effect model analyses was the longitudinal change in cognitive measures of memory, attention/executive function, language, visuospatial skills, and global cognition.
2.5. Statistical analysis
We calculated linear mixed‐effect models with random subject‐specific intercepts and slopes to examine the longitudinal association between NPS at baseline and change in cognitive z‐scores over time. In our models, NPS at baseline were the independent variables (predictors), and the trajectories for individual yearly change in cognitive z‐scores over time were the dependent variables (outcomes). All models included NPS at baseline, time in years from baseline and their interaction. We conducted the analyses for twelve NPS as assessed by the NPI‐Q, and also included clinical depression (BDI‐II score ≥ 13) and clinical anxiety (BAI score ≥ 10) in the models. All models were adjusted for age, sex, education, and whether or not the participant had previously taken the cognitive tests. All statistical analyses were done using the conventional two‐tailed alpha level of 0.05 and performed with SAS 9.4 (SAS Institute, Inc).
3. RESULTS
The sample consisted of 5081 persons (2612 males, 51.4%) of whom 4439 persons (87.4%) were CU and 642 (12.6%) had MCI. The median (interquartile range, IQR) age was 74.4 (67.4, 81.2) years, the median (IQR) years of education were 14.0 (12.0, 16.0), and median (IQR) follow‐up time was 4.5 (1.3, 6.8) years. Participant demographics at baseline are displayed in Table 1.
TABLE 1.
Participant characteristics at baseline
| Variable | CU (N = 4439) | MCI (N = 642) | Total (N = 5081) |
|---|---|---|---|
| Age, years | 73.7 (66.2, 80.3) | 80.6 (73.9, 84.4) | 74.4 (67.4, 81.2) |
| Male sex | 2243 (50.5) | 369 (57.5) | 2612 (51.4) |
| Education, years | 14.0 (12.0, 16.0) | 12.0 (12.0, 15.0) | 14.0 (12.0, 16.0) |
| Race/ethnicity | |||
| White | 4371 (98.5) | 629 (98.0) | 5000 (98.4) |
| Asian | 22 (0.5) | 4 (0.6) | 26 (0.5) |
| Black | 11 (0.2) | 1 (0.2) | 12 (0.2) |
| Othera | 35 (0.8) | 8 (1.2) | 43 (0.9) |
| Delusions | 13 (0.3) | 18 (2.8) | 31 (0.6) |
| Hallucinations | 7 (0.2) | 6 (0.9) | 13 (0.3) |
| Agitation | 113 (2.5) | 55 (8.6) | 168 (3.3) |
| Depression | 470 (10.6) | 167 (26.0) | 637 (12.5) |
| Anxiety | 249 (5.6) | 91 (14.2) | 340 (6.7) |
| Euphoria | 19 (0.4) | 12 (1.9) | 31 (0.6) |
| Apathy | 183 (4.1) | 111 (17.3) | 294 (5.8) |
| Disinhibition | 53 (1.2) | 29 (4.5) | 82 (1.6) |
| Irritability | 336 (7.6) | 128 (19.9) | 464 (9.1) |
| Motor behavior | 28 (0.6) | 11 (1.7) | 39 (0.8) |
| Nighttime behaviorb | 277 (7.3) | 98 (18.2) | 375 (8.7) |
| Appetite | 191 (4.3) | 60 (9.3) | 251 (4.9) |
| BDI‐II score ≥ 13c | 279 (6.4) | 99 (15.9) | 378 (7.6) |
| BAI score ≥ 10d | 277 (6.3) | 87 (13.6) | 364 (7.2) |
| z‐score memorye | 0.20 (−0.46, 0.84) | −1.41 (−1.88, −0.84) | 0.04 (−0.72, 0.74) |
| z‐score attentionf | 0.22 (−0.35, 0.76) | −1.12 (−2.12, −0.32) | 0.12 (−0.54, 0.68) |
| z‐score languageg | 0.18 (−0.38, 0.73) | −0.98 (−1.80, −0.37) | 0.07 (−0.55, 0.67) |
| z‐score visuospatialh | 0.18 (−0.47, 0.80) | −0.99 (−1.64, −0.34) | 0.07 (−0.65, 0.71) |
| z‐score global cognitioni | 0.23 (−0.38, 0.80) | −1.39 (−1.93, −0.88) | 0.08 (−0.62, 0.70) |
Note: Continuous variables are presented as median (IQR) and categorical variables are presented as N (%).
Abbreviations: BAI, Beck Anxiety Inventory; BDI‐II, Beck Depression Inventory‐II; CU, cognitively unimpaired; IQR, interquartile range; MCI, mild cognitive impairment; NPS, neuropsychiatric symptoms.
Other includes: American Indian/Alaska Native (N = 3), more than one race (N = 25), unknown race (N = 15).
Information missing on 751 participants (646 CU, 105 MCI).
Information missing on 92 participants (71 CU, 21 MCI).
Information missing on 12 participants (8 CU, 4 MCI).
Information missing on 69 participants (54 CU, 15 MCI).
Information missing on 208 participants (155 CU, 53 MCI).
Information missing on 164 participants (130 CU, 34 MCI).
Information missing on 221 participants (170 CU, 51 MCI).
Information missing on 328 participants (248 CU, 80 MCI).
Longitudinal analyses revealed statistically significant associations between NPS at baseline and change in global cognitive z‐score over time (Figure 1, Table A1). For apathy as an example, the coefficient for time indicates that each one year increase in time is associated with a decline in global z‐score of 0.071 for participants without apathy. Whereas participants with apathy showed a decline in global cognitive z‐score of 0.128 (i.e., −0.071 + (−0.057)); thus, persons with apathy have an increased degree of global cognitive decline than persons without apathy. The interaction between time since baseline and NPS was statistically significant for delusions, hallucinations, agitation, depression, anxiety, apathy, irritability, motor behavior, nighttime behavior, appetite change (all assessed using NPI‐Q) as well as clinical depression (BDI score ≥ 13; Figure 1, Table A1). The coefficients for the variables we adjusted for ranged, depending on the different NPS, between −0.055 and −0.056 for age, −0.196 and −0.217 for sex, 0.109 and 0.112 for education, and −0.204 and −0.206 for whether or not the participant had previously taken the cognitive tests (data not shown).
FIGURE 1.
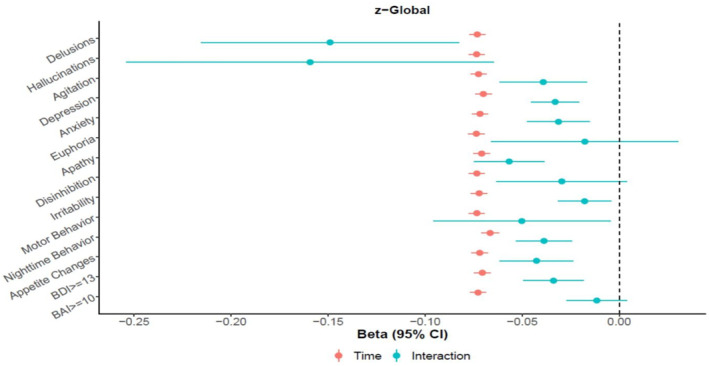
Longitudinal association between presence of Neuropsychiatric symptoms at baseline (IV) and change in global cognition z‐score over time (DV)
Similarly, NPI‐Q‐assessed hallucinations, agitation, depression, apathy, irritability, motor behavior, nighttime behavior and appetite change as well as clinical depression were statistically significantly associated with declining memory z‐scores over time (Figure 2, Table A2). With regard to attention, the interactions were statistically significant for NPI‐Q‐assessed delusions, hallucinations, depression, anxiety, apathy, irritability, motor behavior, nighttime behavior, and appetite change as well as clinical depression (Figure 2, Table A3). Additionally, delusions, agitation, depression, anxiety, apathy, nighttime behavior and appetite change as assessed by the NPI‐Q as well as clinical depression were statistically significantly associated with a decline in language (Figure 2, Table A4). Finally, delusions, depression, anxiety, apathy and nighttime behavior as well as clinical depression were significantly associated with decline in visuospatial skills (Figure 2, Table A5).
FIGURE 2.
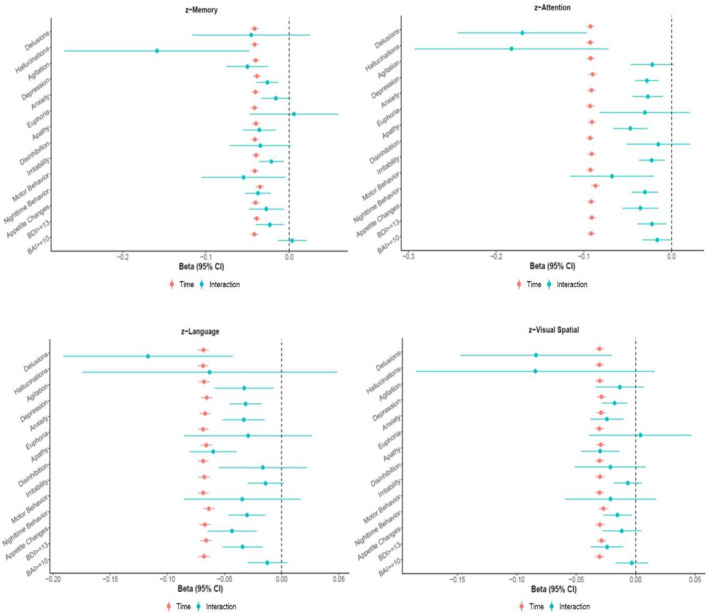
Longitudinal association between presence of Neuropsychiatric symptoms at baseline (IV) and change in domain‐specific cognitive z‐score over time (DV)
4. DISCUSSION
Here we report the association between NPS at baseline and longitudinal decline in cognitive performance in a population‐based sample of CU individuals and persons with MCI over a median follow‐up of 4.5 years. As hypothesized, presence of informant‐reported NPS as assessed by the NPI‐Q, particularly depression, apathy and nighttime behavior, were associated with statistically significant decline in performance of all four cognitive domains as well as global cognition. In addition, clinical depression, as indicated by a BDI‐II score ≥13, was also associated with significant decline in domain‐specific and global cognition, whereas no significant associations were observed for clinical anxiety as indicated by a BAI score ≥10 and changes in cognitive function over time.
In the past, several studies have examined the associations between NPS and incidence of MCI9, 10, 11 and dementia.12, 13, 14, 15, 16 A relatively smaller number of longitudinal studies have investigated associations between NPS and cognitive trajectories as a continuous measure, and most of these studies focused on depression34, 35, 36 or anxiety.37, 38 For example, in a large sample of over 4000 adults aged ≥65 years, depressive symptoms were significantly associated with a decline in global cognitive function over 3 years, and this association existed even when restricting the analysis to participants who were CU at baseline.39 Similarly, researchers from Australia found that mild worry and depressive symptoms were associated with decline in visual learning and memory at 2‐year follow‐up in community‐dwelling older adults.40 Investigators from the Netherlands reported that anxiety symptoms were associated with stronger decline in verbal memory in nondemented individuals aged ≥65 years.41
To date, few studies have examined other NPS except depression and anxiety, for example, a study from the United Kingdom reported that older, nondemented adults with psychotic symptoms experienced a more pronounced decline in non‐memory cognitive functions than individuals without psychotic symptoms after 6 years of follow‐up.42 Furthermore, a study in more than 9000 CU older adults showed that those individuals with mild behavioral impairment (MBI) experienced greater decline in attention and working memory performance after one year as compared to persons without MBI.43 Researchers from Australia conducted a study among 873 community‐dwelling older adults and reported that anxiety was associated with decline in executive function, and that agitation was associated with decline in language over two years of follow‐up.44 Finally, a recently published research derived from the Cache County study among 470 older adults showed that higher NPS burden as assessed by the Neuropsychiatric Inventory total score at baseline was associated with more pronounced decline on word list memory and recall, delayed praxis recall, and animal fluency tasks.17 However, in contrast to our findings, depression was not associated with decline in any cognitive outcome measure, whereas anxiety was associated with more rapid decline in the symbol digit modalities test.17 The differences in results between their study and ours may be due to different samples and different assessment tools for NPS and cognitive function.
Overall, our study expands on the existing body of literature by providing evidence of an association between various self‐ and informant‐reported NPS, particularly depression, apathy and nighttime behavior, with domain‐specific and global cognitive trajectories in a population‐based sample of more than 5000 older adults. While it is expected, based on previous research, to observe associations between various NPS with memory and attention/executive function,44 the associations between NPS with language and visuospatial skills are less established. Indeed, we found that 10 NPI‐Q‐assessed NPS and clinical depression were associated with increased longitudinal decline in global cognition, eight NPI‐Q‐assessed NPS and clinical depression with increased decline in memory, and nine NPI‐Q‐assessed NPS and clinical depression with increased decline in attention/executive function. In contrast, only seven NPI‐Q‐assessed NPS and clinical depression were associated with increased longitudinal decline in language and five NPI‐Q‐assessed NPS and clinical depression with increased decline in visuospatial skills. More research is needed to confirm the observations by our group and others, particularly with regard to the conflicting findings on the associations between depression and anxiety with cognitive decline. In addition, it would also be interesting to examine potential associations between current NPI‐Q or 1‐lagged NPI‐Q with cognitive trajectories, as it is known from literature that NPI‐Q trajectories are quite variable and unlike cognition do not proceed in a straight line in a single direction. Furthermore, it may be useful to also consider NPI‐Q domain scores or NPI‐Q total score in addition to the dichotomized NPI‐Q domain scores as in our current research.
Our study did not examine potential mechanisms that may underlie an association between NPS and cognitive decline over time. However, previous research has suggested that depressive symptoms may moderate cognitive decline associated with changes in brain white matter hyperintensities.45 With regard to an association between anxiety and cognitive decline, potential explanations may include hypercortisolism, low‐grade inflammation, and increased levels of cytokines, or suppression of neurotrophic factors such as brain‐derived neurotrophic factor (BDNF).46
The strengths of our study are the large sample size of community‐dwelling individuals free of dementia and the rigorous assessment of both informant‐based and self‐reported NPS using three validated instruments. This enabled us to not only focus on depression and anxiety as done in many previous studies but also to look at potential associations between other NPS such as apathy, agitation or nighttime behavior with cognitive trajectories. One weakness of our study pertains to its observational nature. Such a design limits etiologic inference that one can make despite the longitudinal nature of our study. Furthermore, as we have consistently observed6, 9 and as expected, the frequency and distribution of NPS, particularly psychotic symptoms such as delusions or hallucinations, is very low in a population‐based sample. Therefore, one needs to be cautious in generalizing our findings to samples derived from behavioral neurology and neuropsychiatry clinics where the prevalence of NPS including clinically significant NPS is considerably higher. Nevertheless, our study findings can be particularly informative to policy makers and stakeholders that make recommendations based on NPS events that occur in a community setting. In addition, we did not adjust our analyses for multiple comparisons which may increase Type I error. However, when considering a Bonferroni correction for our analyses, the alpha significance level would be 0.004 (i.e., 0.05/14 since we have a total of 14 predictor variables). Thus, when taking Table A1 as an example, nine out of the 11 significant p values for the interaction between NPS and change in cognitive z‐scores would still remain significant and none of our major conclusions would be affected by the correction. Finally, when interpreting our results, one needs to keep in mind that statistical significance, particularly given our large sample size, not always translates to clinical significance. Thus, the observed differences in annualized changes in global or domain‐specific cognition z‐scores for participants with compared to without given NPS over a span of 4.5 years may not always be clinically meaningful. However, when taking NPI‐Q‐assessed depression predicting memory change as an example (Table A2), it can be seen that the time coefficient is −0.039 and having depression adds on −0.026 (time × depression interaction coefficient). Thus, having depression almost doubles memory decline over time. An alternative way of interpretation would be to compare to the age coefficient which is −0.049 (data not shown), that is, having depression is comparable to adding about half a year in age extra with each passing year.
In conclusion, our study adds to the growing body of research providing evidence of an association between NPS with longitudinal cognitive decline in community‐dwelling older adults free of dementia. Particularly, presence of depression, apathy, and nighttime behavior at baseline was associated with more pronounced decline in performance in all cognitive domains (i.e., memory, attention, language, and visuospatial function) as well as global cognition.
CONFLICT OF INTEREST
Dr. Machulda receives research funding from NIH. Dr. Kremers receives research funding from the Department of Defense, NIH, Astra Zeneca, Biogen, and Roche. Dr. Mielke served as a consultant to Eli Lilly, received unrestricted research grants from Biogen, Lundbeck and Roche, and receives research funding from the National Institute on Aging, NIH, and the Department of Defense. Dr. Knopman serves on a Data Safety Monitoring Board for the Dominantly Inherited Alzheimer Network (DIAN) study and is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. Dr. Petersen consults for Roche, Inc, Merck, Inc, Genentech, Inc, and Biogen, Inc, and GE Healthcare and receives royalties from Oxford University Press for the publication of Mild Cognitive Impairment. Dr. Vassilaki has received in the past research funding from Roche and Biogen; she currently consults for Roche, receives research funding from NIH and has equity ownership in Abbott Laboratories, Johnson and Johnson, Medronic and Amgen Dr. Geda receives funding from the NIH, Barrow Neurological Institute, and Roche and served on the Lundbeck Advisory Board. No other disclosures were reported.
ETHICS APPROVAL AND PATIENT CONSENT STATEMENT
MCSA study protocols have been approved by the IRB of the Mayo Clinic and Olmsted Medical Center in Rochester, MN. All participants provided written informed consent.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
Support for this research was provided by NIH grants: National Institute on Aging (R01 AG057708; U01 AG006786; P50 AG016574; R01 AG034676), and National Institute of Mental Health (K01 MH068351). This project was also supported by the Robert Wood Johnson Foundation, the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program, the GHR Foundation, the Mayo Foundation for Medical Education and Research, the Edli Foundation and Barrow Neurological Institute. We would like to thank study coordinators and research nurses at Mayo Clinic Study of Aging for their dedicated work in acquiring high quality data.
Krell‐Roesch J, Syrjanen JA, Machulda MM, et al. Neuropsychiatric symptoms and the outcome of cognitive trajectories in older adults free of dementia: The Mayo Clinic Study of Aging. Int J Geriatr Psychiatry. 2021;36(9):1362–1369. 10.1002/gps.5528
DATA AVAILABILITY STATEMENT
Data may be shared per request from a qualified investigator in accordance with the Mayo Clinic Study of Aging data sharing protocol.
REFERENCES
- 1.Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008;25(2):115‐126. [DOI] [PubMed] [Google Scholar]
- 2.Craig D, Mirakhur A, Hart DJ, McIlroy SP, Passmore AP. A cross‐sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer's disease. Am J Geriatric Psychiatry. 2005;13(6):460‐468. [DOI] [PubMed] [Google Scholar]
- 3.Cummings JL, McPherson S. Neuropsychiatric assessment of Alzheimer's disease and related dementias. Aging Clin Exp Res. 2001;13(3):240‐246. [DOI] [PubMed] [Google Scholar]
- 4.Forrester SN, Gallo JJ, Smith GS, Leoutsakos J‐MS. Patterns of neuropsychiatric symptoms in mild cognitive impairment and risk of dementia. Am J Geriatric Psychiatry. 2016;24(2):117‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geda Y, Rocca W, Knopman D, Roberts R, Petersen R. The prevalence of neuropsychiatric symptoms in mild cognitive impairment: a population‐based study. Neurology. 2006;66(Suppl 2):A119. [Google Scholar]
- 6.Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging. Arch Gen Psychiatry. 2008;65(10):1193‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geda YE, Schneider LS, Gitlin LN, et al. Neuropsychiatric symptoms in Alzheimer's disease: past progress and anticipation of the future. Alzheimer's Dementia. 2013;9(5):602‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment. JAMA. 2002;288(12):1475‐1483. [DOI] [PubMed] [Google Scholar]
- 9.Geda YE, Roberts RO, Mielke MM, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population‐based study. Am J Psychiatry. 2014;171(5):572‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment. Arch Neurol. 2006;63(3):435‐440. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085‐2092. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry. 2013.21(7):685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic‐mild cognitive impairment to Alzheimer's disease: the role of depression and apathy. J Alzheimers Dis. 2010;20(1):175‐183. [DOI] [PubMed] [Google Scholar]
- 14.Pink A, Stokin GB, Bartley MM, et al. Neuropsychiatric symptoms, APOE 4, and the risk of incident dementia: a population‐based study. Neurology. 2015;84(9):935‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramakers IHGB, Visser PJ, Aalten P, Kester A, Jolles J, Verhey FRJ. Affective symptoms as predictors of Alzheimer's disease in subjects with mild cognitive impairment: a 10‐year follow‐up study. Psychol Med. 2010;40(7):1193‐1201. [DOI] [PubMed] [Google Scholar]
- 16.Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of dementia and mild cognitive impairment in the oldest old women. Aging Ment Health. 2018;22(4):474‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burhanullah MH, Tschanz JT, Peters ME, et al. Neuropsychiatric symptoms as risk factors for cognitive decline in clinically normal older adults: the cache county study. Am J Geriatric Psychiatry. 2020;28(1):64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rey AL. 'examen clinique en psychologie. Paris, France: Presses Universitaires de France; 1964. [Google Scholar]
- 20.Wechsler D. Wechsler Memory Scale‐Revised. New York, NY: The Psychological Corporation; 1987. [Google Scholar]
- 21.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Ski. 1958;8(3):271‐276. [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale‐Revised. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 23.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. 2nd ed.Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 24.Lucas JA, Ivnik RJ, Smith GE, et al. Mayo's Older Americans Normative Studies: category fluency norms. J Clin Exp Neuropsychology. 1998;20(2):194‐200. [DOI] [PubMed] [Google Scholar]
- 25.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's older Americans normative studies: WAIS‐R norms for ages 56 to 97. Clin Neuropsychol. 1992;6(sup001):1‐30. [Google Scholar]
- 26.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's older Americans normative studies: WMS‐R norms for ages 56 to 94. Clin Neuropsychol. 1992;6(suppl 001):49‐82. [Google Scholar]
- 27.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's older Americans normative studies: updated AVLT norms for ages 56 to 97. Clin Neuropsychol. 1992;6(suppl 001):83‐104. [Google Scholar]
- 28.Malec JF, Ivnik RJ, Smith GE, et al. Mayo's older Americans normative studies: utility of corrections for age and education for the WAIS‐R. Clin Neuropsychol. 1992;6(sup001):31‐47. [Google Scholar]
- 29.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183‐194. [DOI] [PubMed] [Google Scholar]
- 30.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment ‐ beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240‐246. [DOI] [PubMed] [Google Scholar]
- 31.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI‐Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233‐239. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory‐II (BDI‐II). San Antonio, TX: Psychology Corporation; 2001. [Google Scholar]
- 33.Beck AT, Steer RA. BAI, Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corp.: Harcourt Brace Jovanovich; 1990. [Google Scholar]
- 34.Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364‐370. [DOI] [PubMed] [Google Scholar]
- 35.Paterniti S, Verdier‐Taillefer M‐H, Dufouil C, Alpérovitch A. Depressive symptoms and cognitive decline in elderly people. Br J Psychiatry. 2002;181:406‐410. [DOI] [PubMed] [Google Scholar]
- 36.Mewton L, Reppermund S, Crawford J, Bunce D, Wen W, Sachdev P. Cross‐sectional and prospective inter‐relationships between depressive symptoms, vascular disease and cognition in older adults. Psychol Med. 2019;49(13):2168‐2176. [DOI] [PubMed] [Google Scholar]
- 37.Bierman EJM, Comijs HC, Rijmen F, Jonker C, Beekman ATF. Anxiety symptoms and cognitive performance in later life: results from the longitudinal aging study Amsterdam. Aging Ment Health. 2008;12(4):517‐523. [DOI] [PubMed] [Google Scholar]
- 38.Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community‐dwelling men. Int Psychogeriatr. 2017;29(7):1137‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sachs‐Ericsson N, Joiner T, Plant EA, Blazer DG. The influence of depression on cognitive decline in community‐dwelling elderly persons. Am J Geriatric Psychiatry. 2005;13(5):402‐408. [DOI] [PubMed] [Google Scholar]
- 40.Pietrzak RH, Maruff P, Woodward M, et al. Mild worry symptoms predict decline in learning and memory in healthy older adults: a 2‐year prospective cohort study. Am J Geriatric Psychiatry. 2012;20(3):266‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulpers BJA, Oude Voshaar RC, van Boxtel MPJ, Verhey FRJ, Köhler S. Anxiety as a risk factor for cognitive decline: a 12‐year follow‐up cohort study. Am J Geriatric Psychiatry. 2019;27(1):42‐52. [DOI] [PubMed] [Google Scholar]
- 42.Köhler S, Allardyce J, Verhey FRJ, et al. Cognitive decline and dementia risk in older adults with psychotic symptoms: a prospective cohort study. Am J Geriatric Psychiatry. 2013;21(2):119‐128. [DOI] [PubMed] [Google Scholar]
- 43.Creese B, Brooker H, Ismail Z, et al. Mild behavioral impairment as a marker of cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry. 2019.27(8):823–834. [DOI] [PubMed] [Google Scholar]
- 44.Brodaty H, Heffernan M, Draper B, et al. Neuropsychiatric symptoms in older people with and without cognitive impairment. J Alzheimers Dis. 2012;31(2):411‐420. [DOI] [PubMed] [Google Scholar]
- 45.Park JH, Lee SB, Lee JJ, et al. Depression plays a moderating role in the cognitive decline associated with changes of brain white matter hyperintensities. J Clin Psychiatry. 2018;79(5). [DOI] [PubMed] [Google Scholar]
- 46.Gulpers B, Ramakers I, Hamel R, Köhler S, Oude Voshaar R, Verhey F. Anxiety as a predictor for cognitive decline and dementia: a systematic review and meta‐analysis. Am J Geriatric Psychiatry. 2016;24(10):823‐842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data may be shared per request from a qualified investigator in accordance with the Mayo Clinic Study of Aging data sharing protocol.


