Abstract
Objectives
Due to a worldwide increase in drug consumption, oral healthcare professionals are frequently confronted with patients using one or more drugs. A large number of drugs can be accompanied with adverse drug reactions in the orofacial region, amongst others of the tongue. This paper aims to give an overview of drugs that are known to be accompanied with tongue disorders.
Materials and methods
The national drug information database for Dutch pharmacists, composed of scientific drug information, guidelines and summaries of product characteristics, was analysed for drug‐induced tongue disorders. “MedDRA classification” and “Anatomical Therapeutic Chemical codes” were used to categorize the disorders.
Results
The database comprises of 1645 drugs of which 121 (7.4%) are documented to be accompanied with tongue disorders as an adverse effect. Drug‐induced tongue disorders are predominantly observed in the following drug categories: “nervous systems,” “anti‐infectives for systemic use” and “alimentary tract and metabolism”. The most common drug‐induced tongue disorders are glossitis, tongue oedema, tongue discoloration and burning tongue.
Conclusion
Healthcare professionals are frequently confronted with drugs that can cause tongue disorders. The overview of drugs reported in this article supports clinicians in their awareness, diagnosis and treatment of drug‐induced tongue disorders.
Keywords: burning tongue, drug‐induced tongue disorders, glossitis, tongue oedema, tongue discoloration
1. INTRODUCTION
The global consumption of drugs to treat acute and chronic diseases continues to increase (WHO, 2011). Inevitably, healthcare professionals are frequently confronted with patients using one or more drugs on a daily basis. These drugs can cause several adverse effects in the oral region such as a sensation of oral dryness (xerostomia), hyposalivation, mucositis and taste disorders (Rademacher et al., 2019). Due to the large number of drugs available and their wide range of adverse effects, it is difficult and time‐consuming for healthcare professionals to take all the potential consequences into account during their daily practice. To support oral healthcare professionals in their decision making, the Journal of Oral Diseases is publishing a series of articles discussing the most frequent adverse effects of drugs in the oral region. The first paper discussed drug‐induced taste disorders (Rademacher et al., 2019). This paper focuses on drug‐induced tongue disorders.
Tongue disorders, which are rather frequently observed, can be divided into congenital and acquired tongue disorders. Aglossia, ankyloglossia, hypoglossia, macroglossia, cleft tongue and glossoptosis are examples of congenital tongue disorders (Mangold et al., 2016). Drug‐induced tongue disorders belong to the category acquired tongue disorders.
Several studies have reported cases of drug‐induced tongue disorders (Alharbi et al., 2018; Balaji et al., 2014; Braggio et al., 2018; Brown, 1949; Gurvits & Tan, 2014; Healy et al., 2004; Hubiche et al., 2013; Kalogirou et al., 2017), but a comprehensive overview of drugs associated with tongue disorders as an adverse effect is not available. Such an overview will support oral healthcare providers in the recognition, diagnosis and eventual treatment of drug‐induced tongue disorders.
2. MATERIALS AND METHODS
An elaborated description of the Materials and Methods used in the current study is described by Rademacher et al. (2019). In short, the data on oral adverse effects of medications were derived from the Informatorium Medicamentorum of the Royal Dutch Pharmacists Association (KNMP), the leading drug information database and reference work for pharmacists in the Netherlands (KNMP, 2019). This database is composed of scientific drug information, guidelines and summaries of product characteristics. It includes not only entries derived from scientific publications (randomized control trails, observational studies, case reports, etc.), but also data from the Netherlands pharmacovigilance centre LAREB, the Dutch knowledge centre for adverse drug reactions. The Informatorium Medicamentorum is regularly updated with the latest obtainable information from scientific publications, warnings of authorities and summaries of product characteristics of the European Medicines Agency and Medicines Evaluation Board in the Netherlands. The Informatorium Medicamentorum database was last searched on 1 August 2018. All drugs of which was reported that they may cause tongue disorders were extracted from this database. For each drug, the following information was recorded: generic name of the drug, term of the adverse effect, incidence of the adverse effect and Anatomical Therapeutic Chemical (ATC) code of the drug (WHO, 2003). The MedDRA classification was manually applied after the selection of drugs that have been linked to causing tongue disorders (MedDRA, 2018a, 2018b). This system categorizes medical terminology in five levels. The “Lowest Level Term (LLT)” and the “Preferred Term (PT)” were used to categorize drug‐induced tongue disorders (Rademacher et al., 2019). The most common definitions were used to describe drug‐induced tongue disorders. Microsoft® Excel (version 16.16.1) was used to create a database with acquired information on drug‐induced tongue disorders. Descriptive statistics were applied where applicable.
3. RESULTS
The Informatorium Medicamentorum database comprises information on 1645 drugs with approximately 65,000 unique combinations between a drug and an adverse effect as each drug can cause multiple adverse effects. About 2335 (3.5%) of these unique combinations enclose adverse effects of medication in the orofacial region.
In total, 121 (7.4%) drugs out of the 1645 drugs have been associated with tongue disorders as adverse drug reaction (Table 1). Drug‐induced tongue disorders are predominantly reported in the following drug categories: “nervous systems,” “anti‐infectives for systemic use” and “antineoplastic and immunomodulating agents” (Table 2). The most common drug‐induced tongue disorders are glossitis, tongue oedema, tongue discoloration and burning tongue.
TABLE 1.
Number of medications associated with particular tongue disorders
| Adverse effects of medication related to tongue | Number of medication |
|---|---|
| Burning tongue | 10 |
| Dysaesthesia of tongue | 2 |
| Glossitis | 36 |
| Hairy tongue | 4 |
| Hypertrophy of tongue papillae | 1 |
| Pruritus of tongue | 1 |
| Glossodynia | 6 |
| Tongue disorders NOS* | 5 |
| Coated tongue | 4 |
| Irritation of the tongue | 2 |
| Tongue oedema | 22 |
| Tongue ulceration | 4 |
| Tongue discoloration | 21 |
| Tongue numbness | 3 |
| Total | 121 |
NOS, not otherwise specified.
TABLE 2.
Number of drugs associated with tongue disorders per ATC level 1 category
| ATC level 1 Category | Drug‐induced tongue disorders |
|---|---|
| Alimentary tract and metabolism | 13 |
| Anti‐infectives for systemic use | 35 |
| Antineoplastic and immunomodulating agents | 11 |
| Antiparasitic products, insecticides and repellents | 0 |
| Blood and blood‐forming organs | 2 |
| Cardiovascular system | 9 |
| Dermatologicals | 6 |
| Genitourinary system and sex hormones | 1 |
| Musculoskeletal system | 2 |
| Nervous system | 26 |
| Respiratory system | 4 |
| Sensory organs | 1 |
| Systemic hormonal preparations, excl. | 1 |
| Various | 10 |
| Total: | 121 |
A wide variety of terminology is found in the literature to describe a particular tongue disorder related to the use of a drug and vice versa. Some of these terms may even overlap each other. As it was not possible to identify the exact definitions that were used to denominate a reported adverse drug reaction by coders, we have chosen to categorize the drug‐induced tongue disorders as follows:
Alteration in colour of the tongue (glossitis, tongue discoloration, hairy tongue, coated tongue)
Increase in volume of the tongue (tongue oedema, hypertrophy of tongue papillae)
Alteration in sensitivity of the tongue (burning tongue, dysaesthesia of tongue, pruritus of tongue, glossodynia, tongue numbness)
Defect of surface of the tongue ( tongue ulceration)
Other tongue disorders (tongue irritation, tongue disorders NOS)
3.1. Alteration in colour of the tongue
In total, 36 (2.2% of 1645 drugs) drugs were associated with glossitis (Figure 1) as an adverse drug reaction (Table 1). Glossitis was defined as inflammation of the tongue with loss of filiform papillae, leading to pain, swelling and erythema (Byrd et al., 2003). It was reported in 10 of the 14 ATC level 1 categories of the ATC classification. The drug categories “anti‐infectives for systemic use” (36%) and “nervous systems” (13.9%) contain most medications that have been associated with glossitis. Both categories account for almost 50% of drug‐induced glossitis. Drug‐induced glossitis is rather “common” in 11.1% (4 out of 36 drugs), “uncommon” in 41.7% (15 out of 36 drugs), “rare” in 30.5% (11 out of 36 drugs) and “very rare” in 11.1% (4 out of 36 drugs) of the drugs. The frequency of occurring of glossitis was not reported for methotrexate.
FIGURE 1.
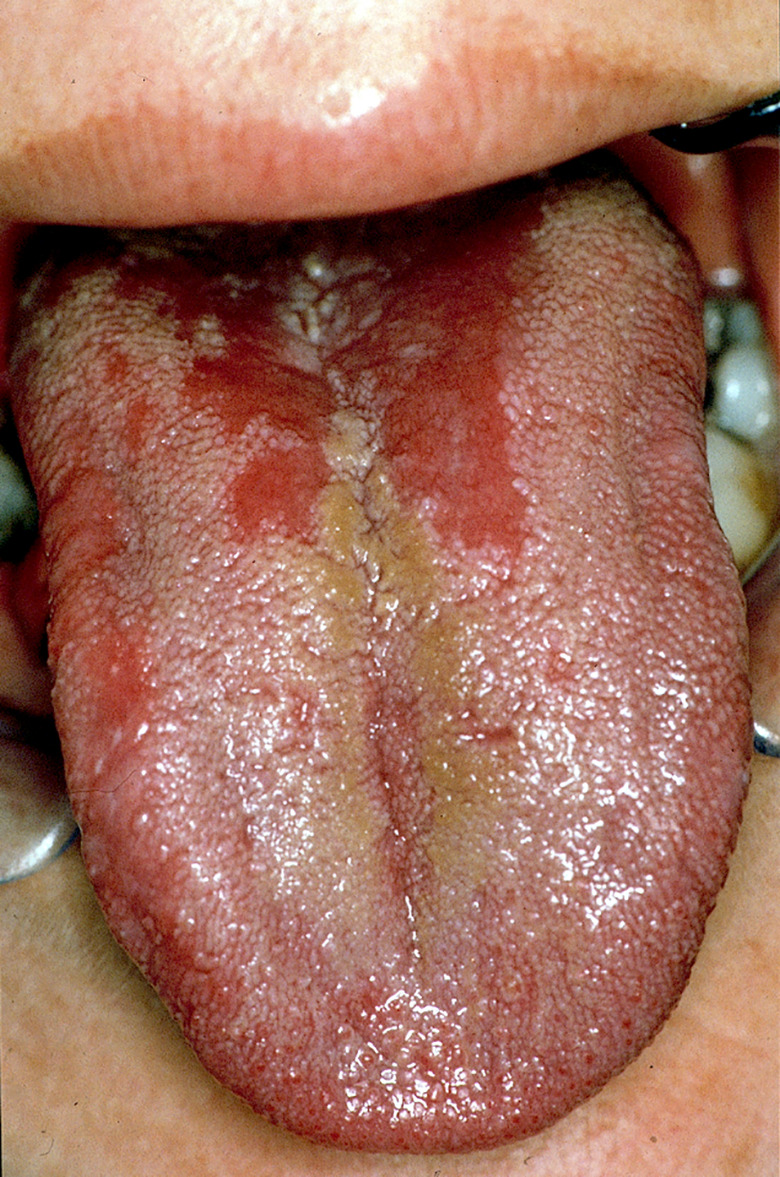
Drug‐induced median rhomboid glossitis (B. Stegenga, 2013). Reprinted with permission
In the Informatorium Medicamentorum database, 21 drugs (1.28 % of 1645 drugs) were associated with the development of tongue discoloration (Figure 2) as an adverse drug reaction. Tongue discoloration was defined as pigmentation of the tongue as a result of the drug or its metabolites deposition or by increasing the production of melanin. The discoloration may be blue, brown, grey or black (Rosebush et al., 2019). Tongue discoloration was reported in 7 of the 14 ATC level 1 categories. Tongue discoloration was predominantly reported in the drug categories “anti‐infectives for systemic use” (52.4%) and “dermatologicals” (19%). Frequency of drug‐induced tongue discoloration was “uncommon” in 19% (4 out of 21 drugs), “rare” in 14.3% (3 out of 21 drugs), “very rare” in 47.6% (10 out of 21 drugs) and “unknown” in 19% (4 out of 21 drugs) of the drugs.
FIGURE 2.
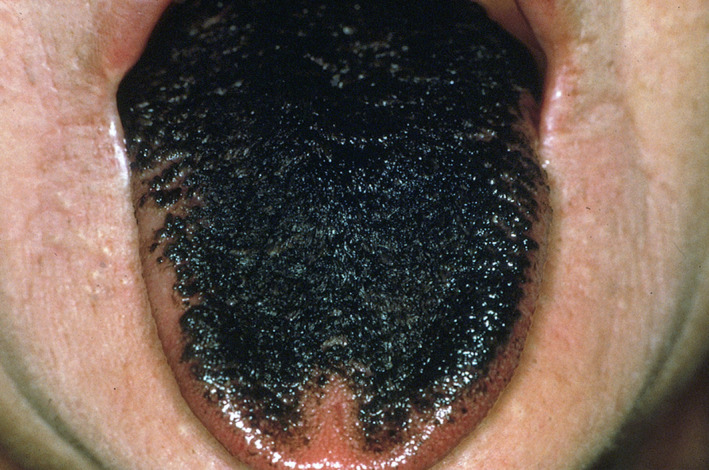
Chlorhexidine‐induced tongue discoloration (B. Stegenga, 2013). Reprinted with permission
Hairy tongue is a transitory and harmless condition characterized by hypertrophy and prolongation of filiform papillae on the surface of the tongue (Figure 3). The colour of the tongue can vary from yellow to brown or black (Reamy et al., 2010). Hairy tongue as an adverse effect was reported for 4 drugs (0.24% of 1645 drugs). Two of these drugs belong to the drug category “anti‐infectives for systemic use.” Coated tongue describes any area of the tongue with a coating on it. Coated tongue as an adverse effect was reported for 4 drugs (0.24% of 1645 drugs). These 4 drugs belong to the drug categories “nervous system,” “anti‐infectives for systemic use,” “dermatologicals” and “alimentary tract and metabolism.” In 3 out 4 drugs is coated tongue a “rare” adverse drug reaction. An overview of all drugs that may alter the colour of the tongue is given in Table 3.
FIGURE 3.
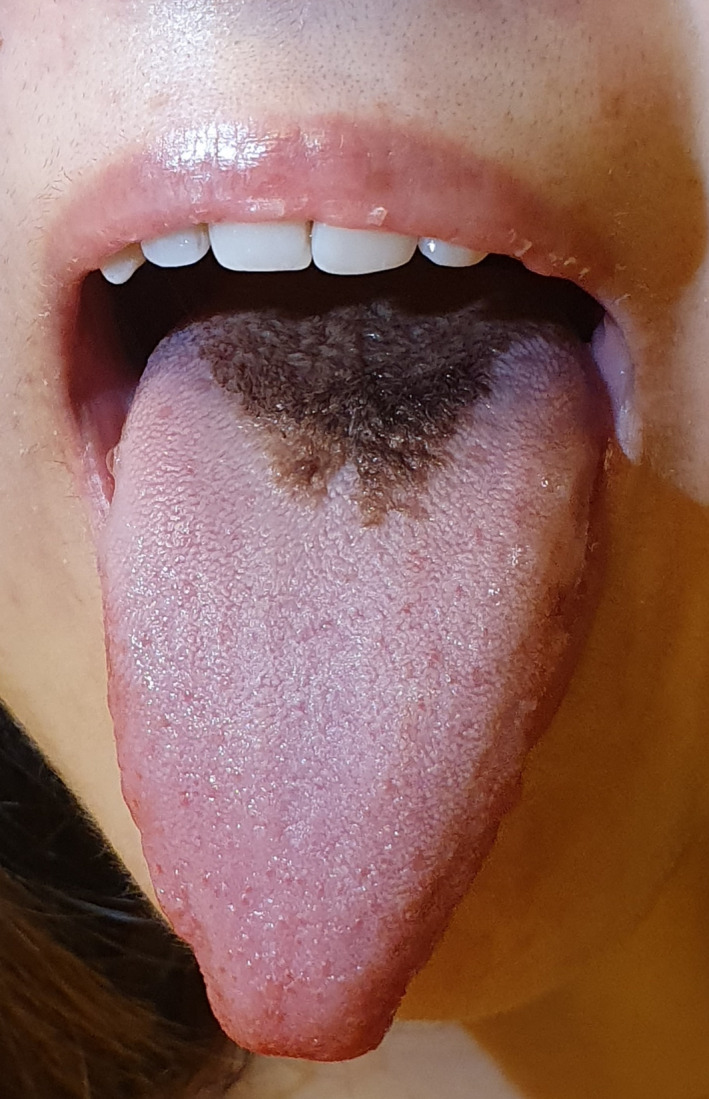
Antibiotic‐induced hairy tongue
TABLE 3.
Alteration in colour of the tongue (glossitis, tongue discoloration, hairy tongue, coated tongue)
| ATC level 1 | ATC level 3 | Generic name | ATC Code | LLT MedDRA* | Frequency | Specific type of administration |
|---|---|---|---|---|---|---|
| ALIMENTARY TRACT AND METABOLISM | STOMATOLOGICAL PREPARATIONS | Tetracycline | A01AB13 | Glossitis | Very rare (<0.01%) | Not given |
| INTESTINAL ANTI‐INFECTIVES | Amphotericin B | A07AA07 | Glossitis | Uncommon (0.1‐1%) | After oral administration | |
| DRUGS FOR PEPTIC ULCER AND | Lansoprazole | A02BC03 | Glossitis | Rare (≥ 0.01% and < 0.1%) | Not given | |
| OTHER ALIMENTARY TRACT AND METABOLISM PRODUCTS | Betaine | A16AA06 | Glossitis | Uncommon (0.1‐1%) | Not given | |
| STOMATOLOGICAL PREPARATIONS | Tetracycline | A01AB13 | Tongue discoloration | Very rare (<0.01%) | After oral or oromucosal administration | |
| INTESTINAL ANTI‐INFECTIVES | Miconazole | A07AC01 | Tongue discoloration | Very rare (<0.01%) | Not given | |
| STOMATOLOGICAL PREPARATIONS | Hydrogen peroxide | A01AB02 | Hairy tongue | Frequency not known | Not given | |
| ANTIEMETICS AND ANTINAUSEANTS | Palonosetron | A04AA05 | Tongue coated | Rare (≥ 0.01% and < 0.1%) | Not given | |
| ANTI‐INFECTIVES FOR SYSTEMIC USE | BETA‐LACTAM ANTIBACTERIALS, PENICILLINS | Benzylpenicillin | J01CE01 | Glossitis | Uncommon (0.1‐1%) | Not given |
| TETRACYCLINES | Minocycline | J01AA08 | Glossitis | Rare (≥ 0.01% and < 0.1%) | Not given | |
| AMINOGLYCOSIDE ANTIBACTERIALS | Tobramycin | J01GB01 | Glossitis | Uncommon (0.1‐1%) | Inhalation liquid | |
| OTHER BETA‐LACTAM ANTIBACTERIALS | Ceftriaxone | J01DD04 | Glossitis | Very rare (<0.01%) | Not given | |
| MACROLIDES, LINCOSAMIDES AND STREPTOGRAMINS | Clarithromycin | J01FA09 | Glossitis | Uncommon (0.1‐1%) | Not given | |
| OTHER ANTIBACTERIALS | Linezolid | J01XX08 | Glossitis | Uncommon (0.1‐1%) | Not given | |
| ANTIMYCOTICS FOR SYSTEMIC USE | Voriconazole | J02AC03 | Glossitis | Uncommon (0.1‐1%) | Not given | |
| OTHER ANTIBACTERIALS | Daptomycin | J01XX09 | Glossitis | Uncommon (0.1‐1%) | Not given | |
| DIRECT ACTING ANTIVIRALS | Raltegravir | J05AX08 | Glossitis | Uncommon (0.1‐1%) | Not given | |
| TETRACYCLINES | Doxycycline | J01AA02 | Glossitis | Uncommon (0.1‐1%) | Not given | |
| ANTIVIRALS | Trifluridine | S01AD02 | Glossitis | Uncommon (0.1‐1%) | In combination with tipiracil | |
| CARBAPENEMS | Imipenem and cilastatin | J01DH51 | Glossitis | Rare (≥ 0.01% and < 0.1%) | Not given | |
| BETA‐LACTAM ANTIBACTERIALS, PENICILLINS | Pheneticillin | J01CE05 | Tongue discoloration | Very rare (<0.01%) | Not given | |
| TETRACYCLINES | Demeclocycline | J01AA01 | Tongue discoloration | Frequency not known | Not given | |
| TETRACYCLINES | Minocycline | J01AA08 | Tongue discoloration | Rare (≥ 0.01% and < 0.1%) | Not given | |
| BETA‐LACTAM ANTIBACTERIALS, PENICILLINS | Amoxicillin | J01CA04 | Tongue discoloration | Uncommon (0.1‐1%) | Not given | |
| DIRECT ACTING ANTIVIRALS | Ribavirin | J05AP01 | Tongue discoloration | Very rare (<0.01%) | Not given | |
| MACROLIDES, LINCOSAMIDES AND STREPTOGRAMINS | Clarithromycin | J01FA09 | Tongue discoloration | Very rare (<0.01%) | After intravenous administration | |
| MACROLIDES, LINCOSAMIDES AND STREPTOGRAMINS | Azithromycin | J01FA10 | Tongue discoloration | Very rare (<0.01%) | Not given | |
| OTHER ANTIBACTERIALS | Linezolid | J01XX08 | Tongue discoloration | Uncommon (0.1‐1%) | Not given | |
| TETRACYCLINES | Doxycycline | J01AA02 | Tongue discoloration | Very rare (<0.01%) | Not given | |
| CARBAPENEMS | Imipenem and cilastatin | J01DH51 | Tongue discoloration | Rare (≥ 0.01% and < 0.1%) | Not given | |
| SULPHONAMIDES AND TRIMETHOPRIM | Sulphamethoxazole and trimethoprim | J01EE01 | Tongue discoloration | Frequency not known | Not given | |
| DIRECT ACTING ANTIVIRALS | Darunavir | J05AE10 | Tongue coated | Rare (≥ 0.01% and < 0.1%) | Not given | |
| COMBINATION OF ANTIBACTERIALS | Combination of antibacterials | J01RA | Hairy tongue | Very rare (<0.01%) | In combination with amoxicillin | |
| SULPHONAMIDES AND TRIMETHOPRIM | Sulphamethoxazole and trimethoprim | J01EE01 | Hairy tongue | Frequency not known | Not given | |
| ANTINEOPLASTIC IMMUNOMODULATING AND AGENTS | IMMUNOSTIMULANTS | Peginterferon alfa‐2a | L03AB11 | Glossitis | Common (1‐10%) | Not given |
| IMMUNOSTIMULANTS | Peginterferon alfa‐2b | L03AB10 | Glossitis | Common (1‐10%) | Not given | |
| OTHER ANTINEOPLASTIC AGENTS | Tivozanib | L01XE34 | Glossitis | Common (1‐10%) | Not given | |
| ANTIMETABOLITES | Methotrexate | L01BA01 | Glossitis | Frequency not known | Not given | |
| IMMUNOSTIMULANTS | Peginterferon alfa‐2b | L03AB10 | Tongue discoloration | Rare (≥ 0.01% and < 0.1%) | Not given | |
| CARDIOVASCULAR SYSTEM | ACE INHIBITORS, PLAIN | Captopril | C09AA01 | Glossitis | Rare (≥ 0.01% and < 0.1%) | Not given |
| ACE INHIBITORS, PLAIN | Enalapril | C09AA02 | Glossitis | Rare (≥ 0.01% and < 0.1%) | Not given | |
| ACE INHIBITORS, PLAIN | Ramipril | C09AA05 | Glossitis | Rare (≥ 0.01% and < 0.1%) | Not given | |
| ACE INHIBITORS, PLAIN | Quinapril | C09AA06 | Glossitis | Rare (≥ 0.01% and < 0.1%) | Not given | |
| ANTIADRENERGIC AGENTS, CENTRALLY ACTING | Methyldopa (levorotatory) | C02AB01 | Tongue discoloration | Very rare (<0.01%) | Not given | |
| DERMATOLOGICALS | ANTIFUNGALS FOR TOPICAL USE | Ketoconazole | D01AC08 | Tongue discoloration | Frequency not known | Not given |
| CHEMOTHERAPEUTICS FOR TOPICAL USE | Metronidazole | D06BX01 | Tongue discoloration | Frequency not known | After cutaneous use | |
| CHEMOTHERAPEUTICS FOR TOPICAL USE | Metronidazole | D06BX01 | Tongue discoloration | Very rare (<0.01%) | Not given | |
| PROTECTIVES AGAINST UV‐RADIATION | Afamelanotide | D02BB02 | Tongue discoloration | Uncommon (0.1‐1%) | Not given | |
| CHEMOTHERAPEUTICS FOR TOPICAL USE | Metronidazole | D06BX01 | Tongue coated | Frequency not known | Not given | |
| CHEMOTHERAPEUTICS FOR TOPICAL USE | Metronidazole | D06BX01 | Hairy tongue | Frequency not known | Not given | |
| GENITOURINARY SYSTEM AND SEX HORMONES | OTHER GYNAECOLOGICALS | Fenoterol | G02CA03 | Glossitis | Rare (≥ 0.01% and < 0.1%) | In combination with ipratropium |
| MUSCULOSKELETAL SYSTEM | ANTI‐INFLAMMATORY AND ANTIRHEUMATIC PRODUCTS, NON‐STEROIDS | Diclofenac | M01AB05 | Glossitis | Rare (≥ 0.01% and < 0.1%) | After systemic use |
| DRUGS AFFECTING BONE STRUCTURE AND MINERALIZATION | Risedronic acid | M05BA07 | Glossitis | Rare (≥ 0.01% and < 0.1%) | Not given | |
| NERVOUS SYSTEM | ANTIEPILEPTICS | Carbamazepine | N03AF01 | Glossitis | Rare (≥ 0.01% and < 0.1%) | Not given |
| ANTIDEPRESSANTS | Sertraline | N06AB06 | Glossitis | Uncommon (0.1‐1%) | Not given | |
| ANTIMIGRAINE PREPARATIONS | Eletriptan | N02CC06 | Glossitis | Uncommon (0.1‐1%) | Not given | |
| PSYCHOSTIMULANTS, AGENTS USED FOR ADHD AND NOOTROPICS | Modafinil | N06BA07 | Glossitis | Uncommon (0.1‐1%) | Not given | |
| ANAESTHETICS, LOCAL | Mepivacaine | N01BB03 | Glossitis | Very rare (<0.01%) | Not given | |
| ANTIDEPRESSANTS | Amitriptyline | N06AA09 | Tongue discoloration | Very rare (<0.01%) | Not given | |
| DRUGS USED IN ADDICTIVE DISORDERS | Varenicline | N07BA03 | Tongue coated | Rare (≥ 0.01% and < 0.1%) | Not given | |
| RESPIRATORY SYSTEM | OTHER DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES, INHALANTS | Tiotropium bromide | R03BB04 | Glossitis | Rare (≥ 0.01% and < 0.1%) | Not given |
| SENSORY ORGANS | OPHTHALMOLOGICALS | Betaxolol | S01ED02 | Glossitis | Very rare (<0.01%) | Not given |
| VARIOUS | ALLERGENS | Allergen extracts | V01AA | Glossitis | Common (1‐10%) | After sublingual administration |
| V01AA02 | ||||||
| ALL OTHER THERAPEUTIC PRODUCTS | Sucroferric oxyhydroxide | V03AE05 | Tongue discoloration | Uncommon (0.1‐1%) | After oral or oromucosal administration |
Definitions :
Glossitis was defined as inflammation of the tongue with loss of filiform papillae, leading to pain, swelling and erythema.
Tongue discoloration was defined as pigmentation of the tongue as a result of the drug or its metabolite deposition or by increasing the production of melanin.
Hairy tongue is a transitory and harmless condition characterized by hypertrophy and prolongation of filiform papillae on the surface of the tongue.
Coated tongue describes any area of the tongue with a coating on it.
3.2. Increase in volume of the tongue
Tongue oedema was reported in 22 drugs (1.3% of 1645 drugs). Tongue oedema was defined as swelling of the tongue due to loss of vascular integrity causing extravasation of fluid into interstitial tissue. This adverse effect was mentioned in 9 out of 14 ATC level 1 categories. Occurrence of tongue oedema (Figure 4) was mainly reported in the drug category “nervous systems” (45.5%). Frequency of drug‐induced tongue oedema was “common” in 13.6% (3 out of 22 drugs), “uncommon” in 31.8% (7 out of 22 drugs), “rare” in 31.8% (7 out of 22 drugs) and “very rare” in 22.7% (5 out of 22 drugs) of the drugs.
FIGURE 4.
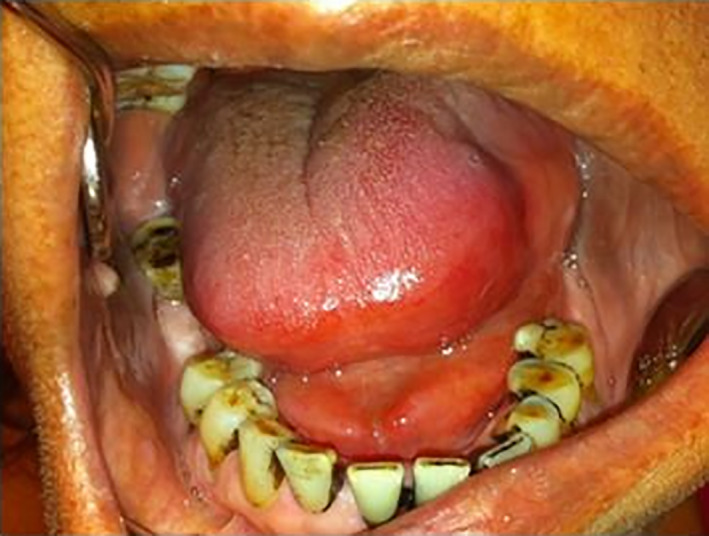
ACE inhibitor‐induced tongue oedema
A rare adverse effect of Imipenem is hypertrophy of tongue papillae. Imipenem, belonging to the drug category “anti‐infectives for systemic use,” is the only drug that causes this adverse drug reaction. An overview of all drugs that may cause tongue oedema and hypertrophy of tongue papillae is shown in Table 4.
TABLE 4.
Increase in volume of the tongue (tongue oedema, hypertrophy of tongue papillae)
| ATC level 1 | ATC level 3 | Generic name | ATC Code | LLT MedDRA* | Frequency | Specific type of administration |
|---|---|---|---|---|---|---|
| ALIMENTARY TRACT AND METABOLISM | OTHER ALIMENTARY TRACT AND METABOLISM PRODUCTS | Idursulfase | A16AB09 | Tongue oedema | Common (1‐10%) | Not given |
| ANTI‐INFECTIVES FOR SYSTEMIC USE | OTHER BETA‐LACTAM ANTIBACTERIALS | Cefazolin | J01DB04 | Tongue oedema | Rare (≥ 0.01% and < 0.1%) | Not given |
| ANTIMYCOTICS FOR SYSTEMIC USE | Voriconazole | J02AC03 | Tongue oedema | Uncommon (0.1‐1%) | Not given | |
| ANTIMYCOTICS FOR SYSTEMIC USE | Posaconazole | J02AC04 | Tongue oedema | Rare (≥ 0.01% and < 0.1%) | Not given | |
| CARBAPENEMS | Imipenem and cilastatin | J01DH51 | Hypertrophy of tongue papillae | Rare (≥ 0.01% and < 0.1%) | Not given | |
| ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS | HORMONE ANTAGONISTS AND RELATED AGENTS | Enzalutamide | L02BB04 | Tongue oedema | Very rare (<0.01%) | Not given |
| BLOOD AND BLOOD‐FORMING ORGANS | VITAMIN K AND OTHER HAEMOSTATICS | Eltrombopag | B02BX05 | Tongue oedema | Common (1‐10%) | In case of patients with immune thrombocytopenic purpura or aplastic anaemia |
| CARDIOVASCULAR SYSTEM | ACE INHIBITORS, PLAIN | Fosinopril | C09AA09 | Tongue oedema | Very rare (<0.01%) | Not given |
| NERVOUS SYSTEM | ANTIDEPRESSANTS | Amitriptyline | N06AA09 | Tongue oedema | Uncommon (0.1‐1%) | Not given |
| ANTIDEPRESSANTS | Doxepin | N06AA12 | Tongue oedema | Rare (≥ 0.01% and < 0.1%) | Not given | |
| ANTIDEPRESSANTS | Nortriptyline | N06AA10 | Tongue oedema | Uncommon (0.1‐1%) | Not given | |
| HYPNOTICS AND SEDATIVES | Melatonin | N05CH01 | Tongue oedema | Very rare (<0.01%) | Not given | |
| ANTIMIGRAINE PREPARATIONS | Rizatriptan | N02CC04 | Tongue oedema | Uncommon (0.1‐1%) | Not given | |
| ANTIEPILEPTICS | Pregabalin | N03AX16 | Tongue oedema | Rare (≥ 0.01% and < 0.1%) | Not given | |
| DOPAMINERGIC AGENTS | Rotigotine | N04BC09 | Tongue oedema | Uncommon (0.1‐1%) | In case of Parkinson’s disease | |
| DOPAMINERGIC AGENTS | Rotigotine | N04BC09 | Tongue oedema | Common (1‐10%) | For restless legs | |
| ANTIPSYCHOTICS | Paliperidone | N05AX13 | Tongue oedema | Uncommon (0.1‐1%) | Not given | |
| ANAESTHETICS, LOCAL | Mepivacaine | N01BB03 | Tongue oedema | Rare (≥ 0.01% and < 0.1%) | Not given | |
| RESPIRATORY SYSTEM | ADRENERGICS, INHALANTS | Indacaterol | R03AC18 | Tongue oedema | Uncommon (0.1‐1%) | Not given |
| SYSTEMIC HORMONAL PREPARATIONS, EXCL. | ANTIPARATHYROID AGENTS | Calcitonin (salmon synthetic) | H05BA01 | Tongue oedema | Rare (≥ 0.01% and < 0.1%) | Not given |
| VARIOUS | MAGNETIC RESONANCE IMAGING CONTRAST MEDIA | Gadoteridol | V08CA04 | Tongue oedema | Rare (≥ 0.01% and < 0.1%) | Not given |
| ALL OTHER THERAPEUTIC PRODUCTS | Palifermin | V03AF08 | Tongue oedema | Very rare (<0.01%) | Not given | |
| ALLERGENS | Allergen extracts | V01AAV01AA02 | Tongue oedema | Very rare (<0.01%) | After subcutaneous administration |
Definition:swelling of the tongue due to loss of vascular integrity causing extravasation of fluid into interstitial tissue.
3.3. Alteration in sensitivity of the tongue
Burning tongue was reported in 10 drugs (0.61% of 1645 drugs) which belong to 5 ATC level 1 categories. Burning tongue was defined as a burning sensation of tongue caused by drugs without specifying the affected region explicitly (Imamura et al., 2019). The appearance of the tongue can be changed, but there is no need for an identifiable change in the appearance of the tongue. The drug category “alimentary tract and metabolism” (30%) consists of most drugs that may cause burning tongue. The frequency of burning tongue was “common” in 30% (3 out of 10 drugs), “uncommon” in 20% (2 out of 10 drugs), “rare” in 10% (1 out of 10 drugs) and “very rare” in 30% (3 out of 10 drugs) of the drugs. The frequency of burning tongue was most frequently (“very common,” 10%) reported for cabozantinib. Dysaesthesia of the tongue is an abnormal unpleasant sensation of the tongue. This adverse effect was reported for metoclopramide and oxaliplatin. These drugs belong to the following drug categories, respectively, “alimentary tract and metabolism” and “antineoplastic and immunomodulating agents.” Numbness of the tongue was defined as loss of sensation in the tongue not due to peripheral nerve injury. Numbness of the tongue was reported in 3 drugs from the drug category “nervous system.” The frequency of this adverse drug reaction is uncommon. Pruritus of tongue was defined as an itchy sensation of the tongue as a result of exposure to medications. It was only reported for allergen extracts and was a common side effect of sublingually administrated allergen extracts. Glossodynia was described as burning sensation of the tongue due to an identifiable cause, viz., drugs. Glossodynia was reported in 6 drugs (0.36% of 1645 drugs) in the following drug categories; “anti‐infectives for systemic use” (33.3%), “antineoplastic and immunomodulating agents” (33.3%), “cardiovascular system” (16.7%) and “various” (16.7%). The frequency of glossodynia was “common” in the drug categories “anti‐infectives for systemic use” and “various” (3 out of 6 drugs). In the drug categories “antineoplastic and immunomodulating agents” and "cardiovascular system" was the frequency "very rare" (3 out of 6 drugs). Table 5 gives an overview of all drugs that may cause alteration in sensitivity of the tongue.
TABLE 5.
Alteration in sensitivity of the tongue (burning tongue, dysaesthesia of tongue, pruritus of tongue, glossodynia, tongue numbness)
| ATC level 1 | ATC level 3 | Generic name | ATC Code | LLT MedDRA* | Frequency | Specific type of administration |
|---|---|---|---|---|---|---|
| ALIMENTARY TRACT AND METABOLISM | INTESTINAL ANTI‐INFECTIVES | Colistin | A07AA10 | Burning tongue | Very rare (<0.01%) | After inhalation |
| ANTIPROPULSIVES | Loperamide | A07DA03 | Burning tongue | Rare (≥ 0.01% and < 0.1%) | Not given | |
| STOMATOLOGICAL PREPARATIONS | Chlorhexidine | A01AB03 | Burning tongue | Very rare (<0.01%) | Not given | |
| PROPULSIVES | Metoclopramide | A03FA01 | Dysaesthesia of tongue | Frequency not known | Not given | |
| ANTI‐INFECTIVES FOR SYSTEMIC USE | BETA‐LACTAM ANTIBACTERIALS, PENICILLINS | Pheneticillin | J01CE05 | Glossodynia | Very rare (<0.01%) | Not given |
| SULPHONAMIDES AND TRIMETHOPRIM | Trimethoprim | J01EA01 | Glossodynia | Very rare (<0.01%) | Not given | |
| ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS | OTHER ANTINEOPLASTIC AGENTS | Cabozantinib | L01XE26 | Burning tongue | Very common (≥10%) | Not given |
| OTHER ANTINEOPLASTIC AGENTS | Oxaliplatin | L01XA03 | Dysaesthesia of tongue | Frequency not known | Not given | |
| OTHER ANTINEOPLASTIC AGENTS | Sorafenib | L01XE05 | Glossodynia | Common (1‐10%) | Not given | |
| OTHER ANTINEOPLASTIC AGENTS | Sunitinib | L01XE04 | Glossodynia | Common (1‐10%) | Not given | |
| CARDIOVASCULAR SYSTEM | ANTIADRENERGIC AGENTS, CENTRALLY ACTING | Methyldopa (levorotatory) | C02AB01 | Glossodynia | Very rare (<0.01%) | Not given |
| NERVOUS SYSTEM | ANTIMIGRAINE PREPARATIONS | Sumatriptan | N02CC01 | Burning tongue | Common (1‐10%) | Not given |
| ANTIEPILEPTICS | Topiramate | N03AX11 | Burning tongue | Uncommon (0.1‐1%) | Not given | |
| ANAESTHETICS, LOCAL | Ropivacaine | N01BB09 | Numbness of tongue | Uncommon (0.1‐1%) | Not given | |
| ANAESTHETICS, LOCAL | Bupivacaine | N01BB01 | Numbness of tongue | Uncommon (0.1‐1%) | Not given | |
| ANAESTHETICS, LOCAL | Prilocaine | N01BB04 | Numbness of tongue | Uncommon (0.1‐1%) | Not given | |
| RESPIRATORY SYSTEM | ADRENERGICS, INHALANTS | Salbutamol | R03AC02 | Burning tongue | Common (1‐10%) | After inhalation |
| THROAT PREPARATIONS | Flurbiprofen | M01AE09 | Burning tongue | Uncommon (0.1‐1%) | Not given | |
| VARIOUS | ALLERGENS | Allergen extracts | V01AAV01AA02 | Burning tongue | Very rare (<0.01%) | After subcutaneous administration |
| ALLERGENS | Allergen extracts | V01AAV01AA02 | Burning tongue | Common (1‐10%) | After sublingual administration | |
| ALLERGENS | Allergen extracts | V01AA | Tongue pruritus | Common (1‐10%) | After sublingual administration | |
| ALLERGENS | Allergen extracts | V01AA | Glossodynia | Common (1‐10%) | After sublingual administration |
Definitions:
Burning sensation of tongue caused by drugs.
Dysaesthesia of the tongue is an abnormal unpleasant sensation of the tongue.
Pruritus of tongue is as an itchy sensation of the tongue.
Glossodynia is a burning sensation of the tongue.
Numbness of the tongue is a loss of sensation in the tongue.
3.4. Defect of surface of the tongue
Four drugs are reported to cause ulceration of the tongue (0.30% of 1645 drugs). These drugs belong to the following drug categories: “antineoplastic and immunomodulating agents” (1 drugs), "cardiovascular system" (1 drug) and "nervous system” (2 drugs). The frequency of tongue ulceration was "rare" in 3 out of 4 drugs (Table 6).
TABLE 6.
Defect of surface of the tongue ( tongue ulceration)
| ATC level 1 | ATC level 3 | Generic name | ATC Code | LLT MedDRA* | Frequency | Specific type of administration |
|---|---|---|---|---|---|---|
| ANTINEOPLASTIC IMMUNOMODULATING AND AGENTS | IMMUNOSUPPRESSANTS | alemtuzumab | L04AA34 | Tongue ulceration | Frequency not known | In case of patients with B‐cell chronic lymphocytic leukaemia |
| CARDIOVASCULAR SYSTEM | VASODILATORS USED IN CARDIAC DISEASES | Nicorandil | C01DX16 | Tongue ulceration | Rare (≥ 0.01% and < 0.1%) | Not given |
| NERVOUS SYSTEM | HYPNOTICS AND SEDATIVES | Melatonin | N05CH01 | Tongue ulceration | Rare (≥ 0.01% and < 0.1%) | Not given |
| ANTIDEPRESSANTS | Sertraline | N06AB06 | Tongue ulceration | Rare (≥ 0.01% and < 0.1%) | Not given |
3.5. Other tongue disorders
Unspecified tongue disorders were reported in 5 drugs (0.30% of 1645 drugs) in the following drug categories: “nervous system” (2 drugs), “antineoplastic and immunomodulating agents” (1 drug), “anti‐infectives for systemic use” (1 drug) and “various” (1 drug). The frequency of tongue disorders NOS was “common” in 20% (1 out of 5 drugs), “uncommon” in 40% (2 out of 5 drugs) and “unknown” in 40% (2 out of 5 drugs) of these drugs. Iloprost and cholestyramine were reported to cause irritation of the tongue. They pertain to the drug category, respectively, “blood and blood‐forming organs” and “cardiovascular system.” An overview of all drugs that may cause irritation of the tongue and tongue disorders NOS can be found in Table 7.
TABLE 7.
Other tongue disorders (tongue irritation, tongue disorders NOS)
| ATC level 1 | ATC level 3 | Generic name | ATC Code | LLT MedDRA* | Frequency | Specific type of administration |
|---|---|---|---|---|---|---|
| ANTI‐INFECTIVES FOR SYSTEMIC USE | SULPHONAMIDES AND TRIMETHOPRIM | Sulphamethoxazole and trimethoprim | J01EE01 | Tongue disorder NOS | Frequency not known | Not given |
| ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS | HORMONES AND RELATED AGENTS | Leuprorelin | L02AE02 | Tongue disorder NOS | Common (1‐10%) | Not given |
| BLOOD AND BLOOD‐FORMING ORGANS | ANTITHROMBOTIC AGENTS | Iloprost | B01AC11 | Tongue irritation | Common (1‐10%) | After inhalation |
| CARDIOVASCULAR SYSTEM | LIPID MODIFYING AGENTS, PLAIN | Cholestyramine | C10AC01 | Tongue irritation | Very rare (<0.01%) | Not given |
| NERVOUS SYSTEM | ANTIDEPRESSANTS | Imipramine | N06AA02 | Tongue disorder NOS | Frequency not known | Not given |
| ANTIDEPRESSANTS | Sertraline | N06AB06 | Tongue disorder NOS | Uncommon (0.1‐1%) | Not given | |
| VARIOUS | ALLERGENS | Allergen extracts | V01AA | Tongue disorder NOS | Uncommon (0.1‐1%) | After sublingual administration |
Tongue disorder NOS: tongue disorder not otherwise specified.
4. DISCUSSION
Drug‐induced tongue disorders were reported in 7.4% (121/1645) of the drugs used in the Netherlands. It was reported in all ATC level 1 drug categories except the drug category “antiparasitic products, insecticides and repellents.” We assume that many oral healthcare providers are confronted with patients that suffer from drug‐induced tongue disorders. Patients using drug from the categories “anti‐infectives for systemic use” and “nervous system” are more likely to endure drug‐induced tongue disorders.
As far as we know, this is the first article that gives a compendious overview of drug‐induced tongue disorders. Most of the articles on this topic are case reports on one particular drug and adverse drug reaction. Till date, there is no study performed that gives a complete overview of drugs that cause tongue disorders. An important note is that the adverse effects reported in our study are not just derived from randomized controlled trials, which bears the hazard of underreporting, but from a mixture of clinical studies and case reports. Furthermore, the data on adverse effects are also extracted from scientific drug information, guidelines and summaries of product characteristics as well as that our study contains entries from LAREB. As the information on adverse drug effects originates from different sources, the hazard of underreporting and inaccurate reporting is minimized in this study.
The drug‐induced tongue disorders reported in the literature are often not well‐defined or a wide range of terminology is used to describe a particular disorder and vice versa. For example, the term glossitis indicates a variety of tongue diseases. Depending upon the underlying cause and symptoms, it can refer to atrophic glossitis or median rhomboid glossitis or benign migratory glossitis or herpetic geometric glossitis, etc. Moreover, tongue conditions like candidiasis or tongue soreness caused by burning mouth syndrome can easily be labelled as glossitis due to their broadly similar clinical presentation and symptoms. As it is not possible to identify the exact definitions of the reported adverse drug reactions, we opted to describe tongue disorders using the most common definitions. Furthermore, to assure data uniformity we standardized the data by using the ATC and MedDRA classification. The use of ATC and MedDRA classification makes our data internationally applicable. As mentioned in the first article of this series, it is recommended to use MedDRA classification for homogenous data collection. We assume that it will improve recording of adverse drug reactions in the future. As discussed in the first article, there will be drugs that are not mentioned in this paper due to difference in local law and regulations on drug per country. But most of the drugs mentioned in this study are available in European countries.
In the recent years, several studies have reported cases of drug‐induced tongue disorders. Drugs like angiotensin‐converting enzyme (ACE) inhibitors(Leung et al., 2012; Stallone et al., 2004), non‐steroidal anti‐inflammatory drugs (NSAIDs) aspirin and certain antibiotics are reported to cause angioedema of the lips, tongue and face. About 25%‐40% of angioedema in orofacial region are induced by ACE inhibitors. Perindopril is one of the ACE inhibitors that is often associated with angioedema of the lips and tongue. The underlying mechanism for ACE inhibitor‐induced angioedema is the enzymatic inhibition of bradykinin degradation (Alharbi et al., 2018). Early recognition of drug‐induced tongue oedema is important as it can be a life‐threatening condition. In this study, tongue oedema was reported in 22 drugs, mainly in the drug category “nervous systems” (45.5%). Fosinopril was the only ACE inhibitor that was reported to cause tongue oedema. Contrary to expectations, the frequency of fosinopril‐induced tongue oedema was very rare (˂0.01%). This discrepancy could be explained by the fact that other studies report on all cases of ACE inhibitor‐induced angioedema in the orofacial region. They do not subdivide the orofacial angioedema into different categories. In this study, however, the focus lied solely on the tongue oedema.
Drugs such as tetracycline, penicillins, anticholinergics and linezolid are reported to cause black hairy tongue (Balaji et al., 2014; Braggio et al., 2018; Gurvits & Tan, 2014; Reamy et al., 2010). Beside the colour black, hairy tongue can also be yellow, green, blue, brown or even colourless. Generally, no treatment is necessary for this condition as it is predominantly asymptomatic. The pathophysiology of drug‐induced black hairy tongue is still unknown. In this study, hairy tongue as an adverse effect was reported for 4 drugs: metronidazole, hydrogen peroxide, antibiotics in combination with amoxicillin and sulphamethoxazole and trimethoprim. On the other hand, 21 drugs were associated with the development of tongue discoloration as an adverse drug reaction. As expected, most of the drugs were antibiotics. The difference is likely due to categorizing the tongue disorders by using the MedDRA classification and ATC codes. In order to collect homogenous data on adverse drug reactions, MedDRA classification is recommended to be used.
The occurrence of severe glossitis after administration of sulphanilamide and sulphathiazole has been reported in the literature. The underlying mechanism for glossitis in those cases was avitaminoses without apparent cause (Brown, 1949). In the present study, glossitis was one of the most frequent adverse effects of drugs. The drug categories “anti‐infectives for systemic use” and “nervous systems” contained most of the medications that can induce glossitis. Nonetheless, both medications are not mentioned in the drug category “anti‐infectives for systemic use.” The reason could be difference in local law and regulations on drug per country. Both antibiotics are not registered in the “farmacotherapeutisch kompas.” Farmacotherapeutisch kompas is an online database in Dutch (FK, 2019) which consist all the medications registered with the Medicines Evaluation Board of the Netherlands. In addition, it also consists drugs that are registered in European Medicines Agency.
Antirheumatic drugs such as leflunomide are reported to cause ulcers in the tongue (Kalogirou et al., 2017). Tongue ulcers are also associated with nicorandil use. The pathophysiology of nicorandil‐induced tongue ulcers is still unclear (Healy et al., 2004). These ulcers usually heal after the discontinuation of the drugs. In the present study, four drugs were reported to cause ulceration of the tongue: alemtuzumab, nicorandil, melatonin and sertraline. Contrary to the literature, tongue ulceration was not reported for leflunomide. Our study might underreport some adverse drug reactions compared to another studies which are not based on MedDRA classification. The LLT term used to categorize the drug‐induced tongue disorders is very specific. According to the farmacotherapeutisch kompas, an adverse effect of leflunomide is ulcers in the mouth which is unspecific compared to tongue ulceration.
5. CONCLUSION
The growing use of drugs is accompanied by a more frequent observation of tongue disorders that may have been induced by the use of drugs. As mentioned before, a wide variety of, partly overlapping, terminology is found in the literature to describe a particular tongue disorder related to the use of a drug and vice versa. The terminology used in this paper might help to bring the terminology used in pharmacology and oral medicine more in line. The overview of drugs reported in this paper helps oral healthcare workers in the recognition, diagnosis and eventual treatment of drug‐induced tongue disorders.
AUTHOR CONTRIBUTIONS
Yalda Aziz: Data curation; Validation; Visualization; Writing‐original draft; Writing‐review & editing. Willem Rademacher: Data curation; Investigation; Methodology; Validation. Atty Hielema: Methodology; Resources. Scott Bradley Patton Wishaw: Methodology; Resources. Denise van Diermen: Supervision. Jan de Lange: Supervision. Arjan Vissink Supervision, Writing‐review & editing. Frederik Rozema Conceptualization, Methodology, Supervision, Writing‐review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/odi.13680.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Royal Dutch Pharmacist Association for providing access to Informatorium Medicamentorum.
Aziz Y, Rademacher WMH, Hielema A, et al. Oral adverse effects: drug‐induced tongue disorders. Oral Dis.2021;27:1528–1541. 10.1111/odi.13680
References
- Alharbi, F. A., Alharthi, A. A., & Alsaadi, F. N. (2018). Perindopril‐induced angioedema of the lips and tongue: A case report. Journal of Medical Case Reports, 12(1), 359. 10.1186/s13256-018-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- B. Stegenga, B., de Bont, A. V. L. G. M., & Spijkervet, F. K. L. (2013). Mondziekten, kaak‐ en aangezichtschirurgie.Handboek voor mondziekten, kaak‐ en aangezichtschirurgie. : Van Gorcum. [Google Scholar]
- Balaji, G., Maharani, B., Ravichandran, V., & Parthasarathi, T. (2014). Linezolid induced black hairy tongue. Indian Journal of Pharmacology, 46(6), 653–654. 10.4103/0253-7613.144942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braggio, C., Bocchialini, G., Ventura, L., Carbognani, P., Rusca, M., & Ampollini, L. (2018). Linezolid‐induced black hairy tongue. Acta Biomedica, 89(3), 408–410. 10.23750/abm.v89i3.7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A. (1949). Glossitis following the administration of sulphanilamide and sulphathiazole; report of two cases. Glasgow Medical Journal, 30(4), 140–143. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18120886. [PMC free article] [PubMed] [Google Scholar]
- Byrd, J. A., Bruce, A. J., & Rogers, R. S.3rd (2003). Glossitis and other tongue disorders. Dermatologic Clinics, 21(1), 123–134. 10.1016/s0733-8635(02)00057-8. [DOI] [PubMed] [Google Scholar]
- FK . (2019). Retrieved from https://www.farmacotherapeutischkompas.nl/.
- Gurvits, G. E., & Tan, A. (2014). Black hairy tongue syndrome. World Journal of Gastroenterology, 20(31), 10845–10850. 10.3748/wjg.v20.i31.10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy, C. M., Smyth, Y., & Flint, S. R. (2004). Persistent nicorandil induced oral ulceration. Heart, 90(7), e38. 10.1136/hrt.2003.031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubiche, T., Valenza, B., Chevreau, C., Fricain, J. C., Del Giudice, P., & Sibaud, V. (2013). Geographic tongue induced by angiogenesis inhibitors. Oncologist, 18(4), e16–e17. 10.1634/theoncologist.2012-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura, Y., Shinozaki, T., Okada‐Ogawa, A., Noma, N., Shinoda, M., Iwata, K., … Svensson, P. (2019). An updated review on pathophysiology and management of burning mouth syndrome with endocrinological, psychological and neuropathic perspectives. Journal of Oral Rehabilitation, 46(6), 574–587. 10.1111/joor.12795. [DOI] [PubMed] [Google Scholar]
- Kalogirou, E. M., Katsoulas, N., Tosios, K. I., Lazaris, A. C., & Sklavounou, A. (2017). Non‐healing tongue ulcer in a rheumatoid arthritis patient medicated with leflunomide. An adverse drug event? Journal of Clinical and Experimental Dentistry, 9(2), e325–e328. 10.4317/jced.53428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNMP . (2019). Retrieved from https:///www.knmp.nl/producten/knmp‐kennisbank.
- Leung, E., Hanna, M. Y., Tehami, N., & Francombe, J. (2012). Isolated unilateral tongue oedema: The adverse effect of Angiotensin converting enzyme inhibitors. Curr Drug Saf, 7(5), 382–383. 10.2174/157488612805076561. [DOI] [PubMed] [Google Scholar]
- Mangold, A. R., Torgerson, R. R., & Rogers, R. S.3rd (2016). Diseases of the tongue. Clinics in Dermatology, 34(4), 458–469. 10.1016/j.clindermatol.2016.02.018. [DOI] [PubMed] [Google Scholar]
- MedDRA . (2018a). MedDRA® Term selection: Points to consider. McLean, (USA): Medical Dictionary for Regulatory Activities. [Google Scholar]
- MedDRA . (2018b). Vision of MedDRA. Retrieved from https://www.MedDRA.,org/about‐MedDRA.,vision. [Google Scholar]
- Rademacher, W. M. H., Aziz, Y., Hielema, A., Cheung, K. C., de Lange, J., Vissink, A., & Reinder Rozema, F. (2019). Oral adverse effects of drugs: Taste disorders. Oral Diseases, 26(1), 213–223. 10.1111/odi.13199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reamy, B. V., Derby, R., & Bunt, C. W. (2010). Common tongue conditions in primary care. American Family Physician, 81(5), 627–634. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20187599. [PubMed] [Google Scholar]
- Rosebush, M. S., Briody, A. N., & Cordell, K. G. (2019). Black and Brown: Non‐neoplastic Pigmentation of the Oral Mucosa. Head and Neck Pathology, 13(1), 47–55. 10.1007/s12105-018-0980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallone, G., Infante, B., Di Paolo, S., Schena, A., Grandaliano, G., Gesualdo, L., & Schena, F. P. (2004). Sirolimus and angiotensin‐converting enzyme inhibitors together induce tongue oedema in renal transplant recipients. Nephrology, Dialysis and Transplantation, 19(11), 2906–2908. 10.1093/ndt/gfh352. [DOI] [PubMed] [Google Scholar]
- WHO . (2003). The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD). Retrieved from http://www.who.int/classifications/atcddd/en/. [Google Scholar]
- WHO . (2011). THE world medicines situation 2011. Retrieved from http://apps.who.int/medicinedocs/en/m/abstract/Js20035en/. [Google Scholar]


