Abstract
Objectives
To compare 2‐year outcome following treatment with drug‐eluting stents (DES) for acute myocardial infarction (MI) versus non‐MI clinical syndromes. In acute MI patients, a stent‐level comparison was performed, comparing Resolute Onyx versus Orsiro stents.
Background
In patients presenting with acute MI, higher adverse event rates have been reported. So far, no clinical results >1 year have been published of acute MI patients treated with Resolute Onyx.
Methods
This post‐hoc analysis of the randomized BIONYX trial(NCT02508714) assessed the main outcome target vessel failure (TVF: cardiac death, target vessel MI, or target vessel revascularization) with Kaplan–Meier methods.
Results
Of all 2,488 trial participants, acute MI patients (n = 1,275[51.2%]) were significantly younger and had less comorbidities than non‐MI patients (n = 1,213[48.8%]). TVF rates were lower in acute MI patients (77/1,275[6.1%] vs. 103/1,213[8.6%], HR:0.70, 95%‐CI 0.52–0.94; plog‐rank = 0.02), mainly driven by target vessel revascularization (4.1 vs. 6.1%, plog‐rank = 0.03). Multivariate analysis showed no independent association of clinical syndrome with TVF (adjusted‐HR: 0.81, 95%‐CI 0.60–1.10; p = .17). In MI patients treated with Resolute Onyx (n = 626) versus Orsiro (n = 649), there was no difference in TVF (6.2 vs. 6.1%; plog‐rank = 0.97) and its components. There was only 1(0.2%) definite‐or‐probable stent thrombosis in RO‐ZES and 8(1.2%) in O‐SES (p = .053).
Conclusions
Two years after treatment with thin‐strut DES in this randomized trial, patients treated for acute MI had lower adverse event rates than non‐MI patients. Yet, these findings were mainly attributable to between‐group differences in patient and lesion characteristics. In patients who underwent PCI for acute MI, both Resolute Onyx and Orsiro showed favorable and similar 2‐year outcomes.
Keywords: clinical trials, drug‐eluting stent, myocardial infarction, percutaneous coronary intervention
1. INTRODUCTION
In patients with acute myocardial infarction (MI), percutaneous coronary intervention (PCI) with drug‐eluting stents (DES) have resulted in higher event rates than seen with other clinical syndromes (i.e., stable and unstable angina).1, 2, 3 While the early DES had substantial safety issues,4, 5 newer devices have more biocompatible durable polymer coatings.6, 7 In addition, the designs of most contemporary DES are further refined and utilize thin or ultrathin stent struts. Yet, it is unclear, whether PCI with such novel DES is still associated with a higher event risk in patients with acute MI.
The thin composite‐wire‐strut durable polymer‐coated zotarolimus‐eluting Resolute Onyx stent (RO‐ZES, Medtronic, Santa Rosa, CA)8 is a recently introduced new‐generation DES. The device was assessed in the randomized BIONYX trial, which is the first trial to examine the RO‐ZES in all‐comers and the first study to report 2‐year clinical outcomes.9, 10 The trial compares RO‐ZES with the ultrathin‐strut biodegradable polymer‐coated sirolimus‐eluting Orsiro stent (O‐SES, Biotronik, Bülach, Switzerland), which is the only contemporary DES with proven superiority over another thin‐strut new‐generation DES in patients with ST‐segment elevation MI (STEMI).11 As of now, no outcome data beyond 1 year have been reported for PCI with RO‐ZES in the setting of STEMI or non‐ST‐segment elevation MI (NSTEMI).
In this secondary analysis of the BIONYX trial, we examined the 2‐year clinical outcome of patients treated for acute MI (i.e., STEMI or NSTEMI) versus all other clinical syndromes (non‐MI), and performed a stent‐level analysis among the acute MI patients.
2. METHODS
2.1. Study design and participants
Study design and 2‐year clinical results have been reported.9, 10 In brief, “BIoresorbable polymer‐coated Orsiro versus durable polymer coated Resolute ONYX stents” (BIONYX; ClinicalTrials.gov:NCT02508714) is an international, investigator‐initiated, randomized clinical trial in all‐comer patients. The study was performed in seven centers in Belgium, Israel, and The Netherlands. The inclusion criteria were broad; patients could be enrolled if they were 18 years and older, capable of providing informed consent, and required PCI for the treatment of significant coronary artery or bypass graft lesions. The only exclusion criteria were: planned surgery within 3 months after randomization necessitating interruption of dual anti‐platelet therapy (DAPT), unlikely adherence to scheduled follow‐up, life expectancy of <1 year, participation in another randomized drug or device trial before reaching primary endpoint, known pregnancy, and known intolerance to components of study DES or anti‐platelet/thrombotic therapy. Patients were enrolled from October 2015 to December 2016 and equally randomized to the novel RO‐ZES or the O‐SES, after stratification for sex and diabetes. During the study period, 6,031 patients were treated with PCI of whom 3,515 were not screened or ineligible for the trial, and another 28 were excluded due to withdrawal of consent or screening failure. The primary outcome was non‐inferiority of the RO‐ZES versus O‐SES at 1‐year follow‐up.9 All patients provided written informed consent. The study complied with the Declaration of Helsinki and was approved by the Medical Ethics Committee Twente, The Netherlands, and the institutional review boards of all participating centers.
2.2. Procedures
Coronary interventions were performed according to standard techniques, and concomitant medical therapy was given according to current international guidelines. The diagnosis of an acute MI at time of index procedure was made by the treating physicians based on medical history, physical examination, cardiovascular biomarkers, electrocardiograms, and coronary angiography, using the standard international definitions of MI.12 In general, patients treated for acute MI received DAPT for 12 months. Quantitative coronary analysis was done according to current standards (QAngio XA, version 7.3). The Resolute Onyx stent has swaged shaped struts and is made from a composite wire in which the platinum‐iridium core is covered with an outer layer of cobalt‐chromium alloy.9 The uncoated strut thickness of stents with diameters ≤4.0 mm is slightly thinner (81 μm) than its predecessor's (91 μm). The struts are covered with a durable polymer‐blend of that elutes zotarolimus for 6 months. The Orsiro stent has cobalt‐chromium struts with a varying strut thickness depending on stent size (60 μm for stents ≤3.0 mm, or 80 μm for stents ≥3.5 mm). The asymmetrical biodegradable polymer‐coating elutes sirolimus within 4 months and resolves within 24 months.9
2.3. Follow‐up and clinical outcome
Clinical follow‐up was obtained at outpatient visits, by telephone follow‐up, or medical questionnaire. There was no routine angiographic follow‐up. The trial was externally monitored (Diagram, Zwolle, The Netherlands) and adverse clinical events were adjudicated by an independent clinical event committee (blinded to the assigned DES). Clinical endpoints, as well as stent thrombosis definitions were prespecified according to the Academic Research Consortium.13, 14 The main endpoint target vessel failure (TVF) is a composite of cardiac death, target vessel‐related MI, or clinically indicated target vessel revascularization. Secondary endpoints include target lesion failure (composite of cardiac death, target vessel‐related MI or target lesion revascularization), target lesion revascularization, and the individual components of TVF.
2.4. Statistical analysis
For between‐group comparisons of continuous variables, the student's t‐test or Wilcoxon Rank Sum test was used, and between‐group differences in categorical variables were examined with the Pearson's χ 2 test. Time to clinical endpoints was assessed by the Kaplan–Meier method, and for between‐group comparisons the log‐rank test was used. Patients were censored at the moment of dropout if they withdrew their consent, were lost to follow‐up, or died. Hazard ratios with two‐sided confidence intervals were computed with Cox proportional hazards analysis. Analyses were done according to the intention‐to‐treat principle. A two‐sided p‐value <.05 was considered significant. For the analysis of acute MI as compared to non‐MI patients, a multivariate model was constructed including the stratification factors sex and diabetes, and all baseline variables that showed between‐group dissimilarities and associations with TVF with p‐values <.15. The final model was constructed using step‐wise backward selection and included sex, diabetes, previous coronary artery bypass grafting, and previous MI. In the same manner, a multivariate model was constructed for the analysis of RO‐ZES versus O‐SES in acute MI patients. The final model included sex, diabetes, and multivessel treatment. Statistical analyses were done with SPSS version 24 (IBM Corp., Armonk, NY).
3. RESULTS
Of all 2,488 BIONYX trial participants, 1,275(51.2%) were treated for an acute MI and 1,213(48.8%) for other clinical syndromes (Figure 1). Patients who were treated for acute MI were younger and more often smokers than the non‐MI patients (62.6 ± 11.5 vs. 65.4 ± 10.3 years, 37.7 vs. 23.2% smokers; both p < .0001). Moreover, patients with acute MI had less often comorbidities (i.e., diabetes, hypertension, and hypercholesterolemia) or a history of MI or coronary revascularization (Table 1). Two‐year follow‐up data were available in 2,460(98.9%) patients (19 lost to follow‐up; 9 withdrew consent).
FIGURE 1.
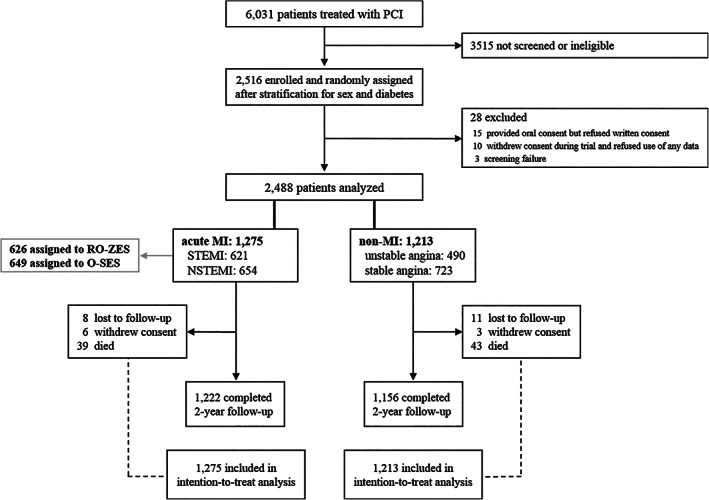
Study flowchart. Abbreviations: MI, myocardial infarction; NSTEMI, non‐ST‐segment elevation myocardial infarction; O‐SES, Orsiro sirolimus‐eluting stent; PCI, percutaneous coronary intervention; RO‐ZES, Resolute Onyx zotarolimus‐eluting stent; STEMI, ST‐segment elevation myocardial infarction
TABLE 1.
Baseline patient, lesion, and procedural characteristics
| Acute MI n = 1,275 | Non‐MI n = 1,213 | p value | |
|---|---|---|---|
| General characteristics | |||
| Age, years | 62.6 (11.5) | 65.4 (10.3) | <.001 |
| Women | 301 (23.6) | 293 (24.2) | .75 |
| Body‐mass index, kg/m2 | 27.8 (4.5) | 28.0 (4.3) | .24 |
| Current smoker | 469/1,245 (37.7) | 272/1,173 (23.2) | <.001 |
| Medical history | |||
| Diabetes, medically treated | 219 (17.2) | 291 (24.0) | <.001 |
| Hypertension | 569/1,257 (45.3) | 693/1,194 (58.0) | <.001 |
| Hypercholesterolemia | 462/1,240 (37.3) | 652/1,187 (54.9) | <.001 |
| Prior MI | 152 (11.9) | 248 (20.4) | <.001 |
| Prior percutaneous coronary intervention | 185 (14.5) | 355 (29.3) | <.001 |
| Prior coronary artery bypass surgery | 48 (3.8) | 128 (10.6) | <.001 |
| Lesion and procedural characteristics | |||
| At least 1 bifurcation | 504 (39.5) | 477 (39.3) | .92 |
| At least 1 severely calcified lesion | 204 (16.0) | 219 (18.1) | .17 |
| At least 1 complex lesion | 1,040 (81.6) | 833 (68.7) | <.001 |
| Total stent length | 30 (18–46) | 30 (18–50) | .73 |
| Multi‐vessel treatment | 219 (17.2) | 222 (18.3) | .46 |
| Direct stenting | 312 (24.5) | 278 (22.9) | .36 |
| Post dilation | 887 (69.6) | 834 (68.8) | .66 |
Note: Values are mean(SD), median (interquartile range), n(%) or n/N(%).
Abbreviation: MI, myocardial infarction.
3.1. Acute MI versus non‐MI patients
Between patients with acute MI and non‐MI patients, there was a significant difference in the main endpoint TVF which occurred in 77/1,275(6.1%) and 103/1,213(8.6%) patients, respectively (HR:0.70, 95%‐CI 0.52–0.94; plog‐rank = .02). There was no significant between‐group difference in the safety endpoints cardiac death, definite‐or‐probable stent thrombosis, and target vessel MI (Table 2; Figure 2). The rate of target vessel revascularization was lower in acute MI patients (4.1 vs. 6.1%, HR:0.67, 95%‐CI 0.47–0.96; plog‐rank = .03). These findings were consistent after adjustment for stratification factors (sex and diabetes). Table 3 displays antiplatelet and oral anticoagulant therapy use at 2‐year follow‐up. After adjusting for confounders, multivariate analysis showed a non‐significant difference in 2‐year rates of TVF, target vessel revascularization, as well as target lesion failure. Adjusted hazard ratios are presented in Table 2.
TABLE 2.
Two‐year clinical outcome of acute MI versus non‐MI patients
| Acute MI n = 1,275 | Non‐MI n = 1,213 | HR (95%‐CI) | Plog‐rank | Adjusted HRa (95%‐CI) | p value | |
|---|---|---|---|---|---|---|
| Target vessel failure | 77 (6.1) | 103 (8.6) | 0.70 (0.52–0.94) | .017 | 0.81 (0.60–1.10) | .17 |
| Cardiac death | 17 (1.3) | 15 (1.3) | 1.08 (0.54–2.16) | .83 | 1.37 (0.68–2.77) | .38 |
| Target vessel MI | 28 (2.2) | 34 (2.9) | 0.78 (0.47–1.28) | .33 | 0.94 (0.57–1.57) | .82 |
| Clinically indicated TVR | 51 (4.1) | 72 (6.1) | 0.67 (0.47–0.96) | .026 | 0.76 (0.52–1.09) | .13 |
| Target lesion failure | 63 (5.0) | 84 (7.0) | 0.70 (0.51–0.98) | .034 | 0.82 (0.59–1.14) | .23 |
| Clinically indicated TLR | 37 (3.0) | 52 (4.4) | 0.67 (0.44–1.03) | .06 | 0.76 (0.49–1.16) | .20 |
| Definite‐or‐probable stent thrombosis | 9 (0.7) | 9 (0.8) | 0.95 (0.38–2.39) | .91 | 1.20 (0.47–3.07) | .71 |
| Definite stent thrombosis | 7 (0.6) | 9 (0.8) | 0.74 (0.28–1.98) | .54 | 0.90 (0.33‐2.45) | .83 |
Note: Values are n(%).
Abbreviations: CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; TLR, target lesion revascularization; TVR, target vessel revascularization.
Adjusted for sex, diabetes, previous MI, and previous coronary artery bypass grafting.
FIGURE 2.
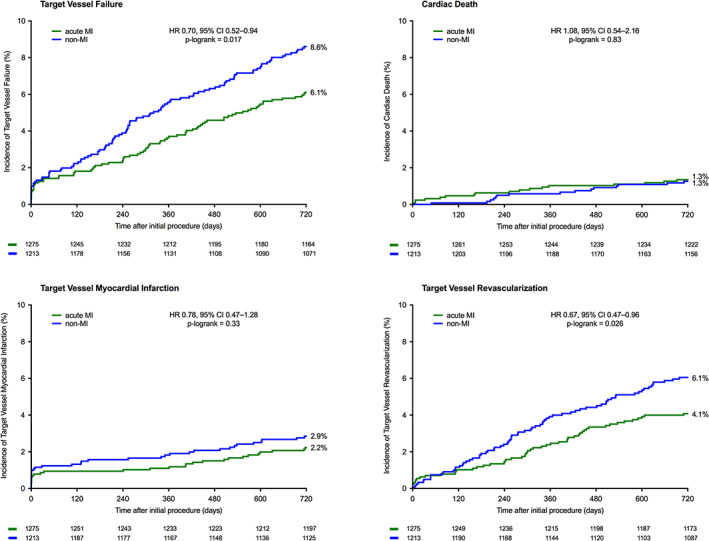
Kaplan–Meier event curves of Target Vessel Failure and components at 2‐year follow‐up in acute MI patients and non‐MI patients. Abbreviations: CI, confidence interval; HR, hazard ratio; MI, myocardial infarction
TABLE 3.
Use of antiplatelet and oral anticoagulant therapy at 2‐year follow‐up
| Acute MI n = 1,218 | Non‐MI n = 1,156 | p value | |
|---|---|---|---|
| Aspirin | 1,059 (86.9) | 936 (81.0) | <.001 |
| Dual antiplatelet therapy | 156 (12.8) | 199 (17.2) | .003 |
| With clopidogrel | 54 (4.4) | 158 (13.7) | <.001 |
| With ticagrelor or prasugrel | 102 (8.4) | 41 (3.5) | <.001 |
| Direct oral anticoagulant | 57 (4.7) | 88 (7.6) | .003 |
| Vitamin K antagonist | 76 (6.2) | 90 (7.8) | .14 |
Note: Values are n(%). Data available in 2,374/2,488 (95.4%) patients.
Abbreviation: MI, myocardial infarction.
3.2. RO‐ZES versus O‐SES in patients with acute MI
Of all 1,275 patients who presented with acute MI, 626(49.1%) were treated with RO‐ZES and 649(50.9%) with O‐SES (follow‐up available in 1,261(98.9%) patients). Table S1 shows the baseline characteristics of patient, lesion, and procedural characteristics of acute MI patients treated with RO‐ZES versus O‐SES. There was no statistically significant between‐stent difference in TVF which occurred in 38/626(6.2%) patients treated with RO‐ZES versus 39/649(6.1%) patients treated with O‐SES (HR:1.01, 95%‐CI 0.65–1.58; plog‐rank = .97, Table 4). Cardiac death occurred in the RO‐ZES group in 5(0.8%) and in the O‐SES group in 12(1.7%) patients (HR:0.43, 95%‐CI 0.15–1.22; plog‐rank = .10). Figure 3 displays Kaplan Meier event curves for TVF and its individual components. In addition, there was only 1(0.2%) definite‐or‐probable stent thrombosis in RO‐ZES and 8(1.2%) in O‐SES (HR:0.13, 95%‐CI 0.02–1.03; p = .053). Table S2 shows details of each case of stent thrombosis, and Table S3 shows subgroup analyses for the acute MI patients. Furthermore, the outcomes of patients treated with RO‐ZES versus O‐SES, separately for each clinical syndrome (i.e., STEMI, NSTEMI, unstable angina, stable angina; and acute coronary syndrome, chronic coronary syndrome) are presented in Tables S4 and S5. In patients with an acute coronary syndrome, RO‐ZES showed a lower rate of definite‐or‐probable stent thrombosis (HR:0.20, 95%‐CI 0.04–0.91; plog‐rank = .02).
TABLE 4.
RO‐ZES versus O‐SES in patients with acute MI at 2‐year follow‐up
| RO‐ZES n = 626 | O‐SES n = 649 | Hazard ratio (95%‐CI) | Plog‐rank | Adjusted HRa (95%‐CI) | p value | |
|---|---|---|---|---|---|---|
| Target vessel failure | 38 (6.2) | 39 (6.1) | 1.01 (0.65–1.58) | .97 | 0.96 (0.62–1.51) | .87 |
| Cardiac death | 5 (0.8) | 12 (1.7) | 0.43 (0.15–1.22) | .10 | 0.40 (0.14–1.14) | .09 |
| Target vessel MI | 12 (2.0) | 16 (2.5) | 0.78 (0.37–1.64) | .50 | 0.76 (0.36–1.60) | .46 |
| Clinically indicated TVR | 29 (4.7) | 22 (3.5) | 1.36 (0.78–2.37) | .27 | 1.31 (0.75–2.28) | .35 |
| Target lesion failure | 32 (5.2) | 31 (4.8) | 1.07 (0.65–1.76) | .79 | 1.04 (0.75–1.43) | .83 |
| Clinically indicated TLR | 23 (3.7) | 14 (2.2) | 1.71 (0.88–3.32) | .11 | 1.64 (0.84–3.18) | .15 |
| Definite‐or‐probable stent thrombosis | 1 (0.2) | 8 (1.2) | 0.13 (0.02–1.03) | .053b | 0.14 (0.02–1.09) | .06 |
| Definite stent thrombosis | 1 (0.2) | 6 (0.9) | 0.17 (0.02–1.43) | .06 | 0.18 (0.02–1.51) | .11 |
Note: Values are n(%).
Abbreviations: CI, confidence interval; MI, myocardial infarction; O‐SES, Orsiro sirolimus‐eluting stent; RO‐ZES, Resolute Onyx zotarolimus‐eluting stent; TLR, target lesion revascularization; TVR, target vessel revascularization.
Adjusted for sex, diabetes mellitus and multivessel treatment.
Wald‐test used because plog‐rank(based on chi‐square) did not correspond with 95%‐CI due to very low event rate in RO‐ZES(plog‐rank = 0.02).
FIGURE 3.
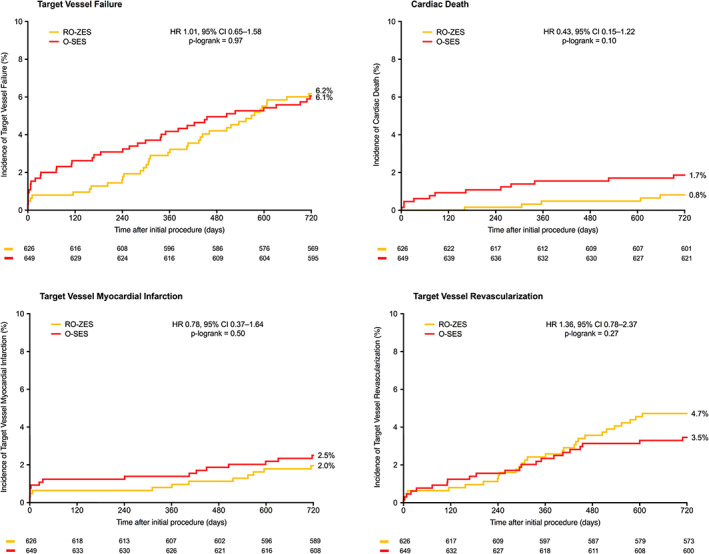
Kaplan–Meier event curves of Target Vessel Failure and components at 2‐year follow‐up in acute MI patients treated with Resolute Onyx versus Orsiro. Abbreviations: CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; O‐SES, Orsiro sirolimus‐eluting stent; RO‐ZES, Resolute Onyx zotarolimus‐eluting stent
4. DISCUSSION
4.1. Main findings
Trial participants with acute MI were found to have significantly lower 2‐year rates of TVF, target vessel revascularization, and target lesion failure than patients with non‐MI clinical syndromes (i.e., stable or unstable angina). Yet, multivariate analysis revealed that the observed between‐group differences were mostly related to dissimilarities in baseline characteristics rather than to the clinical syndrome itself. Our findings suggest that being treated for an acute MI at presentation does not lead to worse 2‐year clinical outcomes.
For the first time this study presents detailed 2‐year outcome data of patients who were treated for an acute MI with the RO‐ZES. In BIONYX trial participants, there was no significant difference between RO‐ZES and O‐SES in various safety and efficacy endpoints. Although the study was not adequately powered to assess stent thrombosis, and overall stent thrombosis rates were low in acute MI patients, it is notable that the 2‐year rate of definite‐or‐probable stent thrombosis for RO‐ZES was particularly low (0.2 vs. 1.2%). The majority of stent thromboses (i.e., six out of nine) occurred while patients were still on DAPT. In addition, the 2‐year DAPT rate (12.8% in acute MI patients) was comparable or lower than in other studies,15, 16, 17, 18 while our study protocol did not formally mandate DAPT termination after 1 year. We speculate that the particularly low stent thrombosis rate in RO‐ZES may be related to its stent design, as the oval strut shape of the RO‐ZES might lower flow disturbance and could facilitate cell migration during endothelialization. A signal for this was found in a pre‐clinical study that showed superior thromboresistance and equivalent endothelial healing of the RO‐ZES versus polymer‐free biolimus‐eluting stents (BioFreedom, Biosensors, Newport Beach, CA).19 Furthermore, in biodegradable polymer DES, a potential relation of stent thrombosis with the duration of polymer degradation cannot be excluded.
4.2. DES studies comparing outcomes in the settings of acute MI versus non‐MI
Several previous studies evaluated new‐generation DES in patients with acute MI versus other clinical syndromes. Yet, when interpreting clinical event rates, it is important to consider the study design, completeness of follow‐up, source of the data, years of enrolment, and the respective in‐ and exclusion criteria of the study. Many randomized DES trials excluded patients who were unable to provide informed consent or had a life expectancy of less than 12 months.1, 2, 7, 20, 21, 22 This applies even to randomized “all‐comer” trials, such as the BIONYX, and it may be most relevant for patients with an acute MI – in particular with STEMI, as these patients present much more often in critical condition than patients with stable or unstable angina. As a consequence, the use of these (more or less unavoidable) exclusion criteria will result in somewhat more favorable event rates in DES trial participants as compared to everyday clinical practice or DES registries in consecutive patients. Nevertheless, many large‐sized DES registries did not enroll consecutive series of patients and therefore may have other limitations.
A pooled analysis of data from registries and randomized studies (with varying in‐ and exclusion criteria) that all assessed the Resolute ZES, a predecessor of the RO‐ZES, compared patients with acute MI (n = 1,716) and stable angina (n = 1,260). That study found at 2‐year follow‐up a higher stent thrombosis rate in the acute MI group, but target vessel revascularization and MI rates were similar.23 Overall, the event rates were somewhat higher than in the RO‐ZES‐treated patients of the present analysis, which may not only be attributed to DES refinement but also to improvements in PCI techniques and differences in concomitant medical therapy.
Two registries compared the outcome of acute MI patients with non‐MI patients who were treated with contemporary24 or first‐generation DES.3 The studies found in the acute MI groups higher rates of cardiac death, target vessel MI, and definite stent thrombosis at 1‐year follow‐up,24 and higher rates of cardiac death and definite stent thrombosis after 5 years.3 Yet, the registry with 5‐year follow‐up showed no between‐group difference in clinical events from 3 months to 5 years. In the acute MI patients of the BIONYX trial we did not find higher adverse event rates, which may be related to between‐study dissimilarities in patient population and design. Nevertheless, there actually were major similarities between patient populations. In the aforementioned registries, patients of the MI groups less frequently had a history of MI, PCI, and coronary artery bypass grafting, and these patients suffered less often from diabetes, hypertension, and hypercholesterolemia. Similar differences between the acute MI and non‐MI patients were found within the study population of our present analysis.
Some other studies with newer‐generation DES have compared the clinical outcome of patients treated for acute coronary syndromes (including unstable angina) to patients treated for stable angina,2, 25, 26, 27 and showed higher adverse event rates in patients with acute coronary syndromes. Yet, this finding, which is in contrast to our own observation, may partly be attributed to dissimilarities between study groups. In BIONYX, patients with unstable angina were categorized as non‐MI patients, while the aforementioned studies categorized patients with unstable angina (together with all patients with acute MI) as part of the acute coronary syndrome group.
4.3. New‐generation DES assessed in patients with acute MI
There is considerable clinical evidence supporting the use of new‐generation DES in patients with acute MI, including STEMI.20, 21, 22, 28, 29, 30, 31 Yet, up to now, clinical outcome data >1 year after treatment with RO‐ZES in patients with acute MI are scarce. The Korean Acute MI Registry32 assessed the short‐term clinical outcome of 1,486 acute MI patients who were treated with either RO‐ZES (n = 402) or everolimus‐eluting stents (n = 1,084). At 6‐month follow‐up, there was no between‐DES difference in target lesion failure (4.0 vs. 3.9%) and definite‐or‐probable stent thrombosis (0.2 vs. 0.3%).32 The current analysis of BIONYX trial participants treated for acute MI also found no difference in 2‐year clinical outcome between RO‐ZES versus O‐SES. In fact, the 2‐year event rates in the RO‐ZES‐treated BIONYX trial participants were relatively low as compared to the 6‐month event rates of the Korean registry.
Two randomized clinical trials previously assessed the O‐SES in the setting of STEMI.11, 33, 34 A secondary analysis of the BIOSCIENCE trial found favorable 2‐year outcomes with O‐SES as compared to the well‐established and widely used durable polymer‐coated everolimus‐eluting Xience stent (Abbott Vascular, Santa Clara, CA).33, 34 BIOSTEMI is the first dedicated randomized clinical trial in STEMI patients, treated with new‐generation thin‐strut DES. The study which randomized 1,300 patients to either O‐SES or Xience11 found superiority of the O‐SES regarding the composite clinical endpoint target lesion failure at 1‐year.
5. LIMITATIONS
This post‐hoc analysis was not powered for the assessment of secondary endpoints and, in particular, infrequent events, such as stent thrombosis. Consequently, the findings of the present study should be considered hypothesis generating. As discussed above, the trial's exclusion criteria prevented the enrolment of frail patients with a life expectancy of less than 12 months. Although cardiogenic shock was not a formal exclusion criterion, many patients in cardiogenic shock and all survivors of an out‐of‐hospital cardiac arrest were not enrolled in this randomized trial due to limited life expectancy or for being unable to provide informed consent. We decided to classify patients based on acute MI or non‐MI, as in patients with STEMI or NSTEMI there is cardiac necrosis that may have an impact on clinical outcome, while there is no myocardial necrosis in patients with stable coronary syndromes and patients with unstable angina. Nevertheless, a comparison of patients with acute coronary syndrome with patients that have chronic coronary syndrome could also be made and may yield different results.
6. CONCLUSIONS
Two years after treatment with novel thin‐strut DES, participants in the randomized BIONYX trial who were treated for an acute MI had lower adverse event rates than non‐MI patients; yet, this finding was mainly attributable to between‐group differences in patient and lesion characteristics. In patients who underwent PCI for acute MI, both RO‐ZES and O‐SES showed favorable and similar 2‐year clinical outcomes.
CONFLICT OF INTEREST
CvB reports that Thoraxcentrum Twente received institutional research grants from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. The other authors have no conflicts of interest to declare.
Supporting information
Table S1. Supporting Information.
Ploumen EH, Buiten RA, Zocca P, et al. Acute myocardial infarction treated with novel Resolute Onyx and Orsiro stents in the randomized BIONYX trial. Catheter Cardiovasc Interv. 2021;98:E188–E196. 10.1002/ccd.29594
Eline H. Ploumen and Rosaly A. Buiten contributed equally to this study.
Funding information Biotronik; Medtronic
DATA AVAILABILITY STATEMENT
The data that support the findings of this study can be made available upon reasonable request. A request can be made to Cardiovascular Research and Education Enschede and will be evaluated by an independent review committee, identified for this purpose.
REFERENCES
- 1.Thim T, Maeng M, Kaltoft A, et al. Zotarolimus‐eluting vs. Sirolimus‐eluting coronary stents in patients with and without acute coronary syndromes: a SORT OUT III substudy. Eur J Clin Invest. 2012;42:1047‐1054. [DOI] [PubMed] [Google Scholar]
- 2.Antonsen L, Thayssen P, Hansen HS, et al. Outcomes after revascularization with everolimus‐ and sirolimus‐eluting stents in patients with acute coronary syndromes and stable angina pectoris: a substudy of the SORT OUT IV trial. EuroIntervention. 2014;10:212‐223. [DOI] [PubMed] [Google Scholar]
- 3.Yamaji K, Natsuaki M, Morimoto T, et al. Long‐term outcomes after coronary stent implantation in patients presenting with versus without acute myocardial infarction (an observation from coronary revascularization demonstrating outcome study‐Kyoto registry Cohort‐2). Am J Cardiol. 2015;116:15‐23. [DOI] [PubMed] [Google Scholar]
- 4.Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study. Lancet. 2007;369:667‐678. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa G, Finn AV, Joner M, et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug‐eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008;118:1138‐1145. [DOI] [PubMed] [Google Scholar]
- 6.Räber L, Magro M, Stefanini GG, et al. Very late coronary stent thrombosis of a newer‐generation everolimus‐eluting stent compared with early‐generation drug‐eluting stents: a prospective cohort study. Circulation. 2012;125:1110‐1121. [DOI] [PubMed] [Google Scholar]
- 7.Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug‐eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR‐TEST 3, ISAR‐TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33:1214‐1222. [DOI] [PubMed] [Google Scholar]
- 8.Price MJ, Shlofmitz RA, Spriggs DJ, et al. Safety and efficacy of the next generation Resolute Onyx zotarolimus‐eluting stents: primary outcomes of the RESOLUTE ONYX core trial. Catheter Cardiovasc Interv. 2018;92:253‐259. [DOI] [PubMed] [Google Scholar]
- 9.von Birgelen C, Zocca P, Buiten RA, et al. Thin composite wire strut, durable polymer‐coated (Resolute Onyx) versus ultrathin cobalt–chromium strut, bioresorbable polymer‐coated (Orsiro) drug‐eluting stents in allcomers with coronary artery disease (BIONYX): an international, single‐blind, randomised non‐inferiority trial. Lancet. 2018;392:1235‐1245. [DOI] [PubMed] [Google Scholar]
- 10.Buiten RA, Ploumen EH, Zocca P, et al. Thin composite‐wire‐strut Zotarolimus‐eluting stents versus ultrathin‐strut Sirolimus‐eluting stents in BIONYX at 2 years. J Am Coll Cardiol Interv. 2020;13:1100‐1109. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias JF, Muller O, Heg D, et al. Biodegradable polymer sirolimus‐eluting stents versus durable polymer everolimus‐eluting stents in patients with ST‐segment elevation myocardial infarction(BIOSTEMI): a single‐blind, prospective, randomised superiority trial. Lancet. 2019;394:1243‐1253. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. The writing group on behalf of the joint ESC/ACCF/AHA/WHF task force for the universal definition of myocardial infarction. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551‐2567. [DOI] [PubMed] [Google Scholar]
- 13.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344‐2351. [DOI] [PubMed] [Google Scholar]
- 14.Vranckx P, Cutlip DE, Mehran R, et al. Myocardial infarction adjudication in contemporary all‐comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5:871‐874. [DOI] [PubMed] [Google Scholar]
- 15.Kandzari DE, Koolen JJ, Doros G, et al. Ultrathin bioresorbable polymer sirolimus‐eluting stents versus thin durable polymer everolimus‐eluting stents. J Am Coll Cardiol. 2018;72:3287‐3297. [DOI] [PubMed] [Google Scholar]
- 16.Kereiakes DJ, Windecker S, Lee Jobe R, et al. Clinical outcomes following implantation of thin‐strut, bioabsorbable polymer‐coated, everolimus‐eluting SYNERGY stents. Circ Cardiovasc Interv. 2019;12:e008152. [DOI] [PubMed] [Google Scholar]
- 17.Zbinden R, Piccolo R, Heg D, et al. Ultrathin strut biodegradable polymer sirolimus‐eluting stent versus durable‐polymer everolimus‐eluting stent for percutaneous coronary revascularization: 2‐year results of the BIOSCIENCE trial. J Am Heart Assoc. 2016;5:e003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen H, Lam MK, Löwik M, et al. Clinical events and patient‐reported chest pain in all‐comers treated with Resolute integrity and Promus Element stents: 2‐year follow‐up of the DUTCH PEERS (DUrable polymer‐based STent CHallenge of Promus ElemEnt versus ReSolute integrity) randomized trial(TWENTE II). JACC Cardiovasc Interv. 2015;8:889‐899. [DOI] [PubMed] [Google Scholar]
- 19.Jinnouchi H, Sato Y, Cheng Q, et al. Thromboresistance and endothelial healing in polymer‐coated versus polymer‐free drug‐eluting stents: implications for short‐term dual antiplatelet therapy. Int J Cardiol. 2020;327:52‐57. [DOI] [PubMed] [Google Scholar]
- 20.Räber L, Yamaji K, Kelbæk H, et al. Five‐year clinical outcomes and intracoronary imaging findings of the COMFORTABLE AMI trial: randomized comparison of biodegradable polymer‐based biolimus‐eluting stents with bare‐metal stents in patients with acute ST‐segment elevation myocardial infarction. Eur Heart J. 2019;40:1909‐1919. [DOI] [PubMed] [Google Scholar]
- 21.Valdes‐Chavarri M, Kedev S, Neskovic AN, et al. Randomised evaluation of a novel biodegradable polymerbased sirolimus‐eluting stent in ST‐segment elevation myocardial infarction: the MASTER study. EuroIntervention. 2019;14:e1836‐42. [DOI] [PubMed] [Google Scholar]
- 22.van Houwelingen KG, Lam MK, Löwik MM, et al. Outcome after myocardial infarction treated with Resolute integrity and Promus Element stents: insights from the DUTCH PEERS(TWENTE II) randomized trial. Rev Esp Cardiol. 2016;69:1152‐1159. [DOI] [PubMed] [Google Scholar]
- 23.Widimsky P, Motovska Z, Belardi J, et al. Long‐term outcomes of patients receiving zotarolimus‐eluting in ST eleveation myocardial infraction, non‐ST elevation acute coronary syndrome, and stable angina: data from the Resolute program. Int J Cardiol. 2013;168:3522‐3526. [DOI] [PubMed] [Google Scholar]
- 24.Moscarella E, Ielasi A, Beneduce A, et al. One‐year clinical outcome of biodegradable polymer sirolimus‐eluting stent in patients presenting with acute myocardial infarction: insight from the ULISSE registry. Catheter Cardiovasc Interv. 2019;94:972‐979. [DOI] [PubMed] [Google Scholar]
- 25.Kalkman DN, Woudstra P, Lu H, et al. Evaluation of clinical outcomes after COMBO stent treatment in patients presenting with acute coronary syndrome. Catheter Cardiovasc Interv. 2017;90:E31‐E37. [DOI] [PubMed] [Google Scholar]
- 26.Planer D, Smits PC, Kereiakes DJ, et al. Comparison of everolimus‐ and paclitaxel‐eluting stents in patients with acute and stable coronary syndromes. J Am Coll Cardiol Interv. 2011;4:1104‐1115. [DOI] [PubMed] [Google Scholar]
- 27.Yoshikawa S, Ashikaga T, Miyazaki T, Kurihara K, Hirao K. Long‐term efficacy and safety of everolimus‐eluting stent implantation in Japanese patients with acute coronary syndrome: five‐year real‐world data from the Tokyo‐MD PCI study. J Interv Cardiol. 2019;2019:3146848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabaté M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST‐segment elevation myocardial infarction treated with everolimus‐eluting stents versus bare‐metal stents (EXAMINATION): 5‐year results of a randomised trial. Lancet. 2016;387:357‐366. [DOI] [PubMed] [Google Scholar]
- 29.Bangalore S, Amoroso N, Fusaro M, Kumar S, Feit F. Outcomes with various drug‐eluting or bare metal stents in patients with ST‐segment elevation myocardial infarction: a mixed treatment comparison analysis of trial level data from 34068 patient‐years of follow‐up from randomized trials. Circ Cardiovasc Interv. 2013;6:378‐390. [DOI] [PubMed] [Google Scholar]
- 30.Sarno G, Lagerqvist B, Nilsson J, et al. Stent thrombosis in new‐generation drug‐eluting stents in patient with STEMI undergoing primary PCI: a report from SCAAR. J Am Coll Cardiol. 2014;64:16‐24. [DOI] [PubMed] [Google Scholar]
- 31.Gąsior P, Gierlotka M, Szczurek‐Katanski K, et al. Safety and efficacy of biodegradable polymer‐coated thin strut sirolimus‐eluting stent vs. durable polymer‐coated everolimus‐eluting stent in patients with acute myocardial infarction. Adv Interv Cardiol. 2018;14:347‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim Y, Oh SS, Jeong MH, et al. Comparison of short‐term clinical outcomes between Resolute Onyx zotarolimus‐eluting stents and everolimus‐eluting stent in patients with acute myocardial infarction: results from the Korea acute myocardial infarction registry (KAMIR). Cardiol J. 2019;26:469‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilgrim T, Piccolo R, Heg D, et al. Biodegradable polymer sirolimus‐eluting stents versus durable polymer everolimus‐eluting stents for primary percutaneous coronary revascularization of acute myocardial infarction. EuroIntervention. 2016;12:e1341‐54. [DOI] [PubMed] [Google Scholar]
- 34.Piccolo R, Heg D, Franzone A, et al. Biodegradable‐polymer sirolimus‐eluting stents versus durable‐polymer everolimus‐eluting stents in patients with acute ST‐segment elevation myocardial infarction: insights from the 2‐year follow‐up of the BIOSCIENCE trial. J Am Coll Cardiol. 2016;9:978‐986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study can be made available upon reasonable request. A request can be made to Cardiovascular Research and Education Enschede and will be evaluated by an independent review committee, identified for this purpose.


