Abstract
Background
The accumulation of advanced glycation end products has been proposed as a causative agent of skin aging, but there are no conventional devices for quantifying advanced glycation end‐product accumulation in facial skin.
Aims
This study aimed to develop a convenient and accurate in situ advanced glycation end‐product measurement system for the human face.
Methods
We developed a facial glycation imaging system, which consisted of illumination (white light‐emitting diode, ultraviolet light‐emitting diode) and image acquisition modules to capture face images. Advanced glycation end product–related autofluorescence and total skin reflectance were calculated to obtain the skin glycation index using an image analysis algorithm. Correlations between the skin glycation index and facial skin elasticity and age were examined in 36 healthy Korean women.
Results
The facial glycation imaging system was validated against a volar forearm skin autofluorescence measurement device, that is, the AGE Reader mu, with forearm skin glycation index (R = 0.64, P < .01). Cheek elasticity was negatively correlated with cheek skin glycation index (R = −0.56, R = −0.57, and R = −0.61, P < .01 for R2, R5, and R7, respectively). Age was significantly correlated with forearm skin glycation index (R = 0.44, P < .01) and cheek skin glycation index (R = 0.48, P < .01).
Conclusion
We successfully developed a novel in situ facial skin glycation index measurement device. Our convenient and accurate system enables in situ skin glycation index monitoring for skin aging studies such as those on anti‐glycation cosmetics.
Keywords: advanced glycation end products, aging, elasticity, noninvasive, skin glycation
1. INTRODUCTION
The skin is the largest organ of the human body, and it gradually accumulates damage over time, with both endogenous and exogenous aging impairing functions at the cellular, tissue, and organ levels.1, 2 Recently, the accumulation of advanced glycation end products (AGEs) has been proposed as a causative agent of aging, and inhibiting such accumulation has been adopted as a potential antiaging strategy in the development of novel cosmetic compounds.3, 4 AGEs are derived from the modification of proteins or lipids that are glycated by reducing sugars,5, 6, 7 and they can change the cell structure and function by modifying extracellular matrices, which also results in the release of free radicals.8 In addition, AGE accumulation in the dermis leads to yellow discoloration of the skin and causes amino acids in collagen, such as lysine and arginine, to form cross‐links between collagen fibers, which results in loss of collagen mobility.8, 9, 10, 11 Therefore, collagen proteins are deteriorated by glycation, and the skin loses its elasticity.12, 13 Further, AGEs display a strong nuclear factor‐κB activation activity and induce inflammatory changes in the skin through pro‐inflammatory cytokines.14, 15
Given the detrimental effects of AGEs on skin aging, evaluating the ability of antiaging products to inhibit AGE accumulation is extremely important. Recent studies have shown noninvasive fluorescence intensity to be significantly correlated with the levels of AGEs, such as carboxymethyl‐lysine, carboxyethyl‐lysine, and pentosidine.10, 16, 17 A commercial device, the AGE Reader mu (Diagnoptics Technologies, NL), is capable of evaluating skin autofluorescence (SAF) for clinical purposes, such as in disease diagnosis.18, 19, 20 The AGE Reader mu irradiates the skin with ultraviolet (UV) light at a wavelength of 300‐420 nm and then measures fluorescent light emitted in the range of 420‐600 nm. However, its measurement area is limited to the forearm, which limits its use in the study of facial skin aging. A technique for evaluating AGE‐related autofluorescence in the facial skin is therefore needed.
In this study, we demonstrated a novel facial glycation imaging system (FGIS) for assessing the skin glycation index (SGI) in the facial skin. We expect that this novel instrument can be conveniently used to obtain in situ facial skin glycation data for cosmetic research.
2. MATERIALS AND METHODS
2.1. Subjects
A total of 36 Korean women aged 20‐59 years were enrolled in the study and distributed by age as follows: 5 subjects, aged 20‐29 years; 8, 30‐39 years; 13, 40‐49 years; and 10, 50‐59 years. The subjects were recruited from the general population, excluding diabetic and obese individuals. Through the lifestyle questionnaire, smokers, and subjects who were exposed to UV rays for more than 3 hours per day were excluded from the experiment to control for external factors of skin aging. On the day of the measurement, all subjects were asked to clean their face and volar forearm to remove skin care products and pollutants. After cleaning, the subjects waited in an air‐conditioned room (22°C and 45% relative humidity) for 20 min to equilibrate their skin status.
2.2. The FGIS
The FGIS was made by modifying the facial aging measurement device Mark‐Vu® (PSIPLUS, KR). The configuration of the FGIS is illustrated in Figure 1. This system consisted of illumination and image acquisition modules. The illumination module comprised white light‐emitting diode (LED) and UV LED light sources for adjusting the position of the light so that the LED light could reach the face evenly. As shown in Figure 1, a 365‐nm short pass filter (Ruisen Optics, CN) was mounted in front of the UV LED light sources to specifically excite skin AGEs, such as pentosidine. The image acquisition module consisted of a monochromatic camera (Canon 100D, JP) and filter in‐out system, in which a 440‐nm fluorescence band‐pass filter (Edmund Optics Inc, USA) was mounted. The switching of the illumination light and filter in‐out system was computer controlled, allowing for image acquisition to be completed in less than 1 s.
FIGURE 1.
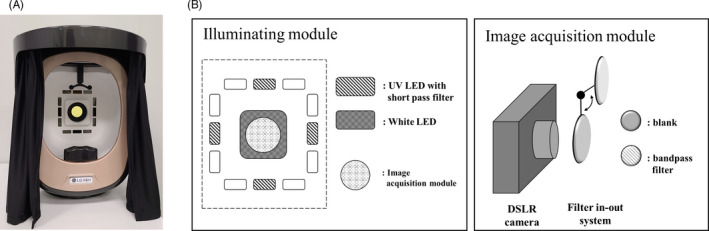
(a) Facial glycation imaging system (FGIS). (b) The configuration of the FGIS
In this study, we tried to predict the facial SGI using the AGE‐related autofluorescence emission intensity induced by UV excitation. However, the amount of autofluorescence depends not only on the amount of AGE accumulation but also on the intensity of skin reflectance. For example, AGE‐related SAF measurements in subjects with darker skin are typically lower than in those with fair skin. Therefore, the SGI was analyzed by empirical optimization using a correlation factor between the average intensity of the AGE‐related autofluorescence light from the skin (IAF) and the average intensity of the total reflected light from the skin (IR) in healthy nondiabetic subjects. The covariates for empirical correction (α and β) differed according to the measurement site. The SGI was calculated as follows:
2.3. Image acquisition and SGI analysis
The following steps describe the flow of SGI analysis by the FGIS (Figure 2):
FIGURE 2.
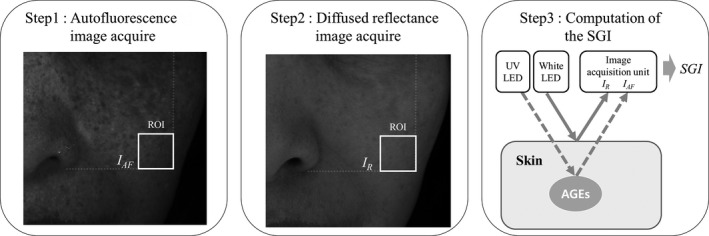
Process of skin glycation index (SGI) evaluation by the facial glycation imaging system (FGIS). UV, ultraviolet; ROI, region of interest; IAF, average intensity of autofluorescence under UV LED illumination; IR, average intensity of the total reflected light from the skin under white LED illumination
Step 1: Acquire the autofluorescence image in the range of 430‐450 nm by UV LED illumination.
Step 2: Acquire the diffused total reflectance image by white LED illumination.
Step 3: Compute the SGI from both acquired images.
In steps 1 and 2, the autofluorescence and diffused total reflectance face images were acquired by the FGIS. In step 3, we selected a 100 × 100‐pixel region of interest (ROI) on the cheek and volar forearm to analyze the SGI. To acquire accurate average intensity data within the ROI, sebum and pigmentation regions were automatically excluded using the intensity cutoff value. After removing the sebum and pigmentation regions within the ROI, the average intensities of the autofluorescence face image (IAF) and diffused reflectance face image (IR) were calculated.
2.4. Measurement of reference AGEs
The commercially available AGE Reader mu (Diagnoptics Technology, NL) was used to obtain the reference measurements from the volar forearm (AFref). All reference AGE data were acquired three times and expressed as a mean. The reference values were compared with the FGIS‐generated SGI values for the forearm (SGIforearm) and the cheek (SGIcheek).
2.5. Skin elasticity measurement
We measured skin elasticity with a noninvasive MPA 580 Cutometer probe (Courage + Khazaka, DE), which used a negative pressure to aspirate a small section of the skin. From each subject, measurements were taken from the skin of the cheek, lateral to the nostril. The time/strain mode was used with three cycles of 3s traction under a negative pressure separated by 3‐s relaxation periods. The skin elasticity parameters were calculated from the measured values, R2, R5, and R7 which represented gross elasticity, net elasticity, and skin firmness, respectively. Data for all skin elasticity parameters were acquired three times and expressed as a mean.
2.6. Statistical analysis
All statistical analyses were performed using SPSS 25.0 (IBM, Armonk, US). Pearson's correlation coefficient was used to examine any correlation between variables, and a P value < 0.05 was considered statistically significant.
3. RESULTS
3.1. Validation of FGIS
We verified the FGIS measurement accuracy using a commercial SAF measuring instrument. Figure 3A shows that reference SAF (AFref) was highly correlated with SGIforearm (R = 0.64, P < .01). Figure 3B shows that SGIcheek had a relatively moderate correlation with AFref (R = 0.40, P < .01). Meanwhile, SGIforearm and SGIcheek were moderately correlated with each other (R = 0.51, P < .01).
FIGURE 3.
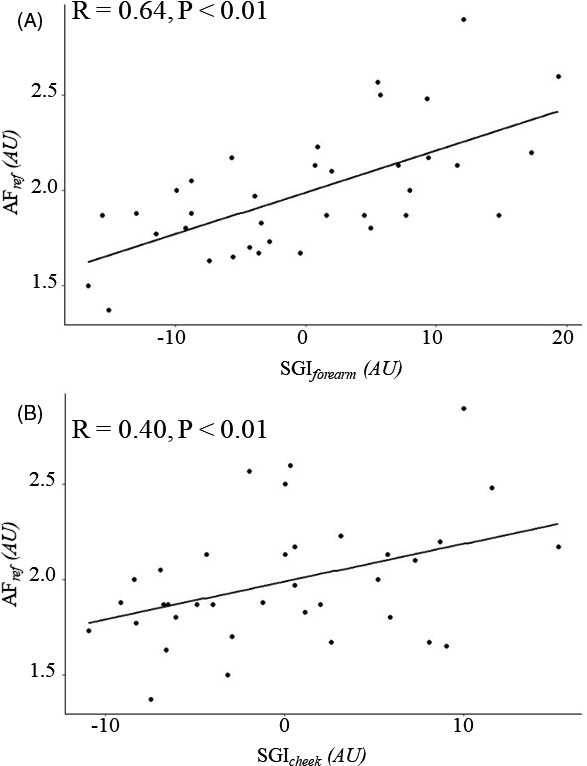
Scatter plots for instrument comparison of the advanced glycation end product (AGE) level to validate the facial glycation imaging system (FGIS). Correlations between AFref and (a) SGIforearm (R = 0.64, P < .01) and (b) SGIcheek (R = 0.40, P < .01). AGEs, advanced glycation end products; AU, arbitrary unit; AFref, skin autofluorescence from the volar forearm measured using the AGE Reader mu; SGIcheek, skin glycation index from the cheek measured using the FGIS; SGIforearm, skin glycation index from the volar forearm measured using the FGIS
3.2. Skin elasticity according to AGE accumulation
We compared the SGI with skin elasticity data to evaluate whether the AGE accumulation was directly related to facial cheek skin elasticity reduction. Figure 4 shows that SGIcheek was negatively correlated with cheek skin elasticity (R2cheek, R5cheek, and R7cheek). Neither AFref nor SGIforearm (both measured in the forearm) was significantly correlated with cheek skin elasticity (Table 1).
FIGURE 4.
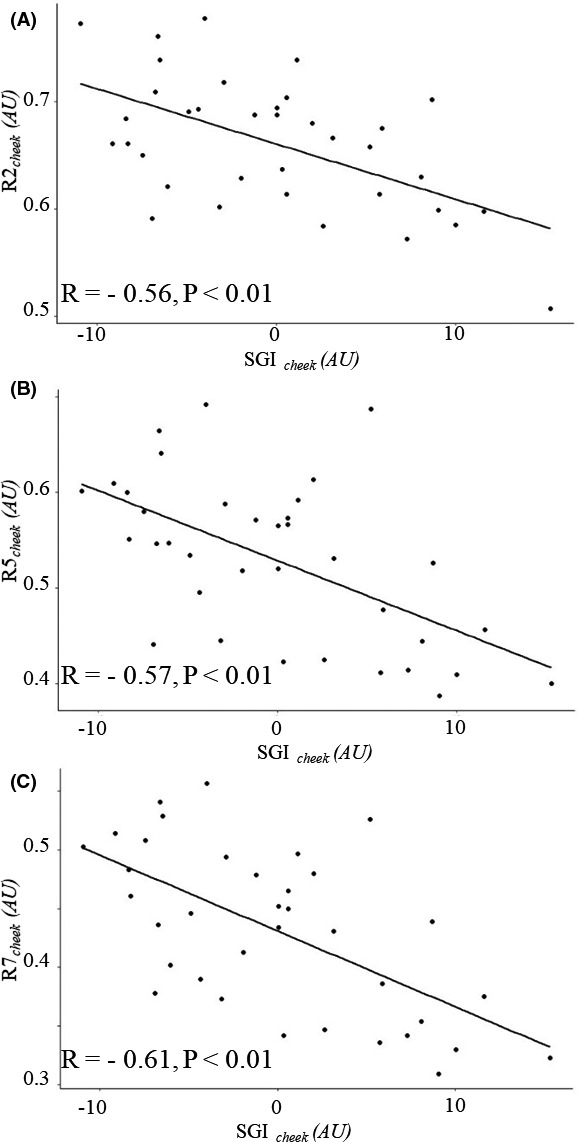
Scatter plots of cheek skin elasticity according to the AGE level. Correlations between SGIcheek and (a) R2 cheek (R = −0.56, P < .01), (b) R5cheek (R = −0.57, P < .01), and (c) R7cheek (R = −0.61, P < .01). AGEs, advanced glycation end products; SGIcheek, skin glycation index from the cheek measured using the facial glycation imaging system; R2 cheek, gross elasticity; R5cheek, net elasticity; R7cheek, skin firmness; AU, arbitrary unit
TABLE 1.
Pearson's correlation coefficients between the skin glycation index and skin elasticity
| Skin glycation index | Skin elasticity | ||
|---|---|---|---|
| R2cheek | R5cheek | R7cheek | |
| SGIcheek | −0.56** | −0.57 ** | −0.61 ** |
| SGIforearm | −0.16 | −0.24 | −0.32 |
| AFref | −0.25 | −0.29 | −0.34* |
SGIcheek, skin glycation index from the cheek measured using the facial glycation imaging system (FGIS); SGIforearm, skin glycation index from the volar forearm measured using the FGIS; AFref, skin autofluorescence from the volar forearm measured using the AGE Reader mu; R2 cheek, gross elasticity; R5cheek, net elasticity; R7cheek, skin firmness.
P < .05.
P < .01.
3.3. Correlation between AGEs and age
Figure 5 shows the correlation between the SGI and age. A significant correlation was observed between age and SGIforearm (R = 0.44, P < .01; Figure 4A) and between age and SGIcheek (R = 0.48, P < .01; Figure 5B). Figure 5C shows that the average AGE level increased with age group. Compared with subjects in their 20s, the average SGIcheek values were significantly higher in those subjects in their 40s and 50s. However, there was no significant difference between subjects in their 20s and those in their 30s.
FIGURE 5.
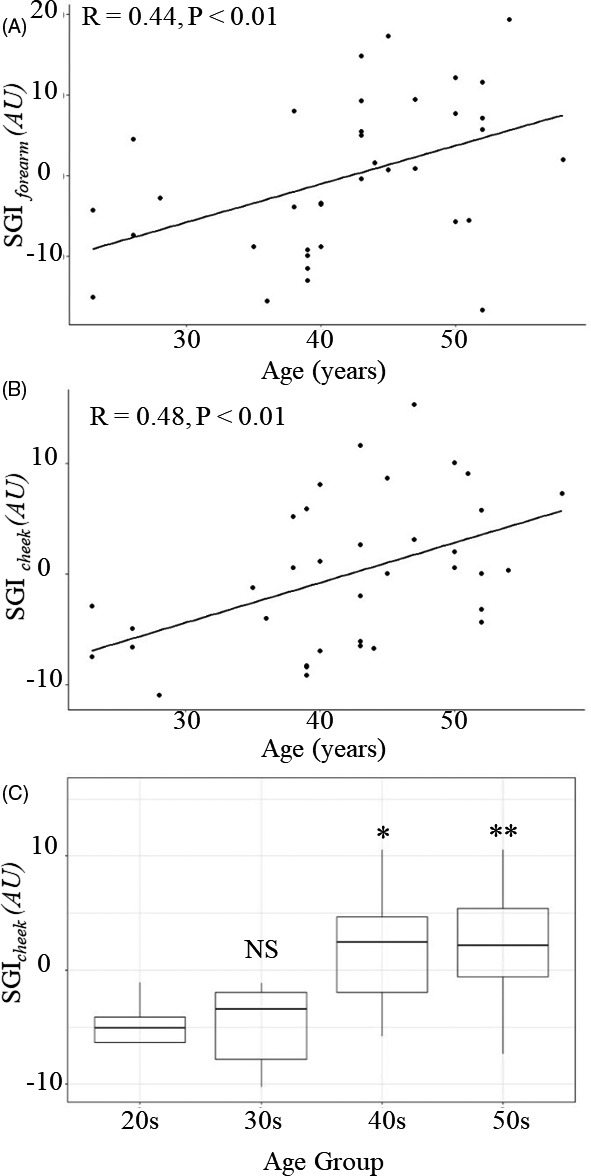
Scatter plots of the AGE level according to age (years). Correlations between age and (a) SGIforearm (R = 0.44, P < .01) and (b) SGIcheek (R = 0.48, P < .01). (c) Average SGIcheek value by age group (NS, no significant difference; *P < .05; **P < .01; compared with the 20s age group). AGEs, advanced glycation end products; SGIcheek, skin glycation index from the cheek measured using the facial glycation imaging system (FGIS); SGIforearm, skin glycation index from the volar forearm measured using the FGIS; AU, arbitrary unit
4. DISCUSSION
In this study, we developed a convenient and accurate FGIS to evaluate facial SGI measurement in situ. Nonfluorescent chromophores in the skin, such as melanin in the epidermis and hemoglobin in the dermis, can selectively absorb excitation light and affect SAF.21 Therefore, various studies have used skin brightness correction to accurately measure the SAF. For example, Hull et al performed a simple calibration for AGE autofluorescence based on empirical optimization using both fluorescence and reflection spectra.22 Meanwhile, Coremans et al initially calculated SAF by dividing the average light intensity of the emission range by the average light intensity of the light reflected from the tissue within the excitation range for skin color correction.23 In this study, the SGI calculation algorithm that corrected skin reflectance was also able to derive an accurate facial SGI with high correlation with the reference device. The correlation between SGIforearm and SGIcheek was only moderate, which is consistent with the findings of a previous study by Larson et al17 Since the volar forearm skin was less frequently exposed to UV rays and the melanocytes were small compared with those in the facial skin, the SGI in the volar forearm seemed to be relatively high.
We tried to directly observe the correlation between AGE accumulation in the facial skin and skin elasticity. In skin aging, the AGE accumulation is mainly formed in collagen residues such as lysine and arginine. Collagen not only serves as a mechanical support frame for cells and tissues in the skin but also represents an active ingredient that can interact with cells and affect various cellular functions, such as migration, differentiation, and proliferation. Collagen glycation forms cross‐links between adjacent collagen fibers, leading to a decrease in overall skin elasticity owing to reduced flexibility.24, 25 As a result, we observed that the cheek SGI was negatively correlated with the skin elasticity values R2cheek, R5cheek, and R7cheek. Furthermore, the SGI as well as other signs of aging sharply increased in subjects who were in their 40s and 50s. These results show that in cosmetic studies, anti‐glycation strategies, such as preventing or inhibiting the formation of AGEs and destroying already formed AGEs, are likely to have a significant correlation with improving skin elasticity. The FGIS can be useful to clarify the in situ degradation of AGEs in the clinical assessment of anti‐glycation cosmetics.
Here, we demonstrated a novel FGIS and identified a correlation between the SGI, skin elasticity, and aging. The SGI showed a good correlation with a decrease in skin elasticity and aging. The FGIS offers great potential for understanding AGE accumulation in facial skin. Ultimately, our novel system allows in situ SGI measurement and the SGI trend to be tracked over time and used to recommend anti‐glycation cosmetics.
AUTHOR CONTRIBUTIONS
All authors participated in the conduct of the research and writing of the manuscript.
ETHICAL STATEMENT
The clinical experimental protocol was approved by the Ethical Committee of LG Household & Health Care (HEET‐20191008, October 31, 2019). Written informed consent was obtained from all subjects included in this study.
ACKNOWLEDGMENTS
We express our appreciation to PSIPLUS for the assistance with device modification for measurement and analysis. We would like to thank Editage (www.editage.co.kr) for English language editing.
Lee J, Jeong ET, Lim J‐M, Park SG. Development of the facial glycation imaging system for in situ human face skin glycation index measurement. J Cosmet Dermatol. 2021;20:2963–2968. 10.1111/jocd.13943
Funding information
LG Household & Health Care.
REFERENCES
- 1.Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462‐1470. [DOI] [PubMed] [Google Scholar]
- 2.Kohl E, Steinbauer J, Landthaler M, Szeimies RM. Skin ageing. J Eur Acad Dermatol Venereol. 2011;25:873‐884. [DOI] [PubMed] [Google Scholar]
- 3.Gkogkolou P, Böhm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinol. 2012;4:259‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hori M, Yagi M, Nomoto K, Shimode A, Ogura M, Yonei Y. Inhibition of advanced glycation end product formation by herbal teas and its relation to anti‐skin aging. Anti Aging Med. 2012;9:135‐148. [Google Scholar]
- 5.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end‐products: A review. Diabetologia. 2001;44:129‐146. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed N. Advanced glycation endproducts–role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3‐21. [DOI] [PubMed] [Google Scholar]
- 7.Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3‐14. [DOI] [PubMed] [Google Scholar]
- 8.Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM. Glucosepane is a major protein cross‐link of the senescent human extracellular matrix. Relationship with diabetes. J Biol Chem. 2005;280:12310‐12315. [DOI] [PubMed] [Google Scholar]
- 9.Pageon H. Reaction of glycation and human skin: the effects on the skin and its components, reconstructed skin as a model. Pathol Biol (Paris). 2010;58:226‐231. [DOI] [PubMed] [Google Scholar]
- 10.Ohshima H, Oyobikawa M, Tada A, et al. Melanin and facial skin fluorescence as markers of yellowish discoloration with aging. Skin Res Technol. 2009;15:496‐502. [DOI] [PubMed] [Google Scholar]
- 11.Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027‐39031. [DOI] [PubMed] [Google Scholar]
- 12.Corstjens H, Dicanio D, Muizzuddin N, et al. Glycation associated skin autofluorescence and skin elasticity are related to chronological age and body mass index of healthy subjects. Exp Gerontol. 2008;43:663‐667. [DOI] [PubMed] [Google Scholar]
- 13.Xin C, Wang Y, Liu M, Zhang B, Yang S. Correlation analysis between advanced glycation end products detected noninvasively and skin aging factors. J Cosmet Dermatol. 2021;20(1):243‐248. [DOI] [PubMed] [Google Scholar]
- 14.Zhu P, Ren M, Yang C, Hu YX, Ran JM, Yan L. Involvement of RAGE, MAPK and NF‐κB pathways in AGEs‐induced MMP‐9 activation in HaCaT keratinocytes. Exp Dermatol. 2012;21:123‐129. [DOI] [PubMed] [Google Scholar]
- 15.Lohwasser C, Neureiter D, Weigle B, Kirchner T, Schuppan D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor‐alpha. J Invest Dermatol. 2006;126:291‐299. [DOI] [PubMed] [Google Scholar]
- 16.Beisswenger PJ, Howell S, Mackenzie T, Corstjens H, Muizzuddin N, Matsui MS. Two fluorescent wavelengths, 440(ex)/520(em) nm and 370(ex)/440(em) nm, reflect advanced glycation and oxidation end products in human skin without diabetes. Diabetes Technol Ther. 2012;14:285‐292. [DOI] [PubMed] [Google Scholar]
- 17.Larsson M, Favilla R, Strömberg T. Assessment of advanced glycated end product accumulation in skin using auto fluorescence multispectral imaging. Comput Biol Med. 2017;85:106‐111. [DOI] [PubMed] [Google Scholar]
- 18.Meerwaldt R, Graaf R, Oomen PHN, et al. Simple non‐invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324‐1330. [DOI] [PubMed] [Google Scholar]
- 19.Gerrits EG, Lutgers HL, Kleefstra N, et al. Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care. 2008;31:517‐521. [DOI] [PubMed] [Google Scholar]
- 20.Stirban A, Heinemann L. Skin autofluorescence ‐ a non‐invasive measurement for assessing cardiovascular risk and risk of diabetes. Eur Endocrinol. 2014;10:106‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koetsier M, Nur E, Chunmao H, et al. Skin color independent assessment of aging using skin autofluorescence. Opt Express. 2010;18:14416‐14429. [DOI] [PubMed] [Google Scholar]
- 22.Hull EL, Ediger MN, Unione AHT, Deemer EK, Stroman ML, Baynes JW. Noninvasive, optical detection of diabetes: model studies with porcine skin. Opt Express. 2004;12:4496‐4510. [DOI] [PubMed] [Google Scholar]
- 23.Coremans JM, Ince C, Bruining HA, Puppels GJ. (Semi‐)quantitative analysis of reduced nicotinamide adenine dinucleotide fluorescence images of blood‐perfused rat heart. Biophys J. 1997;72:1849‐1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pageon H. Reaction of glycation and human skin: The effects on the skin and its components, reconstructed skin as a model. Pathol Biol (Paris). 2010;58:226‐231. [DOI] [PubMed] [Google Scholar]
- 25.Jeanmaire C, Danoux L, Pauly G. Glycation during human dermal intrinsic and actinic ageing: An in vivo and in vitro model study. Br J Dermatol. 2001;145:10‐18. [DOI] [PubMed] [Google Scholar]


