Abstract
The Wnt pathway is upregulated in tendinopathy, affecting inflammation and tenocyte differentiation. Given its potential role in tendinopathy, this signaling pathway may be a relevant target for treatment. The current study examined the therapeutic potential of SM04755, a topical, small‐molecule Wnt pathway inhibitor, for the treatment of tendinopathy using in vitro assays and animal models. In vitro, SM04755 decreased Wnt pathway activity, induced tenocyte differentiation, and inhibited catabolic enzymes and pro‐inflammatory cytokines in human mesenchymal stem cells, rat tendon‐derived stem cells, and human peripheral blood mononuclear cells. Evaluation of the mechanism of action of SM04755 by biochemical profiling and computational modeling identified CDC‐like kinase 2 (CLK2) and dual‐specificity tyrosine phosphorylation‐regulated kinase 1A (DYRK1A) as molecular targets. CLK and DYRK1A inhibition by siRNA knockdown or pharmacological inhibition induced tenocyte differentiation and reduced tenocyte catabolism. In vivo, topically applied SM04755 showed therapeutically relevant exposure in tendons with low systemic exposure and no detectable toxicity in rats. Moreover, SM04755 showed reduced tendon inflammation and evidence of tendon regeneration, decreased pain, and improved weight‐bearing function in rat collagenase‐induced tendinopathy models compared with vehicle control. Together, these data demonstrate that CLK2 and DYRK1A inhibition by SM04755 resulted in Wnt pathway inhibition, enhanced tenocyte differentiation and protection, and reduced inflammation. SM04755 has the potential to benefit symptoms and modify disease processes in tendinopathy.
Keywords: inflammation, repair, stem cells, tendon, therapeutics, treatment
1. INTRODUCTION
Tendinopathy constitutes an estimated 30% of all musculoskeletal ailments in the United States and is a painful injury that is associated with sports and daily tasks.1 Current research suggests that tendinopathy may be a continuum of tendon pathology including inflammation and tendon degeneration and failed healing.2, 3 Tendons are highly prone to injury and, when severely damaged, their intrinsic hypocellularity and hypovascularity make their natural healing process slow and inefficient.4
Despite the high prevalence of chronic tendinopathy, its underlying pathogenesis is not fully understood. Treatments have focused on alleviating symptoms; while most current therapeutic options provide symptomatic relief, some are thought to impair long‐term tendon healing.5 Although cell‐based therapies have demonstrated some success in regenerating tendons,6 efficacy has not been well established. Tissue‐resident mesenchymal stem cells (MSCs) and progenitor cells in the tendon, often referred to as tendon‐derived stem cells (TDSCs),7, 8 can support tendon healing by enhancing vascularization, promoting collagen deposition, and improving extracellular matrix organization.9, 10 Differentiation of endogenous stem cells represents a promising target for the development of tendon‐regenerative therapies.
The Wnt pathway plays an important role in organogenesis, stem cell maintenance, morphogenesis, and tissue remodeling.11 In the canonical Wnt/β‐catenin signaling pathway, binding of Wnt proteins to cell surface receptors leads to inhibition of β‐catenin phosphorylation and proteasome‐mediated degradation. Stabilized β‐catenin then translocates to the nucleus and interacts with the T‐cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors to induce expression of Wnt target genes.11 Wnt signaling is subject to complex modulation at multiple levels, resulting in fine‐tuned signaling. Therefore, modulating this pathway is an attractive therapeutic approach for regenerative medicine.12
Recently, intranuclear CDC‐like kinases (CLKs) and dual‐specificity tyrosine phosphorylation‐regulated kinase 1A (DYRK1A) were validated as novel targets for Wnt pathway modulation.13, 14 Knockdowns of these kinases decreased expression of Wnt target genes independent of β‐catenin while upregulating secreted Wnt pathway inhibitors in hMSCs.13 CLKs have been implicated in splicing regulation through phosphorylation of serine/arginine‐rich splicing factor (SRSF) proteins,15 which is required for SRSF nuclear localization and splicing functions.16 Inhibition of Wnt pathway activity with CLK knockdown and CLK‐specific inhibitors supported the involvement of CLKs in regulating Wnt signaling at a posttranscriptional level,13 possibly by alternative splicing of Wnt target genes. In addition, DYRK1A pharmacological inhibition or knockdown was sufficient for inhibiting inflammatory cytokine production through STAT3, a known DYRK1A target.13, 17
Wnt signaling plays a vital role in the regulation of musculoskeletal homeostasis and tissue‐resident stem cell proliferation and differentiation.11 Wnt pathway activation and its effects on TDSCs might contribute to tissue metaplasia and failed healing in tendinopathy. Indeed, Wnt3a stimulation induced osteogenic marker expression in rat TDSCs (rTDSCs), suggesting erroneous differentiation and depletion of TDSCs for tendon repair.18 Moreover, increased expression of Wnt pathway mediators was observed in healing fibroblast‐like cells, chondrocyte‐like cells, and ossified deposits in an animal model and clinical samples of tendinopathy.18 Wnt signaling has also been implicated in matrix metalloproteinase induction in response to injury and pro‐inflammatory cytokines.19, 20 Together, this suggests that the Wnt pathway plays a central role in tendinopathy pathogenesis, making it a relevant target for treatment.
Here, we describe our findings showing that SM04755 inhibited intranuclear kinases and modulated the Wnt pathway to induce tenocyte differentiation, reduce tendon‐destroying proteases, and decrease inflammatory cytokine production. Moreover, we demonstrate that topical administration of SM04755 reduced inflammation, prevented tendon destruction, and promoted tendon regeneration in a preclinical animal model of tendinopathy.
2. MATERIALS AND METHODS
2.1. Cell culture and assays
Cell culture (Table S1), reporter assays, and cytokine production assays were performed as previously described.13
2.2. Immunostaining, Western blot, and quantitative polymerase chain reaction
Cells were treated as indicated and incubated at 37°C, 5% CO2. Cells were fixed and immunostained with specific antibodies. Secondary antibody alone was used as a negative control. Cells were imaged using a CX5 imager (Thermo Fisher). Total protein was extracted using RIPA buffer (Sigma‐Aldrich). Nuclear and cytoplasmic fractions were prepared using the NE‐PER™ Kit (Thermo Fisher). Western blot was performed as previously described13 using primary and secondary antibodies (Table S2). Quantitative polymerase chain reaction (qPCR) was performed using SYBR® Green or TaqMan™ primers as previously described.13
2.3. Kinase assays and molecular modeling
SM04755 (1 µM) was screened through 318 kinases (Thermo Fisher) and molecular modeling was performed as previously described.13
2.4. siRNA knockdowns
Reverse transfections were performed with siRNA (GE Dharmacon, Table S3) using Lipofectamine RNAiMAX (Thermo Fisher) as previously described.13
2.5. Tendon protection
rTDSCs were differentiated into tenocytes using BMP2 + FGF12 (both at 20 ng/ml) for 14 days followed by a 72‐h treatment with TNF‐α (20 ng/ml) + Oncostatin M (10 ng/ml) or IL‐1β (10 ng/ml)21, 22 and SM04755.
2.6. Pharmacokinetics
Following a single topical application of an aqueous solution of SM04755 (0.3 mg/cm2) on the skin covering the Achilles tendon region (2 cm2) of Sprague‐Dawley rats, tendon and plasma were collected at various timepoints, flash frozen, and stored at −70°C. SM04755 was extracted from plasma or homogenized tendon samples and analyzed as previously described.20
2.7. Rat collagenase‐induced tendinopathy models
At 10 weeks postnatal age, male CD® IGS rats (Charles River) were injected with 50 µl of collagenase (Sigma‐Aldrich, 10 mg/ml Type IA in PBS, pH 7.4, ~469 units/mg) or needle punctured (sham) into the Achilles tendon near the osteotendinous junction. For the repeat‐injection model, collagenase injections were performed at Days 21 and 40 after the first injection. At the indicated time following collagenase injection, rats were randomized and SM04755 or vehicle control was rubbed into the skin near the Achilles tendon using an applicator for 10 s. Rats were periodically observed for pain, illnesses, or abnormalities, and blood was collected for plasma processing. Pain was measured using the Von Frey apparatus (Ugo Basile) with three replicate measurements per limb. Gait was measured using an Incapacitance Meter (Stoelting) with five replicate measurements per rat. Circulating plasma levels of CXCL1 were measured by ELISA per manufacturer's instructions (PeproTech).
At the indicated times, tendons were isolated, fixed in 10% formalin (Thermo Fisher), and embedded in paraffin for hematoxylin and eosin (H&E) staining (Pacific Pathology). Tendons were also flash frozen in liquid nitrogen and stored at −80°C for biochemical analysis. Five‐µm‐thick sections, each 100 µm apart, were stained with either H&E or Sirius Red. Ten slides with two sections per slide were obtained from each tendon, representing 40 total sections. At least 24 sections from each rat were imaged using a light microscope (EVOS® FL, Life Technologies). All animal experiments were performed two times independently.
2.8. Tendon histological scoring
Histological evaluation of H&E‐stained sections was performed by two blinded observers using a modified scoring system9 (Table S4).
3. RESULTS
3.1. Identification of Wnt pathway inhibitor SM04755
Small‐molecule inhibitors of Wnt signaling were identified using a high‐throughput TCF/LEF reporter assay in APC‐mutant SW480 colon cancer cells23 with constitutively active Wnt signaling. Hits were counter screened against cells carrying an EF1α‐driven luciferase reporter to eliminate compounds with off‐target effects. Iterative medicinal chemistry efforts guided by structure‐activity relationship of these compounds generated SM04755 (TCF/LEF EC50 = 150.4 nM), which did not affect the EF1α luciferase reporter (Figure 1A). SM04755 was 4‐ to 100‐fold more potent in the TCF/LEF reporter assay than known Wnt pathway inhibitors (i.e., IWP‐2, IWR‐1, CX‐4945, KY02111, FH535, XAV939, LGK974, ICG001, and iCRT14) and previously reported tenocyte proliferative or regenerative compounds (all‐trans retinoic acid [ATRA], CD1530, [Tyr4]‐Bombesin, Tazarotene, 4‐PPBP maleate, and Oxotremorine M) (Figure S1A–D). Wnt signaling inhibition was confirmed by qRT‐PCR for Wnt pathway genes (i.e., LEF1, TCF4, TCF7, AXIN2, CTNNB1, ASCL1, MYC, and CCND1) in SW480 cells (Figure S1E), rTDSCs (CD90+, CD44+, CD31−, CD34−, CD105−, and CD73−)24 (Figures 1B and S2A), and bone‐marrow‐derived hMSCs (CD29+, CD44+, CD166+, CD105+, and CD45−) (Figure S1F). SM04755 also inhibited the expression of AXIN2, LEF1, TCF4, and TCF7 in hMSCs and rTDSCs when the Wnt pathway was selectively activated using either Wnt3a or CHIR99021 (a GSK‐3β inhibitor)25 (Figure S2B,C).
Figure 1.
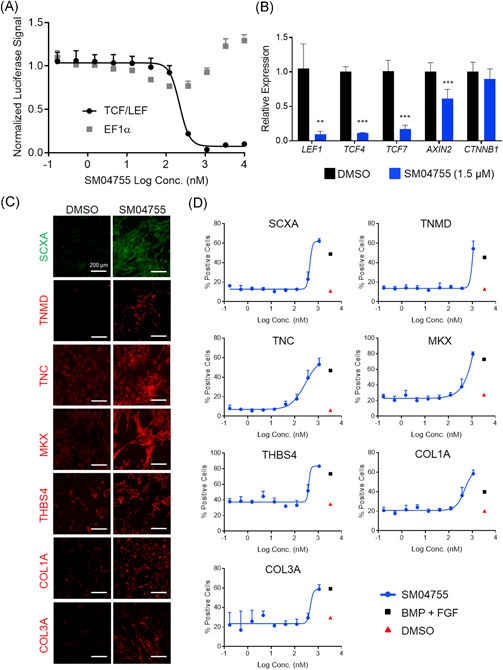
SM04755 was a potent inhibitor of canonical Wnt signaling and induced tenocyte differentiation in rTDSCs. (A) Doseresponse of TCF/LEF promoter‐driven or EF1α promoter‐driven luciferase reporters in SW480 cells treated with SM04755 or DMSO for 48 h (n = 4, mean ± SD). (B) Expression of Wnt pathway genes in rTDSCs measured by qRT‐PCR. Fold change relative to DMSO (n = 3, mean ± SEM). (C) Representative images of immunostaining for tenocyte markers of rTDSCs treated with SM04755 (1.5 μM) or DMSO for 4 days. (D) Dose‐response quantification of the percent of total cells that were positive for the tenocyte markers in (C) (n = 4, mean ± SD). BMP + FGF was used as a positive control. Scale bars: 200 μm; **p < .01, ***p < .001, t‐test
3.2. SM04755 induced tenocyte differentiation in vitro
Tendon regeneration results from the differentiation of MSCs or TDSCs into tenocytes.24, 26 SM04755 promoted dose‐dependent differentiation of hMSCs and rTDSCs into tenocytes expressing tenocyte markers Scleraxis A (SCXA), Tenomodulin (TNMD), Tenascin C (TNC), Mohawk (MKX), Thrombospondin 4 (THBS4), and Type I and Type III collagens produced by the tendon (Figures 1C,D and S3A,B), thus increasing the number of differentiated stained cells by ~4‐ to 10‐fold (p < .0001) compared with DMSO‐treated control. Compared with DMSO control, expression of osteoblast genes (alkaline phosphatase [ALPL], osteocalcin [BGLAP], and RUNX2),27, 28 chondrocyte genes (aggrecan [ACAN], RUNX1, GDF5, and SOX9),20 and adipocyte genes (peroxisome proliferator‐activated receptor gamma [PPARγ], CCAAT/enhancer‐binding protein alpha [C/EBPα], and leptin [LEP])29, 30, 31 was significantly decreased (p < .05) in SM04755‐treated hMSCs (Figure S3C–E). Moreover, expression of adiponectin (ADIPOQ), a hormone shown to increase tendon progenitor cell proliferation and differentiation,32 was significantly increased (p < .05) in cells treated with SM04755 compared with DMSO control (Figure S3E).
In line with their activities in the TCF/LEF reporter assay (Figure S1A–D), several Wnt pathway inhibitors and tendon differentiation‐inducing compounds were less potent than SM04755 in promoting differentiation (Figure S4).
3.3. SM04755 demonstrated anti‐inflammatory effects in vitro
SM04755 dose‐dependently inhibited production of TNF‐α, IL‐6, and IL‐8 in LPS‐stimulated peripheral blood mononuclear cells (PBMCs; EC50 = 169 nM, EC50 = 395 nM, and EC50 = 450 nM, respectively; Figure 2A) and THP‐1 cells (TNF‐α EC50 = 547 nM and IL‐6 EC50 = 356 nM; Figure S5A). SM04755 also inhibited a panel of inflammatory cytokines in LPS‐stimulated PBMCs (Figure 2B). Furthermore, SM04755 decreased IL‐6 production in LPS‐stimulated THP‐1 cells (EC50 = 270.7 nM; Figure S5B); this was comparable to immunosuppressant cyclosporin A and less potent than dexamethasone and prednisolone.13 SM04755 also significantly inhibited (p < .01) the expression of IL‐1β, IL‐6, and IL‐8 in TNF‐α and Oncostatin M‐stimulated rTDSCs compared with DMSO (Figure S5C).
Figure 2.
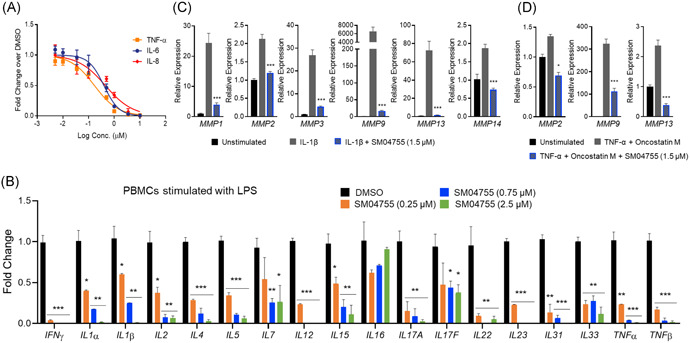
SM04755 inhibited inflammatory cytokine production and protected tenocytes from catabolic breakdown in vitro. (A) Primary human PBMCs stimulated with LPS (500 ng/ml) and treated with SM04755 or DMSO for 24 h. Doseresponse of TNF‐α, IL‐6, and IL‐8 secretion measured by ELISA (n = 3, mean ± SEM). (B) Gene expression of inflammatory cytokines in PBMCs stimulated with LPS (500 ng/ml) and treated with SM04755 for 24 h measured by MSD‐based ELISA. Fold change relative to DMSO (n = 3, mean ± SEM). (C,D) Tenocytes treated with IL‐1β (10 ng/ml; C) or TNF‐α (20 ng/ml) + Oncostatin M (10 ng/ml; D) and DMSO or SM04755 (1.5 μM) for 72 h. Gene expression of proteases measured by qRT‐PCR. Fold change relative to unstimulated control (n = 4, mean ± SEM, *p < .05, **p < .01, ***p < .001, one‐way ANOVA)
3.4. SM04755 inhibited tenocyte catabolism in vitro
The effects of SM04755 on tenocyte catabolism under tendinopathy‐like conditions were evaluated in rTDSC‐derived tenocytes treated with either IL‐1β (10 ng/ml) or a combination of TNF‐α (20 ng/ml) and Oncostatin M (10 ng/ml).33, 34, 35 Treatment with IL‐1β upregulated gene expression of matrix‐degrading enzymes MMP1, MMP2, MMP3, MMP9, MMP13, and MMP14 (Figure 2C), while combined TNF‐α and Oncostatin M treatment upregulated expression of MMP2, MMP9, and MMP13 (Figure 2D), but not MMP1, MMP3, and MMP14 (data not shown), compared with unstimulated tenocytes. Treatment with SM04755 significantly inhibited (p < .05) cytokine‐induced expression of matrix‐degrading enzymes (Figure 2D,E), demonstrating potential for protecting tendons from catabolic breakdown.
3.5. SM04755 potently inhibited CLK2, CLK3, and DYRK1A
Biochemical profiling using SM04755 (1 µM) showed ≥90% inhibition of 12 kinases (out of 318) compared with DMSO. CLK2 was the most potently inhibited kinase (IC50 = 0.822 nM) with six other kinases showing ≥90% inhibition and IC50s within 15‐fold of the IC50 of CLK2. DYRK1A and DYRK1B, which are within the same CMGC kinase group as the CLKs, showed IC50 values of 5.5 nM and 4.6 nM, respectively. Overall, SM04755 demonstrated selectivity against wild‐type kinases (≥90% inhibition or IC50 ≤ 100 nM, representing 3.8% of 318 kinases; Figure 6A and Tables S5–6).
Figure 6.
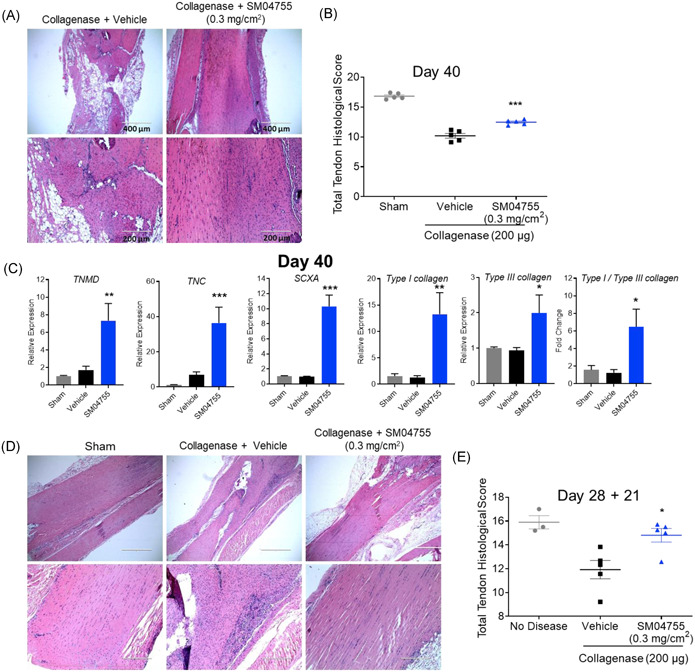
SM04755 inhibited inflammation and promoted in vivo tendon healing in chronic and delayed‐treatment models of tendinopathy in rats. (A) Representative images of rat tendons stained with H&E from the collagenase model on Day 40. (B) Histological score of rat tendons in (A; n = 5, mean ± SEM, ***p < .001, one‐way ANOVA). (C) Expression of tenocyte genes in rat tendons measured by qRT‐PCR. Fold change relative to sham control (n = 12, mean ± SEM, *p < .05, **p < .01,***p< .001,one‐way ANOVA). (D) Representative images of rat tendons stained with H&E from the delayed‐treatment model on Day 28 + 21. (E) Histological score of rat tendons in (D; n = 3 for sham, n = 5 for vehicle or SM04755‐treated, mean ± SEM, *p < .05, one‐way ANOVA)
Computational modeling of SM04755 with CLK2, CLK3, and DYRK1A identified multiple hydrogen‐bond, donor‐acceptor sites that potentially drive SM04755 activity. SM04755 docked to ATP‐binding sites of the kinases, forming hydrogen bonds with GLU244, LEU246, and LYS193 in CLK2, GLU237, LEU239, and LYS186 in CLK3, and GLU239, LEU241, and LYS188 in DYRK1A (Figure S6B). For CLK2, CLK3, and DYRK1A, 25/100 dockings (docking energy [DE] = −11.5 ± 2.0 kcal/mol), 38/100 dockings (DE = −13.5 ± 2.0 kcal/mol), and 49/100 dockings (DE = −10.7 ± 2.0 kcal/mol), respectively, landed within the 2 Å root‐mean‐square distance (RMSD). The predicted binding mode would completely block ATP binding, thus inhibiting CLK2, CLK3, and DYRK1A function.
3.6. SM04755 inhibited CLK‐ and DYRK1A‐mediated signaling
In vitro biochemical kinase assays for CLK2, CLK3, and DYRK1A confirmed potent inhibition of CLK2 (IC50 = 5.0 nM), CLK3 (IC50 = 48.6 nM), and DYRK1A (IC50 = 3.5 nM) by SM04755 (Figure 3A). Compared with CLK2 inhibitors (i.e., CC‐671, TG003, ML167, Leucettine L41, and ML315)36, 37, 38, 39, 40 and DYRK1A inhibitors (i.e., harmine, EGCG, AZ191, ID‐8, INDY, L41, and ML315)37, 38, 41, 42, 43, 44 that are in the development for oncology or as tool compounds, SM04755 was 2‐ to 300‐fold more potent than other compounds.13
Figure 3.
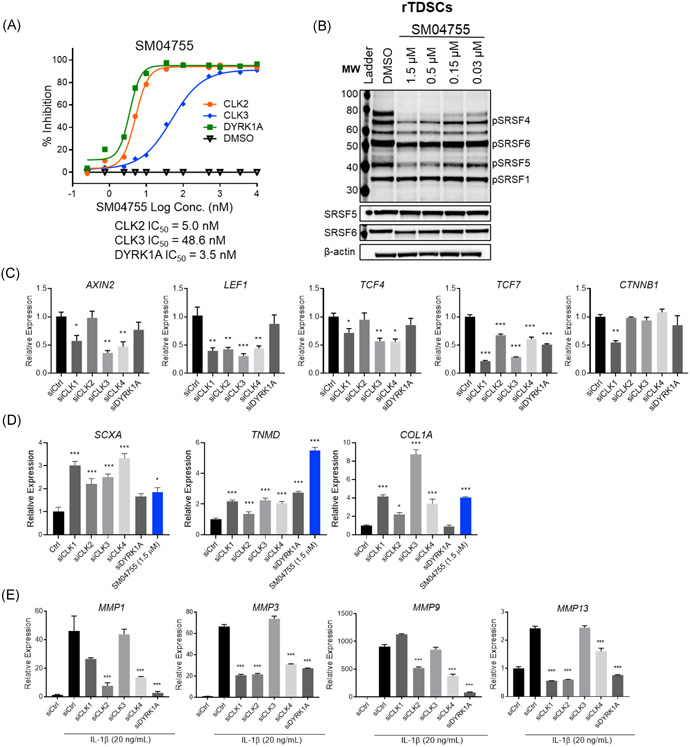
SM04755 was a potent inhibitor of CLKs and DYRK1A. (A) Doseresponse for in vitro biochemical inhibition of CLK2, CLK3, and DYRK1A by SM04755 (n = 4). (B) Western blot of phospho‐SRSFs in rTDSCs following treatment with SM04755 or DMSO for 24 h. (C) Gene expression of Wnt pathway markers in rTDSCs at 96 h following treatment with either nontargeted control siRNA (siCtrl) or specific siRNAs. Fold change relative to siRNA control (n = 3, mean ± SEM, *p < .05, **p < .01, ***p < .001,t‐test). (D) Expression of differentiation markers in hMSCs at 5 days following treatment with siCtrl or specific siRNAs (1.5 µM). Fold change relative to siRNA control (n = 3, mean ± SEM, *p < .05, ***p < .001, t‐test). (E) Gene expression of proteases in tenocytes at 72 h following treatment with siCtrl or specific siRNAs (n = 3, mean ± SEM, ***p < .001, one‐way ANOVA)
Similar to hMSCs, THP‐1 cells, and PBMCs,13 CLK1, CLK2, CLK3, and DYRK1A were predominantly localized in the nucleus, while CLK4 was predominantly localized in the cytoplasm in rTDSCs (Figure S6C). No significant changes in the expression of CLK2, CLK3, CLK4, and DYRK1A were observed in rTDSCs stimulated with Wnt3a or CHIR99021 compared with unstimulated cells; however, CLK1 gene expression was decreased with stimulation (Figure S6D).
CLKs exert their function by phosphorylating SRSF proteins.15, 45 Compared with unstimulated cells, SRSF gene expression was not significantly changed in Wnt3a‐ or CHIR99021‐stimulated rTDSCs (Figure S6E), hMSCs, THP‐1 cells, and PBMCs.13 SM04755 dose‐dependently inhibited the CLK‐mediated phosphorylation of SRSF1, SRSF4, SRSF5, and SRSF6 in rTDSCs (Figure 3B) and hMSCs (Figure S7A) and was more potent than the CLK2/TTK inhibitor CC‐671. In contrast, harmine (a DYRK1A/B inhibitor) had no effects on SRSF phosphorylation (Figure S7B,C). Spliceosome modulation by SM04755 was shown by enlargement of nuclear speckles with immunostaining for the phospho‐epitope of the non‐snRNP factor SC3545 in hMSCs compared with DMSO (Figure S8). SM04755 treatment of hMSCs also led to intron retention in the mRNA of several Wnt pathway genes (Figure S9).
3.7. Inhibition of CLKs and DYRK1A inhibited the Wnt pathway in rTDSCs
In rTDSCs, consistent with the inhibitory effects of SM04755, siRNA knockdowns of CLKs and DYRK1A (Figure S10A) inhibited Wnt pathway gene expression (i.e., AXIN2, LEF1, TCF4, and TCF7), while knockdown of only CLK1 inhibited β‐catenin (CTNNB1) expression compared with nontargeted siRNA control (siCtrl; Figure 3C). Furthermore, while TCF7 and AXIN2 were decreased, no decrease in levels of active and total β‐catenin were observed with SM04755 treatment in CHIR‐stimulated hMSCs and 293T cells (Figure S10B,C).
3.8. Inhibition of CLKs and DYRK1A induced tenocyte differentiation
Knockdowns of CLKs and DYRK1A in rTDSCs increased tenocyte differentiation with ~2‐ to 4‐fold increases in SCXA, TNMD, and COL1A levels compared with siCtrl (Figures 3D and S11A,B), which was comparable to SM04755 treatment (Figures 1C,D and S3A,B). CC‐671 and harmine induced tenocyte differentiation but were less potent than SM04755 (Figure S11C).
3.9. Inhibition of CLK2 and DYRK1A inhibited expression of tenocyte catabolic enzymes
The role of CLKs and DYRK1A in the inhibition of metalloproteinase production was evaluated in rat tenocytes using IL‐1β‐stimulated (10 ng/ml) gene expression of MMP1, MMP3, MMP9, and MMP13. CLK2, CLK4, and DYRK1A knockdowns significantly reduced expression of all four metalloproteinases compared with siCtrl (Figure 3E). While CLK1 knockdown inhibited MMP1, MMP3, and MMP13 expression, it did not reduce MMP9 expression. No inhibition of metalloproteinases was observed with CLK3 knockdown. Additionally, compared with DMSO, SM04755 was more potent than CC‐671 and harmine in reducing MMP1, MMP3, MMP9, and MMP13 expression (Figure S11D).
3.10. SM04755 inhibited inflammatory signaling mediators NF‐κB and STAT3
The effects of SM04755 on STAT3, a known DYRK1A target,17 and other inflammatory mediators were evaluated in LPS‐stimulated THP‐1 monocytes. After 4 h of treatment, SM04755 dose‐dependently decreased phospho‐NF‐κB and total NF‐κB, while phospho‐STAT3, phospho‐FOXO1/3a, phospho‐AKT, phospho‐JNK1, phospho‐cJUN, phospho‐p38/MAPK, and TLR4 were not inhibited compared with DMSO. After 20 h of treatment, SM04755 robustly inhibited phosphorylation of NF‐κB, STAT3, JNK1, and FOXO1/3a, while AKT, cJUN, and p38/MAPK phosphorylation and TLR4 remained unchanged (Figure S12). SM04755 inhibited LPS‐stimulated gene expression of NF‐κB components (Figure 4A). Inhibition of NF‐κB, STAT3, and SRSF proteins by SM04755 was confirmed in PBMCs (Figure 4B,C). SM04755 was more potent than harmine or CC‐671 in inhibiting NF‐κB and STAT3 in LPS‐stimulated PBMCs and THP‐1 cells (Figure S13A,B) and SRSF in THP‐1 cells (Figure S13C). CLK2 and DYRK1A inhibitors demonstrated ~30‐ to 300‐fold less potent anti‐inflammatory activity than SM04755 (Figure S13D).
Figure 4.
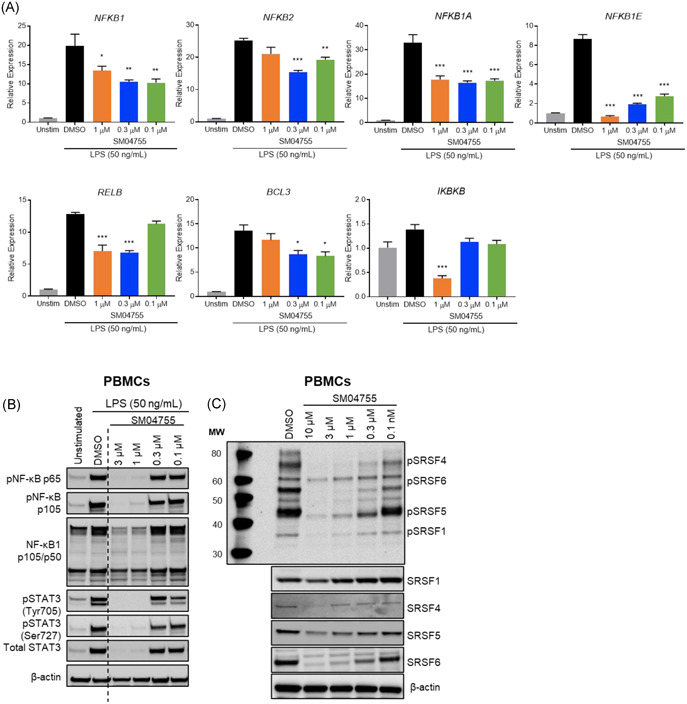
SM04755 inhibited inflammatory responses in PBMCs. (A) Gene expression of NF‐κB components in THP‐1 cells stimulated with LPS and treated with DMSO or SM04755 for 4 or 20 h measured by qRT‐PCR. Fold change relative to unstimulated control (n = 3, mean ± SEM,*p< .05,**p < .01, ***p < .001, one‐way ANOVA). (B) Expression of phospho‐NF‐κB,total NF‐κB,and STAT3 in PBMCs stimulated with LPS and treated with DMSO or SM04755 for 20 h measured by Western blot. β‐actin served as a loading control. (C) Western blot of phospho‐SRSF and total SRSF in PBMCs following treatment with SM04755 or DMSO for 1 h. β‐actin served as a loading control
3.11. A single topical application of SM04755 demonstrated exposure in the tendon over 24 h and low systemic exposure
Pharmacokinetic evaluation of a single topical application of SM04755 (0.3 mg/cm2) in rats demonstrated a tendon residence time of >24 h (Figure S14). Systemic exposure of SM04755 in plasma was approximately 800‐fold lower than tendon exposure (lower limit of quantification = 10 ng/ml) and no weight loss, swelling, and signs of pain or distress were observed in treated rats (data not shown).
3.12. SM04755 decreased inflammatory cells and pro‐inflammatory cytokine production in tendons in vivo
Collagenase‐induced tendinopathy in rats has been utilized to model acute tendon injury in humans.9 Histological analysis of tendons following a single collagenase injection into the Achilles tendon of rats showed the presence of inflammatory cells 2 h after injection with levels that progressively increased over 70 h. Accompanying tendon degeneration was evident and remained stable for 21 days (Figures 5A,B and S15).
Figure 5.
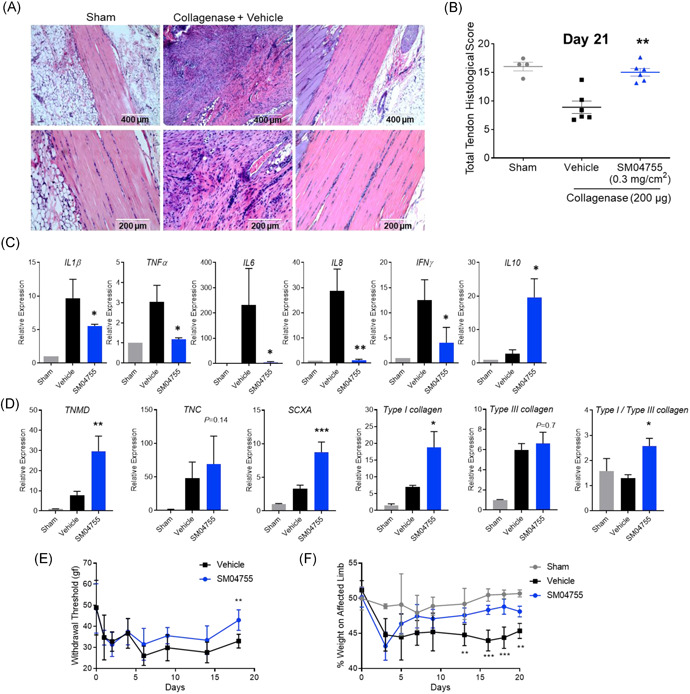
SM04755 inhibited inflammation and promoted in vivo tendon healing in a collagenase‐induced acute tendinopathy model in rats. (A) Representative images of rat tendons stained with H&E on Day 21. (B) Histological score of rat tendons in (A; n = 4 for sham, n = 6 for vehicle and SM04755, mean ± SEM, **p < .01, one‐way ANOVA). (C) Expression of pro‐ and anti‐inflammatory genes in rat tendons measured by qRT‐PCR. Fold change relative to sham control (n = 12, mean ± SEM, *p < .05, **p < .01, one‐way ANOVA). (D) Expression of tenocyte and collagen genes in rat tendons measured by qRT‐PCR. Fold change relative to sham control (n = 12, mean ± SEM, *p < .05, **p < .01, ***p < .001, one‐way ANOVA). (E) Pain measured as paw withdrawal threshold using the Von Frey apparatus in the collagenase‐injected and SM04755‐ or vehicle‐treated limb (n = 6 rats, estimated treatment effect ± 95% CI, **p < .01). (F) Gait of rats injected with collagenase and treated with SM04755 or vehicle, represented as percent weight on the affected limb (n = 3 for sham,n = 6 for treated, estimated treatment effect ± 95% CI, **p < .01, ***p < .001)
The efficacy of SM04755 was evaluated in a collagenase‐induced acute tendinopathy model in rats following daily topical application of SM04755 (0.3 mg/cm2) or vehicle (Figure S16A). SM04755‐treated rats showed decreased inflammatory infiltrates and improved tendon morphology, as indicated by H&E staining, compared with vehicle (Figure 5A). Blinded histopathological analysis of tendons, based on the presence of inflammatory cells, tendon structure, linearity, density, and hemorrhage9 (Table S4), revealed a significant increase (p = .008) in treatment group scores compared with vehicle (Figure 5B), indicating improvements in tendon morphology. Consistent with decreased inflammatory cells in the tendon, significantly reduced pro‐inflammatory (IL1β, TNFα, IL6, IL8, IFNγ; p < .05) and increased immunoregulatory (IL10) cytokine expression was observed in tendons treated with SM04755 for 20 days compared with vehicle (Figure 5C). Serum levels of KC/GRO (CXCL1), an inflammatory biomarker that is associated with tendinopathy,2 were elevated in the vehicle‐treated rats from Days 5 to 20, while SM04755 treatment significantly decreased levels of KC/GRO compared with vehicle (p < .05; Figure 5D).
Histological analysis of tendons at several timepoints (Days 7, 14, and 21) showed significant reduction in inflammation and improved tendon histological scores at Day 14 (p < .05) in the higher‐dose SM04755 (0.9 mg/cm2) group compared with the lower‐dose SM04755 (0.3 mg/cm2) group and vehicle. Both dose groups showed improved tendon histological scores (p < .05) at Day 21 (Figure S17).
3.13. Topical SM04755 promoted tendon differentiation and protection in a rat model of acute tendinopathy
Histopathological analysis of tendons from the collagenase model revealed improved fiber structure and cellular appearance at Day 21 of SM04755 treatment (0.3 mg/cm2) compared with vehicle (Figure 5A). Onset of tenocyte differentiation was confirmed by increased gene expression of TNMD, TNC, and SCXA in SM04755‐treated tendons compared with vehicle (Figure 5E). Twenty‐one days after treatment, Type I collagen levels were significantly (p < .05) increased with a corresponding increase (p < .05) in Type I/Type III collagen ratios (Figure 5E) in tendons of SM04755‐treated rats compared with vehicle. Furthermore, improved tendon fiber structure and increased Type I collagen levels were observed in Sirius Red‐stained tendons of SM04755‐treated rats compared with vehicle (Figure S18).
The effects of SM04755 treatment on pain and function in rats were measured using the Von Frey apparatus and incapacitance test for static weight‐bearing, respectively. Collagenase injection into the Achilles tendon decreased paw withdrawal threshold after 1 day (Figure 5F) and percent of weight on the affected limb after 3 days (Figure 5G). Paw withdrawal threshold was significantly (p < .05) increased following 18 days of SM04755 treatment (Figure 5F), and percent of weight on the affected limb was significantly increased following 13 days of SM04755 treatment, compared with vehicle, with weight‐distribution effects sustained until Day 20 (Figure 5G).
3.14. Topical SM04755 promoted tendon differentiation and improved tendon health in a rat model of chronic failed healing tendinopathy
Failed healing has been suggested as a potentially pathological mechanism of chronic tendinopathy.46 Therefore, a chronic failed healing tendinopathy model was developed using multiple collagenase injections (Days 0, 21, and 40) followed by daily (from Day 1) topical treatment with SM04755 (0.3 mg/cm2; Figure S16B). Histopathological analysis at Days 40 and 60 demonstrated inflammation, tendon degeneration, and appearance of adipose tissue in tendons with multiple collagenase injections and vehicle treatment (Figures 6A and S19A,B). In contrast, SM04755‐treated tendons (0.3 mg/cm2) showed decreased inflammation, decreased adipose tissue, and improved tendon fiber structure (Figures 6A and S19A,B). Tendon health scores were significantly improved for the SM04755 groups at Day 40 (p < .01; Figure 6B), while a general direction of change toward improved scores was observed on Day 60 (p = .317) compared with vehicle (Figure S19C). Expression of tenocyte markers (i.e., TNMD, TNC, and SCXA), Types I and III collagen levels, and Type I/Type III collagen ratios were increased (p < .05) in SM04755‐treated tendons on Day 40 compared with vehicle (Figure 6C).
3.15. Topical SM04755 inhibited inflammation and improved tendon health in a delayed‐treatment rat model of tendinopathy
The ability of SM04755 to reduce inflammation and improve tendon health in a well‐established tendon injury model was evaluated in a collagenase‐induced delayed‐treatment model in rats (Figure S16C). Rats injected with collagenase on Days 0 and 20 and treated with daily topical SM04755 (0.3 mg/cm2) from Day 28 and onward showed improved tendon appearance (Figure 6D) and histologically measured tendon health scores (p < .05) following 21 days of SM04755 treatment compared with vehicle (Figure 6E).
4. DISCUSSION
Wnt signaling plays an important role in tendinopathy by modulating inflammation, tenocyte lineage specification, protease production, and tendon homeostasis (Figure S20A).18, 19, 20 In this study, we used in vitro assays and animal models of tendinopathy to test the effects of SM04755, a novel small‐molecule inhibitor of the Wnt pathway, on these processes. In vitro, SM04755 inhibited Wnt signaling, stimulated tenocyte differentiation, and reduced tendon‐destroying proteases and inflammatory cytokine production. Functional effects in the rat collagenase‐induced tendinopathy models provided evidence for the ability of SM04755 to modulate pathophysiological processes in tendinopathy.
Inhibited or erroneous differentiation of TDSCs contributes to chondrometaplasia and ossification in tendinopathy. This aberrant differentiation may be mediated via the Wnt pathway, as Wnt3a stimulation has been shown to induce osteogenic marker expression in rTDSCs.18 Here, we demonstrated that SM04755 increased tenocyte differentiation marker expression in cultured hMSCs and rTDSCs and tendons in rats with collagenase‐induced tendinopathy compared with vehicle. Stem cell transplantation‐based regenerative strategies have shown promise but have encountered crucial barriers in therapeutic translation such as immune rejection, potential tumorigenesis, and difficulties in regulatory approval and clinical adoption.9, 47, 48 By targeting endogenoustissue‐resident stem cells with regenerative capacity,9, 47, 49 treatment with SM04755 may be an attractive approach to promote tendon regeneration.
Inflammation plays an important role in both acute and chronic tendinopathy.2 The development of treatments for tendinopathy has focused on reducing pain or inflammation with steroids. While steroids provide temporary relief, they may impede long‐term healing.50 Treatments to reduce inflammation, such as nitric oxide patches and growth factor or platelet‐rich plasma (PRP) injections, have been tested with limited success51 and various challenges remain with translation of PRP into the clinic.52 Our data support a role of SM04755 in reducing inflammation in the tendon: SM04755 treatment led to a decrease in inflammatory cells in rat models of acute tendinopathy, chronic failed healing tendinopathy, and delayedtreatment of tendinopathy. Moreover, both in vitro and in the acute tendinopathy model, we observed a decrease in the inflammatory biomarker KC/GRO and several pro‐inflammatory cytokines along with an increase in the immunoregulatory cytokine IL‐10. Thus, SM04755 demonstrated potent anti‐inflammatory activity.
In addition to tendon regeneration and inhibition of inflammation, long‐term healing and prevention of tendon degeneration in chronic tendinopathy would benefit from reduced tendon catabolism.35 SM04755 inhibited the expression of catabolic enzymes in cytokine‐stimulated tenocytes in vitro, thus demonstrating potential for regulating tendon catabolic enzymes in tendinopathy. While additional mechanical and functional studies using chronic animal models and studies evaluating the effects of SM04755 on protease production and matrix catabolism in primary patient‐derived tendons could further support this finding, our data suggest that SM04755 may have potential tendon‐protective effects.
Furthermore, in vitro biochemical and genetic studies demonstrated that the effects of SM04755 were mediated by inhibition of CLKs and DYRK1A (Figure S20B). Inhibition of these kinases by siRNAs and small‐molecule inhibitors validated the role of these kinases in tenocyte differentiation induction as well as reduced inflammation and tenocyte catabolism. CLKs exert their function by phosphorylating SRSF proteins, which modulate RNA splicing,15 and SM04755 dose‐dependently inhibited CLK‐mediated phosphorylation of SRSFs in hMSCs and rTDSCs. Inhibition of Wnt pathway activity with CLK knockdown and CLK‐specific inhibitors supported the involvement of CLKs in regulating canonical Wnt signaling downstream of β‐catenin at a posttranscriptional level by alternative splicing of Wnt target genes in hMSCs and rTDSCs. By modulating splicing kinetics, CLK loss or inhibition may change pre‐mRNA processing, leading to the formation of unstable transcripts and a decrease in overall gene expression.53 CLKs and DYRK1A appear to be the primary targets of SM04755 for Wnt pathway inhibition, tenocyte differentiation and protection, and anti‐inflammation; while these effects of SM04755 appear to be mediated by alternative splicing of Wnt pathway genes, splicing of other genes and contributions from other cellular proteins or pathways cannot be ruled out. Recently, our group identified CLKs and DYRK1A as molecular targets of lorecivivint (LOR; SM04690), an intra‐articular Wnt pathway inhibitor for knee osteoarthritis treatment.13 While LOR has a similar mechanism of action as SM04755, differences in physicochemical properties allowed SM04755 to access tendons following administration on the skin, making it potentially more convenient for the treatment of tendinopathy. Future in vivo studies using tendinopathy models may reveal additional insights into regulation of gene expression by SM04755, CLK2, DYRK1A, and their interactions.
In the clinical context, there is an unmet medical need for patients with acute‐on‐chronic or chronic tendinopathy. SM04755 could be a treatment option that potentially addresses both acute inflammation and long‐term healing. Moreover, the observed efficacy of SM04755 in the delayed‐treatment model demonstrated the compound's potential to treat established tendon injuries in patients. Our in vivo data demonstrated that SM04755 had a positive effect in both acute and chronic tendinopathy. However, this effect may be reduced in extremely severe or chronic cases; we did not observe significantly improved scores on Day 60 in our chronic failed healing tendinopathy model. While several anti‐inflammatory agents have potential long‐term side effects and may harm tendon tissue,50 SM04755 promoted tendon healing, which correlated with reduced pain and improved weight‐bearing function in SM04755‐treated rats. In vivo pharmacokinetic and safety data supported the use of a once‐daily topical application of SM04755 for this study and in vitro efficacy of the compound in human cells supported the rationale to pursue clinical development of SM04755. Together, the data presented here demonstrates the potential of SM04755 as a topical, small‐molecule treatment for tendinopathy.
AUTHOR CONTRIBUTIONS
Vishal Deshmukh: study conception and design, acquisition of data, and analysis and interpretation of data. Sunil KC, Sarah Kennedy, Jeymi Tambiah, John Hood, and Yusuf Yazici: study conception and design. Tim Seo, Alyssa L. O'Green, Joshua Stewart, and Charlene Barroga: acquisition of data and analysis and interpretation of data. Maureen Ibanez, Brian Hofilena, Luis Dellamary, Kevin Chiu, and Abdullah Ghias: acquisition of data. All authors have read and approved the final submitted manuscript.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors thank Andrea C. Carrano, PhD, Elizabeth Orient, MA, and Selin Esener, MS, for assistance with drafting and editing, Mirta Grifman, PhD, for critical revision of the manuscript, Betty Tam, PhD, and Carine Bossard, PhD, for helpful discussion, Arina Alexeeva, MS, for technical support, and Christopher Swearingen, PhD, for assistance with statistical analysis. Financial support for this study and its publication was provided by Samumed, LLC. All authors are employees and/or shareholders of Samumed, LLC.
Deshmukh V, Seo T, O'Green A, et al. SM04755, a small‐molecule inhibitor of the Wnt pathway, as a potential topical treatment for tendinopathy. J Orthop Res. 2021;39:2048–2061. 10.1002/jor.24898
Vishal Deshmukh and Tim Seo contributed equally to this study.
REFERENCES
- 1.Järvinen TAH, Kannus P, Maffulli N, Khan KM. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin. 2005;10(2):255‐266. http://www.ncbi.nlm.nih.gov/pubmed/15922917 [DOI] [PubMed] [Google Scholar]
- 2.Millar NL, Murrell GAC, Mcinnes IB. Inflammatory mechanisms in tendinopathy – towards translation. Nat Rev Rheumatol. 2017;13(2):110‐122. [DOI] [PubMed] [Google Scholar]
- 3.Cook JL, Rio E, Purdam CR, Docking SI. Revisiting the continuum model of tendon pathology: what is its merit in clinical practice and research? Br J Sports Med. 2016;50(19):1187‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonçalves AI, Rodrigues MT, Lee SJ, et al. Understanding the role of growth factors in modulating stem cell tenogenesis. PLOS One. 2013;8(12):e8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller LE, Parrish WR, Roides B, Bhattacharyya S. Efficacy of platelet‐rich plasma injections for symptomatic tendinopathy: systematic review and meta‐analysis of randomised injection‐controlled trials. BMJ Open Sport Exerc Med. 2017;3(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Yin Z, Chen J, et al. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Sci Rep. 2012;2:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Zhao Q, Wang K, et al. Isolation and biological characterization of tendon‐derived stem cells from fetal bovine. In Vitro Cell Dev Biol Anim. 2016;52(8):846‐856. 10.1007/s11626-016-0043-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urdzikova L, Sedlacek R. Human multipotent mesenchymal stem cells improve healing after collagenase tendon injury in the rat. Biomed Eng Online. 2014;13(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for achilles tendon repair. J Orthop Res. 1998;16(4):406‐413. [DOI] [PubMed] [Google Scholar]
- 11.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205):1248012. http://www.ncbi.nlm.nih.gov/pubmed/25278615 [DOI] [PubMed] [Google Scholar]
- 12.Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13(7):513‐532. http://www.ncbi.nlm.nih.gov/pubmed/24981364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshmukh V, O'Green AL, Bossard C, et al. Modulation of the Wnt pathway through inhibition of CLK2 and DYRK1A by lorecivivint as a novel, potentially disease‐modifying approach for knee osteoarthritis treatment. Osteoarthr Cartil. 2019;27(9):1‐14. [DOI] [PubMed] [Google Scholar]
- 14.Tam BY, Chiu K, Chung H, et al. The CLK inhibitor SM08502 induces anti‐tumor activity and reduces Wnt pathway gene expression in gastrointestinal cancer models. Cancer Lett. 2019;19:30473. 10.1016/j.canlet.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 15.Duncan PI, Stojdl DF, Marius RM, Scheit KH, Bell JC. The Clk2 and Clk3 dual‐specificity protein kinases regulate the intranuclear distribution of SR proteins and influence pre‐mRNA splicing. Exp Cell Res. 1998;241(2):300‐308. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh G, Adams JA. Phosphorylation mechanism and structure of serine‐arginine protein kinases. FEBS J. 2011;278(4):587‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khor B, Gagnon JD, Goel G, et al. The kinase DYRK1A reciprocally regulates the differentiation of Th17 and regulatory T cells. eLife. 2015;4:1‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lui PPY, Lee YW, Wong YM, Zhang X, Dai K, Rolf CG. Expression of Wnt pathway mediators in metaplasic tissue in animal model and clinical samples of tendinopathy. Rheumatology. (United Kingdom). 2013;52(9):1609‐1618. [DOI] [PubMed] [Google Scholar]
- 19.Fu SC, Chan BP, Wang W, Pau HM, Chan KM, Rolf CG. Increased expression of matrix metalloproteinase 1 (MMP1) in 11 patients with patellar tendinosis. Acta Orthop Scand. 2002;73(6):658‐662. [DOI] [PubMed] [Google Scholar]
- 20.Deshmukh V, Hu H, Barroga C, et al. A small‐molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthr Cartil. 2018;26(1):18‐27. https://www.sciencedirect.com/science/article/pii/S1063458417311676?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Zhou Z, Taub PJ, et al. BMP‐12 treatment of adult mesenchymal stem cells in vitro augments tendon‐like tissue formation and defect repair in vivo. PLOS One. 2011;6(3):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havis E, Bonnin MA, Esteves de Lima J, Charvet B, Milet C, Duprez D. TGFβ and FGF promote tendon progenitor fate and act downstream of muscle contraction to regulate tendon differentiation during chick limb development. Development. 2016;143(20):3839‐3851. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Zhang W, Evans PM, Chen X, He X, Liu C. Adenomatous polyposis coli (APC) differentially regulates β‐catenin phosphorylation and ubiquitination in colon cancer cells. J Biol Chem. 2006;281(26):17751‐17757. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Zhang W, Liu Z, et al. Characterization and comparison of post‐natal rat Achilles tendon‐derived stem cells at different development stages. Sci Rep. 2016;6:1‐11. 10.1038/srep22946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sineva GS, Pospelov VA. Inhibition of GSK3β enhances both adhesive and signalling activities of β‐catenin in mouse embryonic stem cells. Biol Cell. 2010;102(10):549‐564. [DOI] [PubMed] [Google Scholar]
- 26.Liu SH, Yang RS, Al‐Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing. A current review. Clin Orthop Relat Res. 1995;318:265‐278. http://www.ncbi.nlm.nih.gov/pubmed/7671527 [PubMed] [Google Scholar]
- 27.Arpornmaeklong P, Brown SE, Wang Z, Krebsbach PH. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell‐verived mesenchymal stem cells. Stem Cells Dev. 2009;18(7):955‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieudonne FX, Sévère N, Biosse‐Duplan M, Weng JJ, Su Y, Marie PJ. Promotion of osteoblast differentiation in mesenchymal cells through Cbl‐mediated control of STAT5 activity. Stem Cells. 2013;31(7):1340‐1349. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Han W, Chen L, Tang K. Mechanism of osteogenic and adipogenic differentiation of tendon stem cells induced by sirtuin 1. Mol Med Rep. 2016;14(2):1643‐1648. [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Oh H, Chang JW, Kim SJ. Recovery of tendon characteristics by inhibition of aberrant differentiation of tendon‐derived stem cells from degenerative tendinopathy. Int J Mol Sci. 2020;21(8):2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang H, Chen Y, Chen G, et al. Leptin accelerates the pathogenesis of heterotopic ossification in rat tendon tissues via mTORC1 signaling. J Cell Physiol. 2018;233(2):1017‐1028. [DOI] [PubMed] [Google Scholar]
- 32.Rothan HA, Suhaeb AM, Kamarul T. Recombinant human adiponectin as a potential protein for treating diabetic tendinopathy promotes tenocyte progenitor cells proliferation and tenogenic differentiation in vitro. Int J Med Sci. 2013;10(13):1899‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuzaki M, Guyton G, Garrett W, et al. IL‐1β induces COX2, MMP‐1, ‐3 and ‐13, ADAMTS‐4, IL‐1β and IL‐6 in human tendon cells. J Orthop Res. 2003;21(2):256‐264. [DOI] [PubMed] [Google Scholar]
- 34.John T, Lodka D, Kohl B, et al. Effect of pro‐inflammatory and immunoregulatory cytokines on human tenocytes. J Orthop Res. 2010;28(8):1071‐1077. [DOI] [PubMed] [Google Scholar]
- 35.Del Buono A, Oliva F, Osti L, Maffulli N. Metalloproteases and tendinopathy. Muscles Ligaments Tendons J. 2013;3(1):51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenthal AS, Tanega C, Shen M, et al. An inhibitor of the Cdc2‐like kinase 4 (Clk4). Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda, MD: National Center for Biotechnology Information; 2010. http://www.ncbi.nlm.nih.gov/pubmed/21735605 [PubMed] [Google Scholar]
- 37.Coombs TC, Tanega C, Shen M, et al. Small‐molecule pyrimidine inhibitors of the cdc2‐like (Clk) and dual specificity tyrosine phosphorylation‐regulated (Dyrk) kinases: development of chemical probe ML315. Bioorg Med Chem Lett. 2013;23(12):3654‐3661. 10.1016/j.bmcl.2013.02.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naert G, Ferré V, Meunier J, et al. Leucettine L41, a DYRK1A‐preferential DYRKs/CLKs inhibitor, prevents memory impairments and neurotoxicity induced by oligomeric Aβ25‐35 peptide administration in mice. Eur Neuropsychopharmacol. 2015;25(11):2170‐2182. 10.1016/j.euroneuro.2015.03.018 [DOI] [PubMed] [Google Scholar]
- 39.Muraki M, Ohkawara B, Hosoya T, et al. Manipulation of alternative splicing by a newly developed inhibitor of Clks. J Biol Chem. 2004;279(23):24246‐24254. [DOI] [PubMed] [Google Scholar]
- 40.Riggs JR, Nagy M, Elsner J, et al. The discovery of a dual TTK protein kinase/CDC2‐like kinase (CLK2) inhibitor for the treatment of triple negative breast cancer initiated from a phenotypic screen. J Med Chem. 2017;60(21):8989‐9002. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa Y, Nonaka Y, Goto T, et al. Development of a novel selective inhibitor of the Down syndrome‐related kinase Dyrk1A. Nat Commun. 2010;1(7):86. 10.1038/ncomms1090\n, http://www.nature.com/ncomms/journal/v1/n7/suppinfo/ncomms1090_S1.html [DOI] [PubMed] [Google Scholar]
- 42.Monteiro MB, Ramm S, Chandrasekaran V, et al. A high‐throughput screen identifies DYRK1A inhibitor ID‐8 that stimulates human kidney tubular epithelial cell proliferation. J Am Soc Nephrol. 2018;29(12):2820‐2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De la Torre R, De Sola S, Pons M, et al. Epigallocatechin‐3‐gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutr Food Res. 2014;58(2):278‐288. [DOI] [PubMed] [Google Scholar]
- 44.Göckler N, Jofre G, Papadopoulos C, Soppa U, Tejedor FJ, Becker W. Harmine specifically inhibits protein kinase DYRK1A and interferes with neurite formation. FEBS J. 2009;276(21):6324‐6337. [DOI] [PubMed] [Google Scholar]
- 45.Colwill K, Pawson T, Andrews B, et al. The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J. 1996;15(2):265‐275. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=449941&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 46.Fu SC, Rolf C, Cheuk YC, et al. Deciphering the pathogenesis of tendinopathy: a three‐stages process. Sport Med Arthrosc Rehabil Ther Technol. 2010;2(1):30. http://www.smarttjournal.com/content/2/1/30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CH, Lee FY, Tarafder S, et al. Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest. 2015;125(7):2690‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schon LC, Gill N, Thorpe M, et al. Efficacy of a mesenchymal stem cell loaded surgical mesh for tendon repair in rats. J Transl Med. 2014;12(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thaker H, Sharma AK. Engaging stem cells for customized tendon regeneration. Stem Cells Int. 2012;2012:309187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coombes B, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376(9754):1751‐1767. http://www.ncbi.nlm.nih.gov/pubmed/20970844 [DOI] [PubMed] [Google Scholar]
- 51.Mead MP, Gumucio JP, Awan TM, Mendias CL, Sugg KB. Pathogenesis and management of tendinopathies in sports medicine. Transl Sport Med. 2018;1(1):5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dean BJF, Lostis E, Oakley T, Rombach I, Morrey ME, Carr AJ. The risks and benefits of glucocorticoid treatment for tendinopathy: a systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum. 2014;43(4):570‐576. [DOI] [PubMed] [Google Scholar]
- 53.Funnell T, Tasaki S, Oloumi A, et al. CLK‐dependent exon recognition and conjoined gene formation revealed with a novel small‐molecule inhibitor. Nat Commun. 2017;8(1):7. http://www.ncbi.nlm.nih.gov/pubmed/28232751 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


