Abstract
Background
Baboon syndrome is a rare, type IV hypersensitivity reaction causing a maculopapular rash. Tamoxifen is an antineoplastic agent, working as an estrogen receptor antagonist, also called a selective estrogen receptor modulator. A variety of rashes were reported with Tamoxifen use to‐date except baboon syndrome. The Tamoxifen‐induced baboon syndrome seems to be reversible, as discontinuation of the drug improves clinical outcomes.
Aim
Herein, we present the first case of Tamoxifen‐induced baboon syndrome which occurred 8 years after initiation of Tamoxifen use.
Patients
A 44‐year‐old woman presented with papulovesicular eruption on her body and erythema on her face for a duration of 6 months. There was no evidence of ocular or mucosal involvement. She was diagnosed with breast cancer and treated with tamoxifen 10 mg twice daily over the past 8 years. She was not taking other medications or over‐the‐counter supplements at the time of presentation. The patient underwent urgent skin biopsies of two lesions on her buttock and thigh. No organisms were seen on Gram stain. The patient's skin biopsy revealed extensive hyperorthokeratosis, minimal parakeratosis, hypergranulosis, and lichenoid interface dermatitis in the irregularly acanthotic epidermis supporting diagnosis of fixed drug eruption. Following a multidisciplinary discussion, the patient was diagnosed with baboon syndrome or symmetrical drug‐related intertriginous and flexural exanthema (SDRIFE) associated with Tamoxifen.
Results
Hence, Tamoxifen was immediately discontinued and treated with oral steroid along with topical agents. She showed improvement of clinical abnormalities within days after discontinuation of Tamoxifen.
Conclusions
Given the widespread use of Tamoxifen in the management of patients with breast cancer, it is important that healthcare professionals monitor for rare, however clinically significant, and potentially life‐threatening dermatological manifestations of Tamoxifen use, such as baboon syndrome.
Keywords: Baboon syndrome, drug eruption, tamoxifen
1. INTRODUCTION
Baboon syndrome, also named symmetrical drug‐related intertriginous and flexural exanthema (SDRIFE), is an uncommon type IV hypersensitivity causing a distinct form of fixed drug eruption or systemic contact dermatitis.1 The name Baboon syndrome stems from the rashes on the patient's buttocks resembling the red buttocks of baboons.2, 3 Baboon syndrome is featured by erythematous maculopapular rashes with a diffuse and symmetrical pattern on the flexures, forming a V‐shape on the medial thighs and scattered erythema covering the buttocks. In addition to chronic dermatitis with different etiologies, it can also appear in its acute form only days after systemically administrating substances, including contact allergens such as mercury and nickel and certain drugs.4 In the last few decades, hundreds of drugs have been introduced as the cause of this disease. Amoxicillin, ceftriaxone, penicillin, and erythromycin are among the most common drugs, but many other medications like antihypertensive drugs and contrast agents may also cause Baboon syndrome.5 Tamoxifen is a selective estrogen receptor modulator that blocks the transcriptional activity of estrogen receptors by directly binding to them, creating a complex in the cell nucleus which causes a decrease in estrogen transcription and functional activities. Tamoxifen is widely used as adjuvant therapy in treating the early stages of invasive breast cancer and ductal carcinoma in situ.6
We present the first case of Tamoxifen‐induced baboon syndrome which occurred 8 years after initiation of Tamoxifen use. The Tamoxifen‐induced baboon syndrome seems to be reversible as discontinuation of the drug improves clinical outcomes. Given the widespread use of Tamoxifen in the management of patients with breast cancer, it is imperative that healthcare professionals recognize the rare, however clinically significant, and potentially life‐threatening dermatological manifestations of Tamoxifen use such as baboon syndrome.
2. CASE PRESENTATION
A 44‐year‐old woman presented with papulovesicular eruption on her body and erythema on her face for the duration of 6 months. There was no evidence of ocular or mucosal involvement. The initial skin manifestations were self‐limited; however, the skin eruptions progressively worsened about 1 month prior to her first clinic visit. The rashes were associated with pruritus, but without burning sensation. She denied history of similar skin manifestations in the past.
Her past medical history was significant for history of invasive ductal carcinoma stage 2B breast cancer diagnosed 8 years prior presentation in our clinic. She underwent total mastectomy followed by 28 sessions of chemotherapy with Taxotere over a period of 4 years. She was also treated with Microrelin 3.75 mg for the period of three months and Tamoxifen 10 mg twice daily over the past 8 years. She was not taking other medications or over‐the‐counter supplements at the time of presentation. She denied history of other medical illness, family history, and exposure to radiation or chemical agents. Physical examination was remarkable for papulovesicular rashes on her arms, legs, back, chest, buttocks, axillary, and inguinal areas. In addition, there was an erythema on her face (Figure 1). There was no evidence of ocular or genital mucosal involvement. The patient was conscious, well oriented, and afebrile. Vital signs were stable. The patient underwent skin biopsies of two lesions on her buttock and thigh. No organisms were seen on Gram stain. The patient's skin biopsy revealed extensive hyperorthokeratosis, minimal parakeratosis, hypergranulosis, and lichenoid interface dermatitis in the irregularly acanthotic epidermis. Additionally, necrotic keratinocytes, civatte bodies, melanin incontinence, mild perivascular lymphocytic infiltration, a few eosinophils, and mast cells were also evident. The clinical manifestations and the pathological findings of the skin lesions supported the diagnosis of baboon syndrome associated with Tamoxifen (Figure 2).
Figure 1.
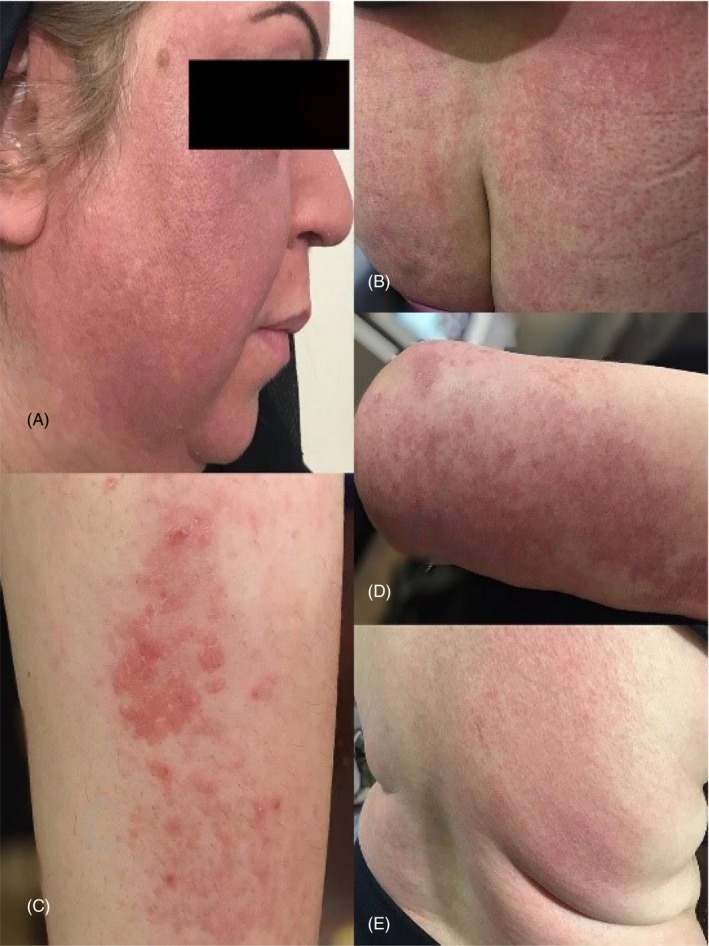
Papulovesicular rash and erythema fixed drug eruption due to Tamoxifen in her body, without mucosal involvement (A) Face, (B) buttock, (C) Front forearm, (D) Arm, (E) Back
Figure 2.
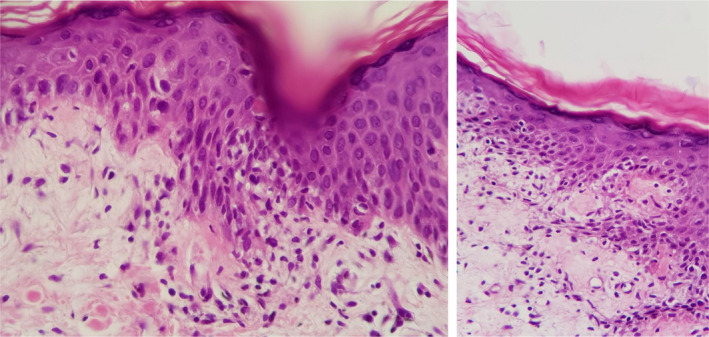
(A) By light microscopy of the skin biopsy, focal hydropic degeneration of dermoepidermal junction along with a few lymphocytic exocytosis (thin arrow) and one necrotic keratinocyte (notched arrow) are in evident. H&E stained slide, 400 × magnification. (B) Focal lichenoid change at basal layer (notched arrows), lymphocytic exocytosis (thin arrows) are shown. Mild dermal perivascular lymphocytic infiltration and edema subjacent to overlying epidermis are seen. H&E stained slide, 400 × magnification
Hence, after consulting with her oncologist, Tamoxifen was immediately discontinued. The patient was also treated with both oral steroid, prednisolone 50 mg once daily for 3 days and tapering doses thereafter, and topical corticosteroid initially with Clobetasol propionate twice a day for 2 weeks and Mometasone once daily for 1 week. She showed a remarkable improvement of skin eruptions within days after discontinuation of Tamoxifen and steroid therapies (Figure 3).
Figure 3.
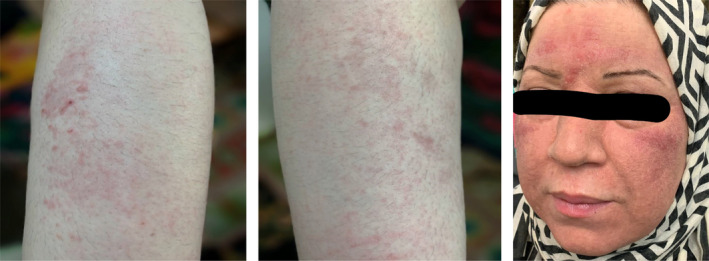
(A) Fixed drug eruption (FDE) after treatment with discontinuation of Tamoxifen and administration of topical corticosteroid. (B) Baboon syndrome (BS) 2 wk after discontinuation of tamoxifen and administration of topical corticosteroid. (C) Baboon syndrome 10 wk after discontinuation of tamoxifen
3. DISCUSSION
Herein, we report the first case of Tamoxifen‐induced baboon syndrome which occurred 8 years after initiation of Tamoxifen use. The Tamoxifen‐induced baboon syndrome seems to be reversible, as discontinuation of the drug improved the clinical outcomes. This diagnosis was strongly supported by clinical presentations, pathological studies of the skin biopsy specimens, and favorable clinical response to the discontinuation of Tamoxifen.
There are several reports of baboon syndrome associated with the systemic exposure to medications such as ampicillin, daptomycin, penicillin, cephalosporins, hydroxyzine, paracetamol, omeprazole, clozapine, ranitidine, infliximab, chemotherapeutic agents, pseudoephedrine, codeine, allopurinol, cimetidine, and nystatin.7 To our knowledge, this is the first report of Tamoxifen‐induced baboon syndrome. The term baboon syndrome or SDRIFE is described as an erythema affecting the gluteal and intertriginous surfaces with a symmetrical pattern.1 The diagnosis of SDRIFE encompasses five criteria: (a) first time administration of a systemic drug or exposure to repeated doses (excluding contact allergens); (b) sharply demarcated erythema of the gluteal/perianal area and/or V‐shaped erythema of the inguinal/perigenital area; (c) presence of at least another affected intertriginous/flexural site; (d) symmetrical localization; and (e) absence of systemic involvement.
The reason of the involvement of flexural areas is not known. There are theories that describe it as a type of recall phenomenon from an unlinked dermatitis in the past or because of excretion of the causative drug metabolites from eccrine glands present in flexural areas. Although the typical histological picture of SDRIFE is characterized by a superficial mononuclear perivascular neutrophilic and eosinophilic infiltration, and CD4 and CD3 T lymphocytes in the dermis, other features including subcorneal pustules, vacuolar degeneration and hydropic change in the basal cells with bullae in the subepidermal area, and necrosis of keratinocytes can be observed. In our case, the diagnosis of Tamoxifen‐induced baboon syndrome was made according to a typical clinical course and the histopathology. The differential diagnoses of SDRIFE include fixed drug eruption (FDE), acute generalized exanthematous pustulosis (AGEP), and drug rash with eosinophilia (DRESS). AGEP and DRESS cause a widespread rash with accompanying systemic changes, while clinically, FDE can be easily differentiated from SDRIFE by round‐oval patches or plaques that are most commonly pigmented and located on acral, genital (penis, vulva, glans penis), mucosal (like vulva mucosa), extremities (50% upper and 50% lower), lips, and face. These can be with or without bullous lesions. Also some cases are reported to have extreme FDE reactions known as baboon syndrome.3, 5, 8, 9 The most important treatment in SDRIFE is to identify and stop the causative agent. The patient should be advised to avoid the causative medication lifelong. Topical steroids may reduce the redness while the reaction resolves. Recovery may take up to 3 weeks.
In summary, baboon syndrome is a relatively common drug hypersensitivity eruption. Here, we report the first case of Tamoxifen‐induced baboon syndrome which occurred several years after initiation of Tamoxifen administration. The resolution of symptoms occurred after cessation of the offending drug, Tamoxifen; thus, early diagnosis is crucial for treatment strategy and improvement of prognosis. The best approach for the diagnosis of drug‐induced adverse effects in clinical practice mostly relied on the physician to quickly evaluate suspicious cases and reach a conclusive diagnosis. This is at present best achieved by the combination of clinical judgment with and without pathological evaluation of skin biopsy specimens. In cases of skin manifestation injury after Tamoxifen administration, Tamoxifen should either be discontinued or attenuated depending on the degree and extent of manifestations. Skin biopsy is useful in establishing a diagnosis and guiding management. Steroids should be considered if dermatological manifestations persists or worsening despite discontinuation of Tamoxifen. Given the widespread use of Tamoxifen in the management of patients with breast cancer, it is important that healthcare professionals monitor for rare, however clinically significant, and potentially life‐threatening dermatological manifestations of Tamoxifen use.
AUTHORS CONTRIBUTION
Ramin Mofarrah has contributed to the conception and design of this paper as well as analysis and interpretation of data and revision of the drafted manuscript. Ramina Mofarrah, Kousar Jahani Amiri, Naghmeh Jallab, and Sueshianth Ghobadiaski have each made substantial contributions to drafting the manuscript. Birger Kraenke, Maziar Rahmani, Narges Hashemi, Maryam Ghasemi, and Nazgol Rahmani have made critical revisions on this manuscript.
All authors have given their approval of the version to be submitted for publication and have participated sufficiently in the work to take public responsibility for appropriate portions of the content and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGMENT
Open Access funding enabled and organized by ProjektDEAL.
Mofarrah R, Mofarrah R, Kränke B, et al. First report of tamoxifen‐induced baboon syndrome. J Cosmet Dermatol.2021;20:2574–2578. 10.1111/jocd.13863
REFERENCES
- 1.Hausermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51(5–6):297‐310. [DOI] [PubMed] [Google Scholar]
- 2.Andersen KE, Hjorth N, Menne T. The baboon syndrome: systemically‐induced allergic contact dermatitis. Contact Dermatitis. 1984;10(2):97‐100. [DOI] [PubMed] [Google Scholar]
- 3.Blackmur JP, Lammy S, Baring DE. Baboon syndrome: an unusual complication arising from antibiotic treatment of tonsillitis and review of the literature. BMJ Case Rep. 2013:2‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akkari H, Belhadjali H, Youssef M, Mokni S, Zili J. Baboon syndrome induced by hydroxyzine. Indian J Dermatol. 2013;58(3):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roopa B, Roopa B, Kumar S, Rohini PM, Prasanna V. Case report‐baboon syndrome with paracetamol. Int J Basic Clin Pharmacol. 2018;7:2061‐2064. [Google Scholar]
- 6.Heery M, Corbett P, Zelkowitz R. Precautions for patients taking tamoxifen. J Adv Pract Oncol. 2018;9(1):78‐83. [PMC free article] [PubMed] [Google Scholar]
- 7.Janjua SA, Pastar Z, Iftikhar N, Ammad S. Intertriginous eruption induced by terbinafine: a review of baboon syndrome. Int J Dermatol. 2017;56(1):100‐103. [DOI] [PubMed] [Google Scholar]
- 8.Bulur I, Keseroglu HO, Saracoglu ZN, Gönül M. Symmetrical drug‐related intertriginous and flexural exanthema (Baboon syndrome) associated with infliximab. J Dermatol Case Rep. 2015;9(1):12‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montazer F, Jahani Amiri K, Mofarrah R, Ahmadi A, Nouripour B, Mofarrah R. A first case of fixed drug eruption due to Tamsulosin. J Cosmet Dermatol. 2020;19(5):1143‐1145. [DOI] [PubMed] [Google Scholar]


