Abstract
Alternaria species are well‐known aggressive pathogens that are widespread globally and warmer temperatures caused by climate change might increase their abundance more drastically. Early blight (EB) disease, caused mainly by Alternaria solani, and brown spot, caused by Alternaria alternata, are major concerns in potato, tomato and eggplant production. The development of EB is strongly linked to varieties, crop development stages, environmental factors, cultivation and field management. Several forecasting models for pesticide application to control EB were created in the last century and more recent scientific advances have included modern breeding technology to detect resistant genes and precision agriculture with hyperspectral sensors to pinpoint damage locations on plants. This paper presents an overview of the EB disease and provides an evaluation of recent scientific advances to control the disease. First of all, we describe the outline of this disease, encompassing biological cycles of the Alternaria genus, favorite climate and soil conditions as well as resistant plant species. Second, versatile management practices to minimize the effect of this pathogen at field level are discussed, covering their limitations and pitfalls. A better understanding of the underlying factors of this disease and the potential of novel research can contribute to implementing integrated pest management systems for an ecofriendly farming system. © 2021 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: fungi, Solanum tuberosum, Solanum lycopersicum, rotation, precision agriculture
Environmental, biological, genetic, chemical and management factors that interact in pathogen systems with Alternaria spp.
1. INTRODUCTION
Early blight (EB), caused by Alternaria species, is one of the major diseases in the production of tomato (Solanum lycopersicum), potato (Solanum tuberosum) and other plants, and is most prevalent on unfertilized or otherwise stressed plants. Infection by this polycyclic disease led to production losses of 35–78% in tomato and 5–40% in potato, respectively.1 In North America, the annual cost for fungicide application is estimated to be $21.4 to $44.8 million.2 Alternaria species are among the few pathogens that can sporulate when exposed to several short‐wet periods alternating with dry periods.3 Phytotoxic metabolites are produced such as alternariol, altersolano, altertoix, macrosporin and solanpyrone.4, 5, 6 Dark brown to black lesions with concentric rings in the senescing leaves of potato and tomato are common symptoms, and others are stem lesions and fruit rots. Alternaria species are abundant in agricultural soils globally7 and it is considered to be one of the most aggressive soil‐borne pathogens in the world.8 Furthermore, increasing attention has been paid to these species nowadays since warmer temperatures due to climate change can favour an increase in their hotspots over the world.8 Alternaria species cause not only plant disease but also exhibit harmful effects on human and animal health. More than 70 toxins produced by the mycotoxin of Alternaria are found in a wide range of food and animal feed products in many countries.
The genus Alternaria is divided into 24 sections based on molecular and morphological traits.9 While A. solani is the most recognized causative agent of EB, other species, including A. alternata, A. arborescens and, A. porri, are also found on leaflets with symptoms of early blight in potato10 as well as A. linariae and A. grandis in tomato.11, 12, 13 A. solani is equally aggressive on both crops, while A. tomatophila is highly aggressive to tomato but weaker on potato.14 It was reported15 that A. solani is more suppressed than A. alternata by fungicides of demethylation inhibitors, the quinone outside inhibitors, a dithiocarbamate and a carboxylic acid amide. Some fungicides used for late blight (LB) control are applicable for EB, but not vice versa.16 EB infection has been the subject of the research over 100 years, but until now only a limited number of review papers regarding EB infection have been published5, 6, 17 and they focus mainly on screening methods and genetics of resistant varieties. However, holistic information about not only underlying factors of the infection but also practical modern strategies at field level to minimize the damage has not been reported. The present work aims to examine a wide range of themes related to EB, and special attention is given to the practical methods and strategies in greenhouse or open‐field conditions to minimize fungicide use for an environmentally benign integrated‐pest management (IPM) approach against EB.
2. HOST–PATHOGEN–ENVIRONMENT INTERACTION
The disease triangle is a bedrock illustrating the factors involved in the emergences and severity of plant disease. The triangle consists of (i) a susceptible host, (ii) a favourable environment for the development of the disease and (iii) a virulent pathogen (Fig. 1). A better understanding of each component allows us to set up more practical strategies for pest management. What environmental factors favour spore germination and dispersion of EB? What kind of underlying factors in the plant can induce resistance? How can the population of pathogens be decreased? Breaking down the complexity of the plant pathogen in nature by using the triangle method enables us to answer these questions.
Figure 1.
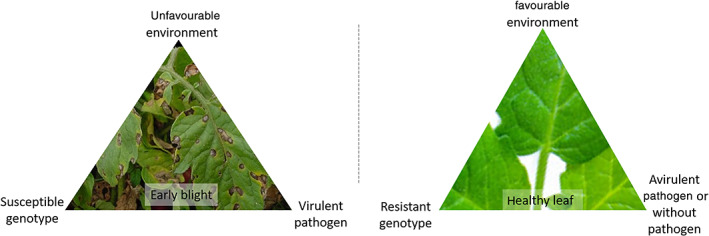
Two contrasting cases of disease triangles illustrating host genotypes, pests and environments for pest management against early blight.
2.1. Biological cycle and climate factors
The optimum temperature for EB epidemics is in the range of 20–30 °C.18 As few as 3 hours of continuous leaf wetness between 21 and 25 °C is sufficient for EB lesion formation19 and at 24 ± 2 °C, the infection appears within 4–6 h of leaf wetness.20 There is variability with different geographic regions, with an optimal temperature in temperate regions of 22–28 °C, while the most conidia production under tropical conditions occurs at 29–35 °C.21 Radiation affects the germination of the spores. The light range between 300 and 500 nm appeared to be responsible for inhibition in germination meanwhile wavelengths above 750 nm do not postpone germination.22 The conidiophores are formed under high humidity and light, whereas conidial formation is favoured by alternate high and low humidity in the dark. Conidia disperse from the soil surface and fall on leaves and infect them. The primary factors for disseminating conidia are rain, wind and insects.19, 21 Relative humidity (RH) is also a crucial parameter for the biological cycle of Alternaria species. At high humidity on leaf surfaces, especially after dew, conidial initiation occurs. A duration of 4–6 hours of leaf wetness after inoculation with conidia is sufficient to ensure the onset of EB.20 The link between lower temperature and higher RH during the night is favourable for the development of sporulation18; the new spores are released during the day when the temperature increases.
An interrupted wet period (IWP) plays an essential role in the biological cycle of A. solani and A. alternata. Unlike other fungi, germinating spores of these fungi can survive dry conditions and after rewetting of the leaf the germination process recommences. A. solani can produce seven times more conidia under IWP than during a continuous wetting period of the same duration.23 This unique characteristic enables it to adapt to different regions such as the northwest of Spain, where alteration in air humidity occurs frequently.
2.2. Dispersal pattern
Compared to P. infestans, the dispersal range of Alternaria solani is limited to within the proximity of the field, whilst dispersal distances of hundreds of metres or kilometres is the case for P. infestans.24 The reduction in the spore density of A. solani starts at a distance of 400 m and from 600 m further away the initial population of EB at crop emergence is negligible.25 A. alternata achieves longer dispersal distances than A. solani, showing seven times higher aerial conidial concentration than A. solani, probably due to the smaller spores.26 New infections of A. alternata in a field probably come from locations surrounding the field, whereas the localized canopy of host plants within a field is the primary source of A. solani conidia.
The unique characteristic of this pathogen is that when RH and wet leaf surface are high, along with prolonged leaf wetness (>12 h) and lower wind speed, the spore dispersion is diminished.27 By contrast, when wind speed is high, together with less wet leaf surface, spore catches increase.21 In another report,28 a strong correlation was found between dispersal and conditions favourable for sporulation in Alternaria spp. The spores of Alternaria spp. belong to aeroallergens and a daily average of the spore concentration (100 m−3) has been widely accepted in aerobiological research as a relevant threshold for respiratory allergy risk.29
Pathogen‐induced foliar damage at the early bulking stages of potato results in more significant yield loss than during potato tuber maturation and late bulking. The importance of fungicide disease management at early bulking growth stage is highlighted in the meta‐analysis work after gathering data from 23 trials.30 The impact of EB severity at early bulking on relative yield loss was 32% in comparison with that at tuber maturation‐late bulking, which was 19% of relative yield loss. There is another report on a crop loss model by this disease using yield loss as a function of disease severity.31
2.3. Susceptibility to EB infection and protection mechanism of wild genotypes
The epidemic development of EB varies mostly depending on the type of plant cultivar.5, 6 Ideally, the later the maturity time, the less susceptible the cultivar is to Alternaria infection.32 This is related to the fact that early maturity cultivars have a trait of determinate growth and have no ability to sprout new foliage continuously, consequently retaining older and senesced foliage on the plant, which is where lesions of EB start to appear. By contrast, the delayed fruit initiation occurs in late‐maturing varieties, and relatively young leaves grow continuously during the cropping season.5 The older or senesced foliage is a hotspot of EB. Incidence of EB is closely related to the nutrient contents in plant tissue and sugar; alkaloid contents and phenols are the primary chemical compounds that trigger the infection.5 A plant enriched with glucose could inhibit the production of cell‐wall‐degrading enzymes by A. solani.33, 34, 35 By contrast, alkaloids, which play an essential role as antimicrobial and antifeedant compounds,36 have been found in laboratory assays to prevent the growth of A. solani in potato leaves. Phenols are involved as secondary metabolite compounds for the plant protection system. Plants resistant against EB have reportedly contained a high content of phenols along with the activities of defence‐related enzymes including phenylalanine ammonia‐lyase (PAL) peroxidase, chitinase and polyphenoxidase, which increase strength to the host cell wall, resulting in the inhibition of pathogen invasion.37, 38, 39
Wild genotypes of potato and tomato in the Solanum genus, originally from South America, are a beneficial resource of germplasm resistance to EB disease.40, 41, 42 The genotypes that belong to S. habrochaites and S. arcanum were found to be moderately or highly resistant to EB.5, 43 The resistant wild‐type cultivars strongly activate several pathways of plant response, including glycol‐alkaloid, flavonoid and lignin biosynthesis by up‐regulating gene expressions.44 However, some others have a wide range of variations in the magnitude of resistance, including S. arcanum, S. peruvianum, S. neorickii, S. chilense and S. habrochaites. This heterogeneity within the same genotype could be related to local adaptations to EB.45
2.4. Molecular research
The species S. lycopersicum is susceptible to numerous diseases. However, many resistance genes have already been identified in genotypes of wild or domesticated species and crossed into commercial genotypes. It is estimated that most cultivars have resistance to a minimum of six diseases in the case of pure lines and up to 10 diseases in hybrids.46 A classic example of durable resistance is to the fungus Verticillium (Ve). The approach of using a Ve‐resistant locus was introduced more than 60 years ago in commercial cultivars and is present in many tomato cultivars on the market.47 Using this technique, research has been developed for more stable and efficient resistance to EB in tomatoes and potatoes.6
Susceptibility genes can also affect the degree of resistance against EB disease in addition to resistance genes.48 The resistance to EB can be achieved by regulating the expression of susceptibility genes in plants using the method of silencing genes.49 The sequencing and resequencing associated with transcriptomics and metabolomics may enable the identification of genes responsible for the most efficient and long‐lasting resistance to EB. In addition, the method of DNA editing techniques (CRISPR/CAS9) is a clear‐cut approach to create commercial and highly resistant genetic material, and the use of CRISPR/CAS9 for resistance to A. alternata has recently been published.50, 51, 52
Quantitative trait loci (QTLs) and marker‐assisted selection can facilitate the identification and characterization of resistant/susceptible genes, and this technique enables the transfer of the resistant genes to commercial varieties more quickly and efficiently. Some wild species that can be crossed with S. Iycopersicon have QTLs associated with resistance to EB.53, 54 By contrast, the classical breeding method requires more time and has some constraints, such as dependency on the donor parent of the resistance gene, that may require the use of a third genotype for transferring the gene of interest. Some species have fruit characteristics that are very different from commercial tomatoes and potatoes. Some wild species are self‐incompatible as S. arcanum,55 which remains with green color at maturity stage, not producing lycopene or being highly pubescent like S. habrochaites.56, 57 Thus, methods such as marker‐assisted selection can accelerate the development of new cultivars resistant to Alternaria spp. Using QTL technologies, more than 30 resistance cultivars with EB resistance genes in tomatoes were found.6 Other approaches for Alternaria species such as next‐generation sequencing technologies58 and the semi‐nested PCR‐based method59 are also attractive tools.
Metabolomics technology is an emerging tool used extensively in the pathogenic research field to identify toxic compounds in host plant cells induced by microorganisms. Metabolomic research related to A. alternata showed that chlorogenic acid is a metabolic acid with higher quantity in the resistant genotype compared to the susceptible. Chlorogenic acid was inoculated into in vitro cultures of A. alternata and the contents of alternariol, alternariol monomethyl ether and tenuazonic acid were analyzed. At 4 days after inoculation, chlorogenic acid almost wholly inhibited the synthesis of mycotoxins due to the presence of alternariol. A direct effect of chlorogenic acid on these mycotoxins could help to explain the mechanism of fungus infection.60
As the disease is greatly influenced by environmental conditions, it is believed that more comprehensive research associating resistance to EB with concomitant resistance to abiotic factors is desirable.
2.5. Soil
Soil is an essential biosphere for the development of EB in the biological cycle. In general, infected plant debris on the soil surface carries over the disease to the following season, mainly when the soil is dry. Hence, soil moisture and temperature are crucial components of survival. A. solani can overwinter with/without a host in the range of −3.3 to 21.2 °C. This fungus can survive in soil from the previous cropping season over 8 months, but not 16 months. Under these circumstances, crop rotation is recommended. An optimal interval for the rotation is 2 years for potato,61, 62 while 3‐ to 5‐year crop rotation is recommended for tomato.5 The underlying reasons for the difference in the duration was not found in the literature.
Plants exposed to nutrient starvation are more susceptible to infection. Nitrogen is vital for EB resistance, and several previous works highlight the importance of nitrogen supply. It is reported63 that nitrogen content and the area under the disease progress curve (AUDPC) have a significant correlation with potato starch yield. By contrast, a potato plant with lower nitrogen has more chance of becoming infected by A. solani. High nitrogen content in plant tissues may prolong vegetative growth and delay ripening, which results in less vulnerability to EB infection. Nitrogen input directly affects healthy leaf area duration and absorption of the radiation, but does not affect the resistance against EB once it appears, implying that adequate application at the right time needs to be implemented.64 It is also recommended to apply the nitrogen fertilizer at two or three different timings rather than make a one‐time application as this allows an increase in potato yield.63, 64, 65 However, the yield would be reduced if application was split more than four times because the limited nitrogen supply at each application is the defining factor rather than the appropriate timings.66
Unlike nitrogen, phosphorous and potassium are not considered as essential factors for EB infection. By contrast, several micronutrients such as boron and zinc are important for plant resilience against Alternaria species given that deficiencies of these nutrients create leaky and unstable cell membranes in plants, releasing a massive number of organic compounds from cells, which could be a suitable food source for Alternaria species.67 In particular, the importance of zinc for plant protection against EB is highlighted in several scientific reports: zinc intervenes with the synthesis of activating metabolic reactions, production of chlorophyll and carbohydrate, and the synthesis of tryptophan, which converts later to auxin. The content of organic carbon in the soil does not affect EB infection.68
Soil texture is essential for control of fungi infection. After heavy rainy days, necrotic EB lesions appear, especially in sandy soils,31 and this tendency is also confirmed in the case of Belgium potato regions after 2‐year trials.69 An underlying factor of this result could be nutrient deficiency. The interlink between rotation duration and soil texture should be highlighted as shorter rotation occurs in a sandy region, in contrast to clay soil, which gives potatoes more chance to become infected by EB. There is a report on increased susceptibility of crops grown in sandy soils under plant‐parasitic nematode attack of about 200 infective juveniles of a root‐knot nematode, Meloidogyne incognita, per plant, which eventually caused severe damage in plant growth and chlorophyll content.70 In general, except for the stem nematode Ditylenchus dipsaci, the nematode population is higher in coarse‐textured soil such as sandy soil compared with clay soil, and this might partly affect EB depending on the crop host suitability and nematode densities at planting.64, 71
There is an interaction between Alternaria species and other microorganisms. Burkholderia bacteria have a co‐habitant relationship with A. alternata under nutrient‐limited conditions, using multiple substrates provided by the fungi which attenuated the starvation response observed when these bacteria are grown alone.72 Concomitantly, this symbiotic mechanism can also limit the phytopathogenic activity of Alternaria species,72, 73 meaning that Burkholderia can play a role as a biological agent of Alternaria species.49, 74 Rhizobacteria in the soil are known as a pathogen control. However, 59% of rhizobacteria failed to enhance resistance against EB.75 Concerning the interaction of A. solani with other fungi groups, it is reported that Arsenicum album, Nitricum acidum and Staphysagaria can inhibit the mycelium growth of A. solani under in vivo conditions.76 A reduction in soil extracellular and intracellular enzymes (e.g. dehydrogenase, phosphatase, β‐glucosidase and urease) is observed in fungus‐infected soil, implying that pathogens probably change the redox activity of plants by nutrient leaching, resulting in altered soil enzyme activity.77
2.6. Functions of existing forecast models and the limitations of model usages
Decision support systems (DSS) for proper pest management with fungicides to minimize the damage from EB infection have been developed for over 40 years. Several simulation models were implemented in the 1970 and 1980s, and the most recognized ones are FAST (Forecasting Alternaria Solani on Tomatoes), TOM‐CAST (Tomato Disease Forecast) and EPIDEM (Alternaria solani on tomatoes and potatoes). To modify and update, other derivate models have been created as decision support78, 79, 80 (e.g. CU‐FAST, PA‐FAST, NJ‐TOM‐CAST, WISDOM and Plant‐Plus).
2.6.1. FAST model
The FAST DSS, known as a long‐run product, was created for forecasting EB in tomato and then adjusted to potato field conditions.81 It comprises two empirical submodels to determine periods when environmental conditions are favourable for EB disease development.82, 83 One submodel includes leaf wetting time and mean air temperature, and the second submodel contains the daily severity‐rating (R), estimated by three environmental parameters: (i) mean air temperature for the past 5 days, (ii) hours of RH higher than 90% for the past 5 days and (iii) total rainfall for the past 7 days. This model enables the following three indicators to be forecast: (i) total of all severity disease values (TS) since the initial stage of the growing season, (ii) 7‐day cumulative severity value (CS) and (iii) 5‐day cumulative rating value (CR), calculated by totalling R values for the past 5 days. Based on the two threshold options of CS and CR values, this support model has successfully reduced the application frequency while the blight severity remained at the same magnitude with the commercial schedule.19, 84
2.6.2. TOM‐CAST model
TOM‐CAST is another long‐run DSS which has a much simpler approach to facilitate implementation than the FAST model. Using a small component of the FAST model (hourly leaf wetness and temperature), disease severity values (DSVs) are determined.82 The value range of the DSVs15, 16, 17, 18, 19, 20 defines the pest control schedule. Subsequent treatment is conducted with a pesticide, originally arranged with chlorothalonil. Other work81 demonstrated the performance of TOM‐CAST for six other fungicides comparing with a weekly schedule, highlighting the marketable yield with this model. The limitation of TOM‐CAST is the fact that it is not a maturity‐based model; no variable related to plant growth is included. Tuning DSVs to adjust to local conditions and varieties is challenging, requiring data from trials done over several years to find the optimal DSV values.85 To improve this model, a new model, the modified‐TOMCAST model, was created by complementing the DSS with a crop maturity module.86, 87 This updated model is useful to adjust the variability of different varieties in crop growth until maturation by changing the thresholds of the model at different crop stages. However, it should be highlighted that there exist some limitations of using these models. For instance, the IWP is not taken into consideration as a component of the model.23, 27 Also, rigorous calibration and validation of temperature intervals are required for different cropping conditions, such as semi‐arid and irrigated systems.
2.6.3. Pitfalls of forecast model application
The pitfall of using these models with fungicides is the ability of microorganisms to avoid sensitivity to the chemicals. Indeed, many different types of fungicides are used for EB, including (i) maneb, mancozeb, difenoconazole, boscalid, fluopyram, boscalid, fluopyram and chlorothalonil in the EU,15 (ii) boscalid and fluopyram in North America,88, 89 and (iii) difenoconazole and ethylhydrogen‐phosphate in Asia.90 However, the high genetic diversity of A. solani enables it to adapt to fungicides and shift the population towards more resistant isolates.15, 91, 92, 93, 94 Fungicide resistance against quinone outside inhibitor (QoI) was described first in the USA92, 93 and a decade later in Germany,95 while resistance against succinate dehydrogenase inhibitors (SDHIs) was found in Idaho. Among 39 strains of A. solani collected in 2009, only three strains were resistant to SDHIs and all to azoxystrobin. A year later, 57% of the isolates were resistant to boscalid.96 In a survey in the USA in 2010 and 2011, approximately, 80% of all A. solani assayed were found to have some level of resistance to boscalid, with about 5% and 75% of the population moderately resistant (to concentrations of 5–20 μg mL–1) and highly resistant (>20 μg mL–1) to the fungicide. Nearly 99% of all boscalid‐resistant isolates possessed the F129L mutation in the cytrochrome b gene, responsible for QoI resistance, indicating that an A. solani population with dual fungicide resistance predominates in the states surveyed.94 In Belgium, 83 A. solani and 53 A. alternata isolates were collected during 2014 and 2015 to assess the prevalence of SDHI mutants. The isolates were screened for the presence of amino acid substitutions in the different subunits of the succinate dehydrogenase gene (SdhB, SdhC and SdhD). The isolate screening revealed that mutations causing a reduced sensitivity towards SDHIs were widespread in the Belgian Alternaria population: 70% of the A. solani and 41% of the A. alternata isolates possessed one or more mutations.97
3. PRACTICAL STRATEGIES
The durability of host resistance is affected by the evolutionary potential of the pathogen and the inoculum pressure. The resistance genes in the plant coevolve with those of the pathogen's virulence, implying that consecutive cultivation of the same cultivars is not appropriate. The importance of proper pest management has to be considered for (i) minimization of the disease on crop leaves, (ii) decrease of potential inoculum in the field and its product, including mycotoxins, and (iii) avoiding the fate of breaking genetic resistance. Elimination of weeds as such as S. nigrum and S. carolinense, considered potential inoculum sources of Alternaria spp., can reduce the risk of sporulation.46 In addition, elimination of senescent leaves, apical pruning and vertical support favour radiation in the canopy and increase ventilation, which reduces water film on the leaf blade and eliminates contaminated debris and senescent leaves with greater susceptibility to the pathogen.
IPM is a holistic approach for sustainable agriculture. It relies on the type of cropping system, geography and development stage of plants, which are discussed in subsequent sections. It is reported6 that EB can be controlled by three measures: fungicide treatment, use of resistant cultivars and cultural practices. The first two measures are described above; in this section, the feasible practices for IPM are addressed (Fig. 2). The following agronomic practices can minimize or prevent possible damage caused by EB.
Figure 2.
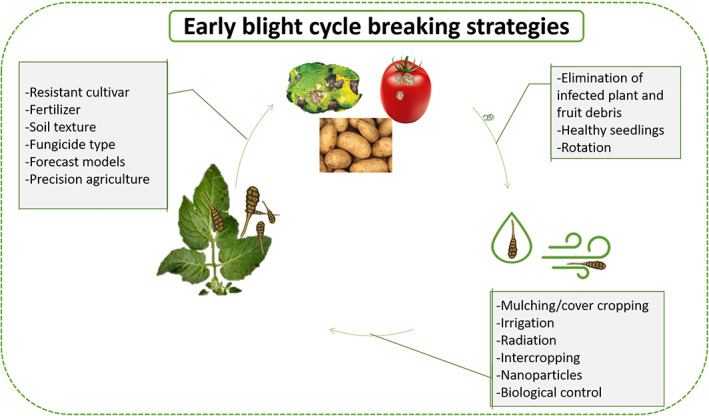
Different practices of pest management against early blight disease.
3.1. Rotation
First and foremost, crop rotation is an essential practice to minimize the possibility of the onset of EB infection, given that continuous potato production in the same field over 2–3 years is risky.98 Two years without potatoes is the minimum interval to delay the onset of EB in the potato field.99 Rotation is an efficient practice not only for reducing pests and diseases but also for balancing soil nutrients, thus effectively improving the physical and chemical properties of soil and regulating soil fertility. However, there exist a couple of pitfalls in the practice: (i) the production schedule needs to be changed every year to increase land utilization and (ii) there is a possible reduction in economic return over several years due to less potato production, which is valuable as a cash crop for farmers.
3.2. Intercropping
The inhibition of the conidial movement of fungi results from the fact that the intercrop provides a physical barrier and microclimate modification to reduce conidial germination and development. Marigold is a recognized plant for the protection against Pratylenchus penetrans and a lower population of this nematode affects crop resistance to infection by pathogens such as A. solani.100 Olfactory species [e.g. marigold (Calendula officinalis) and onion (Allium cepa)] are thought to be used for intercropping due to an antimicrobial allelopathy effect. However, intercropping will increase the difficulty of mechanical harvesting. In addition, the morphological characteristics and fertilizer requirements of intercropping crops and the cropping growth period needed to be taken into account.
3.3. Mulching and cover cropping
Mulching and cover cropping are also useful practices for reduction in EB infection.101, 102 Disease incidence of foliar and fruit rot pathogens can be reduced by creating a physical barrier to keep fruit from coming into direct contact with soil and disrupting the rain splash distribution of inoculum of soil‐borne fruit. Another hypothesis of reduction in splash dispersal is believed to be that (i) sensor wetness duration of the crop is reduced by mulch and (ii) soil particle dispersal is reduced. Moreover, mulching with plastic material is also effective for the prevention of soil evapotranspiration.19 Disadvantages of plastic mulching are cost and the need to use ecofriendly plastic.
3.4. Ultraviolet radiation
Blocking ultraviolet (UV) radiation by mesh or plant cover can retard the development of EB infection. Sporulation is affected by radiation exposure since the conidiophores are formed under high humidity and light.103 It is worth mentioning that the UV blocking method can delay not only disease progression of EB but also other diseases caused by other pathogenic microorganisms.104
3.5. Irrigation
The water regime is interlinked with EB germination. It is reported that sprinkler irrigation systems reduce disease incidence compared with furrow application, which creates excessive water condition in the field.105, 106 The change in microclimate conditions by irrigation reduces the incidence of this disease more in comparison with rainfed water systems.98
3.6. Nanoparticles
Application of nanoparticles is an attractive tool to enhance plant protection against EB.107, 108, 109, 110 Selenium, copper, silica and silver are the candidates for this method, which increases the antioxidant enzymes in plant tissues. Other work77 has demonstrated the impact of biosynthesized silver nanoparticles in reducing EB and increasing plant growth as well as photosynthesis. Finding an optimal dose of a nanoparticle is a crucial point for implementation.
3.7. Plant growth promoting rhizobacteria, biological agents and mycorrhiza
An ecofriendly biological control is useful to minimize EB emergence. A versatile series of different antagonistic agents for control of A. solani and A. alternata, or plant growth promoting reagents (PGPRs), have been reported as biological agents of EB. Streptomycetes spp. are antagonists that reduce spore germination, mycelial growth and sporulation of EB.111 Regarding the Ascomycetes group, Trichoderma species, such as Trichoderma harzianum and Trichoderma viride, are recognized PGPRs and biological agents against EB.112, 113, 114, 115 Pretreatment of potato tubers with Trichoderma decreased the infection of new‐crop tubers.115 It is presumed that Trichoderma has three strategies to enhance protection: (i) it is involved in the phytohormonal mechanisms of auxin and ethylene, (ii) it reduces gene expressions of some proteins involved in pyruvate kinase biosynthesis and (iii) it increases antioxidant enzymes in plant tissues.116
Additionally, rhizobacteria are a practicable microorganism group for protection against A. solani.75, 111, 116, 117, 118, 119, 120, 121, 122 Bacillus sp. show antifungal activity by releasing Indole‐3‐acetic acid (IAA) and lytic enzymes and triggers for plant protection mechanisms.120, 123 The combined application of PGPRs (e.g. Bacillus subtilis and Trichoderma) can strengthen the plant defence system by inducing these plant hormones as well as enhancing antioxidant enzymes including peroxidase and polyphenol oxidase.116, 124, 125
Mycorrhiza reduces susceptibility to A. solani.116 Plant hormone signalling pathways (e.g. the jasmonic and salicylic acid pathways) and enzymatic activities related to phenol compounds63 are activated by mycorrhiza. Supplying phosphorous by mycorrhiza interaction is not essential for the enhancement of the protection system against EB.126 Interestingly, mycorrhiza can mediate plant–plant communication between healthy plants and pathogen‐infected tomato plants63 with the induction of defence signals such as peroxidase, polyphenol oxidase, chitinase, β‐1,3‐glucanase, phenylalanine ammonia‐lyase and lipoxygenase.
Inoculation of a local biological agent against EB is a recommended approach. As an example, the inoculation of Macrolepiota sp., belonging to the Basidiomycete family, is useful not only for biological control against A. solani but also for the enhancement of plant growth.
3.8. Treatment with organic products or biostimulants
Using organic materials and biostimulants for plant protection is also reported for pest management against EB. Biopolymers of sodium alginate,127 extract of seaweed,128 the neem tree129 and the artemisia tree130 were tested to protect plants from infection by EB. Other biostimulants such as humic acid, chitosan and thiamine have also been examined.131, 132 The induction of antifungal enzymes and regulation of gene expression for defence systems involved with plant hormones are the underpinning factors for the reduction in the infection.127
3.9. Precision agriculture
Precision agriculture is an emerging approach that enables farmers to improve accuracy and reduce costs by a pinpoint application of crop protection agents using technologies such as hyperspectral imaging by drones and robotics. These technologies might also help to identify nutrient and water stress in the potato plant, which affects susceptibility to EB. DSS can be useful for forecasting the date of exceeding control thresholds and fungicide application. Using mathematical models, the time requirement for the processing of the data analysis can be minimized. The model FAST, for instance, is a forecast system for A. solani on tomato. BSPcast is a model derived from FAST by adapting it to the aetiology and epidemiology of S. vesicarium on pear. The model TOM‐CAST was also derived from FAST as a weather‐timed fungicide spray forecast, and its potential to reduce spray applications has been tested. Many papers on the detection of EB by hyperspectral images have been published.133, 134, 135, 136, 137 The wavelengths of 715133 and 750 nm,133 which belong to the range of near‐infrared, are the most discriminative range of the spectrum for disease classification. Different algorithms based on deep‐learning and machine‐learning are applied for image processing techniques, and the accuracy of the models for disease detection of EB and LB in tomato leaves varies between 76% and 98%.123 To increase the accuracy, the following points should be considered: (i) larger dataset size, (ii) higher spatial resolution or a broader spectral range and (iii) more advanced image‐processing techniques. Overall, precision agriculture is a promising and conducive approach to protecting the environment and reducing production costs while reducing the use of fungicides and fertilizers.
4. FUTURE APPROACH
Novel technologies and agronomical practices can mitigate the damage of EB by making tomato and potato production more sustainable. Due to the intertwined complexity of EB disease, the idea of using one specific approach as the 'silver bullet’ solution should be avoided (e.g. application of biological control without knowing soil fertility or fungi gene editing without considering field practices). By contrast, a multidisciplinary approach across different scientific domains from laboratory to field experiments can help us to understand more about underlying factors and allow us to co‐design IPM with other stakeholders (e.g. farmers, seed distributors, fungicide companies). Further investigation should be conducted to determine an economic assessment of combined applications.
ACKNOWLEDGEMENTS
Keiji Jindo thanks for his financial support (3710473400‐1). We thank Professor Roland N. Perry for revising the English.
REFERENCES
- 1.Grigolli JFJ, Kubota MM, Alves DP, Rodrigues GB, Cardoso CR, Rodigues GBet al., Characterization of tomato accessions for resistance to early blight. Crop Breed Appl Biot 11:174–180 (2011). [Google Scholar]
- 2.Stevenson WR, James RV, Inglis DA, Johnson DA, Schotzko RT and Thornton RE, Fungicide spray programs for defender, a new potato cultivar with resistance to late blight and early blight. Plant Dis 91:1327–1336 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Al‐Askar AA, Ghoneem KM, Rashad YM, Abdulkhair WM, Hafez EE, Shabana YMet al., Occurrence and distribution of tomato seed‐borne mycoflora in Saudi Arabia and its correlation with the climatic variables. J Microbial Biotechnol 7:556–569 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaerani R and Voorrips RE, Tomato early blight (Alternaria solani): the pathogen, genetics, and breeding for resistance. J Gen Plant Pathol 72:335–347 (2006). [Google Scholar]
- 5.Chaerani R, Groenwold R, Stam P and Voorrips RE, Assessment of early blight (Alternaria solani) resistance in tomato using a droplet inoculation method. J Gen Plant Pathol 73:96–103 (2007). [Google Scholar]
- 6.Adhikari P, Oh Y and Panthee DR, Current status of early blight resistance in tomato: an update. Int J Mol Sci 18:2019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egidi E, Delgado‐Baquerizo M, Plett JM, Wang J, Eldridge DJ, Bardgett RDet al., A few ascomycota taxa dominate soil fungal communities worldwide. Nat Commun 10:2369 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado‐Baquerizo M, Guerra CA, Cano‐Díaz C, Egidi E, Wang J‐T, Eisenhauer Net al., The proportion of soil‐borne pathogens increases with warming at the global scale. Nat Climate Change 10:550–554 (2020). [Google Scholar]
- 9.Woudenberg JH, Truter M, Groenewald JZ and Crous PW, Large‐spored Alternaria pathogens in section Porri disentangled. Stud Mycol 79:1–47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edin E, Liljeroth E and Andersson B, Long term field sampling in Sweden reveals a shift in occurrence of cytochrome b genotype and amino acid substitution F129L in Alternaria solani, together with a high incidence of the G143A substitution in Alternaria alternata . Eur J Plant Pathol 155:627–641 (2019). [Google Scholar]
- 11.Bessadat N, Berruyer R, Hamon B, Bataille‐Simoneau N, Benichou S, Kihal Met al., Alternaria species associated with early blight epidemics on tomato and other Solanaceae crops in northwestern Algeria. Eur J Plant Pathol 148:181–197 (2016). [Google Scholar]
- 12.Tatiana TMS, Rodrigues LAM, Dhingra OD and Mizubuti ESG, In vitro production of conidia of Alternaria solani. Trop Plant Pathol 35:203–212 (2010). https://www.scielo.br/pdf/tpp/v35n4/v35n4a01.pdf [Google Scholar]
- 13.Bessadat N, Hamon B, Bataillé‐Simoneau N, Mabrouk K and Simoneau P, First report of Alternaria dauci causing leaf blight of coriander (Coriandrum sativum) in Algeria. Plant Dis 103:2471–2471 (2019). [Google Scholar]
- 14.Gannibal PB, Orina AS, Mironenko NV and Levitin MM, Differentiation of the closely related species, Alternaria solani and A. tomatophila, by molecular and morphological features and aggressiveness. Eur J Plant Pathol 139:609–623 (2014). [Google Scholar]
- 15.Landschoot S, De Reu J, Audenaert K, Vanhaverbeke P, Haesaert G, De Baets Bet al., Potentials and limitations of existing forecasting models for Alternaria on potatoes: challenges for model improvement. Potato Res 60:61–76 (2017). [Google Scholar]
- 16.Mantecón JD, Potato yield increases due to fungicide treatment in argentinian early blight (Alternaria solani) and late blight (Phytophthora infestans) field trials during the 1996‐2005 seasons. Plant Health Prog 8:28 (2007). [Google Scholar]
- 17.Van der Waals JKE, A review of early blight of potato. Afr Plant Protect 7:91–102 (2001). [Google Scholar]
- 18.Escuredo O, Seijo‐Rodríguez A, Meno L, Rodríguez‐Flores MS and Seijo MC, Seasonal dynamics of Alternaria during the potato growing cycle and the influence of weather on the early blight disease in north‐West Spain. Am J Potato Res 96:532–540 (2019). [Google Scholar]
- 19.Mills DJ, Coffman CB, Teasdale JR, Everts KL, Abdul‐Baki AA, Lydon Jet al., Foliar disease in fresh‐market tomato grown in differing bed strategies and fungicide spray programs. Plant Dis. 86:955–959 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Vloutoglou IKS and Darras A, Effects of isolate virulence and host susceptibility on development of early blight (Alternaria solani) on tomato. Bull OEPP/EPPO Bull 30:263–267 (2000). [Google Scholar]
- 21.Jambhulkar PP, Jambhulkar N, Meghwal M and Ameta GS, Altering conidial dispersal of Alternaria solani by modifying microclimate in tomato crop canopy. Plant Pathol J 32:508–518 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevenson RE, Effect of radiation, temperature, and moisture on conidial germination of Alternaria solani. Phtopathology 78:926–930 (1988). [Google Scholar]
- 23.Meno L, Escuredo O, Rodríguez F and Seijo MC, Interrupted wet period (IWP) to forecast the aerial Alternaria in potato crops of a Limia (Spain). Agronomy 9:585 (2019). [Google Scholar]
- 24.Zaag V, Overwintering and epidemiology of Phytophthora infestans and some new possibilities of control. Tijdschr Plziekt 62:89–156 (1956). [Google Scholar]
- 25.Weisz R, Smilowitz Z and Christ B, Distance, rotation, and border crops affect Colorado potato beetle (Coleoptera: Chrysomelidae) colonization and population density and early blight (Alternaria Solani) severity in rotated potato fields. J Econ Entomol 87:723–729 (1994). [Google Scholar]
- 26.Ding S, Meinholz K, Cleveland K, Jordan SA and Gevens AJ, Diversity and virulence of Alternaria spp. causing potato early blight and brown spot in Wisconsin. Phytopathology 109:436–445 (2019). [DOI] [PubMed] [Google Scholar]
- 27.van der Waals JE, Korsten L, Aveling TAS and Denner FDN, Influence of environmental factors on field concentrations of Alternaria solani conidia above a south African potato crop. Phytoparasitica 31:353–364 (2003). [Google Scholar]
- 28.Chen W‐Q, Morgan DP, Felts D and Michailides TJ, Antagonism of Paenibacillus lentimorbus to Botryosphaeria dothidea and biological control of panicle and shoot blight of pistachio. Plant Dis. 87:359–365 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Gravesen S, Fungi as a cause of allergic disease. Allergy 34:133–154 (1979). [DOI] [PubMed] [Google Scholar]
- 30.Yellareddygari SKR, Pasche JS, Taylor RJ and Gudmestad NC, Individual participant data meta‐analysis of foliar fungicides applied for potato early blight management. Plant Dis. 100:200–206 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Shtienberg D.Alternaria diseases of potatoes: epidemiology and management under Israeli conditions. PPO – Special Report no. 16, pp. 169–180 (2014).
- 32.Abuley IK, Nielsen BJ and Labouriau R, Resistance status of cultivated potatoes to early blight (Alternaria solani) in Denmark. Plant Pathol 67:315–326 (2018). [Google Scholar]
- 33.Lukens DSR, Effect of glucose and adenosine phosphates on production of extracellular carbohydrases of Alternaria solani . Plant Physiol 54:666–669 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaerani R.Early blight resistance in tomato: screening and genetic study. PhD thesis, Wageningen University, Wageningen, The Netherlands (2006).
- 35.Sands PJ, Hackett C and Nix HA, A model of the development and bulking of potatoes (Solanum tuberosum L.) I. derivation from well‐managed field crops. Field Crop Res 2:309–331 (1079). [Google Scholar]
- 36.James G. Roddick and Anna L. Rijnenberg, Effect of steroidal glycoalkaloids of the potato on the permeability of liposome membranes. Physiol Plant 68:436–440 (1986). [Google Scholar]
- 37.Yamunarani K, Jaganathan R, Bhaskaran R, Govindaraju P and Velazhahan R, Induction of early blight resistance in to mato by Quercus infectoria gall extract in association with accumulation of phenolics and defense‐related enzymes. ACTA Physiol Plant 26:281–290 (2004). [Google Scholar]
- 38.Awan ZA and Shoaib A, Combating early blight infection by employing Bacillus subtilis in combination with plant fertilizers. Curr Plant Biol 20:100125 (2019). [Google Scholar]
- 39.Jagadeesh KSJDR, Biological control of early blight of tomato caused by Alternaria solani as influenced by different delivery methods of pseudomonas gladioli B25. Acta Hort 808:327–332 (2009). [Google Scholar]
- 40.Weber BN and Jansky SH, Resistance to Alternaria solani in hybrids between a Solanum tuberosum haploid and S. raphanifolium. Phytopathology 102:214–221 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Upadhyay P, Rai A, Kumar R, Singh M and Sinha B, Microarray analyses during early stage of the tomato/Alternaria solani interaction. Genom Data 6:170–172 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolters PJ, de Vos L, Bijsterbosch G, Woudenberg JHC, Visser RGF, van der Linden Get al., A rapid method to screen wild Solanum for resistance to early blight. Euro . J Plant Pathol 154:109–114 (2019). [Google Scholar]
- 43.Upadhyay P, Sources of resistance against early blight (Alternaria solani) in tomato (Solanum lycopersicum). Indian J Agric Sci 79:752–753 (2009). [Google Scholar]
- 44.Shinde BA, Dholakia BB, Hussain K, Panda S, Meir S, Rogachev Iet al., Dynamic metabolic reprogramming of steroidal glycol‐alkaloid and phenylpropanoid biosynthesis may impart early blight resistance in wild tomato (Solanum arcanum Peralta). Plant Mol Biol 95:411–423 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Stam R, Scheikl D and Tellier A, The wild tomato species Solanum chilense shows variation in pathogen resistance between geographically distinct populations. PeerJ 5:e2910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foolad MR, Merk HL and Ashrafi H, Genetics, genomics and breeding of late blight and early blight resistance in tomato. Crit Rev Plant Sci 27:75–107 (2008). [Google Scholar]
- 47.Fradin EF, Zhang Z, Juarez Ayala JC, Castroverde CD, Nazar RN, Robb Jet al., Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol 150:320–332 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinde BA, Dholakia BB, Hussain K, Aharoni A, Giri AP and Kamble AC, WRKY1 acts as a key component improving resistance against Alternaria solani in wild tomato, Solanum arcanum Peralta. Plant Biotechnol J 16:1502–1513 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarkar D, Maji RK, Dey S, Sarkar A, Ghosh Z and Kundu P, Integrated miRNA and mRNA expression profiling reveals the response regulators of a susceptible tomato cultivar to early blight disease. DNA Res 24:235–250 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenderoth M, Pinecker C, Voss B and Fischer R, Establishment of CRISPR/Cas9 in Alternaria alternata . Fungal Genet Biol 101:55–60 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Igbalajobi OYZ and Fischer R, Red‐ and blue‐light sensing in the plant pathogen Alternaria alternata depends on phytochrome and the white‐collar protein LreA. MBio 10:e00371–e00319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wenderoth M, Garganese F, Schmidt‐Heydt M, Soukup ST, Ippolito A, Sanzani SMet al., Alternariol as virulence and colonization factor of Alternaria alternata during plant infection. Mol Microbiol 112:131–146 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Lin G, Niño‐Liu D and Foolad MR, Mapping QTLs conferring early blight (Alternaria solani) resistance in a Lycopersicon esculentum×L. hirsutum cross by selective genotyping. Mol Breed 12:3–19 (2003). [Google Scholar]
- 54.Odilbekov F, Selga C, Ortiz R, Chawade A and Liljeroth E, QTL mapping for resistance to early blight in a tetraploid potato population. Agronomy 10:728 (2020). [Google Scholar]
- 55.Dinh QD, Dechesne A, Furrer H, Graham T, Visser RGF, Harbinson Jet al., High altitude wild species Solanum arcanum LA385–a potential source for improvement of plant growth and photosynthetic performance at suboptimal temperatures. Front Plant Sci 10:1163 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghani MA, Abbas MM, Amjad M, Ziaf K, Ali B, Shaheen Tet al., Production and characterisation of tomato derived from interspecific hybridisation between cultivated tomato and its wild relatives. J Hortic Sci Biotech 95:506–520 (2020). [Google Scholar]
- 57.Sifres A, Blanca J and Nuez F, Pattern of genetic variability of Solanum habrochaites in its natural area of distribution. Gen Resour Crop Evol 58:347–360 (2011). [Google Scholar]
- 58.Zhang D, He JY, Haddadi P, Zhu JH, Yang ZH and Ma L, Genome sequence of the potato pathogenic fungus Alternaria solani HWC‐168 reveals clues for its conidiation and virulence. BMC Microbiol 18:176 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu Q, Yang ZH, Zhao DM, Zhang D, Wang Q, Ma LSet al., Development of a semi‐nested PCR‐based method for specific and rapid detection of Alternaria solani causing potato early blight in soil. Curr Microbiol 74:1083–1088 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Wojciechowska E, Weinert CH, Egert B, Trierweiler B, Schmidt‐Heydt M, Horneburg Bet al., Chlorogenic acid, a metabolite identified by untargeted metabolome analysis in resistant tomatoes, inhibits the colonization by Alternaria alternata by inhibiting alternariol biosynthesis. Eur J Plant Pathol 139:735–747 (2014). [Google Scholar]
- 61.Abuley IK, Nielsen BJ and Hansen HH, The influence of crop rotation on the onset of early blight (Alternaria solani). J Phytopathol 167:35–40 (2018). [Google Scholar]
- 62.Ravikumar MC, Singh H and Garampalli RH, Comparative evaluation of long‐term storage techniques on viability and virulence of Alternaria solani . J Taibah Univ Sci 10:607–613 (2018). [Google Scholar]
- 63.Abuley IK and Nielsen BJ, Corrigendum to “evaluation of models to control potato early blight (Alternaria solani) in Denmark”. Crop Prot 102:118–128 (2017). [Google Scholar]
- 64.Shah SFA, McKenzie BA, Gaunt RE, Marshall JW and Frampton CM, Effect of early blight (Alternaria solani) on healthy area duration and healthy area absorption of potatoes (Solanum tuberosum) grown in Canterbury New Zealand with different nitrogen application and stress from potato cyst nematode (Globodera rostochiensis). N Z J Crop Hort Sci 32:85–102 (2010). [Google Scholar]
- 65.Blachinski D, Shtienberg D, Dinoor A, Kafkafi U, Sujkowski LS, Zitter ATAet al., Influence of foliar application of nitrogen and potassium on Alternaria diseases in potato, tomato and cotton. Phytoparasitica. 24:281–292 (1996). [Google Scholar]
- 66.Kelling KA, Stevenson WR, Speth PE and James RV, Interactive effects of fumigation and fungicides on potato response to nitrogen rate or timing. Am J Potato Res 93:533–542 (2016). [Google Scholar]
- 67.Machado PP, Steiner F, Zuffo AM and Machado RA, Could the supply of boron and zinc improve resistance of potato to early blight? Potato Res 61:169–182 (2018). [Google Scholar]
- 68.Busnello FJ, Boff MIC, Agostinetto L, Souza ZS and Boff P, Potato genotypes reaction to early blight and late blight in organic cultivation. Ciênc Rural 49:e20180469 (2019). [Google Scholar]
- 69.Vandecasteele M, Landschoot S, Carrette J, Verwaeren J, Höfte M, Audenaert Ket al., Species prevalence and disease progression studies demonstrate a seasonal shift in the Alternaria population composition on potato. Plant Pathol 67:327–336 (2018). [Google Scholar]
- 70.Ahmad L. Siddiqui ZA. Abd_Allah EF , Effects of interaction of Meloidogyne incognita, Alternaria dauci and Rhizoctonia solani on the growth, chlorophyll, carotenoid and proline contents of carrot in three types of soil. Acta Agric. Scand. Sect. B Plant Soil Sci 69:324–331 (2019). [Google Scholar]
- 71.Ahmad F, Raziq F, Ullah N, Khan H and Din N, In vitroand in vivobio‐assay of phytobiocidal effect of plant extracts on Alternaria solani causing agent of early blight disease in tomato. Arch Phytopathol Plant Protect 50:568–583 (2017). [Google Scholar]
- 72.Stopnisek N, Zuhlke D, Carlier A, Barberan A, Fierer N, Becher Det al., Molecular mechanisms underlying the close association between soil Burkholderia and fungi. ISME J 10:253–264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rojas‐Rojas FU, Salazar‐Gomez A, Vargas‐Diaz ME, Vasquez‐Murrieta MS, Hirsch AM, De Mot Ret al., Broad‐spectrum antimicrobial activity by Burkholderia cenocepacia TAtl‐371, a strain isolated from the tomato rhizosphere. Microbiology 164:1072–1086 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Stoyanova M, Pavlina I, Moncheva P and Bogatzevska N, Biodiversity and incidence of Burkholderia species. Biotech Biotechnol Equip 21:306–310 (2014). [Google Scholar]
- 75.Hariprasad P, Venkateswaran G and Niranjana SR, Diversity of cultivable rhizobacteria across tomato growing regions of Karnataka. Biol Control 72:9–16 (2014). [Google Scholar]
- 76.Modolon TA, Boff P, Boff MIC and Miquelluti DJ, Mycelium growth of early tomato blight pathogen, Alternaria solani, subjected to high dilution preparations. Biol Agric Hortic 31:28–34 (2014). [Google Scholar]
- 77.Kumari M, Pandey S, Bhattacharya A, Mishra A and Nautiyal CS, Protective role of biosynthesized silver nanoparticles against early blight disease in Solanum lycopersicum . Plant Physiol Biochem 121:216–225 (2017). [DOI] [PubMed] [Google Scholar]
- 78.Pscheidt JW and Stevenson WR, Comparison of forecasting methods for control of potato early blight in Wisconsin. Plant Dis 70:915–920 (1986). [Google Scholar]
- 79.Cowgill WP, Maletta MH, Manning T, Tietjen WH, Johnston SA and Nitzsche PJ, Early blight forecasting systems: evaluation, modification, and validation for use in fresh‐market tomato production in northern New Jersey. Hortscience 40:85–93 (2005). [Google Scholar]
- 80.Harms T HR, Konschuh M. Evaluation of early blight (Alternaria solani) prediction techniques for Southern Alberta. Available: www.demofarm.ca/pdf/early_blight_prediction.pdf [1 June 2020].
- 81.Shuman JL and Christ BJ, Integrating a host‐resistance factor into the FAST system to forecast early blight of potato. Am J Potato Res 82:9–19 (2005). [Google Scholar]
- 82.Madden L, Pennypacker SP and MacNab AAFAST, A forecast system for Alternaria solani on tomato. Phytopathology 68:1354–1358 (1978). [Google Scholar]
- 83.Pennypacker SP, Madden LV and MacNab AA, Validation of an early blight forecasting system for tomatoes. Plant Dis 67:287–289 (1983). [Google Scholar]
- 84.Batista DC, Lima MA, Haddad F, Maffia LA and Mizubuti ESG, Validation of decision support systems for tomato early blight and potato late blight, under Brazilian conditions. Crop Prot 25:664–670 (2006). [Google Scholar]
- 85.Byrne JM, Efficacy and economics of management strategies to control anthracnose fruit rot in processing tomatoes in the aidwest. Plant Dis 81:1167–1172 (1997). [DOI] [PubMed] [Google Scholar]
- 86.Abuley IK and Nielsen BJ, Evaluation of models to control potato early blight (Alternaria solani) in Denmark. Crop Prot 102:118–128 (2017). [Google Scholar]
- 87.Abuley IK and Nielsen BJ, Integrating cultivar resistance into the TOMCAST model to control early blight of potato, caused by Alternaria solani . Crop Protect 117:69–76 (2019). [Google Scholar]
- 88.Bauske MJ and Gudmestad NC, Parasitic fitness of fungicide‐resistant and ‐sensitive isolates of Alternaria solani . Plant Dis 102:666–673 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Bauske MJ, Yellareddygari SKR and Gudmestad NC, Potential impact of fluopyram on the frequency of the D123E mutation in Alternaria solani . Plant Dis 102:656–665 (2018). [DOI] [PubMed] [Google Scholar]
- 90.Hina Zafar SS and Sheikh A, Detection of antifungal activity of various plant extracts against Alternaria solani, the cause of early blight of tomato. Int J Biol Biotech 11:369–374 (2014). [Google Scholar]
- 91.Holm AL, Rivera VV, Secor GA and Gudmestad N, Temporal sensitivity of Alternaria solani to foliar fungicides. Am J Pot Res 80:33–40 (2003). [Google Scholar]
- 92.Pasche JS, Piche LM and Gudmestad NC, Effect of the F129L mutation in Alternaria solani on fungicides affecting mitochondrial Respiratio. Plant Dis 89:269–278 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Pasche JS and Gudmestad NC, Prevalence, competitive fitness and impact of the F129L mutation in Alternaria solani from the United States. Crop Protect 27:427–435 (2008). [Google Scholar]
- 94.Gudmestad NC, Arabiat S, Miller JS and Pasche JS, Prevalence and impact of SDHI fungicide resistance in Alternaria solani . Plant Dis 97:952–960 (2013). [DOI] [PubMed] [Google Scholar]
- 95.Juergen Leimimger HH.Disease‐orientated threshold values as tool for effective early blight control. PPO‐Special Report no. 99‐106 (2012).
- 96.Fairchild KL, Miles TD and Wharton PS, Assessing fungicide resistance in populations of Alternaria in Idaho potato fields. Crop Prot 49:31–39 (2013). [Google Scholar]
- 97.Landschoot S, Vandecasteele M, Carrette J, De Baets B, Höfte M, Audenaert Ket al., Assessing the Belgian potato Alternaria population for sensitivity to fungicides with diverse modes of action. Eur J Plant Pathol 148:657–672 (2016). [Google Scholar]
- 98.Olanya OM, Honeycutt CW, Larkin RP, Griffin TS, He Z and Halloran JM, The effect of cropping systems and irrigation management on development of potato early blight. J Gen Plant Pathol 75:267–275 (2009). [Google Scholar]
- 99.Abuley IK, Nielsen BJ and Hansen HH, The influence of timing the application of nitrogen fertilizer on early blight (Alternaria solani). Pest Manag Sci 75:1150–1158 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Morgan GD, Stevenson WR, MacGuidwin AE, Kelling KA, Binning LK, and Zhu J , Plant pathogen population dynamics in potato fields. J Nematol 34:189–193 (2002). [PMC free article] [PubMed] [Google Scholar]
- 101.Tusiime SM, Nonnecke GR, Masinde DM and Jensen HH, Evaluation of horticultural practices for sustainable tomato production in eastern Uganda. Hort Sci 54:1934–1940 (2019). [Google Scholar]
- 102.Wyenandt CA, Rideout SL, Gugino BK, McGrath MT, Everts KL and Mulrooney RP, Fungicide resistance management guidelines for the control of tomato diseases in the mid‐Atlantic and northeast regions of the United States. Plant Health Prog 11:32 (2010). [Google Scholar]
- 103.Yadav SM, Singh V and Chand R, Mass sporulation of Alternaria solani causing early blight of tomato. Indian Phytopathol 68:83–86 (2015). [Google Scholar]
- 104.Raviv MAY, UV radiation effects on pathogens and insect pests of greenhouse‐grown crops. Photochem Photobiol 79:219–226 (2004). [DOI] [PubMed] [Google Scholar]
- 105.Olanya OM, Honeycutt CW, He Z, Larkin RP, Halloran JM and Frantz JM, Early and late blight potential on russet Burbank potato as affected by microclimate, cropping systems and irrigation management in northeastern United States, in Sustainable Potato Production: Global Case Studies, ed. by He Z, Larkin RP and Honeycutt CW. Springer, Amsterdam, pp. 43–60 (2012). [Google Scholar]
- 106.Nasr‐Esfahani M, An IPM plan for early blight disease of potato Alternaria solani sorauer and A. alternata (fries.) Keissler. Arch Phytopathol Plant Prot:1–12 (2020). [Google Scholar]
- 107.Derbalah A, Shenashen M, Hamza A, Mohamed A and El Safty S, Antifungal activity of fabricated mesoporous silica nanoparticles against early blight of tomato. Egypt J Basic Appl Sci 5:145–150 (2019). [Google Scholar]
- 108.Zakharova OV, Gusev AA, Zherebin PM, Skripnikova EV, Skripnikova MK, Ryzhikh VEet al., Sodium tallow Amphopolycarboxyglycinate‐stabilized silver nanoparticles suppress early and late blight of Solanum lycopersicum and stimulate the growth of tomato plants. BioNanoScience 7:692–702 (2017). [Google Scholar]
- 109.Adisa IO, Pullagurala VLR, Peralta‐Videa JR, Dimkpa CO, Elmer WH, Gardea‐Torresdey JLet al., Recent advances in nano‐enabled fertilizers and pesticides: a critical review of mechanisms of action. Environ Sci Nano 6:2002–2030 (2019). [Google Scholar]
- 110.Quiterio‐Gutierrez T, Ortega‐Ortiz H, Cadenas‐Pliego G, Hernandez‐Fuentes AD, Sandoval‐Rangel A, Benavides‐Mendoza Aet al., The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternaria solani . Int J Mol Sci 20:1950 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shanmugam V, Atri K, Gupta S, Kanoujia N and Naruka DS, Selection and differentiation of Bacillus spp. antagonistic to Fusarium oxysporum f.sp. lycopersici and Alternaria solani infecting tomato. Folia Microbiol (Praha) 56:170–177 (2011). [DOI] [PubMed] [Google Scholar]
- 112.Selim ME, Effectiveness of trichoderma biotic applications in regulating the related defense genes affecting tomato early blight disease. J Plant Pathol Microbiol 6:10 (2015). [Google Scholar]
- 113.Verma A, Kumar S, Harshita, Shina A and Jaiswal S, Evaluate the efficacy of bio‐control agents and botanicals against early blight of potato caused by Alternaria solani . Pharm Innov J 7:28–30 (2018). https://www.thepharmajournal.com/archives/2018/vol7issue3/PartA/7‐2‐44‐666.pdf [Google Scholar]
- 114.Fontenelle ADB, Guzzo SD, Lucon CMM and Harakava R, Growth promotion and induction of resistance in tomato plant against Xanthomonas euvesicatoria and Alternaria solani by Trichoderma spp. Crop Protect 30:1492–1500 (2011). [Google Scholar]
- 115.Kulikov SN, Alimova FK, Zakharova NG, Nemtsev SV and Varlamov VP, Biological preparations with different mechanisms of action for protecting potato against fungal diseases. Appl Biochem Microbiol 42:77–83 (2006). [PubMed] [Google Scholar]
- 116.Narendra Babu A, Jogaiah S, Ito S, Kestur Nagaraj A and Tran LS, Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci 231:62–73 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Fatima Z, Saleemi M, Zia M, Sultan T, Aslam M, Rehman Ret al., Antifungal activity of plant growth‐promoting rhizobacteria isolates against Rhizoctonia solani in wheat. Afr J Biotechnol 8:219–225 (2009). [Google Scholar]
- 118.Grady EN, MacDonald J, Ho MT, Weselowski B, McDowell T, Solomon Oet al., Characterization and complete genome analysis of the surfactin‐producing, plant‐protecting bacterium Bacillus velezensis 9D‐6. BMC Microbiol 19:5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao Z, Zhang B, Liu H, Han J and Zhang Y, Identification of endophytic bacillus velezensis ZSY‐1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea . Biol Control. 105:27–39 (2017). [Google Scholar]
- 120.Paramanandham P, Rajkumari J, Pattnaik S and Busi S, Biocontrol potential against Fusarium oxysporum f. sp. lycopersici and Alternaria solani and tomato plant growth due to plant growth–promoting Rhizobacteria. Int J Veg Sci 23:294–303 (2017). [Google Scholar]
- 121.Attia MS, El‐Sayyad GS, Abd Elkodous M and El‐Batal AI, The effective antagonistic potential of plant growth‐promoting rhizobacteria against Alternaria solani‐causing early blight disease in tomato plant. Sci Hortic 266:109289 (2020). [Google Scholar]
- 122.Caulier S, Gillis A, Colau G, Licciardi F, Liepin M, Desoignies Net al., Versatile antagonistic activities of soil‐borne Bacillus spp. and Pseudomonas spp. against Phytophthora infestans and other potato pathogens. Front Microbiol 9:143 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karthika S, Midhun SJ and Jisha MS, A potential antifungal and growth‐promoting bacterium Bacillus sp. KTMA4 from tomato rhizosphere. Microb Pathog 142:104049 (2020). [DOI] [PubMed] [Google Scholar]
- 124.Chowdappa P, Mohan Kumar SP, Jyothi Lakshmi M and Upreti KK, Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3 . Biol Control 65:109–117 (2013). [Google Scholar]
- 125.Mane MM, Lal A, Zghair QN and Simon S, Efficacy of certain bio agents and fungicides against early blight of potato (Solanum tuberosum L.). International . J Plant Protect 7:433–436 (2014). [Google Scholar]
- 126.Lawrence CB, Singh NP, Qiu J, Gardner RG and Tuzun S, Constitutive hydrolytic enzymes are associated with polygenic resistance of tomato to Alternaria solani and may function as an elicitor release mechanism. Physiol Mol Plant Pathol 57:211–220 (2000). [Google Scholar]
- 127.Dey P, Ramanujam R, Venkatesan G and Nagarathnam R, Sodium alginate potentiates antioxidant defense and PR proteins against early blight disease caused by Alternaria solani in Solanum lycopersicum Linn. PLoS One 14:e0223216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hernández‐Herrera RM, Virgen‐Calleros G, Ruiz‐López M, Zañudo‐Hernández J, Délano‐Frier JP and Sánchez‐Hernández C, Extracts from green and brown seaweeds protect tomato (Solanum lycopersicum) against the necrotrophic fungus Alternaria solani . J Appl Psychol 26:1607–1614 (2013). [Google Scholar]
- 129.Hassanein NM, Abou Zeid MA, Youssef KA and Mahmoud DA, Control of tomato early blight and wilt using aqueous extract of neem leaves. Phytopathol Mediterr 49:143–151 (2010). [Google Scholar]
- 130.Khan SW, Hussain A, Ali H, Abbas H, Ali S and Hussain A, Spatial distribution of early blight disease on tomato, climatic factors and bioefficacy of plant extracts against Alternaria solani . Acta Sci Pol Hortorum Cultus 18:29–38 (2019). [Google Scholar]
- 131.Abd‐El‐Kareem F, Abd‐El‐Faten FM and Fotouh YO, Integrated treatments between humic acid and sulfur for controlling early blight disease of potato plants under field infection. Res J Agric Biol Sci 5:1039–1045 (2009). [Google Scholar]
- 132.Farouk S, Youssef SA and Ali AA, Exploitation of biostimulatants and vitamins as an alternative strategy to control early blight of tomato plants. Asian J Plant Sci 11:36–43 (2012). [Google Scholar]
- 133.Xie C and He Y, Spectrum and image texture features analysis for early blight disease detection on eggplant leaves. Sensors (Basel) 16:676 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gold KM, Townsend PA, Chlus A, Herrmann I, Couture JJ, Larson ERet al., Hyperspectral measurements enable pre‐symptomatic detection and differentiation of contrasting physiological effects of late blight and early blight in potato. Remote Sens (Basel) 12:286 (2020). [Google Scholar]
- 135.Li Z, Paul R, Ba T, Saville AC, Hansel JC, Yu Tet al., Non‐invasive plant disease diagnostics enabled by smartphone‐based fingerprinting of leaf volatiles. Nat Plants 5:856–866 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Van De Vijver R, Mertens K, Heungens K, Somers B, Nuyttens D, Borra‐Serrano Iet al., In‐field detection of Alternaria solani in potato crops using hyperspectral imaging. Comput Electron Agric 168:105106 (2020). [Google Scholar]
- 137.Xie C, Shao Y, Li X and He Y, Detection of early blight and late blight diseases on tomato leaves using hyperspectral imaging. Sci Rep 5:16564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


