Abstract
Objective
To evaluate the role of the microaxial percutaneous mechanical circulatory support device (Impella® pump) implantation pre‐percutaneous coronary intervention (PCI) versus during/after PCI in cardiogenic shock (CS) and high‐risk PCI populations.
Background
A better understanding of the safety and effectiveness of the Impella and the role of timing of this support initiation in specific clinical settings is of utmost clinical relevance.
Methods
A total of 365 patients treated with Impella 2.5/CP in the 17 centers of the IMP‐IT Registry were included. Through propensity‐score weighting (PSW) analysis, 1‐year clinical outcomes were assessed separately in CS and HR‐PCI patients, stratified by timing of Impella support.
Results
Pre‐procedural insertion was associated with an improvement in 1‐year survival in patients with CS due to acute myocardial infarction (AMI) treated with PCI (p = .04 before PSW, p = .009 after PSW) and HR‐PCI (p < .01 both before and after PSW). Among patients undergoing HR‐PCI, early Impella support was also associated with a lower rate of the composite of mortality, re‐hospitalization for heart failure, and need for left‐ventricular assist device/heart transplantation at 1‐year (p = .04 before PSW, p = .01 after PSW). Furthermore, Impella use during/after PCI was associated with an increased in‐hospital life‐threatening and severe bleeding among patients with AMI‐CS receiving PCI (7 vs. 16%, p = .1) and HR‐PCI (1 vs. 9%, p = .02).
Conclusions
Our findings suggested a survival benefit and reduced rates of major bleeding when a pre‐PCI Impella implantation instead of during‐after procedure was used in the setting of HR‐PCI and AMI‐CS.
Keywords: cardiogenic shock, high‐risk PCI, left ventricular assist device, mechanical cardiac support
Abbreviations
- AKI
acute kidney injury
- AMI
acute myocardial infarction
- CS
cardiogenic shock
- HF
heart failure
- HR‐PCI
high‐risk percutaneous coronary intervention
- HT
heart transplantation
- IABP
intra‐aortic balloon pump
- LVAD
percutaneous left ventricular assist device
- LVEF
left‐ventricular ejection fraction
- MACE
major adverse cardiac event
- MACE
major adverse cardiac events
- MCS
mechanical cardiac support
1. INTRODUCTION
Over the past decade, the short‐term percutaneous mechanical circulatory support (MCS) device, Impella® pump (Abiomed, Danvers, MA, USA), is increasingly used in patients with cardiogenic shock (CS) and high‐risk percutaneous coronary intervention (HR‐PCI).1, 2 Hemodynamic support with Impella increases cardiac output, improves end‐organ perfusion, and unloads the left ventricle, thus reducing myocardial oxygen demand, decreasing ventricular filling pressures and increasing coronary perfusion pressure.3, 4 Following studies advocating safety and suggesting efficacy, at least in a complex PCI setting, the Impella pumps received FDA approval for use in HR‐PCI and CS in 2015 and 2016, respectively.5, 6, 7, 8 However, recent real‐world observational studies report higher rates of adverse events and costs with Impella.9, 10 Of note, the interpretation of these analysis is likely hampered by the uneven distribution of clinically relevant variables, heterogeneity of clinical indications, type and timing of hemodynamic support in relation to patient's clinical status at presentation and type of revascularization. Hence, identification of optimal timing of Impella implantation in specific clinical settings is of utmost clinical relevance. Therefore, we analyzed the temporality interplay between Impella insertion and PCI on all‐cause death and composite of mortality, re‐hospitalization for heart failure (HF), left ventricular assist device (LVAD) or heart transplantation (HT) at 1 year in CS and HR‐PCI patients included in the IMPella Mechanical Circulatory Support Device in Italy (IMP‐IT) Registry.11
2. METHODS
2.1. Study population
The IMP‐IT study is an investigator‐initiated, multicenter, retrospective, national registry study promoted by the Italian Society of Interventional Cardiology (Società Italiana di Cardiologia Interventistica–GISE).11 Consecutive patients treated with Impella 2.5, Impella CP, Impella 5.0 and Impella RP, both for CS and HR‐PCI, in 17 Italian centers were included. Details regarding included centers and collection of records are described elsewhere.11 To keep the population more uniform and limit unmeasured confounding, only patients treated with Impella device on the same day of the index coronary angiography were included and patients who had clinical deterioration or complications requiring MCS placement before arriving in the cath‐lab were excluded.9 No prespecified protocol for Impella use was implemented and device implantation (type and timing) was at the operating physician's discretion. For the purpose of this analysis, patients treated with Impella 5.0 and RP or in whom timing of the device use was not available were excluded. A description of the criteria for CS and HR‐PCI patient‐population inclusion and the devices used in the study is provided in the Appendix. Timing of Impella insertion (before coronary angiography or before coronary guidewire positioning vs during/after the procedure) was pre‐specified in the study protocol. Adjunctive device implantation (extracorporeal membrane oxygenation‐ECMO, intra‐aortic balloon pump‐IABP) was at the operating physician's discretion, the applied implantation sequence was: IABP before Impella placement and ECMO as escalation therapy after Impella use. Information related to medical history, procedural characteristics, 30‐day and 1‐year outcomes (obtained as in‐person visits, telephone interviews, and medical notes from any hospital admission or outpatient visits) were collected and entered in a pre‐specified database. Adverse events were adjudicated by two independent cardiologists (M.A., V.P.) using source documents provided by the hospital site. PCI was performed according to standard clinical practice at the participating site. Collection of data at each participating site was performed according to the local institutional review board/ethics committee policies.
2.2. Study endpoints
The study aimed to evaluate the impact of the timing of Impella 2.5/CP insertion during two different clinical indications (CS and HR‐PCI). These two settings were analyzed separately. Study outcomes of interest included 1‐year mortality and the composite of all‐cause death, rehospitalization for HF, LVAD implantation or HT referred as the major adverse cardiac events (MACE) at 1 year. In addition, in‐hospital mortality, bleeding events, device‐related complications, the occurrence of sepsis, acute kidney injury (AKI), and the need for escalation therapy (defined as the need for ECMO, other left ventricular assist device implantation or heart transplant) were also evaluated.
2.3. Statistical methods
Continuous variables are reported as median (first‐third interquartile range) or mean ± SD as appropriate and categorical variables as percentages (relative frequencies). Differences between groups were assessed using the Kruskal–Wallis test for continuous variables and Chi‐square test for categorical variables. Since not all patients with CS underwent PCI, we provide analyses on the total CS population as well as among patients with CS due to acute myocardial infarction (AMI) treated with a PCI. We compared the timing of Impella implantation (before PCI vs. during/after PCI) among patients undergoing non‐emergent HR‐PCI and those with CS, including those with AMI‐CS treated with PCI. The comparison was performed using a Propensity Score Weighting (PSW) analysis to reduce the imbalance in patient characteristics between groups.12 The individual propensity scores were estimated using the Generalized Boosted Model (GBM), a popular Machine Learning Technique (MLT) used for PSW analysis.13 The following parameters were used: trees, interaction depth (the highest level of variable interactions allowed) and shrinkage respectively equal to 10,000, 3 and 0.01. The balancing was assessed using weighted standardized mean differences using the inverse probability weighting approach.14 The Cox proportional hazard model and likelihood‐ratio test were used to detect differences between the groups in all‐cause mortality and freedom from MACE. The variables that resulted to be not adequately balanced with the PSW were included in the Cox proportional hazard model to assess the effect of residual confounding on the outcomes of interest.13 Weighted estimates were obtained using the sampling weights derived from the estimated PSW. The full list of patients' characteristics used for the PSW and the multivariable weighted Cox model is detailed in the Supplementary appendix. The results of these analyses are reported as Hazard Ratio (HR) and 95% confidence interval (95% CI), together with the p‐value of the likelihood‐ratio test. The survival and freedom from MACE curves were derived using the Cox proportional hazard model.
3. RESULTS
Of the 406 patients included in the IMP‐IT Registry, 365 were eligible for this analysis. Among these, 191 received Impella in the setting of CS (52%) and 174 for HR‐PCI (48%). Most patients were treated with Impella 2.5 in the CS (61%) and HR‐PCI (62%) setting. Overall, mechanical support was initiated before the interventional procedure in 53%, during in 27% and after PCI in 20% patients.
3.1. Impella for CS
The baseline clinical characteristics of the patients presenting with CS stratified by timing of implantation of Impella are reported in Table 1. Most (60%) of these patients were treated with mechanical support during or after the interventional procedure. The etiology of CS was mostly (85%) AMI in both groups.
TABLE 1.
Baseline, procedural, and in‐hospital management characteristics stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure in patients with cardiogenic shock
| Overall (N = 191) | Before (N = 77) | During/after (N = 114) | p‐value | |
|---|---|---|---|---|
| Age | 66 [55;73] | 67 [58;74] | 62 [54;73] | .12 |
| Male | 73% (140) | 73% (56) | 74% (84) | 1 |
| Hypertension | 54% (100) | 56% (42) | 53% (58) | .75 |
| Dyslipidemia | 43% (79) | 48% (36) | 39% (43) | .27 |
| Diabetes mellitus | 35% (65) | 36% (27) | 35% (65) | .88 |
| Chronic pulmonary disease | 11%21 | 13%10 | 10%11 | .64 |
| Prior MI | 36% (67) | 44% (33) | 31% (34) | .08 |
| Prior PCI | 34% (63) | 36% (27) | 33% (36) | .75 |
| Prior CABG | 5%10 | 8%6 | 4%4 | .32 |
| CKDa | 25% (47) | 27%20 | 25% (27) | .86 |
| AF | 10%19 | 11%8 | 10%11 | 1 |
| Prior TIA/stroke | 5%10 | 7%5 | 5%5 | .75 |
| PAD | 14% (26) | 16%12 | 13%14 | .64 |
| Chronic HF | 25% (47) | 31%23 | 22%24 | .22 |
| LVEF, % | 25 [20;30] | 25 [20;30] | 22 [15;32] | .64 |
| RV dysfunction | 24% (42) | 18%13 | 28% (29) | .09 |
| INTERMACS class 1 | 63% (116) | 54% (39) | 69% (77) | .03 |
| Out of hospital cardiac arrest | 25% (46) | 21%16 | 28% (30) | .34 |
| Etiology of cardiogenic shock | ||||
| AMI | 85% (163) | 79% (61) | 90% (102) | .10 |
| Acute myocarditis | 3%5 | 5%4 | 1%1 | |
| Arrhythmias | 4%8 | 4%3 | 4%5 | |
| Other | 8%15 | 12%9 | 5%6 | |
| Laboratory values | ||||
| Ph | 7.4 [7.3;7.5] | 7.4 [7.3;7.5] | 7.4 [7.3;7.4] | .33 |
| HR (bpm) | 100 [80;110] | 100 [77;110] | 98 [80;110] | .86 |
| MAP | 63 [53;78] | 65 [53;80] | 60 [53;77] | .56 |
| Baseline lactate (mmol/l) | 4.7 [2.6;7.5] | 3 [2.2;6.4] | 5 [3.1;7.5] | .04 |
| Baseline hemoglobin (g/dl) | 12.3 [10.6;14.2] | 12.2 [10.7;14.1] | 12.4 [10.4;14.2] | .98 |
| Baseline creatinine (mg/dl) | 1.2 [1;1.8] | 1.3 [0.9;1.8] | 1.3 [1;1.8] | .91 |
| Procedural characteristics | ||||
| PCI performed | 80% (147) | 76% (55) | 82% (92) | .36 |
| Number of diseased vessels | 2 [1;3] | 2.5 [1.3; 3] | 2 [1;3] | .09 |
| Three vessels disease | 42% (75) | 50% (35) | 37% (40) | .09 |
| BCIS myocardial jeopardy score | 8 [6;12] | 10 [6;12] | 8 [6;12] | .42 |
| Left Main stenosis | 36% (60) | 41%25 | 34% (35) | .40 |
| LAD stenosis | 80% (132) | 84% (51) | 78% (81) | .42 |
| CX stenosis | 58% (95) | 69% (42) | 52% (53) | .04 |
| RCA stenosis | 58% (96) | 75% (46) | 48% (50) | <.01 |
| By‐pass occlusion | 1%2 | 2%1 | 1%1 | 1 |
| Number of treated vessels | 1.3 ± 0.9 | 1.6 ± 0.9 | 1.2 ± 0.7 | .01 |
| Three vessels treated | 13%22 | 23%14 | 7%8 | .01 |
| Rotablator | 7%11 | 5%3 | 7%8 | .77 |
| Resuscitation required during index procedure | 21% (40) | 17%13 | 24% (27) | .23 |
| Resuscitation required after index procedure | 31% (58) | 26%20 | 34% (38) | .42 |
| In‐hospital management | ||||
| Impella removed immediately after PCI | 12%21 | 23%16 | 5%5 | <.01 |
| Duration of support (hours) | 53 [24;120] | 48 [8;120] | 72 [24;120] | .06 |
| Use of Impella 2.5 | 61% (117) | 48% (37) | 70% (80) | <.01 |
| Use of Impella CP | 39% (74) | 52% (40) | 30% (34) | <.01 |
| Other cardiopulmonary support used | ||||
| Inotropes | 74% (130) | 69% (50) | 77% (80) | .30 |
| Mechanical ventilation | 75% (139) | 71% (53) | 78% (86) | .30 |
| ECMO | 29% (55) | 22%17 | 33% (38) | .10 |
| IABP | 36% (67) | 23%17 | 45% (50) | <.01 |
Note: Results reported as n (%) for categorical variables and median (interquartile range) or mean ± SD for continuous variables as appropriate.
Abbreviations: AF, atrial fibrillation; AMI, acute myocardial infarction; BCIS, British Cardiovascular Intervention Society; CABG, coronary artery by‐pass graft; CKD, chronic kidney disease; COPD, Chronic pulmonary disease; Cx, circumflex coronary artery; ECMO, ExtraCorporeal Membrane Oxygenation; HF, heart failure; HR, heart rate; IABP, intra‐aortic balloon pump; LAD, left descending artery; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; RCA, right coronary artery; RV, right ventricular; TIA, transient ischemic attack.
Defined as eGFR <60 ml/min/1.73 m2.
Procedural characteristics and in‐hospital management are reported in Table 1. Of those who received Impella 2.5, the majority (70%) where implanted during or after the interventional procedure, while of those requiring Impella CP, 52% where implanted before intervention. AMI‐CS patients were treated with PCI during the index procedure in 90% of the cases, without significant difference between subgroups.
Among the measured variables, the ones related to the pre‐PCI use of Impella support were mostly angiographic (e.g., higher number of vessels diseased and treated); otherwise, the delayed Impella implantation was mainly driven by a worsening hemodynamic decay despite the use of other cardiac support.
In‐hospital outcomes of CS patients are reported in Tables 2 and3. Overall, patients receiving Impella pre‐intervention had a trend toward a lower occurrence of in‐hospital all‐cause death (36 vs. 51%, p = .06). Also, patients receiving Impella pre‐procedure had a lower rate of AKI (39 vs. 56%, p = .04) with no difference in device‐related complications. Notably, among patients with AMI‐CS receiving PCI, in‐hospital death (29 vs. 52%, p < .01) and AKI (38 vs. 61%, p = .02) were significantly lower in the pre‐PCI group while life‐threatening or severe bleeding showed a lower trend (7 vs. 16%, p = .1).
TABLE 2.
In‐hospital outcomes stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure in patients with cardiogenic shock
| Overall (N = 191) | Before (N = 77) | During/after (N = 114) | p‐value | |
|---|---|---|---|---|
| In‐hospital outcomes | ||||
| Death | 45% (85) | 36% (27) | 51% (58) | .06 |
| Life‐threatening or severe bleeding | 16% (30) | 12%9 | 18%21 | .15 |
| Number of red blood cell transfusions | 5 [2;13] | 5 [2;12] | 5 [2;14] | .62 |
| Device‐related complications | 35% (67) | 31%24 | 38% (43) | .35 |
| Access‐site bleeding | 12%22 | 12%9 | 12%13 | 1 |
| Limb ischemia | 12%23 | 8%6 | 15%17 | .17 |
| Need for endovascular intervention | 6%12 | 7%5 | 6%7 | 1 |
| Aortic injury | 1%1 | 0% (0) | 1%1 | 1 |
| Left ventricular perforation | 1%1 | 0% (0) | 1%1 | 1 |
| Others | 5%9 | 5%4 | 4%5 | .33 |
| Sepsis | 28% (52) | 33%25 | 25% (27) | .24 |
| Acute kidney injurya | 49% (84) | 39%25 | 56% (59) | .04 |
| Need for renal replacement therapy | 27% (50) | 23%17 | 31% (33) | .30 |
| Escalation therapyb | 22% (42) | 25%19 | 21%23 | .49 |
| LVEF at discharge, % | 33 [25;43] | 33 [25;42] | 32 [25;45] | .86 |
Note: In‐hospital outcomes are reported as n (%) or median (interquartile range) as appropriate.
Abbreviation: LVEF, left‐ventricular ejection fraction.
Defined as a serum creatinine increase ≥0.3 mg/dl from baseline.
Defined as the need for extracorporeal membrane oxygenation, other left ventricular assist device implantation or heart transplant.
TABLE 3.
In‐hospital outcomes stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure in patients with cardiogenic shock due to acute myocardial infarction and treated with percutaneous coronary intervention (PCI)
| Combined (N = 147) | Before (N = 55) | During/after (N = 92) | p‐value | |
|---|---|---|---|---|
| In‐hospital outcomes | ||||
| Death | 44% (64) | 29%16 | 52% (48) | <.01 |
| Life‐threatening or severe bleeding | 13%19 | 7%4 | 16%15 | .11 |
| Number of red blood cell transfusions | 2 [5;14] | 2 [5;12] | 2 [5;14] | .58 |
| Device‐related complications | 35% (52) | 29%16 | 39% (36) | .29 |
| Access‐site bleeding | 12%17 | 11%6 | 12%11 | 1 |
| Limb ischemia | 15%21 | 17%9 | 19%17 | .46 |
| Need for endovascular intervention | 7%10 | 7%4 | 7%1 | 1 |
| Aortic injury | 1%1 | 0% (0) | 1%1 | 1 |
| Left ventricular perforation | 0% (0) | 0% (0) | 0% (0) | NA |
| Sepsis | 30% (42) | 31%17 | 29%25 | .85 |
| Acute kidney injurya | 53% (70) | 38%18 | 61% (52) | .02 |
| Need for renal replacement therapy | 27% (38) | 21%11 | 31% (27) | .23 |
| Escalation therapyb | 19% (27) | 17%9 | 20%18 | .68 |
| LVEF at discharge, % | 33 [25;42] | 33 [25;42] | 33 [25;45] | .62 |
Note: In‐hospital outcomes are reported as n (%) or median (interquartile range) as appropriate.
Abbreviation: LVEF, left‐ventricular ejection fraction.
Defined as a serum creatinine increase ≥0.3 mg/dl from baseline.
Defined as the need for extracorporeal membrane oxygenation, other left ventricular assist device implantation or heart transplant.
The unadjusted and adjusted outcomes in the overall population at 1‐year are reported in Table 4, Figures 1 and 2 and in Supplementary Figures 1–2. Overall, no differences in outcomes between the groups were observed, especially irrespective of additional mechanical support (ECMO or IABP) use (p = .69). When restricting the analysis to patients with AMI‐CS receiving PCI, the use of Impella pre‐PCI instead of during/after procedure was associated with a significant survival benefit both at unadjusted (hazard ratio (HR) 0.61, [confidence interval (CI)] [0.37–0.99]; p = .04) and adjusted (HR 0.45, CI [0.21–0.99]; p = .009) analysis (Table 4, Figure 3 and Supplementary Figure 1). The MACE rates were not different between the groups (Table 4, Figure 4 and Supplementary Figure 2).
TABLE 4.
Cox proportional hazard analysis of one‐year outcomes stratified by timing of insertion of Impella 2.5 and CP (before vs during/after interventional procedure)
| All‐cause death | MACE | ||||
|---|---|---|---|---|---|
| HR [CI] | p | HR [CI] | p | ||
| CS | Before adjustment | 0.80 [0.53–1.22] | .31 | 0.93 [0.65–1.35] | .71 |
| After adjustment | 1.13 [0.65–1.96] | 1 | 1.12 [0.72–1.74] | .54 | |
| PCI patients with AMI‐CS | Before adjustment | 0.61 [0.37–0.99] | .04 | 0.76 [0.49–1.17] | .21 |
| After adjustment | 0.45 [0.21–0.99] | .009 | 0.79 [0.41–1.52] | .38 | |
| HR‐PCI | Before adjustment | 0.32 [0.14–0.73] | .007 | 0.49 [0.25–0.98] | .04 |
| After adjustment | 0.35 [0.12–0.96] | .001 | 0.51 [0.23–0.96] | .01 | |
Note: Data are reported as hazard ratio (HR) [confidence interval (CI)] before and after propensity score weighting (PSW) and multivariable weighted cox model adjustment. This analysis aims to detect differences between the compared groups in terms of all‐causes mortality and freedom from MACE in patients with cardiogenic shock (CS) and high‐risk percutaneous coronary intervention (HR‐PCI). Since not all patients with CS underwent PCI, we present analyses on the total CS population as well as among acute myocardial infarction (AMI)—CS cases treated with PCI.
FIGURE 1.
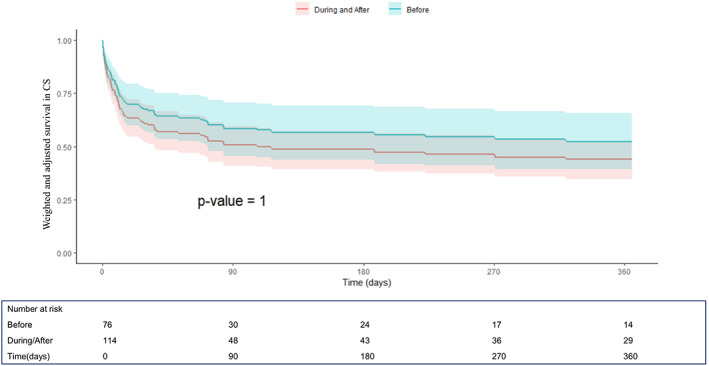
One‐year survival curves derived from the Cox proportional hazard model after propensity score weighting and multivariable weighted Cox model adjustment from the IMP‐IT Registry in patients with cardiogenic shock (CS) (hazard ratio (HR) 1.13, [confidence interval (CI)] [0.65–1.96]; p = 1, before vs during/after interventional procedure), stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure
FIGURE 2.
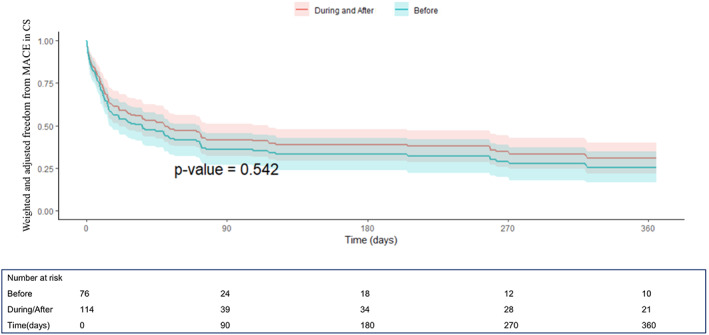
One‐year freedom from MACE (composite of all‐cause mortality, rehospitalization for heart failure, need for left ventricular assist device or heart transplant) curves derived from the Cox proportional hazard model after propensity score weighting and multivariable weighted Cox model adjustment from the IMP‐IT Registry in patients with CS (HR 1.12, CI [0.72–1.74]; p = .54, before vs. during/after interventional procedure), stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure
FIGURE 3.
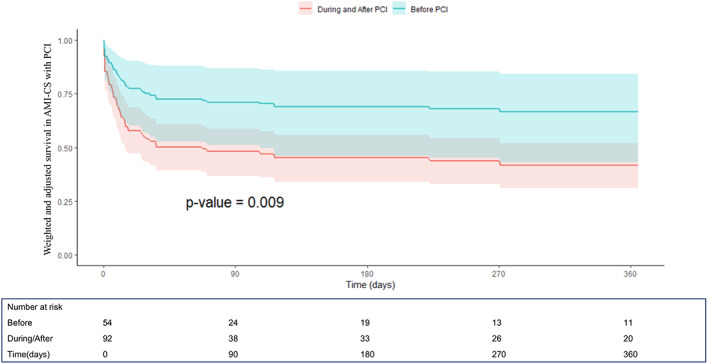
One‐year survival curves derived from the Cox proportional hazard model after propensity score weighting and multivariable weighted Cox model adjustment from the IMP‐IT Registry in patients with acute myocardial infarction (AMI) complicated by CS and treated with percutaneous coronary intervention (PCI) (HR 0.45, CI [0.21–0.99]; p = .009, before vs during/after PCI), stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure
FIGURE 4.
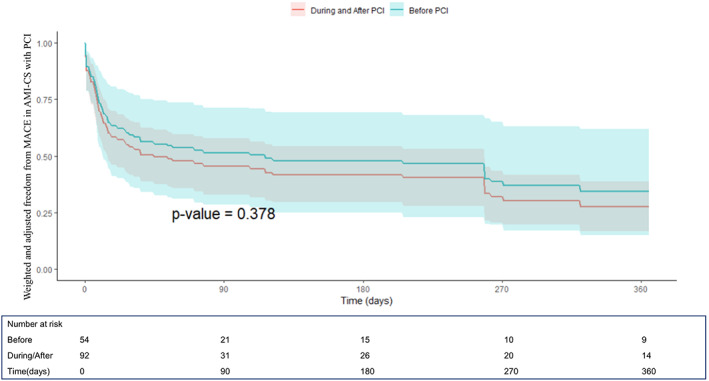
One‐year freedom from MACE curves derived from the Cox proportional hazard model after propensity score weighting and multivariable weighted Cox model adjustment from the IMP‐IT Registry in patients with AMI complicated by CS and treated with PCI (HR 0.79, CI [0.41–1.52]; p = .38, before vs during/after PCI), stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure
3.2. Impella for HR‐PCI
The baseline clinical and procedural characteristics and the in‐hospital management of the patients with HR‐PCI stratified by timing of implantation of the Impella device are reported in Table 5. Most of these patients (67%) were treated with Impella before the interventional procedure. Among the measured variables, the ones related to the pre‐PCI use of Impella support were mostly angiographic (e.g., higher number of vessels diseased and treated, more frequent involvement of the left anterior descending); otherwise, the delayed Impella implantation was mainly driven by a peri‐procedural hemodynamic decay. However, as highlighted by the low prevalence of required resuscitation practice and additional cardio‐pulmonary supports, the device was implanted during or after the PCI due to operating physician's carefulness without any procedural emergency in a considerable set of patients.
TABLE 5.
Baseline, procedural and In‐Hospital management characteristics stratified by timing of insertion of Impella 2.5 and CP in relation to PCI in patients with high‐risk PCI
| Overall (N = 174) | Before (N = 117) | During/after (N = 57) | p‐value | |
|---|---|---|---|---|
| Age | 73 [66;80] | 72 [66;81] | 76 [69;80] | .42 |
| Male | 83% (145) | 87% (102) | 75% (43) | .09 |
| Hypertension | 82% (143) | 84% (98) | 79% (45) | .51 |
| Dyslipidemia | 61% (106) | 62% (72) | 60% (34) | .87 |
| Diabetes mellitus | 46% (80) | 47% (55) | 44%25 | .75 |
| COPD | 20% (35) | 16%19 | 30%16 | .06 |
| Prior MI | 42% (73) | 38% (44) | 51% (29) | .10 |
| Prior PCI | 24% (42) | 21%24 | 32%18 | .13 |
| Prior CABG | 15% (26) | 14%16 | 18%10 | .65 |
| CKDa | 38% (66) | 36% (42) | 43%24 | .40 |
| AF | 17% (29) | 17%20 | 16%9 | 1 |
| Prior TIA/stroke | 11%19 | 10%12 | 12%7 | .79 |
| PAD | 25% (44) | 27% (32) | 21%12 | .46 |
| Chronic HF | 54% (94) | 56% (66) | 50% (28) | .51 |
| LVEF, % | 30 [25;35] | 30 [25;35] | 30 [25;37] | .42 |
| RV dysfunction | 13%21 | 10%12 | 18%9 | .20 |
| Out of hospital cardiac arrest | 1%2 | 1%1 | 2%1 | 1 |
| Laboratory values | ||||
| Ph | 7.40 [7.38;7.45] | 7.45 [7.38;7.45] | 7.40 [7.34;7.44] | .82 |
| HR (bpm) | 75 [69;86] | 77 [70;86] | 74 [68;86] | .48 |
| MAP | 80 [75;90] | 80 [75;90] | 78 [69;90] | .10 |
| Baseline lactate (mmol/l) | 1.5 [1;1.9] | 1.4 [1;1.8] | 1.8 [1.2;2.1] | .10 |
| Baseline hemoglobin (g/dl) | 12.4 [11.3;13.7] | 12.3 [11.4;13.3] | 12.9 [11.3;14] | .14 |
| Baseline creatinine (mg/dl) | 1 [0.8;1.4] | 0.9 [0.8;1.4] | 1.1 [0.9;1.4] | .04 |
| Procedural characteristics | ||||
| PCI performed | 99% (173) | 99% (117) | 100% (56) | 1 |
| Number of diseased vessels | 3 [2;3] | 3 [2;3] | 3 [2;3] | .05 |
| Three vessels disease | 68% (119) | 72% (84) | 61% (35) | .22 |
| BCIS myocardial jeopardy score | 12 [10;12] | 12 [10;12] | 12 [9;16] | .27 |
| Left Main stenosis | 48% (81) | 49% (57) | 46%24 | .73 |
| LAD stenosis | 94% (159) | 97% (113) | 87% (46) | .01 |
| CX stenosis | 88% (147) | 92% (107) | 78% (40) | .02 |
| RCA stenosis | 81% (136) | 85% (99) | 73% (37) | .06 |
| By‐pass stenosis | 10%16 | 8%9 | 14%7 | .41 |
| Number of treated vessels | 1.9 ± 0.9 | 2 ± 0.8 | 1.6 ± 1.0 | <.01 |
| Three vessels treated | 25% (41) | 27% (30) | 21%11 | .46 |
| Rotablator | 25% (43) | 22%25 | 32%18 | .21 |
| Resuscitation required during index procedure | 4%6 | 2%2 | 7%4 | .07 |
| Resuscitation required after index procedure | 4%7 | 3%3 | 7%4 | .23 |
| In‐hospital management | ||||
| Impella removed immediately after PCI | 83% (141) | 83% (96) | 83% (45) | 1 |
| Duration of support (hours) | 1.5 [1.5;3] | 2 [1.5;3] | 1.5 [1.5;2.4] | .49 |
| Use of Impella 2.5 | 62% (108) | 61% (71) | 65% (37) | .61 |
| Use of Impella CP | 38% (66) | 39% (46) | 35%20 | .64 |
| Other cardiopulmonary support used | ||||
| Inotropes | 8%14 | 9%10 | 8%4 | 1 |
| Mechanical ventilation | 17% (29) | 21%24 | 9%5 | .09 |
| ECMO | 0% (0) | 0% (0) | 0% (0) | NA |
| IABP | 2%3 | 3%3 | 0% (0) | .55 |
Note: Results reported as n (%) for categorical variables and median (interquartile range) or mean ± SD for continuous variables as appropriate.
Abbreviations: AF, atrial fibrillation; BCIS, British Cardiovascular Intervention Society; CABG, coronary artery by‐pass graft; CKD, chronic kidney disease; COPD, Chronic pulmonary disease; Cx, circumflex coronary artery; ECMO, ExtraCorporeal Membrane Oxygenation; HF, heart failure; HR, heart rate; IABP, intra‐aortic balloon pump; LAD, left descending artery; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; RCA, right coronary artery; RV, right ventricular; TIA, transient ischemic attack.
Defined as eGFR <60 ml/min/1.73 m2.
In‐hospital outcomes are reported in Table 6. Patients receiving pre‐PCI Impella support had significantly lower in‐hospital death (3 vs. 12%, p = .02) and life‐threatening or severe bleeding (1 vs. 9%, p = .02).
TABLE 6.
In‐hospital outcomes stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure in patients with high‐risk PCI
| Combined (N = 174) | Before (N = 117) | During/after (N = 57) | p‐value | |
|---|---|---|---|---|
| In‐hospital outcomes | ||||
| Death | 6%10 | 3%3 | 12%7 | .02 |
| Life‐threatening or severe bleeding | 3%6 | 1%1 | 9%5 | .02 |
| Number of red blood cell transfusions | 2 [1;3] | 2 [2;3] | 1 [1;2] | .20 |
| Device‐related complications | 10%17 | 7%8 | 16%9 | .11 |
| Access‐site bleeding | 7%12 | 4%5 | 12%7 | .06 |
| Limb ischemia | 2%4 | 3%3 | 2%1 | 1 |
| Need for endovascular intervention | 2%4 | 3%3 | 2%1 | 1 |
| Aortic injury | 0% (0) | 0% (0) | 0% (0) | NA |
| Left ventricular perforation | 0% (0) | 0% (0) | 0% (0) | NA |
| Sepsis | 4%7 | 6%7 | 0% (0) | .10 |
| Acute kidney injurya | 13%19 | 16%14 | 9%5 | .31 |
| Need for renal replacement therapy | 4%6 | 3%3 | 5%3 | .39 |
| Escalation therapyb | 0% (0) | 0% (0) | 0% (0) | NA |
| LVEF at discharge, % | 32 [26;40] | 33 [26;40] | 30 [25;36] | .23 |
Note: In‐hospital outcomes are reported as n (%) or median (interquartile range) as appropriate. One‐year outcomes are reported as number of events (Kaplan–Meier failure estimate [95% confidence interval]).
Abbreviation: LVEF, left‐ventricular ejection fraction.
Defined as a serum creatinine increase ≥0.3 mg/dl from baseline.
Defined as the need for extracorporeal membrane oxygenation, other left ventricular assist device implantation or heart transplant.
Unadjusted and adjusted one‐year outcomes are reported in Table 4, Figures 5 and 6 and in Supplementary Figure 3. Impella support pre‐PCI Impella was associated with a lower 1‐year mortality (HR 0.32, C.I. [0.14–0.73], p < .01) and composite outcome of death, hospitalization for HF, need for LVAD /HT (HR 0.49, C.I. [0.25–0.98], p = .04) compared to those receiving Impella during or after PCI. These results were confirmed in the adjusted analysis for major potential confounding (HR 0.35, C.I. [0.12–0.96], p = .001 and HR 0.51, CI [0.23–0.96]; p = .01, respectively).
FIGURE 5.
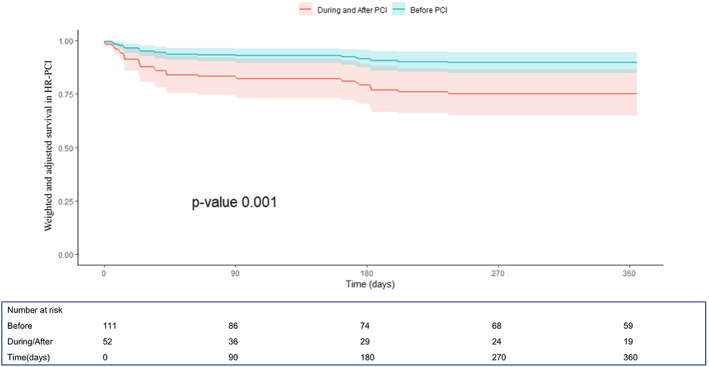
One‐year survival curves derived from the Cox proportional hazard model after propensity score weighting and multivariable weighted Cox model adjustment from the IMP‐IT Registry in high‐risk percutaneous coronary interventions (HR‐PCI) patients (HR 0.35, C.I. [0.12–0.96], p = .001, before vs. during/after PCI), stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure
FIGURE 6.
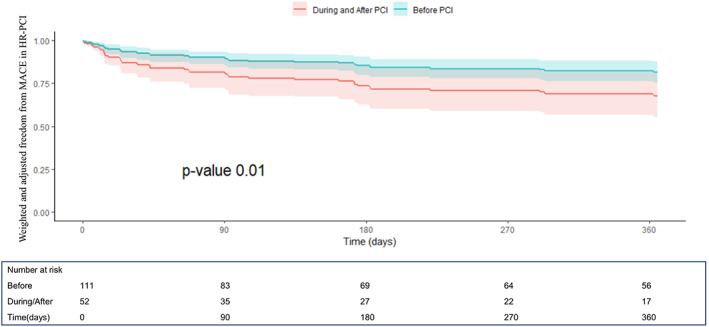
One‐year freedom from MACE curves derived from the Cox proportional hazard model after propensity score weighting and multivariable weighted Cox model adjustment from the IMP‐IT Registry in HR‐PCI patients (HR 0.51, CI [0.23–0.96]; p = .01, before vs. during/after PCI), stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure
4. DISCUSSION
To the best of our knowledge, this is the first study based on IMP IT Registry ‐ the largest European national registry—to address the interplay between the timing of Impella support and 1‐year clinical outcomes in patients with HR‐PCI and CS indications. This real‐world analysis suggests: (a) Impella is frequently used prophylactically in HR‐PCI, while it is mostly implanted during/after the interventional procedure in CS; (b) there is a significant interplay between timing of Impella implantation and clinical outcomes as confirmed by a lower occurrence of in‐hospital and 1‐year mortality with prophylactic Impella use in case of PCI‐procedure; (c) pre‐PCI use of the Impella was safer and associated with lower in‐hospital life‐threatening and severe bleeding rates both in HR‐PCI and AMI‐CS patients receiving PCI.
4.1. Clinical role of Impella
The role of Impella has been recently questioned. Indeed, Impella has been shown to provide superior hemodynamic support than IABP both in CS and HR‐PCI settings and some studies have suggested its safety with a possible survival benefit and reduced MACE rate,2, 3, 4, 5, 6, 7 at least in a complex PCI population. However, more recent analyses have failed to show a prognostic advantage of the Impella versus IABP, raising concerns in terms of higher rates of adverse events.10, 11, 15 Major caveats in the interpretation of these studies are the heterogeneity of the clinical indications, the type and timing of MCS support with respect to clinical status of the patient and the type of revascularization. Given the lack of adequately powered randomized trials, we used the national dataset of IMP‐IP Registry to assess the optimal timing of both 2.5 and CP devices implantation in CS and HR‐PCI on outcomes and identify best practice for management of patients with Impella support. Similar to previous studies based on registries, our study included patients with high clinical and anatomical complexity.9, 10, 11 Contrariwise, our analysis focused on the role of timing of Impella support and associated outcomes in two specific high‐risk clinical subsets of patients revascularized using PCI.16
4.2. Impella in CS
Notably, no clinical benefit of Impella support was observed in the overall CS population according to different timing of device implantation. This finding is in line with previous studies that failed to show a survival benefit of Impella versus IABP.10, 11, 15 The lack of survival benefit with MCS devices seems to be related to several inherent confounders associated with the diagnosis and management of CS. First, MCS devices, including Impella, are generally used late following the onset of refractory CS per guideline recommendation,17 and in those with non‐AMI CS complicated by severe hemodynamic decay.18 Moreover, given the heterogeneity of variables and MCS use in CS, statistical adjustments alone are insufficient to precisely delineate the role of cardiac support devices in this setting. For instance, in the retrospective analysis based on payer codes in the US Premier Healthcare Database, patients receiving Impella were sicker, frequently presented in an unstable clinical condition, and received high risk and complicated PCI, which might have contributed to higher rates of adverse events.9 Another large study based on the American College of Cardiology's National Cardiovascular Data Registry reported a higher in‐hospital adverse events rate with Impella compared to IABP, regardless of the timing of implantation.10 This study performed a sensitivity analysis, which reduces selection bias related to the use of IABP vs Impella, although they did not perform a paired comparison between pre‐ and post‐PCI Impella use.10 In the present study, temporal relationship of the device implantation to the first sign of shock was not captured, most patients received the Impella 2.5 device, 15% of cases had non‐AMI CS and about 10% of those with AMI‐CS did not get any revascularization, which might had hampered our attempt to find a temporality interplay between the device placement and clinical outcomes. Restricting the analysis to the patients with AMI‐CS treated with PCI, the earlier use of Impella was associated with significant early and late survival benefit compared to a delayed implantation. These results suggest that a very high likelihood of a final diagnosis of MI should be required for pre‐procedural implantation in CS and that the bailout use of Impella support during revascularization might have no impact on mortality.16, 19 In addition, it is important to note that pre‐PCI Impella use was associated with a lower trend of life‐threatening or severe bleedings despite the use of a 14‐Fr sheath with Impella CP and a significant lower rate of AKI. Thus, we advocate that obtaining vascular access before PCI than emergent Impella implantation and use of a large‐bore closure device might play a role in reducing vascular complications.20 Previous findings have already shown that CS‐AKI patients are more likely to require MCS than those without AKI and that Impella use during HR‐PCI protect against acute renal failure.21, 22 As a matter of fact, our results reinforce the concept of a renal protective effect through Impella use, suggesting that early implantation may be crucial in the management of AKI before it occurs. To note, differently from survival and AKI incidence, we failed to find any long‐term MACE improvement in the AMI‐CS population setting. However, compared to peri‐procedural Impella use, several adjunctive unmeasured factors, such as concomitant comorbidities, non‐cardiac complications and physicians' conduct, might have played a role on re‐hospitalization for HF occurrence.
4.3. Impella in HR‐PCI
In the HR‐PCI population the use of Impella pre‐PCI compared to during/after procedure was associated with reduced mid‐term mortality and rate of re‐hospitalization for HF, LVAD or HT. Even in the absence of a detailed report on specific periprocedural coronary‐related complication, we showed a not negligible rate of in‐hospital complications (more than 20% in the overall high‐risk cohort) and that the use of Impella prior to PCI was statistically associated with a reduced adverse event rate. Moreover, although we cannot discriminate between a delayed implantation driven by a peri‐procedural complication or merely physician's choice, our results highlighted an increased rate of in‐hospital life‐threatening/severe and access‐site bleeding with Impella use during/after PCI that is not properly related to the hemodynamic decay secondary to cardiac complications. These findings highlighted the role of a careful pre‐procedural planning avoiding to running for a bail‐out use of the device to improve PCI outcomes in a non‐emergent high‐risk setting. Of note, these patients had more diffused CAD, a higher number of vessels treated, and higher rates of complete revascularization. Therefore, our results might support the concept of prophylactic Impella use in the context of complex coronary artery disease and severe left ventricular systolic dysfunction in improving clinical outcomes. These results are in alignment with preliminary data from the interim analysis on 898 patients in the PROTECT III post‐marketing study on HR‐PCI, presented at Transcatheter Cardiovascular Therapeutics 2019. Patients in PROTECT III were older, more frequently women and non‐Caucasians, received extended duration of support, and had more complex procedures with more vessels treated than patients in PROTECT II trial. Compared to the IABP control arm in the PROTECT II trial, PROTECT III patients showed a lower incidence of the composite end‐points at 90 days (16.8 vs. 31%; p < .0001) (Jeffrey J Popma, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, USA at TCT 2019; 24–29 September, San Francisco, USA).
4.4. Future perspectives
Adequately powered RCTs are needed to confirm the temporal relationship of Impella implantation with clinical outcomes observed in our analysis in HR PCI and to further elucidate the understanding in CS. The ongoing randomized DanGer Shock trial will address whether mechanical circulatory LV support with Impella CP prior to PCI can improve survival in AMI with CS patients compared to current guideline‐driven therapy.23
4.5. Limitations
There are several limitations to consider in our study. (a) Given its observational and non‐randomized design, our findings remain hypothesis‐generating. Impella insertion, completeness of revascularization and all other adjunctive therapies were at the operating physician's discretion and, therefore, subject to potential treatment and patient selection bias. Moreover, temporal relationship of the device implantation to the first sign of shock and to other potential MCS was not captured, so we may oversight adjunctive causal associations with outcomes. (b) Even the best efforts at creating balanced comparisons (e.g., PSW analysis) may not account for residual confounding from unmeasured variables and may result in unbalanced comparisons.24 Among them, the operator preference or availability, might have strongly influenced the choice of the type and timing of Impella in both setting. (c) Especially for CS setting, the measurement of hemodynamic invasive parameters was not available. Objective hemodynamic parameters, such as reduced cardiac index and increased pulmonary capillary wedge pressure, are helpful for CS diagnosis confirmation, and are essential in terms of more clearly defining the status of the right heart and systemic vasculature, particularly in those patients who do not respond in the expected manner to initial therapy; however, they are not mandatory in clinical practice.25 (d) The results of the subgroup analysis on AMI‐CS patients receiving PCI should be taken with caution given the increased chance of false‐positive findings. 5) Data collection was retrospective and event monitoring was not standardized across clinical centers; thus, some adverse events may be underreported. However, our adverse events rates were largely aligned with other studies with analogous patient populations.
5. CONCLUSIONS
The results of the IMP‐IT sub‐study suggested a significant clinical benefit in the setting of HR‐ PCI and AMI‐CS of pre‐PCI implantation of Impella instead of during/after procedure for both short‐and long‐term mortality and lower major bleeding rates. The pre‐intervention use of Impella is also associated with favorable impact on the composite of mortality, re‐hospitalization for HF, LVAD implantation and HT in HR‐PCI cases.
CONFLICT OF INTEREST
Alaide Chieffo, Giuseppe Tarantini and Federico Pappalardo received speakers' fees from Abiomed and GADA; Marco B. Ancona received speaker's fees from Cordis; Francesco Burzotta and Carlo Trani received speaker's fees from Abiomed, Abbott and Medtronic, Paolo Pagnotta received speaker's fees from Boston Scientific and Cardia; all the others declared no conflicts regarding this publication.
Supporting information
Appendix S1: Supporting Information
Figure S1 One‐year survival curves from the IMP‐IT Registry in patients with cardiogenic shock (CS), derived from the Cox proportional hazard model before propensity score weighting and multivariable weighted Cox model adjustment and stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure. Survival curves in the overall population with CS (Panel A) (hazard ratio (HR) 0.80, [confidence interval (CI)] [0.53–1.22]; p = 0.31, before vs during/after interventional procedure) and in patients with acute myocardial infarction (AMI) complicated by CS and treated with percutaneous coronary intervention (PCI) (Panel B) (HR 0.61, CI [0.37–0.99]; p = 0.04).
Figure S2 One‐year freedom from the composite of all‐cause mortality, rehospitalization for heart failure, need for left ventricular assist device or heart transplant (MACE) curves from the IMP‐IT Registry in patients with CS, derived from the Cox proportional hazard model before propensity score weighting and multivariable weighted Cox model adjustment and stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure. Freedom from MACE curves in the overall population with CS (Panel A) (HR 0.93, CI [0.65–1.35]; p = 0.71, before vs during/after interventional procedure) and in patients with AMI complicated by CS and treated with PCI (Panel B) (HR 0.76, CI [0.49–1.17]; p = 0.21).
Figure S3 One‐Year outcomes from the IMP‐IT Registry in patients treated with high‐risk percutaneous coronary intervention (HR‐PCI), derived from the Cox proportional hazard model before propensity score weighting and multivariable weighted Cox model adjustment and stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure. Survival curves (Panel A) (HR 0.32, CI [0.14–0.73]; p < 0.01, before vs during/after PCI) and freedom from MACE curves (Panel B) (HR 0.49, CI [0.25–0.98]; p = 0.04).
Tarantini G, Masiero G, Burzotta F, et al. Timing of Impella implantation and outcomes in cardiogenic shock or high‐risk percutaneous coronary revascularization. Catheter Cardiovasc Interv. 2021;98:E222–E234. 10.1002/ccd.29674
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the main text and in the supplementary material of this article.
REFERENCES
- 1.Burzotta F, Trani C, Doshi SN, et al. Impella ventricular support in clinical practice: collaborative viewpoint from a European expert user group. Int J Cardiol. 2015;201:684‐691. [DOI] [PubMed] [Google Scholar]
- 2.Chieffo A, Burzotta F, Pappalardo F, et al. Clinical expert consensus document on the use of percutaneous left ventricular assist support devices during complex high‐risk indicated PCI: Italian Society of Interventional Cardiology Working Group Endorsed by Spanish and Portuguese interventional cardiology societies. Int J Cardiol. 2019;293:84‐90. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe S, Fish K, Kovacic JC, et al. Left ventricular unloading using an Impella CP improves coronary flow and infarct zone perfusion in ischemic heart failure. J Am Heart Assoc. 2018;7:e006462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naidu SS. Novel percutaneous cardiac assist devices: the science of and indications for hemodynamic support. Circulation. 2011;123(5):533‐543. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra‐aortic balloon pump in patients undergoing high‐risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126(14):1717‐1727. [DOI] [PubMed] [Google Scholar]
- 6.Maini B, Naidu SS, Mulukutla S, et al. Real‐world use of the Impella 2.5 circulatory support system in complex high‐risk percutaneous coronary intervention: the USpella registry. Catheter Cardiovasc Interv. 2012;80(5):717‐725. [DOI] [PubMed] [Google Scholar]
- 7.Sjauw KD, Konorza T, Erbel R, et al. Supported high‐risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol. 2009;54(25):2430‐2434. [DOI] [PubMed] [Google Scholar]
- 8.Ouweneel DM, Eriksen E, Seyfarth M, Henriques JP. Percutaneous mechanical circulatory support versus intra‐aortic balloon pump for treating cardiogenic shock: meta‐analysis. J Am Coll Cardiol. 2017;69(3):358‐360. [DOI] [PubMed] [Google Scholar]
- 9.Amin AP, Spertus JA, Curtis JP, et al. The evolving landscape of Impella® use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141(4):273‐284. [DOI] [PubMed] [Google Scholar]
- 10.Dhruva SS, Ross JS, Mortazavi BJ, et al. Association of use of an intravascular microaxial left ventricular assist device vs intra‐aortic balloon pump with in‐hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2020;323(8):734‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chieffo A, Ancona MB, Burzotta F, et al. Observational multicenter registry of patients treated with IMPella mechanical circulatory support device in Italy: the IMP‐IT registry. EuroIntervention. 2020;15(15):e1343‐e1350. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41‐55. [Google Scholar]
- 13.McCaffrey DF, Griffin BA, Almirall D, et al. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388‐3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661‐3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rios SA, Bravo CA, Weinreich M, et al. Meta‐analysis and trial sequential analysis comparing percutaneous ventricular assist devices versus intra‐aortic balloon pump during high‐risk percutaneous coronary intervention or cardiogenic shock. Am J Cardiol. 2018;122(8):1330‐1338. [DOI] [PubMed] [Google Scholar]
- 16.Basir MB, Schreiber TL, Grines CL, et al. Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol. 2017;119(6):845‐851. [DOI] [PubMed] [Google Scholar]
- 17.Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119‐177. [DOI] [PubMed] [Google Scholar]
- 18.O'Neill WW, Grines C, Schreiber T, et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J. 2018;202:33‐38. [DOI] [PubMed] [Google Scholar]
- 19.Iannaccone M, Albani S, Giannini F, et al. Short term outcomes of Impella in cardiogenic shock: a review and meta‐analysis of observational studies. Int J Cardiol. 2020;324:44‐51. [DOI] [PubMed] [Google Scholar]
- 20.Tarantini G, Nai FL. Impella ventricular assist device: a "valvular bypass" to support high‐risk percutaneous coronary intervention or complicated transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2020;95(3):363‐364. [DOI] [PubMed] [Google Scholar]
- 21.Flaherty MP, Moses JW, Westenfeld R, et al. Impella support and acute kidney injury during high‐risk percutaneous coronary intervention: the global cVAD renal protection study. Catheter Cardiovasc Interv. 2020;95(6):1111‐1121. [DOI] [PubMed] [Google Scholar]
- 22.Ghionzoli N, Sciaccaluga C, Mandoli GE, et al. Cardiogenic shock and acute kidney injury: the rule rather than the exception. Heart Fail Rev. 2020. 10.1007/s10741-020-10034-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Udesen NJ, Møller JE, Lindholm MG. Et al; DanGer shock investigators. Rationale and design of DanGer shock:Danish‐German cardiogenic shock trial. Am Heart J. 2019;214:60‐68. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill WW, Ohman EM. Letter by O'Neill and Ohman regarding article, "Impella support for acute myocardial infarction complicated by cardiogenic shock: matched‐pair IABP‐SHOCK II trial 30‐day mortality analysis". Circulation. 2019;140(11):e557‐e558. [DOI] [PubMed] [Google Scholar]
- 25.Chioncel O, Parissis J, Mebazaa A, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock ‐ a position statement from the heart failure Association of the European Society of cardiology. Eur J Heart Fail. 2020;22(8):1315‐1341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Figure S1 One‐year survival curves from the IMP‐IT Registry in patients with cardiogenic shock (CS), derived from the Cox proportional hazard model before propensity score weighting and multivariable weighted Cox model adjustment and stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure. Survival curves in the overall population with CS (Panel A) (hazard ratio (HR) 0.80, [confidence interval (CI)] [0.53–1.22]; p = 0.31, before vs during/after interventional procedure) and in patients with acute myocardial infarction (AMI) complicated by CS and treated with percutaneous coronary intervention (PCI) (Panel B) (HR 0.61, CI [0.37–0.99]; p = 0.04).
Figure S2 One‐year freedom from the composite of all‐cause mortality, rehospitalization for heart failure, need for left ventricular assist device or heart transplant (MACE) curves from the IMP‐IT Registry in patients with CS, derived from the Cox proportional hazard model before propensity score weighting and multivariable weighted Cox model adjustment and stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure. Freedom from MACE curves in the overall population with CS (Panel A) (HR 0.93, CI [0.65–1.35]; p = 0.71, before vs during/after interventional procedure) and in patients with AMI complicated by CS and treated with PCI (Panel B) (HR 0.76, CI [0.49–1.17]; p = 0.21).
Figure S3 One‐Year outcomes from the IMP‐IT Registry in patients treated with high‐risk percutaneous coronary intervention (HR‐PCI), derived from the Cox proportional hazard model before propensity score weighting and multivariable weighted Cox model adjustment and stratified by timing of insertion of Impella 2.5 and CP in relation to the interventional procedure. Survival curves (Panel A) (HR 0.32, CI [0.14–0.73]; p < 0.01, before vs during/after PCI) and freedom from MACE curves (Panel B) (HR 0.49, CI [0.25–0.98]; p = 0.04).
Data Availability Statement
The data that supports the findings of this study are available in the main text and in the supplementary material of this article.


