Abstract
Aims
An effective decontamination procedure of personnel wearing personal protective equipment is required by CBRN responders and healthcare workers when dealing with biological warfare agents or natural outbreaks caused by highly contagious pathogens. This study aimed to identify critical factors affecting the efficacy of peracetic acid (PAA)‐based disinfectants and products containing either hydrogen peroxide or sodium hypochlorite under the same conditions.
Methods and Results
The influence of concentration, application (contact) time, erroneous human behaviour, interfering substance, technical assets and weather conditions on disinfection efficacy against Bacillus subtilis spores were assessed in 14 experimental groups. Residual contamination of protective suits was measured to provide responders with readily understandable information (up to 100 colony forming units classified a suit as disinfected). Weather conditions, short application time and erroneous human behaviour substantially affected the effectiveness of PAAs (P < 0·05). Non‐PAA‐based disinfectants (either liquid or foam) did not reach comparable efficacy (P < 0·001).
Conclusions
Peracetic acid was effective at a concentration of 6400–8200 ppm and an application time of 4 min.
Significance and Impact of the Study
The study provides operationally relevant data for the use of PAA‐based disinfectants in preparedness planning and management of biological incidents and natural outbreaks.
Keywords: antimicrobials, bacillus, bacterial spores, biocontrol, CBRN responders, decontamination, disinfection, erroneous behaviour, peracetic acid, personal protective equipment
Introduction
The intentional release and dissemination of biological warfare agents, together with contagious outbreaks, pose a challenge for authorities worldwide. One of the critical steps to reduce the consequences is a decontamination procedure of responders or healthcare workers (HCW) analogous to a shower decontamination phase in biosafety level 4 laboratories (BSL‐4). Personnel using chemical‐resistant personal protective equipment (PPE) can undergo whole‐body decontamination in a chemical shower before the doffing procedure. Others (e.g. HCW) usually wearing filtering facepiece respirators and goggles proceed only with local disinfection due to ineffective chemical protection. It is not of surprise the latter is associated with a substantial risk of self‐contamination described when various PPE protocols using fluorescent lotion or powder were tested (Kang et al. 2017; Chughtai et al. 2018). An inadequate PPE removal process accounts for a part of HCW‐related infections during the Ebola outbreak in West Africa from 2014 to 2016 (Casalino et al. 2015; Suwantarat and Apisarnthanarak 2015). Thus, in addition to a mere doffing procedure, national authorities generally implement a universal decontamination procedure of personnel wearing PPE into their operational guidelines for natural outbreaks and biological incidents.
As Bacillus anthracis spores are considered extremely resilient and more challenging to eliminate than viruses or bacteria, a manufactured product Persteril® was selected. Persteril, which lists peracetic acid (PAA) as an active ingredient, seemed to be the most optimal solution due to its broad‐spectrum efficacy, accessibility and short contact time. The disinfectant selection was based primarily on theoretical and laboratory evaluation. However, systematic research and verification of the effectiveness of the procedure in the real environment have been missing.
Disinfectant efficacy, in general, is strongly affected by the rate of application (concentration × contact time × applied volume/surface area), especially in situations when small volumes of a product are applied (Springthorpe and Sattar 2005). While residual contamination in the case of chemical/radiological events can be immediately measured, methods instantly evaluating the presence of biological agents have not been developed. To our best knowledge, only a few studies related to biological decontamination of personnel in operationally relevant conditions have been conducted so far (Darby and Glass 2002; Parks et al. 2013; Anon. 2013; Archer et al. 2018). The objectives of this study include assessment of the current decontamination procedure in a field environment, optimization if required, comparison with other disinfectants, and identification of practical aspects mentioned above. An impact of a dry doffing procedure was not tested.
Materials and methods
Suits
Only standard type 3B Microchem 3000 Model 122 suits (Ansell Microguard Ltd, Kingston upon Hull, UK) of M, L, XL, 2XL and 3XL sizes were selected for this study. By cutting an XL suit into pieces, we determined the suit surface area to be approximately 3 m2. Other PPEs (e.g. masks, gloves, boots) were not included in the study design.
Disinfectants
Persteril 36® at 2% concentration (groups A–F; Oqema s.r.o., Sokolov, Czech Republic) and Persteril 15® at 4·8% concentration (groups M–O; Oqema s.r.o.) were used, both containing 6400–8200 ppm of PAA.
To compare Persteril 36® efficacy, Savo Original® at 66·7% concentration (Bochemie a.s., Bohumin, Czech Republic) containing sodium hypochlorite solution with 30 000 ppm of active chlorine, Hvezda AB + CC® (MPD plus s.r.o., Rakovnik, Czech Republic) representing a mixture of hydrogen peroxide, sodium hydroxide and surfactant at 10% concentration and Vanodox® (Evans Vanodine International plc, Preston, Lancashire, UK) being a PAA‐based disinfectant at 14·5% concentration were selected.
Tap water was used for dilution of Persteril when field tests were conducted at firefighter brigade stations. The degree of hardness was recorded and ranged from 110 to 288 ppm CaCO3. In other cases, all disinfectants were diluted with demineralized water (Ro 2310, Earth Resources, Prague, Czech Republic). The disinfectants mentioned above were prepared under civilian CBRN unit guidelines and sprayed as aqueous solutions. Hvezda AB+CC® was additionally utilized as a foam using a special device (see below).
Experimental setup
Volunteers were randomly divided into 14 groups (Table 1). Experimental groups A–F and H–M were conducted in the biosafety level 4 (BSL‐4) facility of the Biological Defence Department (Military Health Institute, Techonin, Czech Republic). In addition to water hardness, other values were monitored, including relative humidity and air temperature. All tests were carried out in a walk‐through closed stainless decontamination chamber to prevent cross‐contamination and accelerate the course of tests. A connection to the stationary air‐pressure system provided compressed air into the spraying devices. Due to laboratory capacity and availability of volunteers and operators, only 50 decontamination tests were carried out per 2 days.
Table 1.
Experimental groups
| Group | Disinfectant | Concentration | Contact time (minutes) | Preceding rehearsal | Interfering substance | Asset | Area |
|---|---|---|---|---|---|---|---|
| A | Persteril 36® | 2% | 2 | Not tested | No | fogger* | BSL 4 |
| B | Persteril 36® | 2% | 4 | Not tested | No | fogger* | BSL 4 |
| C | Persteril 36® | 4% | 2 | Not tested | No | fogger* | BSL 4 |
| D | Persteril 36® | 2% | 4 | No | No | fogger* | BSL 4 |
| E | Persteril 36® | 2% | 4 | Yes | No | fogger* | BSL 4 |
| F‡ | Persteril 36® | 2% | 4 | Yes | Clean conditions | fogger* | BSL 4 |
| H | Savo Original® | 30 000 ppm | 4 | Yes | Clean conditions | fogger* | BSL 4 |
| I | Hvezda AB + CC® | 10% | 4 | Yes | Clean conditions | fogger* | BSL 4 |
| J | Hvezda AB + CC® | 10% | 4 | Yes | Clean conditions | foamer† | BSL 4 |
| K | Vanodox® | 14·5% | 4 | Yes | Clean conditions | fogger* | BSL 4 |
| L | Vanodox® | 14·5% | 4 | Yes | Dirty conditions | fogger* | BSL 4 |
| M | Persteril 15® | 4·8% | 4 | Yes | Dirty conditions | fogger* | BSL 4 |
| N | Persteril 15® | 4·8% | 4 | Yes | Clean conditions | fogger (see table 2) | U2‐U3 open area |
| O | Persteril 15® | 4·8% | 4 | Yes | Clean conditions | fogger (see table 1) | U1, U4‐7 closed tent |
534 Thumb Gun Fogger.
FI‐5NV.
Group G was not experimental.
The experimental groups were designed to add more variables incrementally (Table 1). We tested concentration over application (application time) in groups A–C. Once these groups were evaluated, D and E groups (rehearsal influence) were performed based on the group B results. Group F (clean conditions) followed test parameters of groups B and E. Group G was produced artificially by summing the results from groups E and F. Groups H, I, J and K were designed to compare the efficacy of various disinfectants with group G (application time, rehearsal, clean conditions). Groups L and M challenged the decontamination efficacy with dirty conditions while other parameters (rehearsal and application time) remained unchanged. Experimental groups N and O were performed at seven selected fire stations within the Czech Republic. The experimental flow chart is depicted in Fig. 1.
Figure 1.
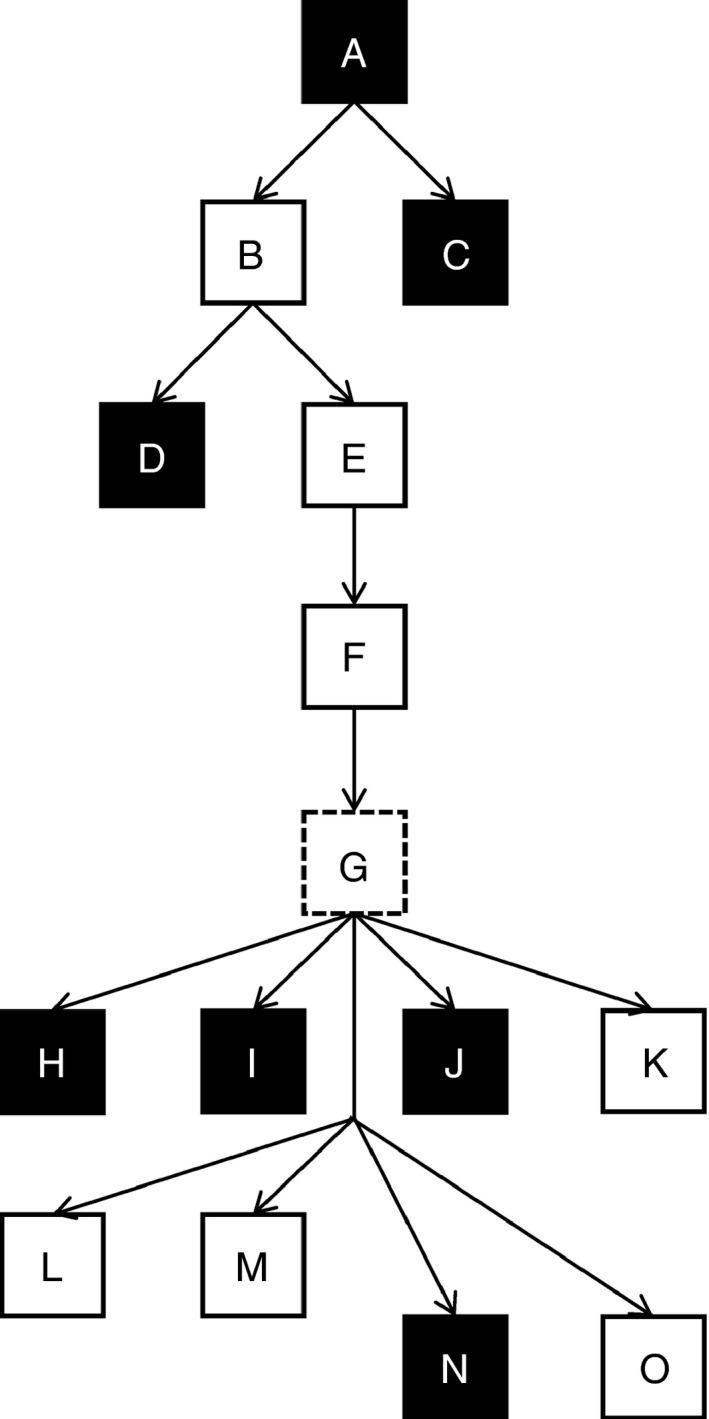
Development of experimental study design (black square: ineffective procedure, white square: effective procedure, dashed square: summed results of groups E and F).
Experiments performed in the field conditions (groups N and O) followed the test parameters of group F (application time, rehearsal and clean conditions). In contrast to the BSL 4 facility, the decontamination tests were challenged by external factors such as weather conditions, water hardness and various technical equipment. Approximately five personnel and at least five volunteers were required to attend the testing day. All the units (U) were tasked to prepare and deploy their own decontamination assets, including application devices. Suits, spores, disinfectants and tools for microbiological evaluation were strictly provided by project leaders. Swabbed samples were immediately processed in a mobile laboratory provided by the Population Protection Institute (Lazne Bohdanec, Czech Republic).
Weather conditions (relative humidity, air temperature and wind speed) were recorded from official sources and compared with a deployed meteorological device (model WS‐3600‐11; TechnoLine s.r.o, Chomutov, Czech Republic). Wind speed not exceeding 4 m s−1 was measured in U2‐3 locations (Table 2) because only these units carried out the decontamination tests in open areas. The tested cluster was reduced to 12 suits per unit, not to interfere with each unit’s regular tasks or curb their operational capabilities. Once the preliminary results from U1–U3 locations had been analysed, brigades U4–U7 were requested to perform the decontamination procedure only in the full‐closed tents. All tests were conducted in 2 years.
Table 2.
Description of technical assets and weather conditions at firefighter brigade stations
| Unit | Application device | Manufacturer | Working pressure (kPa) | Measured flow rate (L.min−1) | Period of year | Air temperature (°C) | rH (%) |
|---|---|---|---|---|---|---|---|
| U1 | REO 893 | Unknown | Unknown | 0·5 | May 2018 | 13 | 83·6 |
| U2 | Gloria Prima 5 | GLORIA Haus‐ und Gartengeräte GmbH | 300 (manual) | 0·55 | June 2018 | 24 | 50 |
| U3 | PZ 18s | Komma‐est, s.r.o. | 400 (air powered) | 1·1 | June 2018 | 24 | 62 |
| U4 | STIHL SG 51 | STIHL s.r.o. | 200–600 (manual) | 1 (300 kPa) | June 2018 | 25·8 | 39 |
| U5 | Solo 425 COMFORT 15L | SOLO® KLEINMOTOREN GmbH | 400 (manual) | 1·5 | June 2018 | 14·5 | 75 |
| U6 | Mary 10 | DiMartino SpA | 300 (manual) | 0·715 | September 2018 | 22 | 57 |
| U7 | PZ 18s | Komma‐est, s.r.o. | 400 (air powered) | 1·15 | September 2018 | 24 | 60 |
Test organism
Bacillus subtilis spores were prepared using a protocol developed at the BSL‐4 facility. In brief, B. subtilis subsp. subtilis ATCC 6051 (supplied by Czech National Collection of Type Cultures, National Reference Laboratory, Prague, Czech Republic) was grown on blood agar (LabMediaServis s.r.o., Jaromer, Czech Republic) for 24 h. A colony of B. subtilis was collected, transferred on AK Agar #2 in the Roux flask (LabMediaServis s.r.o.), and incubated at 35°C for 24 h and then at 20°C for 7 days. Subsequently, spores of B. subtilis were rinsed with physiological saline (Penta s.r.o., Prague, Czech Republic) and centrifuged (5000 g at 3°C for 15 min; JOUAN BR4i, Jouan S.A., Saint‐Herblain, France). The obtained sediment was resuspended in 5 ml of physiological solution and shock‐heated at 70°C for 30 min to destroy residual vegetative cells. The final product was filtered over a fourfolded cotton gauze (17 threads per cm2; BATIST Medical a.s., Cerveny Kostelec, Czech Republic). The presence of spores was confirmed by Wirtz and Conklin staining technique. An average of ~9 × 107 colony forming units (CFU) per ml was estimated by the 10‐fold serial dilution.
Spots and spore dispersion
Spots, 10 × 10 cm in size, were drawn on an originally folded suit. Only three areas were marked on the left shoulder, back, and left thigh and sprayed with the tested micro‐organism in groups A–C. For the remaining groups, 90 spots were drawn on each suit in the following locations: chest and abdomen (spots 1–16), right lower extremity (spots 17–32), left lower extremity (33–50), right upper extremity (spots 51–60), left upper extremity (61–70), hood and back (71–90). Of these, only three spots were randomly selected by the computer and contaminated. Neither volunteers nor volunteer‐operators were informed about the choice before sampling.
At least 24 h before the testing, spore suspension was dispersed onto the suit spots by using a hand‐held sprayer (model #100192; Alfachem s.r.o., Letovice, Czech Republic) at a volume of 0·5 ml, and the suits were left undisturbed overnight. The rationale for such dose resulted from the total volume of spore suspension required for two consequent testing days (50 suits, 150 spots, ~75 ml in total). Higher spore production was not available. Only one delegated person was responsible for the spore application procedure conducted in the BSL‐4 facility.
Interfering substance
The spore suspensions mixed with 0·3 and 3 g l−1 of bovine serum albumin (Reanal Zrt., Budapest, Hungary) were used to simulate clean conditions in accordance with the European standard CSN EN 13704 (groups F, H–K, N, and O) and to challenge dirty conditions (groups L and M), respectively (Votava et al. 2005).
Spraying devices
A hand‐held, air‐powered chemical atomizer (550 kPa; 534 Thumb Gun Fogger, model #950411, Lafferty Equipment Manufacturing, Inc., North Little Rock, AR) was used to apply aqueous disinfectant. An applied volume of disinfectant was regularly measured by the weighing of a supply canister between refillings. The actual gross flow rate estimate was calculated to 0·73 l min−1.
Foam application was carried out with an air‐powered foam unit (300–500 kPa; FI‐5NV, Foam‐It, Grand Rapids, MI). The compressed air was distributed from a stationary system of the BSL‐4 facility. The required pressure degree (500–550 kPa) was controlled with a reduction valve. Dry foam with a flow rate of ~2·27 l min−1 was produced in our study.
Sporicide neutralization
Adequate neutralizers were selected based on CSN EN 13704 and a previously published paper (Calfee et al. 2012). Sodium thiosulphate (LACH‐NER s.r.o, Neratovice, Czech Republic) at a concentration of 5% was used to inactivate PAA‐based disinfectants and Savo Original® and Dey‐Engley neutralization broth M1062 (LabMediaServis s.r.o.) to neutralize Hvezda AB+CC®. To accelerate the inactivation process under field conditions, we sprayed sodium thiosulphate using a hand‐held spraying device (model #100192) immediately onto examined spots and their surroundings after disinfectant application.
Sample processing
A total of 1815 samples were taken in this study. Dry‐cotton swabs (Nerbe plus GmbH, Winsen, Germany) were used for the sampling procedure. The swabs were immersed into sterilized tubes containing 5% sodium thiosulphate and immediately transported to a laboratory. Swabs remained immersed for 10 min to neutralize sporocides. Subsequently, the tubes were vortexed for 30 s. Aliquots (0·2 ml) were plated on blood agar plates, incubated at 37°C, and enumerated after 24 h. Negative plates or plates with a low number of colonies were further incubated for 48 and 72 h.
Quality control (QC)
Quality control checks were randomly performed to demonstrate the accuracy and precision of the results. Sampling before the suit decontamination (positive control) confirmed the correct inoculation of the selected spots. Random sample duplicates were collected by another sampler to estimate sampling precision. Cultivation of sodium thiosulphate solution and agar plates excluded false‐positive results due to contamination.
Results expression
Bacillus subtilis colonies of each sampled area were enumerated. When the number of residual viable spores crossed the strict threshold of 100 CFU, a sampled area (a spot) was marked as positive (the rationale discussed later). Only suits containing all three negative spots (below 100 CFU) were counted as negative, and the decontamination procedure was considered successful. Such binary coding simplifies the evaluation of suits. It also provides practical information for the first responders and their rehearsals because first responders are interested in residual contamination more than logarithmic reduction values.
Statistical analysis
Data analyses were performed with R software, ver. 3.5.2 (R Foundation for Statistical Computing), including GenBinomApps package. A 95% confidence interval (CI) for binomial proportion was calculated as the Clopper–Pearson interval (‘clopper.pearson.ci’ function in R). Experimental results were compared using Two‐Proportions Z‐Test (‘prop.test’ function in R) to determine whether a parameter played a significant role in the decontamination efficacy. Yates’s correction for continuity was applied if the number of expected positive or negative cases was less than five. If Z‐test could not be used, the Fisher’s exact test in a 2 × 2 contingency table was applied (‘fisher.test’ function in R). The level of significance was set to 0·05 for all tests. All P values are one‐tailed.
Results
The average recovery calculated from positive controls was ~4·4 × 105 ± 2·6 × 105 CFU (mean ± standard deviation). Of 88 positive controls, all exhibited massive growth of viable spores, confirming a correctly sampled area.
Two of 213 duplicate samples exceeded the study limit between standard and duplicate samples (up to 10 CFU). Hence, a 0·9% false‐negative rate was determined. Additionally, these two sampling areas were marked as positive. Sodium thiosulphate solution and agar plate controls remained negative throughout the project period.
CIs and tested hypotheses are summarized in Table 3.
Table 3.
Decontamination efficacy of peracetic acid‐based disinfectants under different conditions and its comparison with products containing hydrogen peroxide or sodium hypochlorite
| Group | N | Positive | Negative | Negative proportion (%) (95% CI)* | Test | P value |
|---|---|---|---|---|---|---|
| A | 49 | 29 | 20 | 40·8 (27·0–55·8) | – | – |
| B | 100 | 5 | 95 | 95·0 (88·7–98·4) | A < B | <0·001 |
| C | 50 | 24 | 26 | 52·0 (37·4–66·3) | A < C | 0·132 |
| D | 50 | 13 | 37 | 74·0 (59·7–85·4) | – | – |
| E | 50 | 4 | 46 | 92·0 (80·8–97·8) | D < E | 0·017 † |
| F | 50 | 2 | 48 | 96·0 (86·3–99·5) | E < F | 0·339 ‡ |
| G | 100 | 6 | 94 | 94·0 (87·4–97·8) | – | – |
| H | 25 | 21 | 4 | 16·0 (4·5–36·1) | H < G | <0·001 |
| I | 25 | 25 | 0 | 0·0 (0·0–13·7) | I < G | <0·001 |
| J | 25 | 25 | 0 | 0·0 (0·0–13·7) | J < G | <0·001 |
| K | 50 | 2 | 48 | 96·0 (86·3–99·5) | K < G | 0·551 † |
| L | 25 | 1 | 24 | 96·0 (79·6–99·9) | L < G | 0·500 † |
| M | 24 | 0 | 24 | 100·0 (85·8–100·0) | M < G | 0·758 † |
| N | 23 | 7 | 16 | 69·6 (47·1–86·8) | N < O | 0·015 † |
| O | 59 | 5 | 54 | 91·5 (81·3–97·2) | O < G | 0·393 † |
Used 95% Clopper–Pearson’s confidence interval.
Used Yates’s continuity correction.
Fisher’s exact test.
The current decontamination procedure conducted by experienced BSL‐4 operators (group A) yielded unsatisfactory results. Only 40·8% of suits achieved the desired level of decontamination (≤100 residual viable spores). Decontamination efficacy significantly increased to 95·0% (P < 0·001) when the contact time was increased (group B). In contrast, twofold concentration (group C) did not significantly improve the disinfection process (52·0%, P = 0·132).
After the effective decontamination procedure had been established, erroneous human behaviour and the need for a practical rehearsal were tested in trainees undergoing the decontamination course. Without rehearsal (group D), the decontamination yielded 74·0% efficacy. It significantly increased to 92·0% (P = 0·017) after the rehearsal (group E). The presence of the interfering substance did not affect the disinfection efficacy of PAA (group F) when compared with group E (P = 0·339). Therefore, the results of both groups E and F were summed into group G to provide more sensitive figures. Compared with this group, the decontamination efficacy of Savo Original® (group H), Hvezda AB + CC® either in liquid (group I) or foam form (group J) and Vanodox® (group K) was tested. Using Savo Original® or Hvezda AB + CC® both in liquid and foam form, the efficacy significantly decreased to 16·0, 0·0 and 0·0%, respectively (all P < 0·001). Only Vanodox® disinfection reached 96·0% of negative results (P = 0·551). Additionally, compared with group G, the efficacy of the established procedure remained unchanged when Vanodox® (group L) and Persteril (group M) were challenged with the presence of the interfering substance, providing 96·0 and 100·0% (P = 0·500 and 0·758) of negative results, respectively.
Finally, the decontamination procedure was conducted in the field environment either in an open area (group N) or in a closed tent (group O), yielding 69·6 and 91·5% efficacy, respectively. A significant difference was found between both groups (P = 0·015). No difference was observed when the decontamination procedure was conducted in a closed tent compared with group G (P = 0·393).
Discussion
One of our study’s critical issues was defining an understandable interpretation to all responders and decision‐makers. The frequently used logarithmic reduction in the number of viable spores might not be acceptable for responders or safe enough for the highly contaminated area. While a 103 reduction required by the CSN EN 13704 might appear to low (Votava et al. 2005), a 106 reduction, as proposed by several studies on contaminated surfaces (Anon. 2011; Archer et al. 2018), would pose a real challenge for first responders due to prolonged contact time, vertically orientated surface or an active movement of a person. By contrast, the non‐extensive spore contamination represents a more realistic scenario because the pre‐treatment of massive visible contamination (e.g. soil, vomits, blood) usually foregoes a decontamination process.
Thus, a level of acceptable residual contamination was defined to provide a more straightforward explanation and analogy. Nevertheless, a safe level of residual contamination has not yet been determined. Given that the exposition to ~600 B. anthracis spores per day does not necessarily pose a threat of inhalational anthrax (Cohen and Whalen 2007), we determined a strict threshold of 102 residual viable spores in our study. Due to the possible limitations of the approach and the potential need to shift the threshold, raw data are provided in Table S1. On the other hand, the proposed assessment of residual contamination and the non‐extensive initial spore concentration identified the most critical factors negatively affecting the results of a decontamination procedure (groups A, C, D, H, I, J and N).
Another critical aspect is the reliable detection of pathogens that may be impaired by sampling and processing methods resulting in false‐negative results (Piepel et al. 2016). A standard cotton swab was used due to practical limitations (working hours of all participants, daily processing of ~100 samples). The limit of detection for swab methods varies across several studies and depends on sampled surfaces, surrogate agents or sampling and extraction methods (Estill et al. 2009; Lutz et al. 2013). To mitigate the disadvantages of the previously mentioned procedures, samples were taken and processed only by four project leaders and two laboratory technicians.
Due to unsatisfactory results achieved in group A (total sprayed volume ~1·5 l on a suit of an average area of 3 m2), the contact time was increased up to 4 min in group B resulting in a higher volume of the disinfectant applied (~2·8 l). The rationale for such a short time limit was that CBRN responders wearing a self‐contained breathing apparatus are limited by compressed‐air supply. In contrast to group B, the concentration of Persteril was increased twice in group C, while the contact time remained unchanged (2 min, total volume ~1·5 l). The achieved results support the theory that the disinfectant efficacy should not be perceived only as of the dose (concentration × contact time) but more likely as the rate of application (concentration × contact time × volume/area) (Springthorpe and Sattar 2005). The generally accepted paradigm (higher concentration = shorter contact time = greater efficacy) (Rutala and Weber 2008) appears to be rejected for PAA disinfectants with this application mean. Because of the vertical hydrophobic surface of a protective suit, the application time had to be identical with the contact time to follow the application rate theory.
Human error is a decisive factor determining the success of every process. When a single‐nozzle sprayer is used, both an operator and a contaminated response member can be sources of a decontamination failure. The human error was tested in groups D and E. The group D comprised theoretical briefing about the project and instructions about the sprayer. Seven volunteers attended this phase. Within 2 days, they sprayed 50 times each other (3 of them applied a disinfectant ten times, 4 of them just five times). Each volunteer‐operator adopted a different approach without any correction by project leaders. Without any practical training and correction, they did not substantially modify their approach over the testing period. Mostly they struggled to keep a systematic approach within the 4‐min timeframe and frequently skipped sides and peripheral parts of a suit. However, they were informed regularly about the remaining time.
The practical decontamination exercise preceding the real test was implemented in groups E–O. Here, the project leaders additionally demonstrated a systematic procedure that included: dividing the body into eight zones (front/back upper parts, front/back middle parts, front/back low extremities and left/right side parts), one‐word instructions to guide volunteers, and the optimal number of spraying repetitions of each body zone (twice at least). Following the advanced theoretical briefing, they practiced the decontamination procedure using water while the project leaders played the role of contaminated response members. Similar to group D, seven volunteers joined two consecutive testing days alternating the roles of the operators and the response members.
Once the risk of a human error was identified and adjusted, the interfering substance simulating clean conditions (CSN EN 13704) was incorporated into the study design (group F). Despite the substance, PAA‐based disinfectant preserved the decontamination effect. Subsequently, the efficacy of other disinfectants was assessed. Chlorine and liquid hydrogen peroxide‐based disinfection (groups H and I) did not yield acceptable results when applied at recommended concentrations for 4 min. We did not reveal any critical factors determining the efficacy but the inadequately short contact time in the presence of the interfering substance, which is in accordance with earlier studies (Majcher et al. 2008; Omidbakhsh 2010; Humphreys et al. 2013). Group J was the only case when Hvezda AB+CC® as the foam was tested with identical results to the group I. Although a foaming disinfectant may be favoured due to its ability to increase the contact time compared to liquid solutions and visual inspection of a decontaminated surface, advantages were outbalanced. Our tested device generated a recommended thickness of the foam layer (1–1·5 mm) only when a needle‐valve was fully opened for maximal dryness (Rybka et al. 2019). It is impractical and costly to evaluate the desired foam properties for each of the many devices and chemicals. Furthermore, a substantial risk of blocking a full‐face mask chemical filter by foam must be considered.
Only Vanodox® (group K), another PAA‐based product, achieved acceptable results comparable to Persteril. However, lower dilution of the original product must be used to obtain 6400–8200 ppm of the active ingredient. Additionally, Persteril 15® and Vanodox® were challenged by dirty conditions (groups L and M). No significant changes were observed, supporting previous conclusions that the efficacy of PAA‐based disinfections was not significantly affected by interfering substances even if lower concentrations of 2250–3000 ppm were tested (Majcher et al. 2008; Humphreys et al. 2013).
Spray deposition on a vertical hydrophobic surface is a critical factor determining both the efficient and safe application of a disinfectant. Previous research has shown that the deposition is substantially affected by droplet size. The American Society of Agricultural and Biological Engineers (ASABE) has developed a scheme classifying droplets according to their volume median diameter (VMD) from extremely fine to ultra‐coarse (Anon. 2018). Comparing ASABE S572.2 flow rates and operating pressure with our data, it may be roughly estimated that the assets used in our study generated very fine/fine/medium droplets. Unfortunately, only one supplier provided detailed information on installed nozzles (group P, VMD ~70 μm, flow rate 0·125 l min−1, 500 kPa). If a fine spray method is being used, up to 50% of disinfectant may be retained on the surface (Nasr et al. 2007). Comparing groups N and O, we demonstrated and quantified the influence of climate conditions on the disinfectant efficacy when the decontamination procedure is conducted in an open area. Drifting small droplets off a target by wind is one plausible explanation (Nuyttens 2007). If very fine/fine/medium particles are sprayed in a full closed room/tent, the high surface coverage of a contaminated person will be achieved. Moreover, the risk of cross‐contamination among responders and their equipment and the contamination of the downwind area is reduced (Archer et al. 2018).
CBRN units are equipped with a wide range of technical, mostly commercial assets for applying a decontamination solution. By analysing the results of groups O and G, it seems that such variability is not of any significant importance in the field if other factors are either eliminated (weather conditions) or implemented (rehearsal, adequate contact time, spray quality). A single‐nozzle device is cheap, easily operated, but the application of sufficient volume and consistent coverage require longer spray duration (Archer et al. 2018) and previous training of an operator.
PAA‐based disinfectant is the most appropriate solution when a broad‐spectrum product is considered for a decontamination procedure of responders or HCW in the field environment. PAA yielded acceptable results when continuously applied using a single‐nozzle sprayer within a 4‐ min time frame (contact time) at a concentration of 6400–8200 ppm and volume ~2·8 l per decontaminated person (surface area of 3 m2). Regular training of both operators and volunteers is necessary to achieve the desired efficacy. Nevertheless, one should bear in mind that a decontamination procedure only reduces the risk of contamination before PPE is taken off. Therefore, a correct doffing procedure should be regularly practiced and performed when necessary.
We provide evidence‐based data in an operationally relevant context that will be assessed by Czech national authorities to implement safe and efficacious biological decontamination procedures of personnel wearing PPE.
Funding
This work was supported by the Ministry of the Interior of the Czech Republic (identification number VI20172020095) and by the grant of Ministry of Defence of the Czech Republic (Long‐term development plan Medical Aspects of Weapons of Mass Destruction of the Faculty of Military Health Sciences, University of Defence).
Conflict of Interests
Authors declare no competing interests.
Authors’ contribution
A.R. designed all the experiments. A.R., A.G., T.K. and J.M. participated as project leaders. O.P., L.K. and P.K. provided the laboratory support. P.P. and J.D. performed the statistical analyses. A.R. and J.P. have drafted the first version of the manuscript and have incorporated all the modifications recommended by the other authors.
Supporting information
Table S1. The number of colony‐forming units (CFU) of residual viable spores of Bacillus subtilis after decontamination.
Acknowledgements
The authors would like to thank Lada Hejtmankova, Bara Faltusova, Josef Dolejs, Pavel Dvoulety, Romana Klicova and Karel Tittl for technical and administrative support and to volunteers from the firefighter brigade and Biological Defense Department Techonin for their enthusiasm.
References
- Anon. (2011) Technical Brief: Evaluation of Liquid and Foam Decontamination Technologies for Surfaces Contaminated by Bacillus anthracis Spores, vol No. 600S11003. Washington, D.C.: U.S. Environmental Protection Agency. [Google Scholar]
- Anon. (2013) Bio‐Response Operational Testing and Evaluation (BOTE) Project ‐ Phase 1: Decontamination Assessment, vol No. 600/R–13/168. Washington, D.C.: U.S. Environmental Protection Agency. [Google Scholar]
- Anon. (2018) Spray Nozzle Classification by Droplet Spectra. (No. 572.2). ASABE. https://webstore.ansi.org/Standards/ASABE/ANSIASABES572FEB2020. Accessed 28 Nov 2020.
- Archer, J., Karnik, M., Touati, A., Aslett, D. and Abdel‐Hady, A. (2018) Evaluation of Electrostatic Sprayers for Use in a Personnel Decontamination Line Protocol for Biological Contamination Incident Response Operations, No. EPA/600/R‐18/283. Washington, D.C.: U.S. Environmental Protection Agency. [Google Scholar]
- Calfee, M.W., Ryan, S.P., Wood, J.P., Mickelsen, L., Kempter, C., Miller, L., Colby, M., Touati, A.et al. (2012) Laboratory evaluation of large‐scale decontamination approaches. J Appl Microbiol 112, 874–882. [DOI] [PubMed] [Google Scholar]
- Casalino, E., Astocondor, E., Sanchez, J.C., Díaz‐Santana, D.E., Del Aguila, C. and Carrillo, J.P. (2015) Personal protective equipment for the Ebola virus disease: a comparison of 2 training programs. Am J Infect Control 43, 1281–1287. [DOI] [PubMed] [Google Scholar]
- Chughtai, A.A., Chen, X. and Macintyre, C.R. (2018) Risk of self‐contamination during doffing of personal protective equipment. Am J Infect Control 46, 1329–1334. [DOI] [PubMed] [Google Scholar]
- Cohen, M.L. and Whalen, T. (2007) Implications of low level human exposure to respirable B. anthracis . Appl Biosaf 12, 109–115. [Google Scholar]
- Darby, S.M. and Glass, M.J. (2002) Formal Test Report for the Tactical Personnel Biological Decontamination Validation (No. WDTC‐TR‐02‐072). U.S. Army Dugway Proving Ground.
- Estill, C.F., Baron, P.A., Beard, J.K., Hein, M.J., Larsen, L.D., Rose, L., Schaefer, F.W., Noble‐Wang, J.et al. (2009) Recovery efficiency and limit of detection of aerosolized Bacillus anthracis sterne from environmental surface samples. Appl Environ Microbiol 75, 4297–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys, P.N., Finan, P., Rout, S., Hewitt, J., Thistlethwaite, P., Barnes, S. and Pilling, S. (2013) A systematic evaluation of a peracetic‐acid‐based high performance disinfectant. J Infect Prev 14, 126–131. [Google Scholar]
- Kang, J., O’Donnell, J.M., Colaianne, B., Bircher, N., Ren, D. and Smith, K.J. (2017) Use of personal protective equipment among health care personnel: results of clinical observations and simulations. Am J Infect Control 45, 17–23. [DOI] [PubMed] [Google Scholar]
- Lutz, J.K., Crawford, J., Hoet, A.E., Wilkins, J.R. and Lee, J. (2013) Comparative performance of contact plates, electrostatic wipes, swabs and a novel sampling device for the detection of Staphylococcus aureus on environmental surfaces. J Appl Microbiol 115, 171–178. [DOI] [PubMed] [Google Scholar]
- Majcher, M.R., Bernard, K.A. and Sattar, S.A. (2008) Identification by quantitative carrier test of surrogate spore‐forming bacteria to assess sporicidal chemicals for use against Bacillus anthracis . Appl Environ Microbiol 74, 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr, G.G., Yule, A.J., Lloyd, S.E. and Whitehead, A. (2007) The application of fine sprays for chemical, biological, and radiological or nuclear (CBRN) decontamination. In Proceedings of the 21th ILASS‐Europe Meeting. Mugla, Turkey. [Google Scholar]
- Nuyttens, D. (2007). Drift from field crop sprayers: the influence of spray application technology determined using indirect and direct drift assessment means (dissertation). Katholieke Universiteit Leuven, Belgium, Leuven. https://www.researchgate.net/publication/28360407_Drift_from_field_crop_sprayers_the_influence_of_spray_application_technology_determined_using_indirect_and_direct_drift_assessment_means. Accessed 29 Nov 2020. [Google Scholar]
- Omidbakhsh, N. (2010) Evaluation of sporicidal activities of selected environmental surface disinfectants: carrier tests with the spores of Clostridium difficile and its surrogates. Am J Infect Control 38, 718–722. [DOI] [PubMed] [Google Scholar]
- Parks, S., Gregory, S., Fletcher, N., Pottage, T., Thompson, K.‐A., Lakeman, J., Jhutty, A., Walker, J.T.et al. (2013) Showering BSL‐4 suits to remove biological contamination. Appl Biosaf 18, 162–171. [Google Scholar]
- Piepel, G., Hutchison, J., Kaiser, B.D., Amidan, B., Sydor, M. and Barrett, C. (2016) Recovery Efficiency, False Negative Rate, and Limit of Detection Performance of a Validated Macrofoam‐Swab Sampling Method with Low Surface Concentrations of Two Bacillus anthracis Surrogates (No. PNNL‐23955, Rev. 1). Richland, WA: Pacific Northwest National Laboratory. [Google Scholar]
- Rutala, W.A. and Weber, D.J. (2008) Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Atlanta: Centers for Disease Control and Prevention. [Google Scholar]
- Rybka, A., Gavel, A., Prazak, P., Meloun, J. and Pejchal, J. (2019) Decontamination of CBRN units contaminated by highly contagious biological agents. Epidemiol Mikrobiol Imunol Cas Spolecnosti Epidemiol Mikrobiol Ceske Lek Spolecnosti JE Purkyne 68, 40–45. [PubMed] [Google Scholar]
- Springthorpe, V.S. and Sattar, S.A. (2005) Carrier tests to assess microbicidal activities of chemical disinfectants for use on medical devices and environmental surfaces. J AOAC Int 88, 182–201. [PubMed] [Google Scholar]
- Suwantarat, N. and Apisarnthanarak, A. (2015) Risks to healthcare workers with emerging diseases: lessons from MERS‐CoV, Ebola, SARS, and avian flu. Curr Opin Infect Dis 28, 349–361. [DOI] [PubMed] [Google Scholar]
- Votava, M., Slitrová, J. and Matusková, Z. (2005) Microbicidal efficacy of a new foam disinfectant [in Czech]. Epidemiol Mikrobiol Imunol 54, 84–89. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The number of colony‐forming units (CFU) of residual viable spores of Bacillus subtilis after decontamination.


