Abstract
Anti‐müllerian hormone (AMH) produced by granulosa cells (GCs), reserves the ovarian follicle pool for future recruitment and ovulation. However, women who have undergone cyclophosphamide (Cy) treatment have decreased AMH levels due to damaged GCs. This study establishes flow cytometry protocols for identification of GCs and investigates the cause of the Cy‐induced AMH decrease by analyzing the number of GCs and their AMH production at the single cell level. Over 2 weeks, C57BL/6 mice were intraperitoneally injected 6 times with 100 mg/kg Cy and sacrificed either immediately or 4 weeks after Cy treatment. Twenty‐four hours post‐Cy exposure, a decrease in serum AMH levels was seen due to a reduction in the number of follicle‐stimulating hormone receptor (FSHR)+AMH+ GCs and their ability to produce AMH. However, 4 weeks after Cy treatment, serum AMH levels were still decreased due to the decreased number of FSHR+AMH+ GCs, however, their AMH‐producing ability was unaltered. Consistently, in vitro, Cy‐induced low AMH production in FSHR+AMH+ hGL5 cells (immortalized human GCs) was restored 24 h after Cy treatment, although their numbers remained decreased. Thus, the surviving GCs after Cy exposure had intact AMH‐producing ability. In future, an effort to minimize GC death by Cy treatment is required, while maintaining its therapeutic effects.
Keywords: anti‐müllerian hormone, cyclophosphamide, granulosa cells, ovarian follicle
1. INTRODUCTION
Anti‐müllerian hormone (AMH) is produced by granulosa cells (GCs) in the ovaries, where it inhibits the recruitment of primordial follicles and activates GC differentiation in response to follicle‐stimulating hormone (FSH), reserving the follicle pool for future recruitment and ovulation.1 However, in women who have undergone anticancer chemotherapies, serum AMH levels are extremely low or undetectable.2 In particular, cyclophosphamide (Cy) is an alkylating agent used in breast cancer treatment that causes irreversible damage to the ovaries, leading to premature ovarian failure or early menopause in young women.3 One well‐known mechanism of action for Cy is that it directly induces GC apoptosis and follicular atresia, resulting in a marked decrease in AMH, which accelerates the recruitment of remnant primordial follicles diminishing the ovarian reserve and leading to a poor reproductive prognosis.4, 5, 6 The underlying cytotoxic mechanisms in GCs exposed to an activated form of Cy (4‐hydroperoxycyclophosphamide, 4HC) include increased oxidative stress and DNA adduct formation.7, 8
Previous research has reported that decreased AMH levels after Cy treatment are associated with rarely surviving GCs and growing follicles,9, 10 and these chemotherapy‐induced decreases in AMH have continued effects on fertility. However, it is still unclear whether this decline is caused by reductions in the number or abnormalities in the function of GCs. Additionally, the effects of Cy on GC AMH production have not been examined at the single cell level, partly due to the lack of effective assays. Measurements of serum AMH levels by enzyme‐linked immunosorbent assay (ELISA) reflect collective AMH production from all GCs and do not indicate GC quantity, while measurements of AMH protein expression by immunohistochemistry do not necessarily reflect the ability of all GCs in the ovaries. Real time‐polymerase chain reaction (RT‐PCR) assays cannot rule out the possibility of contamination by other cells. To avoid these issues, our study aimed to establish a flow cytometry protocol that measures the number of GCs' and their AMH production. To examine why serum AMH levels were low after rigorous Cy treatment, we analyzed the GCs isolated from growing follicles directly exposed to Cy immediately after treatment and from growing follicles that had matured from Cy‐exposed primordial or primary follicles 4 weeks after Cy treatment.
2. MATERIALS AND METHODS
2.1. Mice
All experiments and analyses were in accordance with the “Guide for the Care and Use of Laboratory Animals” (NIH publication No. 86‐23, revised in 1996). Experimental animal protocols were approved by the Institutional Animal Care and Use Committee of Korea Institute of Oriental Medicine (19‐019, Daejeon, Korea). Eight‐week‐old C57BL/6 female mice were obtained from Nara Biotech (Pyeongtaek, Korea) and housed under specific pathogen‐free conditions. Animals were administered intraperitoneal injections of either saline (n = 15) or Cy (n = 16, 100 mg/kg; Sigma‐Aldrich, St. Louis, MO) 6 times over 2 weeks. The body weight of mice was measured immediately after or 4 weeks after Cy cessation. Mice were sacrificed at 24 h after intraperitoneal injections of pregnant mare's serum gonadotropin (PMSG, 5 IU, ProSpec‐Tany TechnoGene, Rehovot, Israel) to synchronize the menstrual cycle affecting GCs proliferation in growing ovarian follicles. The ovaries were weighed, then placed immediately into puncture medium to isolate the GCs, containing minimum essential medium (MEM) with GlutaMAX (Gibco, Paisley, UK), 1× insulin‐transferrin‐selenium (ITS, Zen‐Bio, NC), recombinant human FSH (Sigma‐Aldrich), and 10% fetal bovine serum (Gibco). To measure AMH levels, the serum was separated from whole blood and frozen at –80°C.
2.2. AMH ELISAs
AMH levels were measured by ELISA (Cloud‐Clone Corp, Houston, TX) in triplicate and calculated according to the manufacturer's instructions. The intra‐ and interassay coefficient of variation values were 10% and 12%, respectively, with a sensitivity of 52.5 pg/ml.
2.3. GC isolation
The ovaries were removed from the animals and placed in puncture medium. GCs were collected by puncturing the growing follicles of the isolated ovaries with a 25‐gauge needle as previously described.11 GC suspensions were centrifuged at 400g for 4 min and resuspended in 0.2 ml of Cell Staining Buffer (BioLegend, CA).
2.4. Flow cytometry for intracellular staining of GCs
GCs were stained with 1:10,000 BD Horizon Fixable Viability Stain 660 (BD Biosciences, CA) to discriminate live from dead cells. After washing with Cell Staining Buffer (BioLegend), the GCs were incubated in a modified fixation/permeabilization buffer (FP buffer) containing Fixation Buffer (BioLegend) and Intracellular Staining Permeabilization Wash Buffer (BioLegend) at a 1:1 ratio. GCs were stained with 0.5 μg/ml of primary antibodies against follicle‐stimulating hormone receptor (FSHR) (rabbit poly IgG, Proteintech, IL) and AMH (mouse mono IgG2a, Santa Cruz Biotechnology, Dallas, TX) at room temperature for 30 min. After washing with FP buffer, GCs were stained with 0.5 μg/ml of Alexa Fluor 488‐conjugated anti‐rabbit IgG (Abcam, Cambridge, UK) and 0.5 μg/ml of allophycocyanin‐cyanine7‐conjugated anti‐mouse Ig light chain (BioLegend) in FP buffer for 30 min at room temperature in the dark. Labeled cells were washed with FP buffer and analyzed by flow cytometry on a BD LSRFortessa Cell Analyzer (BD Biosciences, CA). Isotype control antibodies for FSHR (0.5 μg/ml; rabbit IgG, Invitrogen) and AMH (0.5 μg/ml; mouse IgG2a, Biolegend) were used to differentiate nonspecific background signals from specific antibody signals. Prior to flow cytometry, 50 μl of CountBright Absolute counting beads (Invitrogen, CA) were mixed with cell samples to absolutely calculate their quantities according to the manufacturer's instructions (target cell count in a tube = number of target cell/number of counting beads in each sample × assigned bead count of 50 μl counting beads, number of collecting beads ≥10,000). Approximately 100,000 cells were analyzed in the in vivo experiments and 10,000–50,000 cells were analyzed in the in vitro experiments to calculate the total number of target cells.
2.5. In vitro GC culture
The hGL5 cell line was purchased from Applied Biological Materials (Richmond, BC, Canada) and cultured in Prigrow IV Medium (Applied Biological Materials) supplemented with 1× ITS, 4% Ultroser G Serum Substitute (Pall Corporation, Port Washington, NY), 5% calf serum (Hyclone, UT) and 1% penicillin/streptomycin (P/S, Gibco) according to the manufacturers' instructions. Cells (2 × 104/well) were seeded in 6‐well collagen‐coated plates (Corning, Corning, NY) for 24 h. The cells were treated with 0 or 0.5 μg/ml 4HC (Sigma‐Aldrich) in culture media for 4 h, then washed and cultured with low serum medium containing 1× ITS, 0.4% Ultroser G serum substitute, and 1% P/S. Cells were collected in Cell Staining Buffer for flow cytometry immediately after 4HC treatment and 24 h later.
2.6. Statistical analyses
Statistical significance between the two experimental groups was analyzed by Student's t‐test using Graphpad Prism ver 8.4.0 (Graphpad Software Inc., La Jolla, CA). p <0.05 was considered statistically significant.
3. RESULTS
3.1. Identification of GCs obtained from mouse ovaries using flow cytometry
We identified GCs based on FSHR expression as previously described.12, 13 To examine why serum AMH levels were low after rigorous Cy treatment, we assessed the quantity and quality of the FSHR+AMH+ GCs by flow cytometry (Figure 1). The unstained control (gray line) was used to set the negative population, which determined the background autofluorescence, and the isotype antibody‐stained control was used to confirm the specificity of the binding of the primary antibody in flow cytometry. Measurement of the mean fluorescence intensity (MFI) of FSHR and AMH at the single cell level was performed in live FSHR+AMH+ GCs. This enabled us to examine the effects of Cy on both the number of FSHR+AMH+ GCs and their AMH‐producing ability.
FIGURE 1.
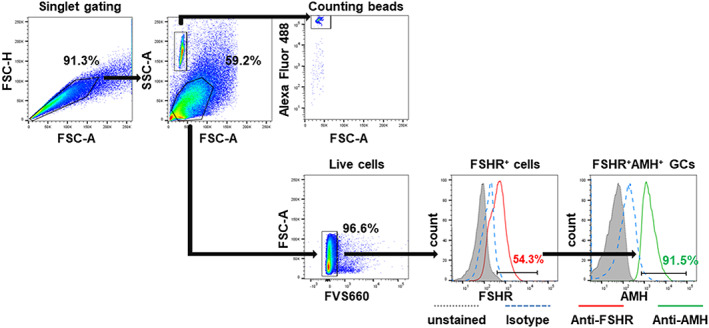
Strategies for identification of GCs by flow cytometry. GCs isolated from mouse ovaries and immortalized hGL5 cells were identified based on FSHR expression using isotype control antibody‐stained cells after live cell gating. Counting beads were gated on the forward scatter (FSC)/side scatter (SSC) plot, and Alexa Fluor 488 positivity was used to quantitate GCs. To assess their quality and AMH production, the mean fluorescence intensity (MFI) of FSHR and AMH in FSHR+AMH+ live GCs were analyzed at the single cell level. Unstained control, gray line; Isotype antibody‐stained control, blue line; anti‐FSHR antibody‐stained control, red line; anti‐AMH antibody‐stained control, green line. AMH, anti‐müllerian hormone; FSHR, follicle‐stimulating hormone receptor; GC, granulosa cells; hGL5, immortalized human granulosa cells [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Cy treatment immediately induced an AMH decrease due to a reduction in the number and function of FSHR + AMH + GCs
To investigate the immediate effect of Cy on ovarian reserves, mice were injected with Cy 6 times over 2 weeks and subsequently sacrificed at 24 h post‐Cy exposure (Figure 2(A)). The body weights of the Cy‐treated mice decreased during the 2 weeks of Cy administration (Figure 2(B)). Significant ovarian atrophy was also observed in the Cy‐treated mice (Figure 2(C)). Moreover, we observed a decrease in serum AMH levels (Figure 2(D)). Rigorous Cy treatment immediately affects the physiological function of the ovaries. To investigate the cause of the Cy‐induced AMH decrease, GCs were isolated from Cy‐exposed growing follicles and identified by FSHR+ and AMH+ expression in order to analyze their numbers and AMH production by flow cytometry. The number of FSHR+AMH+ GCs per ovary decreased significantly after Cy treatment (Figure 2(E)). Both the FSHR expression and AMH production in the GCs decreased after Cy treatment (Figure 2(F,G)). These results indicate that a decline in the number and AMH‐producing ability of the surviving FSHR+AMH+ GCs is responsible for the low serum AMH levels after Cy exposure.
FIGURE 2.
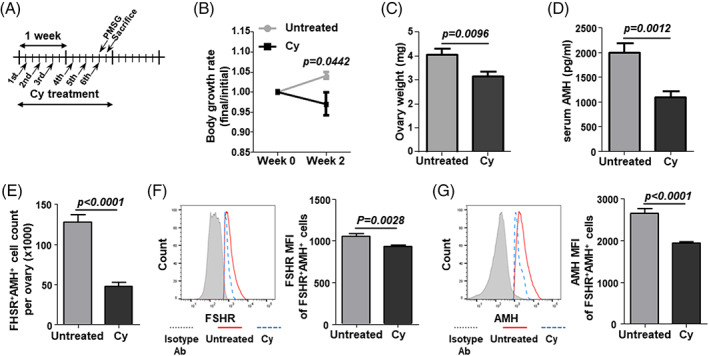
AMH production and number of FSHR+AMH+ GCs at 24 h post‐Cy exposure. (A) Schematic of the in vivo study. Cy (100 mg/kg) was intraperitoneally injected into C57BL/6 mice 6 times over 2 weeks. GCs were isolated from PMSG‐stimulated ovaries at 24 h post‐Cy exposure. (B) Body growth was measured during the 2 weeks of Cy administration. (C) Ovary weight was measured. (D) Serum AMH levels were measured by ELISA. (E) The number of FSHR+AMH+ GCs per ovary were counted using counting beads. (F,G) The mean fluorescence intensity (MFI) of FSHR and AMH in FSHR+AMH+ GCs were calculated using the isotype control antibody‐stained cells (gray line). Untreated: untreated control mice; Cy: cyclophosphamide‐treated mice. Data represent the mean ± s.e.m. (n = 7–8/group). Ab, antibody; AMH, anti‐müllerian hormone; Cy, cyclophosphamide; ELISA, enzyme‐linked immunosorbent assay; FSHR, follicle‐stimulating hormone receptor; GC, granulosa cells; PMSG, pregnant mare's serum gonadotropin [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Cy treatment induced an AMH decrease due to reduction in the number of GCs with intact AMH‐producing ability 4 weeks after Cy exposure
To investigate the delayed effect of Cy on ovarian reserves, mice were injected with Cy 6 times over 2 weeks, then housed for 4 weeks to ensure that Cy affected follicles were replaced with newly growing follicles. The mice were sacrificed at 24 h post‐PMSG injection (Figure 3(A)). The body growth was slower over the 4 weeks after Cy cessation when compared to untreated mice (Figure 3(B)). We observed a significant decrease in the ovarian weight and serum AMH levels (Figure 3(C),(D)). To investigate the cause of the Cy‐induced AMH decrease, the number, and AMH production of FSHR+AMH+ GCs from ovaries 4 weeks after Cy treatment were analyzed by flow cytometry. The number of FSHR+AMH+ GCs had decreased whereas their FSHR expression and AMH production were unchanged compared to the untreated control group (FIGURE 3(E)–(G)). These results indicate that only a decline in the number of surviving FSHR+AMH+ GCs is responsible for the low serum AMH levels 4 weeks after Cy exposure.
FIGURE 3.
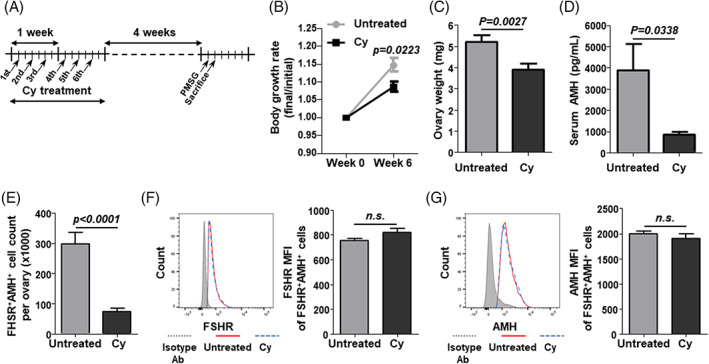
AMH production and number of FSHR+AMH+ GCs at 4 weeks after Cy exposure. (A) Schematic of the in vivo study. Cy (100 mg/kg) was intraperitoneally injected into C57BL/6 mice 6 times over 2 weeks. GCs were isolated from PMSG‐stimulated ovaries at 4 weeks after Cy exposure. (B) Body growth was measured for 6 weeks. (C) Ovary weight was measured. (D) Serum AMH levels were measured by ELISA. (E) The number of FSHR+AMH+ GCs per ovary were counted using counting beads. (F,G) The mean fluorescence intensity (MFI) of FSHR and AMH in FSHR+AMH+ GCs were calculated using the isotype control antibody‐stained cells (gray line). Untreated: untreated control mice; Cy: cyclophosphamide‐treated mice. Data represent the mean ± s.e.m. (n = 8/group). n.s., not significant. Ab, antibody; AMH, anti‐müllerian hormone; Cy, cyclophosphamide; FSHR, follicle‐stimulating hormone receptor; GC, granulosa cells; PMSG, pregnant mare's serum gonadotropin [Color figure can be viewed at wileyonlinelibrary.com]
3.4. In vitro restoration of AMH production but not GC numbers 24 h after Cy exposure
Since the AMH production of GCs was restored 4 weeks after Cy treatment, we performed in vitro experiments using hGL5 cells to investigate the effects of Cy on GCs shortly after Cy exposure. The hGL5 cell line was treated with 4HC for 4 h, after which the number of cells and AMH production was analyzed by flow cytometry (Figure 4(A)). Immediately after treatment, both the number of FSHR+AMH+ hGL5 cells and their AMH production had decreased (Figure 4(B),(C)). Interestingly, the AMH production recovered 24 h after 4HC treatment, while the GC numbers did not (Figure 4(D),(E)). This demonstrates that AMH production can quickly be restored in surviving FSHR+/AMH+ hGL5 cells after Cy exposure.
FIGURE 4.
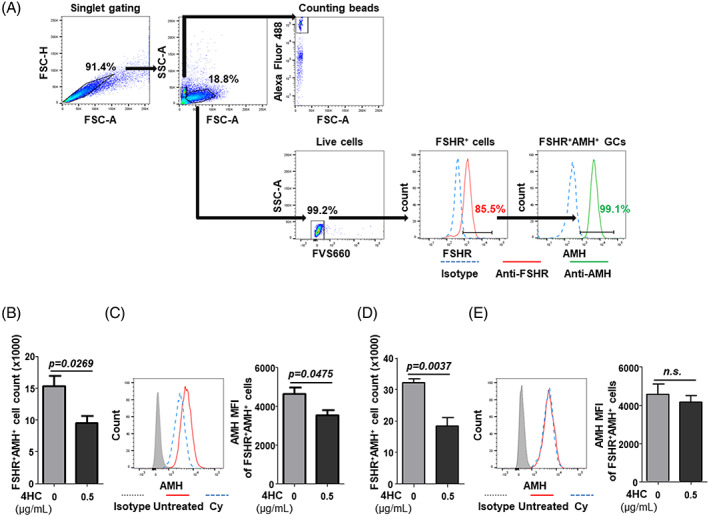
AMH production and number of FSHR+AMH+ hGL5 cells after 4HC treatment. The hGL5 cell line was treated with 4HC for 4 h and analyzed for the number of FSHR+AMH+ live hGL5 cells and their AMH production by flow cytometry. (A) The hGL5 cells were identified based on FSHR expression using isotype control antibody‐stained cells after live cell gating. Counting beads were gated on the forward scatter (FSC)/side scatter (SSC) plot, and Alexa Fluor 488 positivity was used to quantitate GCs. To assess their quality and AMH production, the mean fluorescence intensity (MFI) of FSHR and AMH in FSHR+AMH+ live GCs were analyzed at the single cell level. Isotype antibody‐stained control, blue line; anti‐FSHR antibody‐stained control, red line; anti‐AMH antibody‐stained control, green line. (B) Immediately after 4HC treatment, the number of FSHR+AMH+ live hGL5 cells and (C) mean fluorescence intensity of (MFI) of their AMH expression were calculated using isotype control antibody‐stained cells (gray line). (D) 24 h after 4HC treatment, the number of FSHR+AMH+ live hGL5 cells and (E) mean fluorescence intensity (MFI) of their AMH expression were calculated using the isotype control antibody‐stained cells (gray line). Data represent the mean ± s.e.m. (n = 4/group). n.s., not significant. 4HC, 4‐hydroperoxycyclophosphamide; Ab, antibody; AMH, anti‐müllerian hormone; FSHR, follicle‐stimulating hormone receptor; hGL5, immortalized human granulosa cells [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In 2019, approximately 268,600 American women were newly diagnosed with invasive breast cancer, and the 2% of them who are younger than 40 years of age will have a 40% risk of amenorrhea after chemotherapy, causing significant concerns regarding their future fertility.14, 15 Fertility preservation is an important issue for young women with cancer, and ideally, physicians should recommend options to preserve their fertility, such as the cryopreservation of oocytes, embryos, or ovarian tissues, prior to cancer treatment.16 Women who have already been treated with anticancer drugs display extremely low or undetectable AMH levels, resulting in a decreased fertility reserve that is difficult to recover.17
Interestingly, a case report of a patient with premature ovarian insufficiency (POI) exhibited a slight increase in AMH prior to spontaneous ovulation after chemotherapy for breast cancer, and she became pregnant 3 years later.18 Furthermore, GCs can respond to controlled ovarian hyperstimulation in patients with POI, despite their undetectable AMH levels.19 This phenomenon suggests that GCs surviving chemotherapy could maintain their function in ovulation. This suggests that the ovarian stimulation protocol should be individually flexible regarding the GCs' responses to hormonal stimulation because these protocols affect the incidence of apoptotic GCs, affecting the clinical outcomes of IVF.20, 21 However, studies on the surviving GCs in patients undergoing Cy treatment is lacking, and the method for assessing GCs is still sub‐optimal. To allow for easy or individual analysis of GCs, we constructed a strategy for the identification of GCs obtained from ovarian follicles. Our constructed flow cytometry analysis may be used to easily evaluate and clinically manage GCs.
With flow cytometry analysis, we investigated the immediate or delayed effects of Cy on the number and AMH‐producing ability of GCs after chemotherapy in a mouse model. It is already known that Cy metabolites damage GCs of primordial and growing follicles even after Cy cessation. Our study suggests that the AMH‐producing ability of GCs from Cy‐exposed primordial or primary follicles was intact when they matured into growing follicles even though there was still a low number of GCs at 4 weeks after Cy treatment. In addition, in vitro experiments found that the AMH‐production of FSHR+AMH+ hGL5 cells was restored 24 h after Cy exposure. These results possibly indicate that the restored AMH‐producing ability of GCs is needed to maintain follicle growth by regulation of their own proliferation and growth in vivo. The restoring mechanism was not defined in this study. Gurgen SG et al. reported that oral administration of antioxidants protects Cy‐exposed degenerative ovaries by upregulation of proliferating cell nuclear antigen (PCNA) expression in GCs of developing follicles.22 After Cy treatment exposure ceased, the general endogenous antioxidant system and redox homeostasis could rescue the impaired AMH‐producing ability in damaged GCs of surviving follicles to maintain ovarian reserves and their oocyte growth. Further research on the protection of GCs from Cy exposure should be conducted; protecting GCs during chemotherapy may be important for the increase in serum AMH levels afterward.23, 24 It could also slow the depletion of fertility reserves by inhibiting the recruitment of primordial follicles into the growing follicle pool and prevent the development of POI or early menopause. A clinical study revealed a positive correlation between the cumulative Cy dose received and the incidence of menopause.25 Therefore, physicians should make an effort to manage the amount of Cy administered to younger patients while still maintaining its therapeutic effects.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Jihyun Kim: Conceptualization; data curation; formal analysis; investigation; methodology; resources; writing‐original draft; writing‐review and editing. Sooseong You: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; supervision; writing‐original draft; writing‐review and editing.
Supporting information
MIFlowCyt: MIFlowCyt Item Checklist.
Kim J, You S. After cyclophosphamide exposure, granulosa cells recover their anti‐müllerian hormone‐producing ability but not their numbers. Cytometry. 2021;99:807–813. 10.1002/cyto.a.24297
Funding information Korea Institute of Oriental Medicine, Grant/Award Number: KSN2013240
REFERENCES
- 1.Verma AKRS, Mishra J, Gupta M, Sharma M, Deshmukh G, Ali W. Anti‐mullerian hormone: A marker of ovarian reserve and its association with polycystic ovarian syndrome. J Clin Diagn Res. 2016;10:qc10–2. 10.7860/jcdr/2016/20370.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freour TBP, Masson D. Anti‐mullerian hormone levels and evolution in women of reproductive age with breast cancer treated with chemotherapy. Eur J Cancer. 2017;74:1–8. 10.1016/j.ejca.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RA, Wallace WHB. Antimullerian hormone, the assessment of the ovarian reserve, and the reproductive outcome of the young patient with cancer. Fertil Steril. 2013;99:1469–75. 10.1016/j.fertnstert.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Detti LUR, Lu M, Diamond MP, Saed GM, Fletcher NM, Zhang J, et al. Serum markers of ovarian reserve and ovarian histology in adult mice treated with cyclophosphamide in pre‐pubertal age. J Assist Reprod Genet. 2013;30:1421–9. 10.1007/s10815-013-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durlinger ALKP, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti‐mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–96. 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 6.Sonigo C, Beau I, Grynberg M, Binart N. AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide‐treated mice. FASEB J. 2019;33:1278–87. 10.1096/fj.201801089R. [DOI] [PubMed] [Google Scholar]
- 7.Ganesan SKA. Phosphoramide mustard exposure induces DNA adduct formation and the DNA damage repair response in rat ovarian granulosa cells. Toxicol Appl Pharmacol. 2015;282:252–8. 10.1016/j.taap.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai‐Turton MLB, Tan Y, Luderer U. Cyclophosphamide‐induced apoptosis in COV434 human granulosa cells involves oxidative stress and glutathione depletion. Toxicol Sci. 2007;98:216–30. 10.1093/toxsci/kfm087. [DOI] [PubMed] [Google Scholar]
- 9.Luan YEM, Woodruff TK, Kim SY. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J Endocrinol. 2019;240:243–56. 10.1530/joe-18-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen QNZN, Liew SH, Findlay JK, Hickey M, Hutt KJ. Cisplatin‐ and cyclophosphamide‐induced primordial follicle depletion is caused by direct damage to oocytes. Mol Hum Reprod. 2019;25:433–44. 10.1093/molehr/gaz020. [DOI] [PubMed] [Google Scholar]
- 11.Liu JZW. Methods for evaluation of ovarian granulosa cells with exposure to nanoparticles. Methods Mol Biol. 1894;2019:73–81. 10.1007/978-1-4939-8916-4_5. [DOI] [PubMed] [Google Scholar]
- 12.Clavero ACJ, Núñez AI, García‐Peña ML, Maldonado V, Fontes J, Mendoza N, et al. Apoptosis in human granulosa cells after induction of ovulation in women participating in an intracytoplasmic sperm injection program. Eur J Obstet Gynecol Reprod Biol. 2003;110:181–5. 10.1016/s0301-2115(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 13.Kossowska‐Tomaszczuk KDGC, De Geyter M, Martin I, Holzgreve W, Scherberich A, Zhang H. The multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells. 2009;27:210–9. 10.1634/stemcells.2008-0233. [DOI] [PubMed] [Google Scholar]
- 14.Amercian Cancer Society . In: 2019‐2020 BCFF, editor. Atlanta: Amercian Cancer Society, Inc; 2019.
- 15.Hulvat MC, Jeruss JS. Maintaining fertility in young women with breast cancer. Curr Treat Options Oncol. 2009;10:308–17. 10.1007/s11864-010-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahajan N. Fertility preservation in female cancer patients: an overview. J Hum Reprod. 2015;8:3–13. 10.4103/0974-1208.153119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peigne MDC. Serum AMH level as a marker of acute and long‐term effects of chemotherapy on the ovarian follicular content: A systematic review. Reprod Biol Endocrinol. 2014;12:26. 10.1186/1477-7827-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakimoto YFA, Wakimoto G, Ikezawa Y, Matsuoka M, Omote M, Sugiyama Y, et al. Association between spontaneous ovulation and serum anti‐mullerian hormone levels in a premature ovarian insufficiency patient after a multimodal treatment for breast cancer. J Obstet Gynaecol Res. 2019;45:2297–301. 10.1111/jog.14101. [DOI] [PubMed] [Google Scholar]
- 19.Maclaran KPN. Current concepts in premature ovarian insufficiency. Womens Health (Lond). 2015;11:169–82. 10.2217/whe.14.82. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko TSH, Takahashi T, Ohta N, Saito T, Hiroi M. Effects of controlled ovarian hyperstimulation on oocyte quality in terms of the incidence of apoptotic granulosa cells. J Assist Reprod Genet. 2000;17:580–5. 10.1023/a:1026439409584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brannian JEK, Mueller BA, Bietz MG, Hansen K. Differential gene expression in human granulosa cells from recombinant FSH versus human menopausal gonadotropin ovarian stimulation protocols. Reprod Biol Endocrinol. 2010;8:25. 10.1186/1477-7827-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gürgen SGED, Elmas C, Kaplanoğlu GT, Ozer C. Chemoprotective effect of ascorbic acid, α‐tocopherol, and selenium on cyclophosphamide‐induced toxicity in the rat ovarium. Nutrition. 2013;29:777–84. 10.1016/j.nut.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 23.van Houten ELTA, Visser JA. Anti‐mullerian hormone (AMH): Regulator and marker of ovarian function. Ann Endocrinol (Paris). 2010;71(3):191–197. 10.1016/j.ando.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 24.La Marca AVA. Anti‐mullerian hormone (AMH) in female reproduction: Is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf). 2006;64:603–10. 10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- 25.Maltaris TWM, Mueller A, Schmidt M, Seufert R, Fischl F, Koelbl H, et al. Cancer and fertility preservation: Fertility preservation in breast cancer patients. Breast Cancer Res. 2008;10:206. 10.1186/bcr1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MIFlowCyt: MIFlowCyt Item Checklist.


