Abstract
Background
Almonds are a rich source of phenolic and polyphenolic compounds, which have antioxidant activity. In vitro and in vivo studies have demonstrated that topical application of almond oil and almond skin extract reduces UVB‐induced photoaging. Ultraviolet‐B (UVB) protection by oral almond consumption has not been previously studied in humans.
Objectives
To investigate whether oral almond consumption can increase resistance to UVB radiation and reduce skin aging in healthy Asian women.
Methods
Thirty‐nine female participants (18‐45 years) with Fitzpatrick skin type II‐IV were randomly assigned to consume either 1.5 oz of almonds or 1.8 oz of pretzels daily for 12 weeks. Minimal erythema dose (MED) was determined using a standardized protocol, which determined the minimal radiation needed to induce erythema on the inner arm following UVB exposure. Facial skin texture was evaluated by two dermatologists using the Clinician's Erythema Assessment scale and Allergan Roughness scale. Facial melanin index, hydration, sebum, and erythema were determined using a cutometer.
Results
The MED was increased in the subjects consuming almonds compared to the control group consuming pretzels. There were no differences noted between the groups consuming almonds versus pretzels in Allergan roughness, melanin, hydration, or sebum on facial skin.
Conclusions
Our findings suggest that daily oral almond consumption may lead to enhanced protection from UV photodamage by increasing the MED.
Keywords: almond, facial skin texture, minimal erythema dose, randomized controlled trial, UVB skin exposure
1. INTRODUCTION
Several intrinsic and extrinsic factors affect skin radiance, skin aging, and formation of wrinkles. It has been suggested that extrinsic skin aging manifests differently in Caucasians compared to East Asians.1 In particular, previous studies have suggested that Caucasians are more likely to develop wrinkles, whereas East Asians are more likely to exhibit pigment spot formation. Ultraviolet light (UV) radiation appears to play a key role in pigment changes occurring with age, the major sign of skin photoaging in Asians.2
Skin susceptibility to photoaging also depends on constitutive pigmentation and skin color type, with Caucasians and Asians being more sensitive to photoaging.2 A common cause of both hyperpigmentation and photoaging is exposure to UV light such as UVA (320‐400 nm) and UVB (290‐320 nm). Overexposure of the skin to UVA or UVB generates oxidative stress, which leads to inflammation and erythema of the skin.3, 4, 5 Therefore, it is crucial to find more ways to protect against UV damage, in addition to wearing protective clothing and applying topical sunscreens.
Almonds (Prunus dulcis) belong to the family Rosaceae and are the most widely produced tree nut on a global basis, with the United States and specifically California, as the major producer.4, 6 Almonds are nutrient‐dense and provide an excellent source of vitamin E, β‐sitosterol, squalene, manganese, magnesium, copper, fiber, riboflavin, and protein.7, 8, 9 Almonds are a good source of the mono and polyunsaturated fatty acids oleic and linoleic acids.10 Additionally, recent studies have shown that almonds, specifically their skins, are a rich dietary source of phenolic and polyphenolic compounds, which are believed to account for a major portion of their antioxidant capacity.9, 11, 12 In vitro and in vivo studies on almond skins have reported antioxidant and anti‐inflammatory properties. An almond‐enriched high monounsaturated fat diet reduced some markers of inflammation including C‐reactive protein.13 Other studies have shown that almond consumption can reduce markers of oxidative stress and inflammation in healthy individuals, male smokers and patients with type 2 diabetes.14, 15, 16
In addition, limited animal and in vitro studies have demonstrated that topical application of almond oil in mice and almond skin extract in a 3‐D human skin in vitro model exposed to UV radiation ameliorated and partially inhibited some of the histologic damage associated with photoaging of skin.17, 18 However, Ultraviolet‐B (UVB) protection by oral almond consumption has not been clinically studied in humans.
We investigated whether daily almond consumption compared to pretzel consumption for twelve weeks provide protection from UVB irradiation, reduce photodamage, or improve skin texture in healthy Asian women.
2. MATERIALS AND METHODS
2.1. Subjects
Thirty‐nine healthy women completed the study. Inclusion criteria were the following: 18‐45 years of age, female, Asian, nonsmokers, Fitzpatrick skin type II‐IV with a BMI between 18 and 30 kg/m2. Exclusion criteria were the following: pregnant, use of skin‐related prescription medication or supplements, or use of cosmetic agents with skin lightening/whitening. Throughout the study, participants were instructed to avoid eating almonds or pretzels outside of the study protocol, or any polyphenol‐rich foods (eg, strawberries, raspberries). The clinical protocol was approved by the Institutional Review Board. All subjects gave written informed consent prior to the start of the study.
2.2. Study design
At baseline (2 weeks after screening), participants were randomized to one of two groups: 1) one serving of almonds per day (1.5 oz, 246 kcal) (n = 18) or 2) one serving of pretzels (1.8 oz, 200 kcal) (n = 21) daily for 12 weeks. Participants were provided with a monthly supply of study product. Participants were supplied with either almonds or pretzels at each study visit and compliance was assessed. Height, weight, Fitzpatrick skin type, and skin characteristics (melanin, hydration, sebum, erythema, and texture), were evaluated at baseline, and at weeks 4, 8, and 12. Minimal erythema dose (MED) was evaluated on the inner arm at baseline and 12 weeks.
2.3. Study outcomes
The primary outcome of this intervention study was the quantification of the MED of UVB exposure to inner arm skin. Secondary outcomes were Allergan roughness, erythema, melanin, hydration, and sebum in facial skin.
2.4. Inner arm minimal erythema dose (MED)
We determined the lowest dose of UVB radiation capable of inducing erythema (MED) as described in detail in our previous publication.19 The MED was determined for each subject before (week 0) and after (week 12) intervention. Prior to testing, the skin type was evaluated based on the Fitzpatrick Skin Type scale. Asian skin type ranges from III‐IV, and therefore participants with Fitzpatrick skin type II – IV were included in the study.20 Background erythema (T0) was measured in all test areas before treatment using a mexameter probe attached to Cutometer dual MPA 580 (Courage&Khazaka Electronic GmbH). Skin UVB dose and treatment time were determined based on overall skin type classification. Using the dosing guideline for narrow band ultraviolet‐B (NB‐UVB) and the National Biological UVB mJ chart, we determined the sequential exposure times for each skin patch. A sleeve with 6 cut out patches was placed on the subjects arm. Using the Dermalight 90 handheld device (National Biological) the test area on the inner arm of subjects was irradiated with a defined dose of NB‐UVB light delivered by the UV radiation between 310 and 313 nm, peaking at 310 nm from a fluorescent UVB lamp (Philips TL20 W/12). The lamp is equipped with a guard of about 2 inches. The lamp guard was directly placed on the sleeve with the 6 cut out patches, keeping the lamp always at the same distance to the skin. Depending on skin type, a dose range of 260‐555 mJ/cm2 for a time of 100‐290 seconds selected from the National Biological UVB mJ chart was used. This dose range is safe and typically used in other studies and for treatment of skin conditions like psoriasis and vitiligo.21 To evaluate MED the subjects returned to the clinic 24 hours later and a photograph was taken to determine which skin patch showed minimal erythema (pink color). The lowest dose and time of the occurrence of pink color were determined and used as MED.
2.5. Facial erythema and allergan roughness evaluation
Photos of the face with standardized lighting taken at baseline and 12‐week visit were reviewed by two physicians. The Clinician's Erythema Assessment (CEA) was used to measure erythema; this validated tool applies a scale of 0‐4 (0 = clear skin with no signs of erythema, 1 = almost clear and slight redness, 2 = mild erythema and definite redness, 3 = moderate erythema and marked redness, and 4 = severe erythema and fiery redness).22, 23 Additionally, the Allergan Skin Roughness Scale was used to assign a grade from none (0) to extreme (4) that described the severity of skin coarseness, crosshatching, and elastosis in the midface area based on the photographs in Supporting Information S1.24 Each physician completed photo review independently and submitted their scores to the study coordinator.
2.6. Facial Melanin Index, hydration, and sebum evaluation
Melanin index, hydration, and sebum on facial skin surface were evaluated at baseline (prior to almond intake), visit 3 (4 weeks of intervention), visit 4 (8 weeks of intervention), and visit 5 (12 weeks of intervention) using the mexameter MA18, corneometer CM825, and sebumeter SM815 probes attached to the Cutometer dual MPA 580 (Courage&Khazaka Electronic GmbH). Sebumeter SM 815 uses the difference of light intensity through a plastic strip to indicate the amount of absorbed sebum. The sebum level is expressed in μg/cm2. Corneometer CM 825 uses the high dielectric constant of water for analyzing the water‐related changes in the electrical capacitance of the skin. It displays hydration measurements in system‐specific arbitrary units. A melanin index is calculated by Mexameter MX 18 from the strength of the absorbed and the reflected light at, respectively, 660 and 880 nm. An erythema index is processed similarly at, respectively, 568 and 660 nm.
2.7. Statistical evaluation
The sample size per treatment group was calculated based on our previously published data investigating the effect of pomegranate consumption on MED.19 Linear Mixed effects model was used to analyze the repeated measurements within subjects and to evaluate the change within and between almond and pretzel control groups. Within the intervention groups, the percentage change of each outcome between two time points (baseline to 12 weeks) was compared.
3. RESULTS
3.1. Characteristics of participants
Thirty‐nine participants completed the study. Ten participants were omitted from data analysis because five of the almond participants and four of the pretzel participants did not develop redness on the inner arm after UV irradiation, and deemed UV resistant at the dose and exposure time selected. One participant did not finish the final assessment. We therefore present data from 29 participants (almond N = 13, pretzel N = 16). There were no statistically significant differences at baseline for age, height, weight, or BMI (Table 1). All participants identified as Asian. In the almond group, there were more participants with skin type IV; this difference was not statistically significant. Since dose and timing of UVB exposure were adjusted to skin type, we did not anticipate that this difference between almond and pretzel group would have an effect on the study outcome. No adverse effects were reported. There was no difference in compliance with almond/pretzel consumption between the two groups (Table 2).
TABLE 1.
Baseline demographics of study participants included in the final analysis
| Almond (n = 13) | Control (n = 16) | |
|---|---|---|
| Age (years) | 27.5 ± 6.3 | 28.4 ± 8.7 |
| Height (inches) | 63.2 ± 2.5 | 63.1 ± 2.0 |
| Weight (lbs) | 130.2 ± 16.5 | 125.5 ± 16.5 |
| BMI | 22.9 ± 2.4 | 22.1 ± 2.42 |
| Ethnicity | ||
| Cambodian | 2 (15) | 0 (0) |
| Chinese | 4 (31) | 6 (38) |
| Filipino | 2 (15) | 1 (6) |
| Korean | 2 (15) | 5 (31) |
| Taiwanese | 0 (0) | 1 (6) |
| Vietnamese | 1 (8) | 1(6) |
| Bi‐racial | 2 (15) | 2 (13) |
| Skin type | ||
| III | 4 (31) | 8 (50) |
| IV | 9 (69) | 8 (50) |
Data are means ± SD. Ethnicity, numbers in parenthesis are percentage.
TABLE 2.
Almond/Pretzel consumption compliance at different time points
| Almond (n = 13) | Control (n = 16) | |
|---|---|---|
| Compliance Week 4 | 96.3 ± 5.1 | 97.2 ± 2.8 |
| Compliance Week 8 | 97.0 ± 4.6 | 94.4 ± 6.9 |
| Compliance Week 12 | 97.0 ± 4.8 | 95.6 ± 6.9 |
Data are means ± SD.
3.2. Effects of almond consumption on minimal erythema dose
At baseline, there was no significant difference in MED between the groups (Figure 1). For the women who consumed almonds, we observed a significant increase in MED from 415 ± 64 to 487 ± 59 (18.7 ± 19.2%, P = .006) from baseline to week 12 compared to women in the pretzel group from 415 ± 67 to 421 ± 67 (1.8 ± 11.1%) (Figure 1). The exposure time to reach minimal erythema was also increased significantly in the almond group from 160 ± 23 to 187 ± 25 (17.5 ± 22.2%) compared to the pretzel group from 165 ± 27 to 166 ± 25 (1.7 ± 14%) (p‐0.026) (Figure 1).
FIGURE 1.
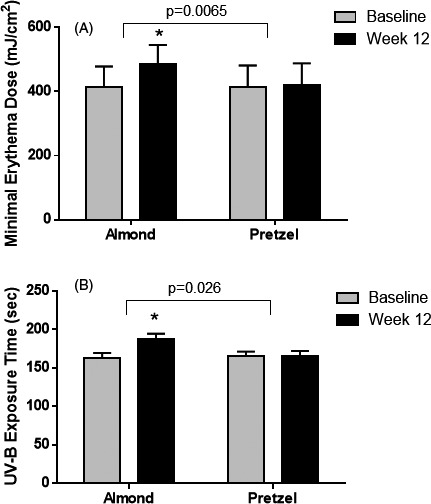
Minimal erythema dose in healthy Asian women consuming almond or pretzels for 12 weeks. Data are means ± SD. *compared to baseline (P ≤ .05); p‐value indicates comparison between almond and pretzel group; almond N = 13, pretzel N = 16
3.3. Effects of almond consumption on erythema and allergan roughness
The facial photos at the baseline and final visits were evaluated independently by two dermatologists using an erythema and Allergan roughness scale (Figure S1). There was no significant difference observed between groups (Figure 2).
FIGURE 2.
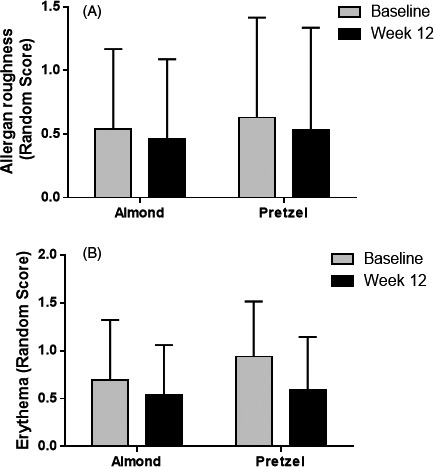
Effect of almond consumption on facial erythema and Allergan roughness compared to pretzel consumption at baseline and week 12. Data are means ± SD; almond N = 13, pretzel N = 16
3.4. Melanin index, erythema, sebum, and hydration
There were also no significant differences in melanin index, erythema, sebum, or hydration determined by cutometer reading (Table 3).
TABLE 3.
Effect of almond consumption on facial skin characteristics (hydration, melanin content, erythema, sebum)
| Time Period | Almond (n = 13) | Control (n = 16) | |
|---|---|---|---|
| Hydration (RU) | Baseline | 60.75 (13.01) | 69.74 (8.78) |
| Week 4 | 63.56 (13.12) | 68.45 (12) | |
| Week 8 | 58.57 (12.89) | 66.98 (10.51) | |
| Week 12 | 58.45 (12.33) | 62.41 (12.17) | |
| Melanin Content (RU) | Baseline | 268.65 (51.56) | 247.13 (50.49) |
| Week 4 | 284.44 (42.67) | 261.79 (39.25) | |
| Week 8 | 264.46 (38.65) | 257.37 (36.87) | |
| Week 12 | 257.28 (35.94) | 247.76 (34.4) | |
| Erythema | Baseline | 346.53 (54.97) | 364.89 (70.51) |
| Week 4 | 357.7 (61.07) | 368.25 (69.13) | |
| Week 8 | 366.13 (49.59) | 371.22 (66.02) | |
| Week 12 | 356.7 (55.58) | 383.06 (66.32) | |
| Sebum | Baseline | 68 (31.58) | 77.21 (37.3) |
| Week 4 | 61.84 (40.1) | 67.59 (35.96) | |
| Week 8 | 57.17 (33.84) | 67.83 (34.85) | |
| Week 12 | 46.78 (28.36) | 60.48 (30.31) |
Data are means ± SD.
4. DISCUSSION
We found that daily consumption of 1.5 ounces of raw almonds improved UVB resistance in Asian female inner arm skin as higher doses of UVB and longer exposure times were required to induce erythema after 12 weeks of almond compared to pretzel consumption. These findings are supported by previous preclinical studies in which topical almond oil and almond skin extract were able to improve protection from UVB‐induced photodamage in mice and in an in vitro 3D human skin model.17, 18 This current study now extends these in vitro observations to humans and provides the first clinical evidence that oral intake of almonds can protect against UVB radiation. Our study did not show any change in other skin markers including Allergan roughness, erythema, melanin index, sebum, or hydration. A recent study by Foolad et al found a decrease in wrinkle severity and width after 16 weeks of almonds consumed at a dose of 20% of daily energy consumption (2.1 oz/340 kcal) in postmenopausal females.24 However, no improvement in skin barrier function was found.
Although we observed an improvement in UV resistance in the almond group, the precise mechanism for this photoprotection of human skin by consuming almonds is currently unknown. However, given the fact that almonds are rich in mono and polyunsaturated fatty acids, vitamin E, quercetin, and other phenolic and polyphenolic compounds, daily consumption of almonds may improve the antioxidant and anti‐inflammatory capacity of human skin, which could be responsible for the increased photoprotection against UVB radiation,6, 11, 12, 16 since UVB radiation is known to induce cellular DNA damage through an oxidative stress response.25
This study demonstrated that almond consumption increased the UBV‐induced MED by about 20%. Skin photodamage is induced by UVB and UVA exposure. However, we did not investigate whether almond consumption protects from UVA‐induced photodamage or damage from general sunlight. Therefore, almond consumption can contribute to UVB‐photoprotection, but does not replace additional sun protection methods such as the application of sunscreen or wearing protective closing.
The skin is the biological barrier against an array of chemical and physical pollutants (eg, UV light, ozone, etc). Most of the effort to protect the skin has been focused on topical application of antioxidants, including vitamins C and E, carotenoids, resveratrol, and pycnogenol. Our prior study has demonstrated that oral consumption of pomegranate juice with strong antioxidant capability can protect skin from UVB damage.19
The current pilot study has shown that almonds, another antioxidant‐rich food can protect the skin as well. It is essential to combine topical skin care with a healthy diet rich in antioxidant compounds to protect against outdoor stressor‐induced skin damage, including the damage associated with aging.
There were some limitations of this study. This study discussed the effect of almond consumption on protection from UVB radiation and not sunlight in general nor UVA exposure. The study population used for the final analysis was limited once we omitted those who were UV resistant. In future studies, the absence of erythema after the baseline UVB exposure should be an exclusion criterion. The subjects in the presented study were under 30 years of age and the amount of photodamage occurring may have been too low to permit the detection of changes in markers of photoaging. Future research should extend the study population to other ethnicities and older subjects with moderate to severe photodamaged skin.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
JNL recruited participants, performed skin tests and drafted the manuscript, SMH evaluated the data and prepared manuscript, GT attained IRB approval and provided administrative assistance, OB and OT performed visual Allergan Roughness test and evaluation of erythema by applying the Clinician's Erythema Assessment, CHT performed statistical analysis, JK designed study, supervised skin evaluation tests and contributed to writing, DH and ZL designed study and reviewed manuscript. All authors read and approved the final manuscript.
Supporting information
Figure S1
ACKNOWLEDGMENTS
Funding was provided by the Almond Board of California.
Li JN, Henning SM, Thames G, et al. Almond consumption increased UVB resistance in healthy Asian women. J Cosmet Dermatol. 2021;20:2975–2980. 10.1111/jocd.13946
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Vierkotter A, Huls A, Yamamoto A, et al. Extrinsic skin ageing in German, Chinese and Japanese women manifests differently in all three groups depending on ethnic background, age and anatomical site. J Dermatol Sci. 2016;83(3):219‐225. [DOI] [PubMed] [Google Scholar]
- 2.Battie C, Jitsukawa S, Bernerd F, Del Bino S, Marionnet C, Verschoore M. New insights in photoaging, UVA induced damage and skin types. Exp Dermatol. 2014;23(Suppl 1):7‐12. [DOI] [PubMed] [Google Scholar]
- 3.Smit N, Vicanova J, Pavel S. The hunt for natural skin whitening agents. Int J Mol Sci. 2009;10(12):5326‐5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishigori C, Hattori Y, Arima Y, Miyachi Y. Photoaging and oxidative stress. Exp Dermatol. 2003;12(Suppl 2):18‐21. [DOI] [PubMed] [Google Scholar]
- 5.Cadet J, Douki T, Ravanat JL. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem Photobiol. 2015;91(1):140‐155. [DOI] [PubMed] [Google Scholar]
- 6.Wijeratne SS, Abou‐Zaid MM, Shahidi F. Antioxidant polyphenols in almond and its coproducts. J Agric Food Chem. 2006;54(2):312‐318. [DOI] [PubMed] [Google Scholar]
- 7.Alasalvar C, Bolling BW. Review of nut phytochemicals, fat‐soluble bioactives, antioxidant components and health effects. Br J Nutri. 2015;113(Suppl 2):S68‐S78. [DOI] [PubMed] [Google Scholar]
- 8.Milbury PE, Chen CY, Dolnikowski GG, Blumberg JB. Determination of flavonoids and phenolics and their distribution in almonds. J Agric Food Chem. 2006;54(14):5027‐5033. [DOI] [PubMed] [Google Scholar]
- 9.Bolling BW, Dolnikowski G, Blumberg JB, Oliver Chen CY. Quantification of almond skin polyphenols by liquid chromatography‐mass spectrometry. J Food Sci. 2009;74(4):C326‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguire LS, O'Sullivan SM, Galvin K, O'Connor TP, O'Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004;55(3):171‐178. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52(12):4026‐4037. [DOI] [PubMed] [Google Scholar]
- 12.Gu L, Kelm MA, Hammerstone JF, et al. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutri. 2004;134(3):613‐617. [DOI] [PubMed] [Google Scholar]
- 13.Rajaram S, Connell KM, Sabate J. Effect of almond‐enriched high‐monounsaturated fat diet on selected markers of inflammation: a randomised, controlled, crossover study. Br J Nutri. 2010;103(6):907‐912. [DOI] [PubMed] [Google Scholar]
- 14.Liu JF, Liu YH, Chen CM, Chang WH, Chen CY. The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: a randomized crossover controlled feeding trial. Eur J Nutr. 2013;52(3):927‐935. [DOI] [PubMed] [Google Scholar]
- 15.Li N, Jia X, Chen CY, et al. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. J Nutri. 2007;137(12):2717‐2722. [DOI] [PubMed] [Google Scholar]
- 16.Chen CO, Milbury PE, Blumberg JB. Polyphenols in Almond skins after blanching modulate plasma biomarkers of oxidative stress in healthy humans. Antioxidants. 2019;8(4):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sultana Y, Kohli K, Athar M, Khar RK, Aqil M. Effect of pre‐treatment of almond oil on ultraviolet B‐induced cutaneous photoaging in mice. J Cosmet Dermatol. 2007;6(1):14‐19. [DOI] [PubMed] [Google Scholar]
- 18.Evans‐Johnson JA, Garlick JA, Johnson EJ, Wang XD, Oliver Chen CY. A pilot study of the photoprotective effect of almond phytochemicals in a 3D human skin equivalent. J Photochem Photobiol B. 2013;126:17‐25. [DOI] [PubMed] [Google Scholar]
- 19.Henning SM, Yang J, Lee RP, et al. Pomegranate juice and extract consumption increases the resistance to UVB‐induced erythema and changes the skin microbiome in healthy women: a randomized controlled trial. Sci Rep. 2019;9(1):e14528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye J, Huang H, Luo G, et al. NB‐UVB irradiation attenuates inflammatory response in psoriasis. Dermatol Ther. 2020;33(4):e13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan J, Liu H, Leyden JJ, Leoni MJ. Reliability of clinician erythema assessment grading scale. J Am Acad Dermatol. 2014;71(4):760‐763. [DOI] [PubMed] [Google Scholar]
- 22.Eichenfield LF, Del Rosso JQ, Tan JKL, et al. Use of an alternative method to evaluate erythema severity in a clinical trial: difference in vehicle response with evaluation of baseline and postdose photographs for effect of oxymetazoline cream 1.0% for persistent erythema of rosacea in a phase IV study. Br J Dermatology. 2019;180(5):1050‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donofrio L, Carruthers A, Hardas B, et al. Development and validation of a photonumeric scale for evaluation of facial skin texture. Dermatol Surg. 2016;42(Suppl 1):S219‐S226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foolad N, Vaughn AR, Rybak I, et al. Prospective randomized controlled pilot study on the effects of almond consumption on skin lipids and wrinkles. Phytother Res. 2019;33(12):3212‐3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swindells K, Rhodes LE. Influence of oral antioxidants on ultraviolet radiation‐induced skin damage in humans. Photodermatol Photoimmunol Photomed. 2004;20(6):297‐304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


