ABSTRACT
Background
It remains unclear whether and how the isolated rapid eye movement (REM) sleep behavior disorder (iRBD)‐related metabolic pattern (RBDRP) changes with disease progression in iRBD.
Objective
To examine longitudinal changes in RBDRP expression in iRBD patients and to explore trajectories of relative metabolic activities of individual brain regions constituting RBDRP.
Methods
In this cohort study, 25 iRBD patients (mean age [±standard deviation], 69.2 ± 5.3 years; 12 [48%] patients were men) and 24 age‐matched healthy controls were included. The patients underwent at least two 18F‐fluorodeoxyglucose positron emission tomography scans at baseline and at the 2‐year and/or 4‐year follow‐ups. We measured the RBDRP expression of the patients and controls which was validated by reproduction in a separate iRBD cohort (n = 13).
Results
At baseline, the RBDRP expression discriminated iRBD patients from healthy controls. However, the RBDRP expression z scores tended to decrease over time in the patients, especially with longer follow‐ups, and this tendency was observed even in patients with high‐risk of phenoconversion. Furthermore, the degree of RBDRP expression at baseline did not predict the disease conversion. The RBDRP breakdown was mainly provoked by the attenuation of relative hypermetabolism in the frontal cortex including premotor areas and relative hypometabolism in the occipital cortex. The putaminal metabolic activity increased steadily with the disease progression.
Conclusions
The RBDRP expression in iRBD patients was altered significantly over time. Some of the brain metabolic changes seem to represent attempted functional compensation against ongoing neurodegeneration. The RBDRP expression measurement at one time point may not be a reliable biomarker for predicting disease conversion. © 2021 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: REM sleep behavior disorder, RBD, metabolic, α‐synucleinopathy, PET
Isolated RBD (iRBD) has been widely recognized as a prodromal stage of α‐synucleinopathies, such as Parkinson's disease (PD), dementia with Lewy bodies (DLB), and less frequently multiple system atrophy (MSA).1 Longitudinal studies have revealed that over 80% of iRBD patients develop an overt α‐synucleinopathy in a decade or more.2, 3 These observations provide an opportunity to detect the earliest stages of the disease and to investigate the efficacy of early neuroprotective strategies. However, individual risk of subsequent phenoconversion varies among iRBD patients.4 In this regard, searching for sensitive predictors of phenoconversion not only enables the stratification of the patients in future neuroprotective trials but also increases our understanding of the underlying pathomechanisms.
Numerous studies have tried to establish neuroimaging markers to identify neurodegenerative changes in iRBD patients.5 One potential candidate neuroimaging marker is the disease‐specific metabolic brain network that is known as the RBD‐related metabolic pattern (RBDRP) derived from the iRBD patients.6, 7, 8 Previous studies using 18F‐fluorodeoxyglucose (18F‐FDG) positron emission tomography (PET) have shown that the RBDRP is typically characterized by relative metabolic increases in the frontal cortex, hippocampus, and pons, and by metabolic decreases in occipital and temporal cortices, although there are some inconsistencies in topographic patterns across the studies.6, 7, 8 However, neurodegeneration in iRBD is an ongoing process, and accordingly brain metabolic patterns might change over time. No longitudinal studies have yet assessed the temporal evolution of the RBDRP in iRBD patients; thus, it remains unclear whether and how the RBDRP expression changes with disease progression in iRBD.
The main aim of the current study was to determine the longitudinal change in RBDRP expression over 4 years of follow‐up in iRBD patients. In addition, we explored the progression of relative metabolic activities of individual brain regions constituting RBDRP.
Methods
Study Design and Participants
We constructed a prospective iRBD cohort between 2013 and 2015 in which all patients were required to be free of parkinsonism/dementia. The diagnosis of RBD was confirmed by video‐polysomnography based on the second edition of the International Classification of Sleep Disorders. The exclusion criteria were set to rule out secondary causes for RBD, as described previously.9 Healthy controls with a similar age distribution to that of the patients were also recruited during the study period. In this cohort, 28 iRBD patients and 24 healthy controls underwent 18F‐FDG PET after enrollment, and their baseline data were presented in our previous study.8 In the present study, we additionally collected 18F‐FDG PET data from the patients at 2‐year and/or 4‐year follow‐ups, and subsequently excluded 3 patients who had no follow‐up data on 18F‐FDG PET. No controls were excluded because we used their baseline data only. Finally, a total of 25 iRBD patients and 24 healthy controls were included for this analysis.
Clinical and dopamine transporter (DAT) imaging variables assessed at baseline and the follow‐ups are described in Appendix S1. In the current study, the high risk of phenoconversion at baseline was defined when a patient had three or more risk factors among mild parkinsonian sign (MPS),10 constipation,9 mild cognitive impairment,11 hyposmia,9 and reduced DAT activity.12 To identify disease phenoconversion, we followed the iRBD patients on a quarterly basis for a maximum of 7 years' observation period. The phenoconversion was defined as the development of PD, DLB, or MSA.
This study was registered at clinicaltrials.gov (NCT02984137), and the study protocol was approved by the Institutional Review Board of our institution (No. 16‐2013‐101). All subjects provided written informed consent before enrolling in this study, in accordance with the Declaration of Helsinki.
FDG PET Image Analysis
18F‐FDG PET was performed using a PET/computed tomography (CT) (Philips Gemini TF‐64 PET/CT scanner, Philips Healthcare, Best, The Netherlands). Detailed imaging protocols have been described elsewhere.8 To identify a spatial covariance pattern of iRBD, we applied an automated algorithm according to the scaled subprofile modeling/principal component analysis method,13 which was done using ScAnVP 7.0w package running on MATLAB 7.11 (MathWorks, Natick, MA). The RBDRP was determined among the principal components (PCs) explaining the top 50% of the total variance in the dataset. We included subject scores for the selected PCs both singularly and in all possible combinations in a series of linear regression models. The model with the lowest Akaike information criterion value was selected to represent the disease‐related pattern. Detailed protocols are described in Appendix S2. Our RBDRP expression was validated by reproduction in a separate polysomnography‐confirmed iRBD cohort (n = 13; mean age [standard deviation], 71.8 [7.5] years; 7 [54%] patients were men) (Fig. 1A). To better explain the longitudinal changes in the RBDRP expression, we additionally obtained the Parkinson's disease‐related metabolic pattern (PDRP) expression in our iRBD patients (Appendix S3).
FIG. 1.
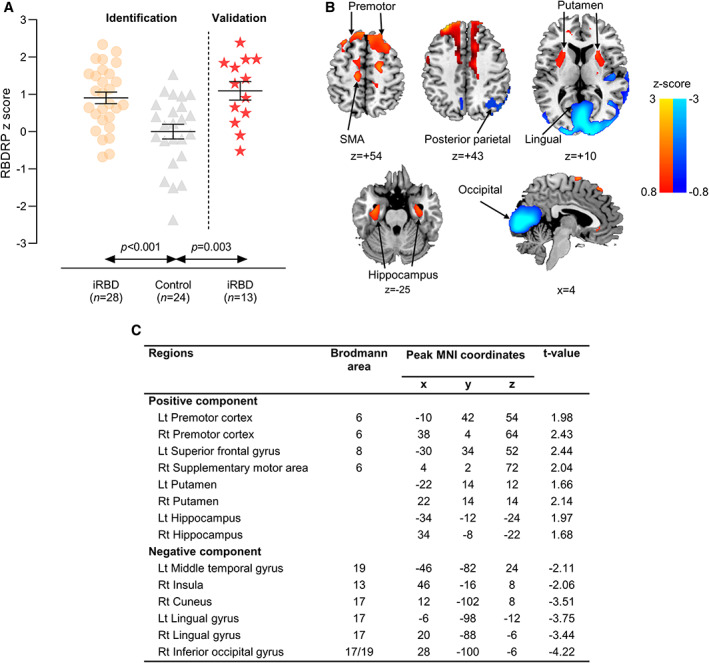
Rapid eye movement (REM) sleep behavior disorder‐related metabolic pattern (RBDRP) identified by network analysis of 18F‐fluorodeoxyglucose positron emission tomography (18F‐FDG PET) (modified from Yoon et al, 20198) and its validation in a separate isolated RBD (iRBD) cohort (n = 13). (A) Bars represent the mean values, and T bars indicate the standard errors. P values were calculated by the Mann–Whitney U test. (B) Voxels with warm colors represent positive regions (metabolic increases) and those with cool colors denote negative regions (metabolic decreases). (C) The regions contribute significantly (P < 0.05) to the topography of RBDRP according to the bootstrapping resample procedure. SMA, supplementary motor area; MNI, Montreal Neurological Institute; Lt, left; Rt, right. [Color figure can be viewed at wileyonlinelibrary.com]
For the subregional metabolic activity analysis, the 18F‐FDG PET images were scaled to the global mean activity to measure relative regional metabolism. To do this, we used a third version of the automated anatomical labeling atlas, parcelating the brain into 166 anatomical regions of interest.14 To minimize potential quantification bias of relative glucose metabolism, normalization was additionally performed using white matter reference regions.
Outcomes
The primary outcome was the longitudinal change in the RBDRP expression over 4 years. The secondary outcomes were the longitudinal changes in metabolic activities of RBDRP‐relevant brain regions. Our RBDRP topography included increased metabolic activity in the premotor cortex, superior frontal gyrus, supplementary motor area, putamen, and hippocampus, and decreased metabolic activity in the middle temporal gyrus, insula, cuneus, lingual gyrus, and inferior occipital gyrus (Fig. 1B,C). Accordingly, metabolic alterations in these regions were included as secondary outcomes. The exploratory outcomes included associations of brain metabolic activities with clinical and DAT imaging variables.
Statistical Analysis
Analyses were performed using R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria). Values are reported as the means, standard deviations, and frequencies. The normality of the data was tested with the Shapiro–Wilk test. Continuous data were analyzed using the Mann–Whitney U test and categorical data were analyzed using the chi‐square test. We investigated primary and secondary outcomes using generalized linear mixed‐effects (GLME) models (follow‐up time as a fixed effect and participants as a random effect). In these models, the covariates were age, sex, and disease duration. Spearman's rank correlation test was used to investigate bivariate correlations between metabolic activities and clinical and DAT imaging variables. The Cox proportional hazards models adjusting for those covariates were used to determine whether RBDRP expression at baseline could predict the development of phenoconversion.
All P values were two‐sided, and a P value of less than 0.05 was considered statistically significant. Post hoc analyses to assess the difference between baseline and the follow‐up visits in the primary outcome were adjusted with the use of a Bonferroni correction. We addressed multiple comparisons of the secondary outcomes using the Benjamini−Hochberg procedure15 with a 5% false discovery rate (FDR). An FDR of 5% would imply that 5% of significant results (P < 0.05) are false‐positives.
Results
Among the 25 iRBD patients, we obtained data for 24 (96%) and 20 (80%) patients for 2‐year and 4‐year follow‐ups, respectively. Baseline characteristics are shown in Table 1. There were no differences in age (P = 0.941) and sex (P = 0.270) between the iRBD and control groups. DAT availability in the anterior and posterior putamen was significantly lower in the iRBD group compared to the control group (P < 0.05 in all).
TABLE 1.
Baseline characteristics
| Variable | iRBD (n = 25) | Control (n = 24) | P value |
|---|---|---|---|
| Age, y | 69.2 (5.3) | 69.5 (4.3) | 0.941 |
| Male sex, % | 12 (48%) | 7 (29%) | 0.270 |
| RBD duration, y | 4.5 (4.0) | − | − |
| MDS‐UPDRS score | |||
| Part I | 7.3 (3.8) | − | − |
| Constipation, % | 9 (36%) | − | − |
| Part II | 3.4 (3.5) | − | − |
| Part III | 5.1 (4.1) | − | − |
| Mild parkinsonian sign, % | 13 (52%) | − | − |
| K‐MMSE score | 27.4 (2.2) | 28.4 (1.5) | 0.176 |
| Neuropsychological test, z score | |||
| TMT‐A | −0.57 (1.05) | − | − |
| TMT‐B | −0.90 (1.80) | − | − |
| CWST (color reading) | −0.80 (0.83) | − | − |
| COWAT (semantic) | −0.29 (1.16) | − | − |
| COWAT (phonemic) | −0.65 (0.82) | − | − |
| RCFT (copy) | −2.02 (1.57) | − | − |
| SVLT (immediate recall) | −0.22 (0.98) | − | − |
| SVLT (delayed recall) | −0.60 (0.94) | − | − |
| SVLT (recognition) | −0.38 (1.03) | − | − |
| K‐BNT | −0.08 (1.15) | − | − |
| Mild cognitive impairment, % | 11 (44%) | − | − |
| Olfactory test | |||
| BTT score | 5.5 (2.6) | − | − |
| Hyposmia, % | 6 (24%) | − | − |
| DAT imaging, binding ratio | |||
| Caudate nucleus | |||
| More affected side | 3.40 (1.05) | 3.91 (0.82)a | 0.096 |
| Less affected side | 3.52 (1.00) | − | − |
| Anterior putamen | |||
| More affected side | 4.87 (1.20) | 5.59 (0.89)a | 0.041 |
| Less affected side | 5.01 (1.22) | − | − |
| Posterior putamen | |||
| More affected side | 4.18 (1.28) | 4.96 (0.87)a | 0.032 |
| Less affected side | 4.37 (1.34) | − | − |
| Reduced DAT availability, % | 5 (20%) | − | − |
Data are shown as n (%) and mean (standard deviation).
DAT imaging data were presented in a subgroup of 19 healthy controls. Their values are the average of left and right DAT binding ratios.
iRBD, isolated rapid eye movement (REM) sleep behavior disorder; y, year; MDS‐UPDRS, Movement Disorder Society Unified Parkinson's Disease Rating Scale; K‐MMSE, Korean version of the Mini‐Mental State Examination; TMT, Trail Making Test; CWST, Color Word Stroop Test; COWAT, Controlled Oral Word Association Test; RCFT, Rey−Osterrieth Complex Figure Test; SVLT, Seoul Verbal Learning Test; K‐BNT, Korean version of the Boston Naming Test; BTT, Butanol Threshold Test; DAT, dopamine transporter.
Longitudinal Changes in RBDRP Expression
At baseline, the RBDRP expression significantly differentiated the iRBD group (z score of 0.91 ± 0.86) from controls (P = 0.006). However, this significant difference was not observed at the 2‐year (z score of 0.52 ± 1.11; P = 0.132) and 4‐year follow‐ups (z score of −0.39 ± 1.10; P = 0.150) (Fig. 2A). The GLME model showed that the RBDRP z score changed significantly over time (P < 0.001). Post hoc testing revealed a significant decrease in the RBDRP expression from baseline to the 4‐year follow‐up (Bonferroni‐adjusted P < 0.001) and between the 2‐year and 4‐year follow‐ups (Bonferroni‐adjusted P = 0.003). The RBDRP expression did not change significantly between baseline and the 2‐year follow‐up (Bonferroni‐adjusted P = 0.537). When looked at individually, the RBDRP expression decreased in about two‐thirds of the patients, but there was an increase in 37% of the patients at the 2‐year follow‐up and 15% at the 4‐year follow‐up compared to the baseline. The decreasing tendency in the RBDRP expression over time was observed even in patients with high‐risk phenoconversion, although their baseline RBDRP z score was significantly higher than those with non‐high‐risk phenoconversion (z score of 1.79 ± 0.45 vs. 0.63 ± 0.77 at baseline; P = 0.003) (Fig. 2B). Among the 6 patients with high‐risk of phenoconversion, the RBDRP expression increase was found in 2 (33%) at the 2‐year follow‐up but none (0%) at the 4‐year follow‐up, as compared with baseline.
FIG. 2.
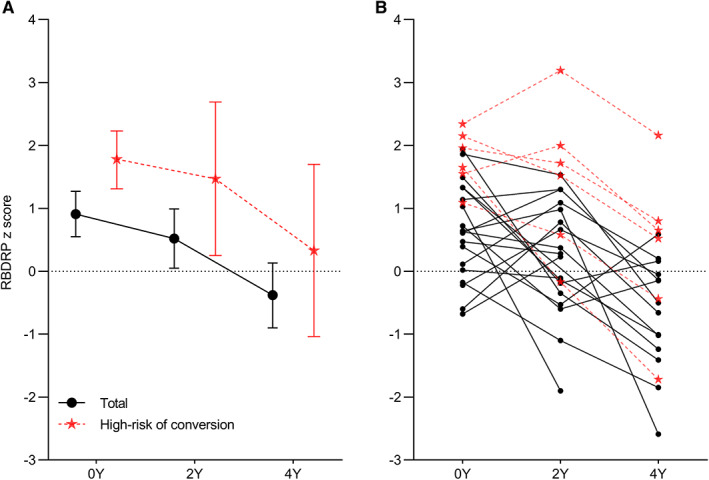
Rapid eye movement (REM) sleep behavior disorder‐related metabolic pattern (RBDRP) expression changes between baseline and the follow‐up visits. (A) Bars represent the mean values, and T bars indicate the 95% confidence intervals. (B) The lines represent individual patient data. Y, year. [Color figure can be viewed at wileyonlinelibrary.com]
The results of correlations between RBDRP expression and clinical and DAT imaging variables are shown in Appendix S4. At baseline, RBDRP expression was only correlated with reduced olfactory function. At longitudinal analyses, the RBDRP z score change per year was 0.32 ± 0.41, which was not associated with any variable.
Longitudinal Changes in the Relative Activity of Regional Metabolism Constituting RBDRP
Next, we analyzed longitudinal metabolic changes in RBDRP‐related individual brain regions using the global mean normalization method. Among the positively contributed regions at baseline (Fig. 3A–E), significant metabolic increases over time were observed in both left and right putamen (P = 0.011 and P < 0.001 after FDR adjustment, respectively). Compared to baseline, 20 patients (80%) showed increased metabolic activities in both putamen at the last follow‐up. This tendency was also observed in all patients with a high‐risk of phenoconversion. Conversely, there was a decreasing tendency of relative metabolic activities in the bilateral premotor cortex, bilateral superior frontal gyrus, and bilateral supplementary motor area (FDR‐adjusted P < 0.01 in all) by repeated scans over 4 years. There was no significant change in the hippocampus. Among the negatively contributed regions at baseline (Fig. 3F–J), relatively increasing tendency of regional metabolic activities was detected in the bilateral cuneus and bilateral lingual gyrus (FDR‐adjusted P < 0.01 in all) over 4 years. Metabolic activities in the middle temporal gyrus, insula, and inferior occipital gyrus did not change. The RBDRP expression decrease was significantly correlated with longitudinal metabolic changes in the bilateral superior frontal gyrus, left supplementary motor area, bilateral cuneus, and bilateral lingual gyrus (P < 0.05 in all) (Appendix S5). When using the white matter normalization method, the significant results were consistently observed in the left promotor cortex, bilateral supplementary motor area, right putamen, bilateral cuneus, and right lingual gyrus (FDR‐adjusted P < 0.05 in all) (Appendix S6).
FIG. 3.
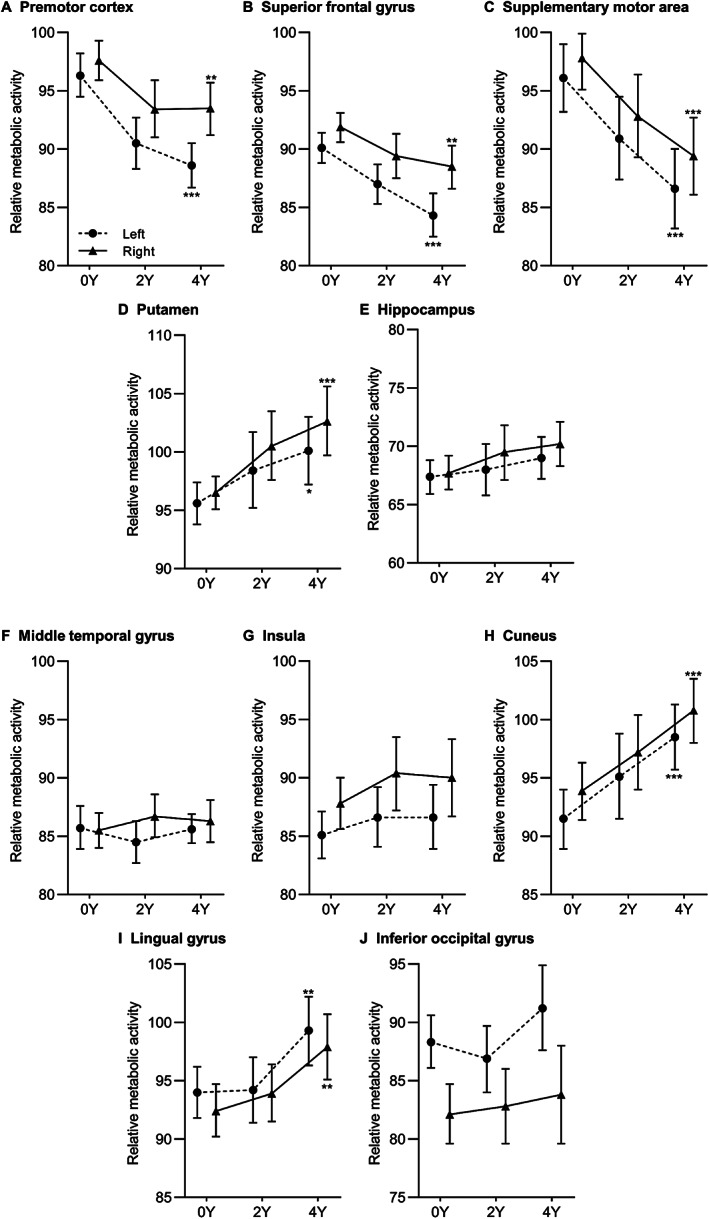
Longitudinal changes in relative metabolic activities of individual brain regions constituting rapid eye movement (REM) sleep behavior disorder‐related metabolic pattern (RBDRP) with positive activity (A–E) and negative activity (F–J) when using the global mean normalization method. *P < 0.05; **P < 0.01; ***P < 0.001 after false discovery rate adjustment. Y, year.
We further examined associations of longitudinal changes in frontal and putaminal metabolism with the progression of prodromal markers. Among the frontal areas, a significant correlation was observed between longitudinal metabolic decrease in the right premotor cortex and longitudinal decline in the Trail Making Test (TMT)‐B z scores (r = 0.56, P = 0.013) (Appendix S7). With respect to putaminal metabolic alterations most of the patients with putaminal metabolic increase showed the progression of parkinsonian motor symptoms and the DAT availability reduction at the last follow‐up, which were more prominent in the patients with MPS or reduced DAT availability at baseline (Appendix S8).
RBDRP Expression in Relation to Disease Conversion
The median follow‐up duration from baseline examination to last contact or disease conversion was 5.4 years. During this period, a total of 8 iRBD patients (32%) evolved into neurodegenerative diseases. Of those, 3 patients developed PD, 4 with DLB, and 1 with MSA‐parkinsonian type (MSA‐P). Multivariable Cox regression analysis showed that the RBDRP expression at baseline did not predict phenoconversion overall (P = 0.061). As shown in Figure 4, a decreasing tendency in the RBDRP expression was observed in 4 converters (50%) at the 2‐year follow‐up compared with baseline. However, this trend was more pronounced at the 4‐year follow‐up when all converters showed decreased RBDRP expression compared to the baseline expression level. Interestingly, all converters showed steadily increasing metabolic activities in both putamen over time when we analyzed regional metabolic activities. The converters tended to have a greater metabolic activity increase in the putamen in both right (1.8 ± 1.1 vs. 0.9 ± 1.4; P = 0.082) and left (1.0 ± 1.1 vs. 0.5 ± 1.2; P = 0.179) sides, although these results were not statistically significant. With respect to frontal areas, PD and DLB converters showed an overall decrease in metabolic activities in the premotor cortex, superior frontal gyrus, and supplementary motor area over time, whereas 1 MSA‐P converter showed increased metabolic activities in these regions. With respect to occipital areas, metabolic activities in the cuneus, lingual gyrus, and inferior occipital gyrus were slightly increased compared to baseline in PD converters. However, overall hypometabolic activities in occipital areas persisted during the follow‐up period in the MSA‐P converter and all DLB converters except for one. Representative data are presented in Appendix S9.
FIG. 4.
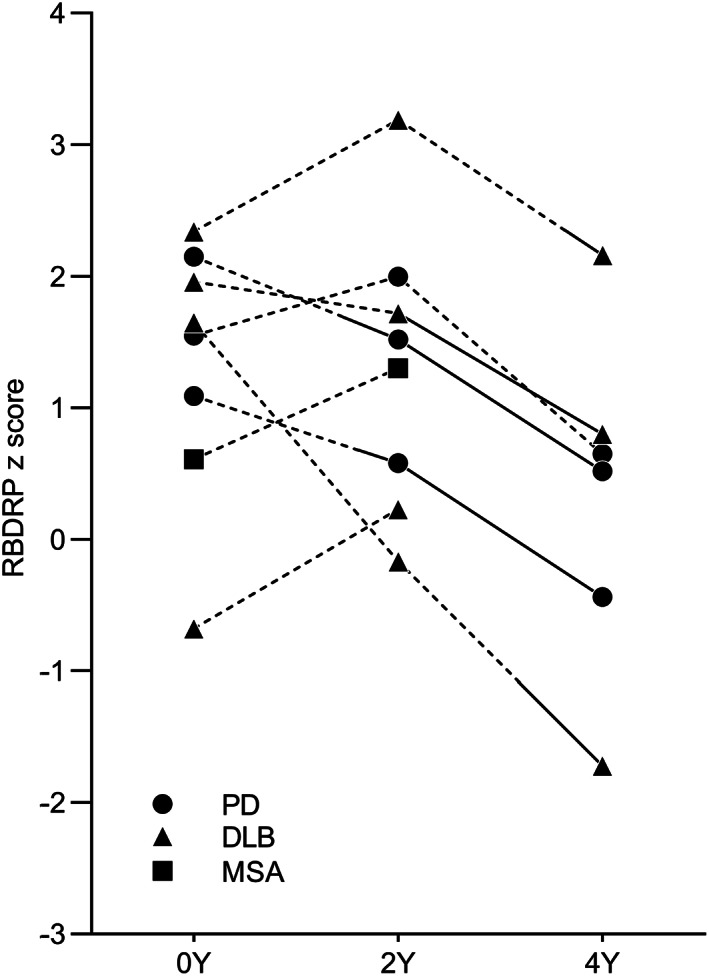
Rapid eye movement (REM) sleep behavior disorder‐related metabolic pattern (RBDRP) longitudinal changes in converters who developed an α‐synucleinopathy during the follow‐up period. The dotted and solid lines represent before and after phenoconversion, respectively. PD, Parkinson's disease; DLB, dementia with Lewy bodies; MSA, multiple system atrophy; Y, year.
Association with PDRP
At baseline, the PDRP expression significantly separated the iRBD group (z score of 0.35 ± 0.52) from controls (P = 0.012). There was a moderate correlation between the baseline PDRP and RBDRP z scores (r = 0.52; P = 0.005). Although longitudinal changes in the PDRP expression showed some heterogeneity between the patients, the expression appeared relatively stable compared to the RBDRP (Appendix S10). Among the patients with RBDRP expression decrease at the 2‐year and 4‐year follow‐ups, 5 (33%) and 4 (24%) showed the PDRP expression increase at each period, respectively. In only 1 patient, the RBDRP activity increased but the PDRP activity decreased during the follow‐up period. With respect to disease conversion, the baseline PDRP expression tended to be higher in the converters than the nonconverters (z score of 0.63 ± 0.36 vs. 0.22 ± 0.57 at baseline; P = 0.091). Longitudinal changes in the PDRP expression showed trends similar to those in the RBDRP expression in the DLB and MSA converters. However, among the 3 PD converters, the PDRP expression was stably elevated in the 2 PD converters, whereas the RBDRP expression was remarkably decreased at the last follow‐up in all.
Discussion
Our results revealed that the disease‐specific metabolic pattern expression in iRBD patients was altered significantly over time, especially with longer follow‐ups, and this alteration was breaking down its original metabolic pattern related to iRBD. Most of the patients with a high risk of phenoconversion including disease converters showed a considerable breakdown of RBDRP over time. The prospective longitudinal investigation of the RBDRP expression and related regional metabolic activity changes in our iRBD population revealed several interesting findings that are discussed below.
The RBDRP topographic patterns of previously reported studies and ours are consistent in most regions,6, 7 but there were some differences regarding whether several specific regions, including the frontal cortex and putamen, contribute significantly to the RBDRP. Compared to the previous RBDRPs,6, 7 our RBDRP included relative hypermetabolism in the putamen, which is consistent with reported regional abnormalities measured by 18F‐FDG PET or perfusion single‐photon emission computed tomography (SPECT) studies in iRBD patients.16, 17, 18 Conversely, hypermetabolism in the frontal cortex contributed to the RBDRP in our study and the study by Wu et al,6 but not in the other study by Meles et al.7 In fact, these discrepancies may not be surprising given that the intricate metabolic patterns of iRBD are possibly affected by both ongoing neurodegeneration and functional compensation, as described below and discussed in a previous article.19 In this article, iRBD patients had increased perfusion in the anterior frontal, lateral parietal, and occipitotemporal cortex relative to healthy controls at about 17 months' follow‐up, while the patients had lower relative perfusion in the anterior frontal and lateral parietotemporal cortex at baseline.19 However, the direction of perfusion changes in several brain regions, particularly the frontal cortex, are not in agreement with our FDG PET data, although there is a possibility of differences between perfusion and metabolism.
Whether RBDRP is a reliable marker reflecting neurodegenerative progression in iRBD is controversial. Meles et al7 found that the RBDRP was significantly expressed in both patients with early and more advanced PD compared with controls, and that subject scores on the RBDRP expression were strongly correlated with those on the PDRP (r = 0.94). By contrast, Wu et al6 reported that the RBDRP expression decreased in early to moderate PD patients, and that there was a weak correlation between subject scores on the RBDRP and PDRP expressions (r = 0.39). However, no definitive conclusion has been drawn from these two studies due to their cross‐sectional design. Our longitudinal data indicate that RBDRP breaks down with disease progression, supporting the findings of Wu et al.6
There are potential mechanisms for the RBDRP breakdown over time in iRBD. At the regional level, frontal hypermetabolic activity and occipital hypometabolic activity were attenuated during the follow‐up period, and such changes were significantly correlated with the RBDRP decrease. Thus, longitudinal metabolic changes in these areas could have significantly contributed to the RBDRP breakdown. The hypometabolism in the occipital cortex is a typical feature of DLB.20, 21 In PD, previous studies showed reduced metabolic activity in the parieto‐occipital association regions in the early stage,22 and more pronounced occipital hypometabolism in the later stages.23 Accordingly, the attenuation of occipital hypometabolism may provide insight into the functional compensation against the neurodegenerative process over time.
In this context, we can postulate that the same mechanism causes initial hypermetabolism in the frontal cortex. The attenuation of frontal hypermetabolic activity over time was found in PD and DLB converters, but not in the MSA‐P converter who distinctively showed an increased metabolic activity in the frontal areas over 2 years. This pattern was also observed in Chinese and American patient cohorts where the metabolic topography of MSA‐P includes hypermetabolism in the superior frontal gyrus.24 However, considering that iRBD patients rarely develop MSA instead of PD or DLB,10 the impact of metabolic patterns associated with MSA needs to be further investigated in large sample data. Interestingly, metabolic decrease in the right premotor cortex correlated with decline in the TMT‐B performance in iRBD patients. Because impairment in the TMT‐B test was reported to be a reliable and early prodromal cognitive marker of Lewy body disease,25 this correlation supports that the changes in the premotor cortex in our patients are likely to be driven by neurodegeneration toward Lewy body disease.
Another explanation for the RBDRP breakdown is that it may be associated with the control of RBD symptoms because most of the patients received treatment for RBD. There is growing evidence that sleep disturbances affect brain perfusion in the waking state.26 Thus, the brain metabolic pattern expression in iRBD is possibly affected by RBD severity itself, and abnormal metabolism may normalize with RBD symptom improvement. However, a previous study showed that the RBDRP did not differ between PD patients with and without RBD symptoms,7 which suggests that the RBDRP does not purely reflect RBD per se. Further studies with a well‐validated RBD severity assessment and its longitudinal change are necessary to clarify this issue.
There was a trend toward elevated PDRP expression in the converters than in the nonconverters, which is grossly similar to a prior study by Holtbernd et al.17 More importantly, most PD converters showed the increased PDRP expression along with the decreased RBDRP expression, and this tendency was not observed in patients who developed DLB or MSA. The PDRP expression in our PD converters are in line with a recent longitudinal study by Kogan et al27 who reported that all 4 iRBD patients who developed PD had suprathreshold PDRP expression at baseline and its greater increase over approximately 4 years of follow‐up. Given the current results, one could speculate that the RBDRP tends to fade away, being replaced by the disease‐specific pattern of the phenoconversion diagnosis. Accordingly, the PDRP or possibly other disease‐specific metabolic brain network such as DLB‐ or MSA‐related metabolic pattern may be a better prognostic marker of disease phenoconversion than the RBDRP itself in the iRBD population.
We observed longitudinal increments of putaminal metabolic activity in iRBD patients. Of note, our data suggest that putaminal metabolic increase is related to nigrostriatal dopaminergic degeneration in iRBD. Similarly, most PD22, 28, 29, 30 and DLB21, 31 patients have been reported to show increased metabolic activity in the putamen. In these disorders, putaminal hypermetabolism is regarded to be due to a pacemaker center in the basal ganglia that compensates dopaminergic loss with increased neuronal firing rate and consequently increased metabolic activity.32, 33 Interestingly, smaller decrease in putaminal DAT availability over time appeared to be a greater increase in putaminal metabolism, which can be explained by previous findings that the temporal pattern of nigrostriatal DAT loss fits with negative exponential curve.34, 35 This corresponds with our previous observations that iRBD patients with reduced DAT availability at baseline had a tendency towards smaller DAT decline.36 The MSA‐P converter also showed slightly increased metabolic activity in the putamen at 2 years before conversion when the patient did not have clinical progression of MPS or reduced DAT availability. Accordingly, it is less likely that the metabolic pattern of this patient was affected by postsynaptic striatal degeneration at that time.
Although hypometabolic activity in the occipital cortex was attenuated over 4 years at a group level, most of our DLB converters showed persistent occipital hypometabolism. One exceptional dementia converter showed an increasing tendency in the occipital metabolic activity, but this patient was suspected of mixed DLB and Alzheimer's‐type dementia. Moreover, the patient also developed overt parkinsonism 3 years later than the appearance of overt cognitive impairment. A recent study found that iRBD and DLB patients shared the same hypometabolism patterns in the occipital areas, whereas there was no overlapping hypometabolism between the iRBD and PD patients.37 These observations suggest that vulnerability for the occipital cortex is associated with the progression toward DLB, which is supported by our findings. Another study by Han et al38 reported that metabolism in the medial parts of the occipital lobe was significantly lower in iRBD patients compared to controls, and there was no significant difference between the iRBD and PD patients. However, the results were limited because they did not assess the intra‐subject longitudinal changes in the regional occipital metabolic activity in iRBD. Therefore, the findings of that study do not need to be confused with our longitudinal analysis results. Actually, occipital hypometabolism was also found in our iRBD cohort that distinguished iRBD patients from healthy controls.
The present study needs to be interpreted cautiously due to the following limitations. First, the number of iRBD patients was not large enough for subgroup analyses, and some patients were lost to follow‐up. Accordingly, we could not statistically compare longitudinal data between the subgroups in most cases. Instead, we described these data qualitatively. Second, we did not have follow‐up data for healthy controls. Age‐related metabolic changes might have a partial overlap with the PDRP,29 which could have affected our results. However, the findings of several brain regions showing increased metabolic activity over time cannot be explained entirely by age‐related effects. Lastly, considering that the evolution of prodromal symptoms of α‐synucleinopathy can start more than 10 years before phenoconversion,39 our follow‐up duration may be too short to comprehend the metabolic changes during the prodromal stages fully.
In conclusion, the RBDRP expression in iRBD patients showed significant alterations over time both individually and at a group level, suggesting that the RBDRP expression status at one time point is not a reliable biomarker predicting disease conversion. Some brain metabolic changes may represent attempted functional compensation against ongoing neurodegeneration. Further research with a larger sample size and longer follow‐up duration is needed to validate our findings and characterize the progression of metabolic network patterns in the prodromal stage of each α‐synucleinopathy.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
R.K.: 1A, 1B, 1C, 2A, 2B, 3A
J.Y.L.: 1A, 1B, 1C, 2C, 3B
Y.K.K.: 1A, 1B, 1C, 2C, 3B
H.K.: 1C, 2A, 2B
E.J.Y.: 1C, 2A, 2B
J.H.S.: 1C, 3B
D.Y.: 1C, 3B
H.N.: 2C, 3B
B.J.: 2C, 3B
Financial Disclosures for the Previous 12 Months
J.Y.L. has received a research grant from National Research Foundation funded by the Ministry of Education, Science and Technology in Korea and a multidisciplinary research grant‐in‐aid and a public clinical research grant‐in‐aid from the Seoul Metropolitan Government Seoul National University Boramae Medical Center. Y.K.K. has received a research grant from the National Research Foundation funded by the Ministry of Education, Science and Technology in Korea. R.K., H.K., E.J.Y., J.H.S., D.Y., H.N., and B.J. have nothing to report.
Supporting information
Appendix S1. Supporting Information
[Corrections added on 05 April 2021 after first online publication: The word alternation has been changed to alteration throughout the article.]
Relevant conflicts of interest/financial disclosures: All authors report no disclosures relevant to the manuscript.
Funding agencies: This study was supported by a research grant from the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology (MEST) in Korea (NRF‐2018R1C1B3008971, 2018R1A5A2025964, and 2016R1D1A1B03936159).
Contributor Information
Jee‐Young Lee, Email: wieber04@snu.ac.kr.
Yu Kyeong Kim, Email: yk3181@snu.ac.kr.
References
- 1.Högl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration ‐ an update. Nat Rev Neurol 2018;14(1):40–55. [DOI] [PubMed] [Google Scholar]
- 2.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16‐year update on a previously reported series. Sleep Med 2013;14(8):744–748. [DOI] [PubMed] [Google Scholar]
- 3.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post‐mortem pathology in idiopathic rapid‐eye‐movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 2013;12(5):443–453. [DOI] [PubMed] [Google Scholar]
- 4.Iranzo A, Santamaria J, Tolosa E. Idiopathic rapid eye movement sleep behaviour disorder: diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol 2016;15(4):405–419. [DOI] [PubMed] [Google Scholar]
- 5.Heller J, Brcina N, Dogan I, et al. Brain imaging findings in idiopathic REM sleep behavior disorder (RBD) ‐ a systematic review on potential biomarkers for neurodegeneration. Sleep Med Rev 2017;34:23–33. [DOI] [PubMed] [Google Scholar]
- 6.Wu P, Yu H, Peng S, et al. Consistent abnormalities in metabolic network activity in idiopathic rapid eye movement sleep behaviour disorder. Brain 2014;137(Pt 12):3122–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meles SK, Renken RJ, Janzen A, et al. The metabolic pattern of idiopathic REM sleep behavior disorder reflects early‐stage Parkinson disease. J Nucl Med 2018;59(9):1437–1444. [DOI] [PubMed] [Google Scholar]
- 8.Yoon EJ, Lee JY, Nam H, et al. A new metabolic network correlated with olfactory and executive dysfunctions in idiopathic rapid eye movement sleep behavior disorder. J Clin Neurol 2019;15(2):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JY, Yoon EJ, Kim YK, et al. Nonmotor and dopamine transporter change in REM sleep behavior disorder by olfactory impairment. J Mov Disord 2019;12(2):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 2019;142(3):744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iranzo A, Santamaría J, Valldeoriola F, et al. Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol 2017;82(3):419–428. [DOI] [PubMed] [Google Scholar]
- 13.Spetsieris PG, Eidelberg D. Scaled subprofile modeling of resting state imaging data in Parkinson's disease: methodological issues. Neuroimage 2011;54(4):2899–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolls ET, Huang CC, Lin CP, Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage 2020;206:116189. [DOI] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57(1):289–300. [Google Scholar]
- 16.Vendette M, Gagnon JF, Soucy JP, et al. Brain perfusion and markers of neurodegeneration in rapid eye movement sleep behavior disorder. Mov Disord 2011;26(9):1717–1724. [DOI] [PubMed] [Google Scholar]
- 17.Holtbernd F, Gagnon JF, Postuma RB, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology 2014;82(7):620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazza S, Soucy JP, Gravel P, et al. Assessing whole brain perfusion changes in patients with REM sleep behavior disorder. Neurology 2006;67(9):1618–1622. [DOI] [PubMed] [Google Scholar]
- 19.Baril AA, Gagnon JF, Pelletier A, et al. Changes in regional cerebral perfusion over time in idiopathic REM sleep behavior disorder. Mov Disord 2020;35(8):1475–1481. [DOI] [PubMed] [Google Scholar]
- 20.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 2017;89(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morbelli S, Chincarini A, Brendel M, et al. Metabolic patterns across core features in dementia with lewy bodies. Ann Neurol 2019;85(5):715–725. [DOI] [PubMed] [Google Scholar]
- 22.Schindlbeck KA, Lucas‐Jiménez O, Tang CC, et al. Metabolic network abnormalities in drug‐naïve Parkinson's disease. Mov Disord 2020;35(4):587–594. [DOI] [PubMed] [Google Scholar]
- 23.Meles SK, Renken RJ, Pagani M, et al. Abnormal pattern of brain glucose metabolism in Parkinson's disease: replication in three European cohorts. Eur J Nucl Med Mol Imaging 2020;47(2):437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen B, Wei S, Ge J, et al. Reproducible metabolic topographies associated with multiple system atrophy: network and regional analyses in Chinese and American patient cohorts. Neuroimage Clin 2020;28:102416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genier Marchand D, Postuma RB, Escudier F, et al. How does dementia with Lewy bodies start? Prodromal cognitive changes in REM sleep behavior disorder. Ann Neurol 2018;83(5):1016–1026. [DOI] [PubMed] [Google Scholar]
- 26.Dang‐Vu TT, Desseilles M, Petit D, Mazza S, Montplaisir J, Maquet P. Neuroimaging in sleep medicine. Sleep Med 2007;8(4):349–372. [DOI] [PubMed] [Google Scholar]
- 27.Kogan RV, Janzen A, Meles SK, et al. Four‐year follow‐up of [18F]fluorodeoxyglucose positron emission tomography‐based Parkinson's disease‐related pattern expression in 20 patients with isolated rapid eye movement sleep behavior disorder shows prodromal progression. Mov Disord 2021;36(1):230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang CC, Poston KL, Dhawan V, Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson's disease. J Neurosci 2010;30(3):1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews DC, Lerman H, Lukic A, et al. FDG PET Parkinson's disease‐related pattern as a biomarker for clinical trials in early stage disease. Neuroimage Clin 2018;20:572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schindlbeck KA, Eidelberg D. Network imaging biomarkers: insights and clinical applications in Parkinson's disease. Lancet Neurol 2018;17(7):629–640. [DOI] [PubMed] [Google Scholar]
- 31.Huber M, Beyer L, Prix C, et al. Metabolic correlates of dopaminergic loss in dementia with Lewy bodies. Mov Disord 2020;35(4):595–605. [DOI] [PubMed] [Google Scholar]
- 32.Burkhardt JM, Jin X, Costa RM. Dissociable effects of dopamine on neuronal firing rate and synchrony in the dorsal striatum. Front Integr Neurosci 2009;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plenz D, Kital ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature 1999;400(6745):677–682. [DOI] [PubMed] [Google Scholar]
- 34.de la Fuente‐Fernández R, Schulzer M, Lisa Kuramoto L, et al. Age‐specific progression of nigrostriatal dysfunction in Parkinson's disease. Ann Neurol 2011;69(5):803–810. [DOI] [PubMed] [Google Scholar]
- 35.Nandhagopal R, Kuramoto L, Michael Schulzer M, et al. Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson's disease. Brain 2011;134(Pt 11):3290–3298. [DOI] [PubMed] [Google Scholar]
- 36.Shin JH, Lee JY, Kim YK, Shin SA, Kim H, Nam H, Jeon B. Longitudinal change in dopamine transporter availability in idiopathic REM sleep behavior disorder. Neurology 2020;95(23):e3081–e3092. [DOI] [PubMed] [Google Scholar]
- 37.Carli G, Caminiti SP, Galbiati A, et al. In‐vivo signatures of neurodegeneration in isolated rapid eye movement sleep behaviour disorder. Eur J Neurol 2020;27(7):1285–1295. [DOI] [PubMed] [Google Scholar]
- 38.Han X, Wu P, Alberts I, et al. Characterizing the heterogeneous metabolic progression in idiopathic REM sleep behavior disorder. Neuroimage Clin 2020;27:102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB. Evolution of prodromal Parkinson's disease and dementia with Lewy bodies: a prospective study. Brain 2019;142(7):2051–2067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information


