Summary
Background
Effective topical treatment options for patients with primary axillary hyperhidrosis (PAHH) are limited. A phase I trial showed promising results regarding the efficacy and safety of a topical cream containing glycopyrronium bromide (GPB).
Objectives
To assess the efficacy, safety and tolerability of a 4‐week topical treatment of GPB 1% cream in patients with PAHH vs. placebo.
Methods
In total, 171 patients (84 receiving placebo; 87 receiving GPB 1%) with PAHH were included in the 4‐week, multicentre, randomized, double‐blind, placebo‐controlled phase IIIa part of the pivotal study. Sweat production was measured by gravimetry. Patients rated the impact of disease with the Hyperhidrosis Disease Severity Scale (HDSS) and Hyperhidrosis Quality of Life Index (HidroQoL©).
Results
Absolute change in sweat production from baseline to day 29 in logarithmic values was significantly larger in the GPB 1% group compared with the placebo group (P = 0·004). The improvement in HidroQoL exceeded the minimal clinically important difference of 4. The proportion of responders was twofold higher for sweat reduction (–197·08 mg GPB 1% vs. –83·49 mg placebo), HDSS (23% GPB 1% vs. 12% placebo) and HidroQoL (60% GPB 1% vs. 26% placebo). Treatment was safe: most treatment‐emergent adverse effects were mild or moderate, and transient. Local tolerability was very good, with 9% of patients having only mild or moderate application‐site reactions. The most reported adverse drug reaction was dry mouth (16%), an expected anticholinergic effect of the treatment.
Conclusions
GPB 1% cream may provide an effective new treatment option exhibiting a good safety profile for patients with PAHH. The long‐term open‐label part (phase IIIb) is ongoing.
Short abstract
What is already known about this topic?
Hyperhidrosis (HH) places a great burden on patients.
Topical application of anticholinergic substances may provide an effective treatment for focal HH with an improved safety profile vs. oral application.
A phase I dose‐finding study on the topical application of a formulation containing glycopyrronium bromide (GPB) showed that a 1% cream could be an effective new treatment option with a good safety profile for patients with primary axillary HH (PAHH).
What does this study add?
In this part of the pivotal phase III trial, compared with those given placebo, patients with PAHH using a GPB 1% cream achieved a significant reduction in sweat production and improved quality of life.
GPB 1% had a good safety profile and excellent tolerability at the application site.
Topical GPB is effective for PAHH and that a cream formulation is beneficial in terms of local tolerability and systemic safety.
Plain language summary available online
Hyperhidrosis (HH) is a chronic condition characterized by disproportionate and excessive sweating. It can be primary or secondary to diseases or medication use.1 Typically, sweating in primary HH is bilaterally symmetrical and localized, and patients do not sweat during sleep.2, 3 The prevalence of primary HH has been reported to be 16·3% in Germany, 4·8% in the USA and 12·8% in Japan.3, 4, 5
The disproportionate sweating experienced by patients with HH has severe and pervasive impacts on patients’ quality of life (QoL).4 Barely tolerable or intolerable severe sweating affects 70% of patients, and frequently or always interferes with their daily activities.4 Patients with HH suffer from high rates of anxiety and depression,6 yet > 50% of all patients with HH receive no treatment.3, 4
Patients with primary axillary HH (PAHH) suffer from an excessive amount of sweat production in the armpits beyond what is needed for thermoregulation. There is no dysfunction of the sweat glands, but a dysregulation of the autonomic nervous system with pathological hyperactivity of the sympathetic system and decoupling of hyperhidrotic regions from central thermoregulation.7
Eccrine sweat glands express various muscarinic acetylcholine (ACh) receptor subtypes and can therefore be activated by ACh and effectively blocked by muscarinic antagonists.2, 8
Topical, oral and injectable medications are available for the treatment of HH.9 Current first‐line treatments are antiperspirants containing aluminium salts (in concentrations ranging from 10% to 35%), the use of which is associated with dermatitis.9, 10 Other possible treatment options include pharmacological inhibition or reduction of ACh release by intradermal injections of botulinum toxin type A or invasive procedures, for example inactivation of sweat glands using microwave thermolysis and surgical sweat gland removal.9
Anticholinergic substances [oxybutynin, methantheline bromide or glycopyrronium (GP)] have been investigated for the oral and topical treatment of HH. Oral use is associated with improvements in QoL and clinical symptoms but at the cost of considerable systemic adverse events.11, 12 Topical formulations containing oxybutynin, GP, umeclidinium or sofpironium have also been used successfully in the treatment of HH but have not yet been approved in the European Union.13, 14, 15, 16
The safety and efficacy of topical formulations containing GP bromide (GPB) for the treatment of PAHH were investigated in a phase I clinical trial in 2020.17 The results confirmed the concept of a locally applied, locally acting product with a low potential for systemic side‐effects. The observed safety profile corresponded to that seen in nonclinical studies.18 According to the literature (which shows the efficacy of topical formulations with GPB concentrations ≥ 1%),13, 19, 20, 21, 22, 23 and based on clinical efficacy results, GPB 1% has been chosen as the effective and safe dose for further development. Here, we report the results obtained after 4 weeks of treatment with GPB 1% cream vs. placebo.
Materials and methods
Study design
In this multicentre, randomized, double‐blind, dose‐confirming part of the phase III study (phase IIIa) with a parallel design, patients with PAHH were randomized to once‐daily treatment with GPB 1% cream or placebo (vehicle without GPB 1%) applied to both axillae for 4 weeks. Efficacy and safety were assessed. The study was conducted from September 2018 to August 2019 in compliance with the Declaration of Helsinki and International Council for Harmonisation guideline of Good Clinical Practice. The trial is registered with ClinicalTrials.gov (NCT03658616).
Patients enrolled in the study were required to sign a written informed consent form before taking part. At the time of providing their informed consent, patients had to be between 18 and 65 years old with a body mass index of 18–32 kg m–2. Patients were only included if they had severe PAHH characterized by a Hyperhidrosis Disease Severity Scale (HDSS) score of 3 or 4, and with resting axillary sweat production in each axilla of > 50 mg in 5 min.
Exclusion criteria included hypersensitivity to GPB, secondary HH, previous surgical treatment for HH, as well as botulinum toxin treatment in the 4 months prior to the study. Detailed inclusion and exclusion criteria can be found in Appendix S2 (see Supporting Information).
Study procedure
The initial screening visit was followed by a washout phase of 2 weeks. Safety and efficacy were assessed on days 15 and 29. Use of a dispenser ensured the exact dosing of 0·54 g cream to each axilla per day. At the end of the study period, dispensers were returned and weighed. As a special precaution, no shaving/depilation of armpits was allowed 14 days before the study or during it; trimming to 1 cm was permitted.
Measurements
Gravimetric measurements (screening, baseline and day 29)
Gravimetric measurements were conducted at room temperature and at a humidity consistent with the normal local climate. After an acclimatization period of at least 30 min, axillary hair was trimmed and axillae were dried with an absorbent paper towel. Standardized filter paper was placed on both axillae for 5 min. Weighing of the standardized filter paper before and after the gravimetric measurements was performed in a central laboratory.
Hyperhidrosis Disease Severity Scale
The HDSS is a disease‐specific diagnostic tool for measuring the the severity of HH. Each of four possible answers is assigned a value on a 4‐point scale ranging from 1 to 4.
Hyperhidrosis Quality of Life Index
The Hyperhidrosis Quality of Life Index (HidroQoL©) is a validated patient‐reported outcome measure (PROM) used to capture the QoL of patients with HH.24, 25 Two domains – daily life activity and psychosocial life – are assessed, with six and 12 questions, respectively. Questions are rated on a 3‐point scale and a summary score for each domain and overall score is calculated. In 2020, the HidroQoL was revalidated specifically for primary axillary HH and the minimally clinically important difference (MCID) for treatment response has been defined as an improvement of ≥ 4 points.26
Dermatology Life Quality Index
The Dermatology Life Quality Index (DLQI) is a validated questionnaire used to measure the impact of skin disease on the QoL of the affected person. It consists of 10 questions that are answered on a 4‐point scale from 0 to 3.
Safety and tolerability
The frequency and severity of adverse events (AEs), serious AEs (SAEs) and treatment‐emergent AEs (TEAEs) were recorded. Using a skin reaction score, local tolerability at the application site was assessed by the investigator.
Endpoints
The primary endpoint was defined as the absolute change in sweat production with the GPB 1% cream vs. placebo from baseline to day 29, as assessed by gravimetry. Key secondary endpoints were the comparison between GPB 1% cream and placebo regarding absolute change in HidroQoL score from baseline to day 29 and the percentage of responders based on HDSS score at day 29 (improvement of ≥ 2 points).
Statistics
Using a two‐sided significance level of 5%, confirmatory hypothesis tests were performed for the primary and key secondary efficacy endpoints. Hierarchically ordered hypotheses testing was performed for key secondary endpoints. Further secondary endpoints were analysed descriptively in an exploratory way. For all performed tests, a two‐sided significance level of 5% (α = 0·05) was applied, unless otherwise noted. More details can be found in Appendix S2.
Results
A total of 171 patients were randomized to receive either GPB 1% cream (n = 87) or placebo (n = 84) once daily for 28 days. Patient demographics and baseline characteristics were well balanced between groups (Table 1 and Figure 1).
Table 1.
Patient demography and baseline characteristics
| Placebo (n = 84) | GPB 1% (n = 87) | |
|---|---|---|
| Sex | ||
| Male | 43 (51) | 44 (51) |
| Female | 41 (49) | 43 (49) |
| Skin type | ||
| White | 81 (96) | 86 (99) |
| Black | – | 1 (1) |
| Asian | 2 (2) | – |
| Other | 1 (1) | – |
| Mean (SD) age (years) | 37·8 (12·3) | 37·4 (11·9) |
| Age range (years) | 18–65 | 18–65 |
| Median (range) BMI (kg m–2) | 25·0 (19·5–32·0) | 25·5 (18·4–32·0) |
Data are n (%) unless otherwise stated.
Figure 1.
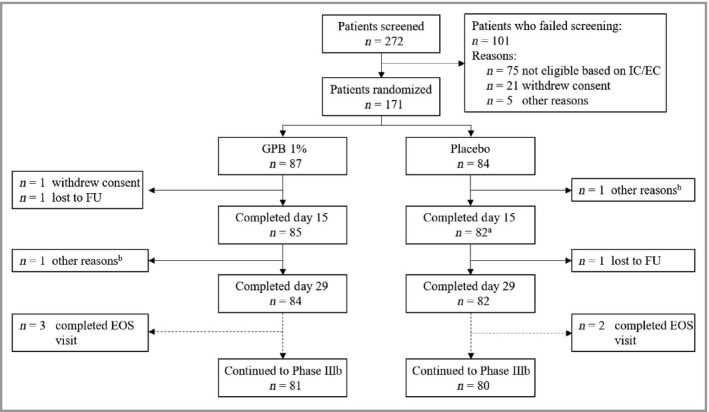
CONSORT flow diagram for the phase IIIa trial. aOne patient missed the day 15 visit but returned for day 29. b‘Other reasons’ indicated no or not enough effect of treatment. EC, exclusion criterion; EOS, end of study; FU, follow‐up; GPB, glycopyrronium bromide; IC, inclusion criterion.
Gravimetrically assessed sweat production
Mean sweat production was reduced by 197·08 mg for the GPB 1% cream group and 83·49 mg for the placebo group (Table 2 and Figure 2). Absolute reduction in sweat production from baseline to day 29 in logarithmic values was statistically significantly larger in the GPB 1% cream group than in the placebo group (P = 0·004; mixed‐effects model). Hence, the primary endpoint of the study was met.
Table 2.
Absolute change in sweat production from baseline to day 29
| Placebo (n = 84) | GPB 1% (n = 87) | GPB 1% vs. placebo P‐valuea | |
|---|---|---|---|
| Baseline | 284·64 (212·47) | 306·97 (249·33) | – |
| Change to day 29 | –83·49 (168·21)b | –197·08 (252·41)c | 0·004 |
Data are mean (SD) absolute values (mg). aTwo‐sided, α = 0·05; bdata available for 78 patients; cdata available for 77 patients.
Figure 2.
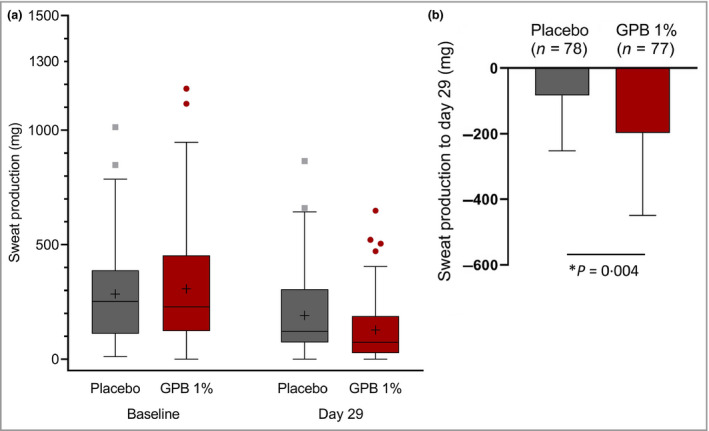
(a) Absolute sweat production (mg) in 5 min as measured by gravimetry at baseline and day 29. Data are shown for the full analysis set (n = 171). Boxes represent the lower and upper quartile; median values are indicated by the horizontal lines, mean values by a ‘+’, and upper and lower whiskers indicate the maximum and minimum values (excluding outliers). Outliers are shown as grey rectangles (placebo) or red circles [glycopyrronium bromide (GPB) 1%]. (b) Change in sweat production from baseline to day 29. Data are shown as mean (SD) for the full analysis set (n = 171: 84 in the placebo group and 87 in the GBP 1% group). *Statistically significant (P‐value for treatment effect is based on the mixed model using the absolute change in logarithmic values of sweat production).
Patient‐reported outcomes
This study used several validated questionnaires to evaluate patient‐reported disease severity and QoL. HDSS showed a change from baseline that clearly favoured GPB 1% cream treatment over placebo at day 15 (difference in median –1·0; P = 0·002) and day 29 (P = 0·014; Table 3). Median improvement in HidroQoL total score – a key secondary endpoint – was significantly greater for GPB 1% cream (–6·0 points) than for placebo (–1·0 point; P < 0·001) on day 29. Similar results were observed for the individual domains of daily life activity and psychosocial life. The impact of axillary HH on QoL was also determined using the DLQI. Here, the median improvement at day 15 was larger for patients in the GPB 1% group (–5·0 points) than for placebo (–2·0 points). The improvement seen for the GPB 1% cream was upheld until day 29. The difference in median between the GPB 1% cream and placebo was statistically significant at both timepoints (P = 0·002 and P = 0·003, respectively).
Table 3.
Absolute change in patient‐reported outcome measure tool from baseline to days 15 and 29
| Change from baseline median (95% CI) | GPB 1% vs. placebo P‐value | ||
|---|---|---|---|
| Placebo (n = 84) | GPB 1% (n = 87) | ||
| HDSS | |||
| Median (range) baseline | 4·0 (3–4) | 3·0 (2–4)a | |
| Day 15 | 0·0 (0·0 to 0·0)b, * | –1·0 (–1·0 to 0·0)c, * | 0·002 |
| Day 29 | 0·0 (0·0 to 0·0)d, * | 0·0 (–1·0 to 0·0)e, * | 0·014 |
| HidroQoL© | |||
| Median (range) baseline | 30·0 (11·0–36·0)f | 29·0 (10·0–36·0)g | |
| Day 15 | –1·0 (–2·0 to –1·0)b, * | –5·0 (–8·0 to –2·0)h, * | < 0·001 |
| Day 29 | –1·0 (–2·0 to –1·0)b, * | –6·0 (–9·0 to –4·0)c, * | < 0·001 |
| DLQI | |||
| Median (range) baseline | 15·0 (0·0–28·0)e | 14·0 (0·0–30·0) | |
| Day 15 | –2·0 (–3·0 to –1·0)b, * | –5·0 (–7·0 to –2·0)h, * | 0·002 |
| Day 29 | –3·0 (–4·0 to –1·0)b | –5·0 (–8·0 to –4·0)c | 0·003 |
CI, confidence interval; DLQI, Dermatology Life Quality Index; GPB, glycopyrronium bromide; HDSS, Hyperhidrosis Disease Severity Scale; HidroQoL, Hyperhidrosis Quality of Life Index. a n = 86; b n = 79; c n = 84; d n = 80; e n = 83; f n = 81; g n = 87; h n = 85. *P < 0·0001.
Responder analysis
The proportion of responders to treatment was determined based on gravimetrically measured sweat production, as well as on the HDSS and HidroQoL questionnaires. At day 29, significantly more patients achieved a reduction in sweat production of > 50%, 75% or 90% with GPB 1% cream than with placebo. More than half of patients achieved a 50% reduction in sweating with the GPB 1% cream [57% (n = 50) vs. 34% (n = 29) with placebo], while nearly one in four achieved a reduction in sweat of 90% [23% (n = 20) vs. 10% (n = 8) with placebo] (Table 4). Overall, the proportion of patients achieving a certain degree of sweat reduction was approximately twofold higher for the GPB 1% cream than for placebo (1·7‐fold for a 50% reduction and 2·4‐fold for a 90% reduction). Based on the HDSS (a key secondary endpoint), more patients in the GPB 1% group experienced a response to treatment (for day 29). At day 15 there was a significantly higher proportion of patients with an improvement of ≥ 2 points than for placebo [25% (n = 22) vs. 9% (n = 8); P = 0·007], while at day 29 the responder rate was similar [23% (n = 20) vs. 12% (n = 10)] and the difference between the groups approached statistical significance (P = 0·054). The proportion of HidroQoL responders with GPB 1% cream [60% (n = 52)] vs. placebo [26% (n = 22); P < 0·001] was significant, as determined in a post hoc analysis (MCID ≥ 4).26
Table 4.
Responder rate assessed by gravimetric measurement at day 29
| Placebo (n = 84) | GPB 1% (n = 87) | P‐value for GPB 1% vs. placebo | |
|---|---|---|---|
| Sweat reduction from baseline (%) | |||
| ≥ 50 | 29 (35) | 50 (57) | 0·003 |
| ≥ 75 | 14 (17) | 28 (32) | 0·011 |
| ≥ 90 | 8 (10) | 20 (23) | 0·012 |
| HDSS improvement | |||
| ≥ 1 | 27 (32) | 41 (47) | 0·045 |
| ≥ 2 | 10 (12) | 20 (23) | 0·054 |
| HidroQoL© ≥ 4 | 22 (26) | 52 (60) | < 0·001 |
GPB, glycopyrronium bromide; HDSS, Hyperhidrosis Disease Severity Scale; HidroQoL, Hyperhidrosis Quality of Life Index.
Safety and tolerability
About half of patients in both study cohorts had at least one TEAE during the study (GPB 1% cream: 49%; placebo: 44%). Most TEAEs were mild or moderate, and there was no discontinuation due to TEAEs. Most patients did not experience an adverse drug reaction (87% placebo and 72% GPB 1%). The most common ADR was dry mouth (16%) and only a few other anticholinergic ADRs were reported (Table 5). Application‐site reactions [application‐site dermatitis (1% in the GPB 1% group), application‐site erythema (5% GPB 1% vs. 5% placebo), application‐site pain (1% GPB 1% vs. 1% placebo), application‐site papules (1% in the GPB 1% group) and application‐site pruritus (1% GPB 1% vs. 1% placebo), none of which was treated] were reported in 9% of patients in the GPB 1% group and 7% of patients receiving placebo and were primarily mild‐to‐moderate in severity. Application‐site erythema was the most common reaction (5%). Further, most patients in both treatment groups had a skin reaction score of 0 (no evidence of irritation) on both axillae at baseline, day 15 and day 29, showing a similar local tolerability between the treatment groups.
Table 5.
Adverse drug reaction (ADR) in the placebo and glycopyrronium bromide (GPB) 1% groups
| Patients with at least one ADR (%) | ||
|---|---|---|
| Placebo | GPB 1% | |
| Drug‐related AEs | 13 | 28 |
| Mild | 12 | 21 |
| Moderate | 0 | 7 |
| Severe | 1 | 0 |
| Anticholinergic AEs | 5 | 20 |
| Dry mouth | 4 | 16 |
| Dry nose | 0 | 2 |
| Mydriasis | 0 | 0 |
| Dry eyes/red eyes | 1 | 1 |
| Blurred vision | 0 | 0 |
| Urinary hesitation | 0 | 0 |
| Urinary retention | 0 | 0 |
| Constipation | 0 | 1 |
| Local skin reactions | 7 | 9 |
AE, adverse effect.
Discussion
The disproportionate sweating of patients with PAHH is a burden on their overall wellbeing. The benefit achieved with current first‐line treatment antiperspirants is very often associated with dermatitis.27 In addition, the use of aluminium salts is undesirable owing to media speculation on their link to Alzheimer disease and cancer.28, 29, 30 Safety concerns, cost or invasiveness also limit the use of other available procedures.
A recent phase I study showed promising results for a GPB‐containing cream.17 Based on the available literature and the risk–benefit profile, a concentration of 1% was selected for further clinical development of GPB. This phase IIIa study investigating the efficacy and safety of GPB 1% cream applied once daily for 4 weeks showed that the cream significantly reduced gravimetrically assessed sweat production compared with placebo (P = 0·004). In addition, use of the HDSS and HidroQoL questionnaires confirmed very good efficacy and a significant improvement in QoL for patients. Treatment with GPB 1% cream was safe, with most TEAEs being mild or moderate, and transient. Local tolerability was very good; 9% of patients experienced local skin reactions, which were all mild or moderate. The most reported drug‐related AE was dry mouth, which was in line with the expected anticholinergic effect of the treatment.
Currently, there is only one topical glycopyrronium‐containing product approved for the treatment of PAHH. These glycopyrronium tosylate (GPT) wipes (Qbrexza®; Dermira, Menlo Park, CA, USA) were approved by the US Food and Drugs Administration in June 2018 and are, at the time of writing, commercially available only in the USA. Pooled efficacy data comparing GPT 3·75%‐containing wipes to placebo over 4 weeks from two trials – ATMOS‐1 (n = 344) and ATMOS‐2 (n = 353) – were published in 2018.31 Mean absolute reduction in sweat production was 107·6 mg vs. 92·1 mg for placebo,31 whereas the mean absolute reduction in sweat production vs. placebo in the present study was considerably higher [197·08 mg (GPB 1%) vs. 83·49 mg (placebo)]. A 50% reduction in sweat vs. baseline was achieved in 75% of patients using GPT wipes and in 53% of patients in the placebo group.31 In the phase IIIa study presented here, the absolute percentage of patients achieving a reduction in sweat of 50% was lower for the group receiving GPB 1% cream. However, the magnitude of the benefit for the GPB 1% cream vs. placebo was similar to that reported for GPT wipes. This is supported by the responder analysis of the respective PROMs, which showed similar results regarding absolute values and magnitude [52·8% GPT vs. 28·3% placebo/66·1% GPT vs. 26·9% placebo (Axillary Sweating Daily Diary item 2)32 vs. 59·8% 1% GPB vs 26·2% placebo (HidroQoL)]. This indicates comparable efficacy of the treatments with glycopyrronium independent of the vehicle (cream vs. wipes) and despite the different concentrations used (0·8% GP vs. 2·4% GP).
A structural analogue to GP in clinical development – sofpironium as bromide salt (SB)16 – in a gel containing SB 5%, 10% or 15% showed an improvement of ≥ 1 point on the Hyperhidrosis Disease Severity Measure‐Axillary (HDSM‐Ax). Improvement was 15–20 percentage points (depending on concentration) higher in patients under SB than with placebo (but without a clear dose response).16 However, the trial was not powered to detect changes in gravimetric sweat production and treatment was carried out over 6 weeks. In 2020, positive results of a phase III study over 6 weeks of treatment with a SB 5%‐containing gel were reported and the product received approval for the treatment of PAHH in Japan (ECCLOCK®; Kaken Pharmaceutical, Tokyo, Japan).33, 34 Change in total sweat production for both axillae from baseline to the end of treatment (EOT) was –157·6 mg (SB) vs. –127·6 mg (placebo; P = 0·015).33 Moreover, the results of the PROM (HDSM‐Ax) after 6 weeks of treatment showed slightly lower efficacy for the GP‐containing wipes and cream. The percentage of PROM responders at the EOT was 48·2% (SB) vs. 26·4% (vehicle; P < 0·001), while the proportion of subjects with a ≥ 50% reduction in the rate of gravimetric sweat production from baseline to EOT was 77·3% (SB) vs. 66·4% (vehicle; P = 0·042).33, 35
HDSS is a qualitative measure of the severity of the patient’s condition based on how it affects daily activities, assessed by a 4‐point scale. In the ATMOS‐1 and ATMOS‐2 trials, there were twice as many HDSS responders, i.e. patients reporting an improvement of ≥ 2 points, with GPT wipes (59·1%) than with placebo (25·7%).31 In the current study, the ratio of responders for GPB 1% cream and placebo was similar to that for GPT wipes (23·0% vs. 11·9%). Yet, the overall lower proportion of patients achieving an HDSS response with GPB 1% cream vs. GPT wipes was somewhat surprising. In the literature, a 2‐point improvement in HDSS is associated with an 80% reduction in sweat production.36 However, in our study a correlation between gravimetrically assessed sweat production and HDSS response was not seen (even patients with a ≥ 90% reduction in sweat were not necessarily HDSS responders). Furthermore, in a phase III study with SB 5%, the proportion of patients whose HDSS was improved to a score of 1 or 2 at the EOT and with a > 50% reduction in gravimetrically assessed sweat production at the EOT was 53·9% (SB) vs. 36·4% (placebo).33 Only minor positive trends between gravimetry and HDSS scores were found in primary focal HH.37 The poor correlation may be due to the combination of distinct concepts of tolerability and interference with daily activities in a single, patient self‐reported rating question.36, 38 Despite being disease‐specific, validated and frequently used, the HDSS could be flawed in its interpretation.36, 38 Furthermore, it is unclear from the literature what measures were used to design or validate the HDSS tool. As a consequence, it is not highly regarded as a comprehensive tool for measuring QoL.38
By using an appropriately validated patient‐reported outcomes tool (HidroQoL)24, 25 for PAHH, improvement in QoL was significantly higher in patients treated with GPB 1% cream compared with placebo, on both the total score and the subscores for the domains of daily life activities and psychosocial life. Moreover, responder analysis of HidroQoL (MCID ≥ 4)26 showed that there were twice as many responders in the GPB 1% group than in the placebo group after only 2 weeks of treatment (day 15) and on day 29 (60% GPB 1% vs. 26% placebo). These results are consistent with recently published results of GPT with the Axilliary Sweating Daily Diary (ASDD),39 a patient‐reported outcomes tool with an emphasis on disease severity and a 24‐h recall timeframe.40 These results confirm that the HidroQoL is a more sensitive measure of the therapeutic impact of GPB on QoL in an interventional setting than the HDSS.38
Confirmation with randomized controlled trials is needed, but the results of published trials show that GPB cream has a lower incidence of local reactions or side‐effects than GPT wipes. Local skin reactions occurred in 30·8% of patients with GPT wipes and 30·3% in a placebo group.31 With regard to systemic safety, more drug‐related AEs were reported for GPT wipes and were typical anticholinergic reactions (dry mouth, mydriasis, urinary hesitation, blurred vision, constipation).31 Discontinuation of GPT wipes occurred infrequently (< 4%),31 while no patients discontinued application of GPB 1% cream in this study after experiencing a TEAE. While data on local tolerability and systemic safety show that GP has, in general, a good safety profile,31 they highlight that, compared with a cream, there are drawbacks to the application of an active pharmaceutical ingredient via an alcoholic solution in terms of tolerability. A study comparing both products should be performed in order to confirm these statements.
GPB 1% cream significantly reduced excessive sweat production in patients with PAHH and significantly improved QoL. Use of GPB 1% cream is safe and well tolerated, indicating that a cream formulation of GPB is a patient‐friendly route of administration. The long‐term open‐label part (phase IIIb) over 72 weeks will gather further data to support these placebo‐controlled results.
Author Contribution
Christoph Abels: Conceptualization (lead); Data curation (equal); Funding acquisition (lead); Supervision (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Michael Soeberdt: Conceptualization (supporting); Writing‐original draft (equal); Writing‐review & editing (equal). Ana Kilic: Conceptualization (supporting); Writing‐original draft (equal); Writing‐review & editing (equal). Hubert Reich: Conceptualization (supporting); Writing‐review & editing (equal). Ulrich Knie: Conceptualization (supporting); Resources (lead); Writing‐original draft (equal). Carolin Jourdan: Formal analysis (equal); Validation (equal); Writing‐review & editing (equal). Katharina Schramm: Formal analysis (equal); Validation (equal); Writing‐review & editing (equal). Susanne Heimstaedt‐Muskett: Validation (equal); Writing‐review & editing (equal). Clarissa Masur: Conceptualization (equal); Data curation (equal); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Rolf‐Markus Szeimies: Investigation (lead); Methodology (lead); Project administration (equal); Supervision (lead); Writing‐review & editing (equal).
Supporting information
Appendix S1 List of investigators and centres.
Appendix S2 Extended materials and methods.
Powerpoint S1 Journal Club Slide Set.
Acknowledgments
This research was sponsored by Dr. August Wolff GmbH & Co. KG Arzneimittel. ClinicalTrials.gov identifier: NCT03658616. The authors thank the patients for their participation in this study; and their colleagues and investigators (see Appendix S1) for their support. Medical writing was provided by Dr Alexander Boreham (co.medical, Berlin, Germany).
Funding sources This study was sponsored by Dr. August Wolff GmbH & Co. KG Arzneimittel, Bielefeld, Germany.
Conflicts of interest C.M., M.S., A.K., U.K. and C.A. were employees of Dr. August Wolff GmbH & Co. KG Arzneimittel at the time of the study. R.‐M.S. is the Principal Investigator and represents the study group (Appendix S1; see Supporting Information). He is also vice president of EURO‐PDT; has been a member of advisory boards for Almirall, Biofrontera, Galderma, LEO Pharma, Novartis, Photonamic and Dr. August Wolff GmbH & Co. KG Arzneimittel; and has received speakers’ honoraria from the aforementioned companies. U.K. and C.A. are named as inventors on a patent application claiming glycopyrronium salt‐containing oil‐in‐water emulsions. C.M. and C.A. are named as inventors on a patent application claiming a topical emulsion of an anticholinergic compound. Dr. August Wolff GmbH & Co. KG Arzneimittel is developing a topical glycopyrronium bromide formulation for the treatment of primary axillary hyperhidrosis.
Plain language summary available online
References
- 1.Connolly M, de Berker D. Management of primary hyperhidrosis. Am J Clin Dermatol 2003; 4:681–97. [DOI] [PubMed] [Google Scholar]
- 2.Rzany B, Hund M. Fokale Hyperhidrose. Hautarzt 2003; 54:767–80. [DOI] [PubMed] [Google Scholar]
- 3.Augustin M, Radtke MA, Herberger Ket al. Prevalence and disease burden of hyperhidrosis in the adult population. Dermatology 2013; 227:10–13. [DOI] [PubMed] [Google Scholar]
- 4.Doolittle J, Walker P, Mills Tet al. Hyperhidrosis: an update on prevalence and severity in the United States. Arch Dermatol Res 2016; 308:743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujimoto T, Kawahara K, Yokozeki H. Epidemiological study and considerations of primary focal hyperhidrosis in Japan: from questionnaire analysis. J Dermatol 2013; 40:886–90. [DOI] [PubMed] [Google Scholar]
- 6.Bahar R, Zhou P, Liu Yet al. The prevalence of anxiety and depression in patients with or without hyperhidrosis (HH). J Am Acad Dermatol 2016; 75:1126–33. [DOI] [PubMed] [Google Scholar]
- 7.Schick CH. Pathophysiology of hyperhidrosis. Thorac Surg Clin 2016; 26:389–93. [DOI] [PubMed] [Google Scholar]
- 8.Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol 2015; 24:644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rzany B, Bechara FG, Feise Ket al. Update of the S1 guidelines on the definition and treatment of primary hyperhidrosis. J Dtsch Dermatol Ges 2018; 16:945–52. [DOI] [PubMed] [Google Scholar]
- 10.Perera E, Sinclair R. Hyperhidrosis and bromhidrosis: a guide to assessment and management. Aust Fam Physician 2013; 42:266–9. [PubMed] [Google Scholar]
- 11.Cruddas L, Baker DM. Treatment of primary hyperhidrosis with oral anticholinergic medications: a systematic review. J Eur Acad Dermatol Venereol 2017; 31:952–63. [DOI] [PubMed] [Google Scholar]
- 12.Sammons JE, Khachemoune A. Axillary hyperhidrosis: a focused review. J Dermatolog Treat 2017; 28:582–90. [DOI] [PubMed] [Google Scholar]
- 13.Hyun MY, Son IP, Lee Yet al. Efficacy and safety of topical glycopyrrolate in patients with facial hyperhidrosis: a randomized, multicentre, double‐blinded, placebo‐controlled, split‐face study. J Eur Acad Dermatol Venereol 2015; 29:278–82. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen NV, Gralla J, Abbott Jet al. Oxybutynin 3% gel for the treatment of primary focal hyperhidrosis in adolescents and young adults. Pediatr Dermatol 2018; 35:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasir A, Bissonnette R, Maari Cet al. A phase 2a randomized controlled study to evaluate the pharmacokinetic, safety, tolerability and clinical effect of topically applied Umeclidinium in subjects with primary axillary hyperhidrosis. J Eur Acad Dermatol Venereol 2018; 32:145–51. [DOI] [PubMed] [Google Scholar]
- 16.Kirsch B, Smith S, Cohen Jet al. Efficacy and safety of topical sofpironium bromide gel for the treatment of axillary hyperhidrosis: a phase II, randomized, controlled, double‐blinded trial. J Am Acad Dermatol 2020; 82:1321–7. [DOI] [PubMed] [Google Scholar]
- 17.Masur C, Soeberdt M, Kilic Aet al. Safety and efficacy of topical formulations containing 0·5, 1 and 2% glycopyrronium bromide in patients with primary axillary hyperhidrosis: a randomized, double‐blind, placebo‐controlled study. Br J Dermatol 2020; 182:229–31. [DOI] [PubMed] [Google Scholar]
- 18.Chabicovsky M, Winkler S, Soeberdt Met al. Pharmacology, toxicology and clinical safety of glycopyrrolate. Toxicol Appl Pharmacol 2019; 370:154–69. [DOI] [PubMed] [Google Scholar]
- 19.Shaw JE, Abbott CA, Tindle Ket al. A randomised controlled trial of topical glycopyrrolate, the first specific treatment for diabetic gustatory sweating. Diabetologia 1997; 40:299–301. [DOI] [PubMed] [Google Scholar]
- 20.May JS, McGuirt WF. Frey's syndrome: treatment with topical glycopyrrolate. Head Neck 1989; 11:85–9. [DOI] [PubMed] [Google Scholar]
- 21.Cladellas E, Callejas MA, Grimalt R. A medical alternative to the treatment of compensatory sweating. Dermatol Ther 2008; 21:406–8. [DOI] [PubMed] [Google Scholar]
- 22.Kim W, Kil H, Yoon Ket al. Topical glycopyrrolate for patients with facial hyperhidrosis. Br J Dermatol 2008; 158:1094–7. [DOI] [PubMed] [Google Scholar]
- 23.Kim WO, Kil HK, Yoon DMet al. Treatment of compensatory gustatory hyperhidrosis with topical glycopyrrolate. Yonsei Med J 2003; 44:579–82. [DOI] [PubMed] [Google Scholar]
- 24.Kamudoni P, Mueller B, Salek M. The development and validation of a disease‐specific quality of life measure in hyperhidrosis: the Hyperhidrosis Quality of Life Index (HidroQOL©). Qual Life Res 2015; 24:1017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamudoni P, Mueller B, Halford Jet al. The impact of hyperhidrosis on patients' daily life and quality of life: a qualitative investigation. Health Qual Life Outcomes 2017; 15:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabes M, Jourdan C, Schramm Ket al. Hyperhidrosis Quality of Life Index (HidroQoL©): further validation and clinical application in patients with axillary hyperhidrosis using data from a phase III RCT. Br J Dermatol 2020. Epub ahead of print 8 June 2020. 10.1111/bjd.19300. [DOI] [PubMed] [Google Scholar]
- 27.Grabell DA, Hebert AA. Current and emerging medical therapies for primary hyperhidrosis. Dermatol Ther 2017; 7:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darbre PD. Aluminium, antiperspirants and breast cancer. J Inorg Biochem 2005; 99:1912–19. [DOI] [PubMed] [Google Scholar]
- 29.Darbre PD. Aluminium and the human breast. Morphologie 2016; 100:65–74. [DOI] [PubMed] [Google Scholar]
- 30.Maya S, Prakash T, Madhu KDet al. Multifaceted effects of aluminium in neurodegenerative diseases: a review. Biomed Pharmacother 2016; 83:746–54. [DOI] [PubMed] [Google Scholar]
- 31.Glaser DA, Hebert AA, Nast Aet al. Topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: results from the ATMOS‐1 and ATMOS‐2 phase 3 randomized controlled trials. J Am Acad Dermatol 2019; 80:128–38. [DOI] [PubMed] [Google Scholar]
- 32.US Food and Drugs Administration . NDA multi‐disciplinary review and evaluation – NDA 210361 QBREXZA (glycopyrronium) cloth, 2.4%. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210361Orig1s000MultidisciplineR.pdf (last accessed December 2018).
- 33.Intrado . Brickell Biotech announces positive phase 3 pivotal study results for sofpironium bromide in Japan. Available at: https://www.globenewswire.com/news‐release/2020/06/15/2047897/0/en/Brickell‐Biotech‐Announces‐Positive‐Phase‐3‐Pivotal‐Study‐Results‐for‐Sofpironium‐Bromide‐in‐Japan‐Released‐by‐Development‐Partner‐Kaken‐Pharmaceutical.html (last accessed July 2020).
- 34.Intrado . Brickell Biotech announces approval of sofpironium bromide gel, 5% in Japan for treatment of primary axillary hyperhidrosis received by its development partner, Kaken Pharmaceutical. Available at: https://www.globenewswire.com/news‐release/2020/09/25/2099220/0/en/Brickell‐Biotech‐Announces‐Approval‐of‐Sofpironium‐Bromide‐Gel‐5‐in‐Japan‐for‐Treatment‐of‐Primary‐Axillary‐Hyperhidrosis‐Received‐by‐its‐Development‐Partner‐Kaken‐Pharmaceutical.html (last accessed September 2020).
- 35.Yokozeki H, Fujimoto T, Abe Yet al. A phase 3, multicenter, randomized, double‐blind, vehicle‐controlled, parallel‐group study of 5% sofpironium bromide (BBI‐4000) gel in Japanese patients with primary axillary hyperhidrosis. J Dermatol 2021; 10.1111/1346-8138.15668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solish N, Bertucci V, Dansereau Aet al. A comprehensive approach to the recognition, diagnosis, and severity‐based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg 2007; 33:908–23. [DOI] [PubMed] [Google Scholar]
- 37.Gibbons M, Armbrecht E, Dudzinski Jet al. Comparison of patient‐reported disease severity and sweat measurements in primary focal hyperhidrosis. J Am Acad Dermatol 2019; 81:1209–11. [DOI] [PubMed] [Google Scholar]
- 38.Wade R, Jones‐Diette J, Wright Ket al. Hyperhidrosis quality of life measures: review and patient perspective. J Dermatol Treat 2019; 30:303–8. [DOI] [PubMed] [Google Scholar]
- 39.Pariser DM, Hebert AA, Drew Jet al. Topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: patient‐reported outcomes from the ATMOS‐1 and ATMOS‐2 phase III randomized controlled trials. Am J Clin Dermatol 2019; 20:135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson L, DiBenedetti D, Pariser Det al. Development and validation of the Axillary Sweating Daily Diary: a patient‐reported outcome measure to assess sweating severity. J Patient Rep Outcomes 2019; 59:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 List of investigators and centres.
Appendix S2 Extended materials and methods.
Powerpoint S1 Journal Club Slide Set.


