Abstract
Intracerebral hemorrhage (ICH) is a common and severe neurological disorder associated with high morbidity and mortality rates. Despite extensive research into its pathology, there are no clinically approved neuroprotective treatments for ICH. Increasing evidence has revealed that inflammatory responses mediate the pathophysiological processes of brain injury following ICH. Experimental ICH was induced by direct infusion of 100 μL fresh (non‐heparinized) autologous whole blood into the right basal ganglia of Sprague–Dawley rats at a constant rate (10 μL/min). The simvastatin group was administered simvastatin (15 mg/kg) and the combination therapy group was administered simvastatin (10 mg/kg) and ezetimibe (10 mg/kg). Magnetic resonance imaging (MRI), the forelimb use asymmetry test, the Morris water maze test, and two biomarkers were used to evaluate the effect of simvastatin and combination therapy. MRI imaging revealed that combination therapy resulted in significantly reduced perihematomal edema. Biomarker analyses revealed that both treatments led to significantly reduced endothelial inflammatory responses. The forelimb use asymmetry test revealed that both treatment groups had significantly improved neurological outcomes. The Morris water maze test revealed improved neurological function after combined therapy, which also led to less neuronal loss in the hippocampal CA1 region. In conclusion, simvastatin–ezetimibe combination therapy can improve neurological function, attenuate the endothelial inflammatory response and lead to less neuronal loss in the hippocampal CA1 region in a rat model of ICH.
Keywords: hippocampus, intracerebral hemorrhage, neurological outcome, perihematomal edema, simvastatin, simvastatin–ezetimibe
Abbreviations
- ICH
Intracerebral hemorrhage
- MRI
Magnetic resonance imaging
- TBI
Traumatic brain injury
- LDL
Low‐density lipoprotein
- ICAM‐1
Intercellular adhesion molecule‐1
- BDNF
Brain‐derived neurotrophic factor
- Hb
Hemoglobin
- BBB
Blood–brain barrier
- NGF
Nerve growth factor
- NF‐κB
Nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- MAPK
Mitogen‐activated protein kinase
Introduction
Spontaneous intracerebral hemorrhage (ICH) accounts for approximately 10% of all strokes and is one of the most lethal forms of stroke, featuring a mortality rate of 30–50%. ICH is often associated with debilitating neurological deficits in survivors [1]. To date, surgical approaches to the treatment of ICH have not been very effective, and no satisfactory drug treatments are currently available.
Cholesterol‐lowering drugs such as 3‐hydroxy‐3‐methylglutaryl‐coenzyme, A reductase inhibitors, and statins have beneficial effects in various experimental brain injury models including ischemic stroke [2, 3], traumatic brain injury (TBI) [4, 5, 6], subarachnoid hemorrhage [7], and ICH [8] models. Statins reduce inflammation, superoxide free radicals, and thrombus formation; and increase cell survival, endothelial cell function, and nitric oxide bioavailability [6]. Intensive statin therapy incrementally lowers low‐density lipoprotein (LDL) cholesterol levels and nonfatal cardiovascular event rates [9, 10].
Because of the residual risk of recurrent cardiovascular events and safety concerns associated with high‐dose statin therapy [11], additional lipid‐modifying therapies have been sought [12, 13]. Ezetimibe targets the Niemann‐Pick C1‐like 1 protein, thereby reducing the absorption of cholesterol from the intestine [14, 15]. When combined with statins, ezetimibe reduces LDL cholesterol levels by an additional 23–24%. Ezetimibe also enhances the systemic anti‐inflammatory effects of simvastatin and potentiates the impact of simvastatin on plasma adipokine levels [16].
In addition to the mechanical tissue damage caused by initial hematomas, injured brain cells and the extravasated components of blood clots trigger a complex sequence of parallel and sequentially deleterious mechanisms, including inflammatory and oxidative stress pathways [17]. In the present study, we evaluated whether simvastatin and simvastatin–ezetimibe combined therapy improved neurological outcomes and endothelial inflammatory responses in an experimental ICH model.
Because of its multiparametric nature, magnetic resonance imaging (MRI) has become the standard imaging methodology for assessing ischemic and hemorrhagic stroke [17, 18]. MRI is highly sensitive temporal evolution of intraparenchymal hemorrhage and cerebral edema [19]. Depending on study timing, the hematoma core and surrounding regions may become hyperintense or hypointense on susceptibility‐weighted images and T2‐weighted (T2W) images relative to normal brain tissue. These indices are associated with the various states of hemoglobin. These types of MRI findings can be used to assess ICH evolution [20].
According to the studies on spinal cord injury cited here and in our previous work, both simvastatin and simvastatin–ezetimibe combination therapy could attenuate the endothelial inflammatory response. In the present study, we investigated the effects of simvastatin and simvastatin–ezetimibe combination therapy in a rat model of ICH on hematoma volume, cerebral edema, endothelial inflammatory response, neurological recovery, and neuron loss in the hippocampus. We further used temporal MRI measurements to evaluate tissue damage profiles. Furthermore, given that our previous work demonstrated that intercellular adhesion molecule‐1 (ICAM‐1) is a good biomarker of the endothelial inflammatory response and accumulating evidence demonstrates that brain‐derived neurotrophic factor (BDNF) is a potential biomarker of TBI and stroke [6, 21], we chose these proteins as biomarkers in the present study.
Materials and Methods
ICH model
All experiments were approved (IACUC‐103034) by the Institutional Animal Care and Use Committee (IACUC) of E‐DA Hospital and complied with the IACUC Guide for the Care and Use of Laboratory Animals. Adult male Sprague–Dawley rats (n = 30; 250–300 g body weight) were group‐housed with a 12/12‐h light‐dark cycle and provided a standard diet. Animals were anesthetized using isoflurane (3.5% induction, 1.0–1.5% maintenance) at a 2 : 1 ratio with N2O/O2. Core animal temperature was maintained at 36–37 °C throughout all surgical and MRI procedures. Autologous whole blood was aspirated from the tail vein. Primary ICH was induced by direct infusion of 100 μL of fresh (non‐heparinized) autologous whole blood into the right basal ganglia (coordinates: 0.2 mm anterior, 5.5 mm ventral, and 3.5 mm lateral to the midline) while the animal’s head was mounted to a stereotaxic frame. Next, 100 μL of autologous blood was infused (10 μl/min) with a micro‐infusion pump, as previously [22]. In order to avoid backflow, the microsyringe was retained in situ for another 10 min before being slowly withdrawn. For the acute post‐ICH time point, the right femoral artery and vein were cannulated to monitor blood pressure.
Simvastatin and combination therapy
The simvastatin group was administered simvastatin (15 mg/kg) via an oral gastric tube [23]. The combination treatment group was administered simvastatin (10 mg/kg) and ezetimibe (10 mg/kg) (Merck, Darmstadt, Germany), as previously [24].
Experimental protocol
Animals were divided into four groups: (i) sham, which underwent a scalp incision only (n = 6); (ii) controls, which were administered ICH without therapy (n = 8); (iii) simvastatin (n = 8); and (iv) simvastatin–ezetimibe combination therapy (n = 8).
MRI imaging
A conventional MRI scanning protocol was used. All subjects underwent conventional MRI with a 3.0 T scanner (MAGNETOM Skyra; Siemens Medical Solutions, Erlangen, Germany) using a 16‐channel coil for high‐resolution imaging. T2 mapping of the brain was performed using an axial multi‐echo spin echo sequence with the following parameters: TR = 5 000 ms; TE, 15, 30, 45, 60, 75, and 90 ms; Nex = 6; FOV, 35 mm; 128 × 64 matrix; slices = 20; thickness, 1 mm without spacing; duration, 18 min, 07 s.
Hematoma and perihematomal edema volume
Regions of interest (ROIs) were drawn around any blood clots and edematous tissue. ROI sizes were approximately equivalent to those of blood clots or edematous cross‐sectional areas on MRI images, as well as the thickness of each layer in the brain. The ROIs were positioned on T2 maps using T2‐weighted images as references. ROI conversion was performed on a pixel‐by‐pixel basis. A single observer drew and placed all of the ROIs. Multiple ROIs were placed over the blood clots and edematous areas in each layer in each sample. The volumes of all ROIs were calculated to assess blood clots and edematous areas and to generate quantitative data using the OsiriX digital analysis program on a Mac OS X computer (OsiriX Imaging Software, v8.5.0; OsiriX Foundation, Geneva, Switzerland).
Forelimb use asymmetry test
Forelimb use during exploratory activity was analyzed by videotaping the rats in a transparent cylinder (20‐cm diameter, 30‐cm height) for 3–10 min, depending on the degree of activity during the trial. A mirror was placed to the side of the cylinder at an angle to enable recording forelimb movements even when the animal was not facing the camera. Scoring was done by an experimenter blinded to animal condition used a video cassette recorder with slow‐motion and clear stop frame capabilities. behaviors were scored according to the following criteria: (i) independent use of the left or right forelimb for contacting the wall during a full rear to initiate a weight‐shifting movement or to regain the animal’s center of gravity while moving laterally in a vertical posture; and (ii) simultaneous use of the left and right forelimbs for contacting the cylinder wall during a full rear and for alternating lateral stepping movements along the wall.
Occasions when the unimpaired (ipsilateral) forelimb was used as a percentage of the total number of limb use observations on the wall (I) were quantified. Occasions when the impaired forelimb (contralateral to the blood injection site) was used as a percentage of the total number of limb use observations on the wall (C) were quantified. Furthermore, occasions when both forelimbs were used simultaneously (or nearly simultaneously during lateral side‐stepping movements) as a percentage of the total number of limb use observations on the wall (B) were also quantified. As previously (Hua et al. [25]), a single overall limb use asymmetry score was then calculated as follows: (I/[I + C + B]) − (C/[I + C + B]).
Morris water maze test
Animal spatial memory was tested via the Morris water maze 28 days after ICH induction. The water maze pool was 210 cm in diameter and 51 cm in height, had non‐reflective interior surfaces, and had no corrugated surfaces or other features that would provide proximal spatial cues. The training process consisted of two steps. In step 1 (day 1), the rats were placed on the platform (11 cm) for 1 min and then made to swim freely to the platform. If the rats did not reach the platform within 2 min, they were guided to the platform by the experimenter. Over the next four days, the platform was submerged to 1 cm below the surface of the water and the rats had to find and remember the location of the platform. Training consisted of two sessions per day. A different entry site was used for each daily session, and the rats were placed underwater to search for the platform. If the rats did not reach the platform within 2 min, they were guided to the platform by the experimenter and allowed to stay there for 10 s. The maximum search time was 2 min. In step 2 (day 7), the platform was removed, and the rats were placed in the entry site used for the most recent training period. The latency to reach the platform location was recorded with a maximum of 2 min.
Determination of ICAM‐1 and BDNF levels
Blood samples were collected in tubes with potassium acetate before injury and at selected times after the injury (24, 48, and 72 h; 7 days). Samples were then centrifuged at 3 000 g for 5 min, immediately frozen, and then stored at −80 °C. ICAM‐1 and BDNF levels were measured using commercially available quantitative sandwich enzyme‐linked immunosorbent assay kits (R&D System, Inc, Minneapolis, MN, USA).
Immunohistochemistry
Brain sections were dewaxed with xylene and dehydrated by ethanol at graded concentrations followed by distilled water. Sections were then incubated for 10 min in 3% hydrogen peroxide to block endogenous peroxidase activity. High‐temperature (boiled buffer) antigen retrieval was performed in a 0.01 m citrate buffer at a pH of 6.0 for 20 min. Brain sections were then incubated overnight at 4 °C with mouse anti‐ICAM1 (CD54), immunoglobulin G (IgG) (diluted 1 : 200, Thermo, Carlsbad, CA, USA), rabbit anti‐NeuN (diluted 1 : 500, Proteintech, Rosemont, IL, USA), or mouse anti‐BDNF IgG (diluted 1 : 200, Abcam, Cambridge, UK) in PBS containing 0.3% (v/v) Triton X‐100, followed by incubation in 37 °C EnVision (Zymed, South San Francisco, CA, USA) solution for 30 min. Finally, sections were incubated with peroxidase substrate diaminobenzidine until a desired staining intensity developed, followed by slight counterstaining with hematoxylin, dehydration, and cover‐slipping with permount. Between incubations, the tissue was washed with PBS three times for 10 min each. Photographing was performed using a microscope (Olympus BX43F, Tokyo, Japan) and ProgRes CapturePro software (Jenoptik Laser GmbH, Jena, Germany).
Cell counting
Counting of NeuN‐positive cells was done in the dorsal region of the CA1 region of the hippocampus. For each animal, at least four microphotographs of the whole hippocampus of both the left and right hemispheres were obtained (Figure 1). Images were captured with an Aperio AT2 (Leica Biosystems, Nußloch, Germany) and analyzed via Aperio software.
Figure 1.

Photomicrographs showing immunohistochemical staining (NeuN) of the rat hippocampal CA1 region at different magnification. The survival neurons with different intensity of NeuN staining in the hippocampal CA1.
Statistical analyses
Data are expressed as means ± standard errors in all plots. Difference between post‐ and pre‐injury outcomes were calculated and compared between the groups (control, simvastatin, and combination) using one‐way analyses of variance. Bonferroni’s post hoc comparisons were used to investigate pairwise comparisons between any two groups. Values of P < 0.05 were statistically significant. Data analyses were conducted using SPSS 22 (IBM, Armonk, NY, USA).
Results
ICAM‐1 and BDNF analyses
ICAM‐1 levels did not differ between groups (Figure 2a) and no intragroup differences (compared to pre‐ICH) were observed at any time point. Compared to the control group, significantly elevated BDNF levels were noted in the simvastatin and combination therapy groups (simvastatin–ezetimibe) at 24 h and 72 h (P = 0.026 and 0.044 for simvastatin; P = 0.002 and 0.024 for combined therapy, both respectively). BDNF levels were significantly elevated at 7 days in the simvastatin group as compared to the control group (P = 0.043) (Figure 2b).
Figure 2.

Analyses of ICAM‐1 revealing no significant differences among the control, simvastatin, and combination therapy groups (a). The simvastatin group exhibited significantly elevated BDNF levels at all time points, while the combination therapy group exhibited significantly elevated BDNF levels at the 24‐h and 72‐h time points (b). *P < 0.05 for Bonferroni post hoc comparison vs. the simvastatin group; P < 0.05 for Bonferroni post hoc comparison vs. the combination group. Data are presented as means ± standard errors. ICAM‐1, intercellular adhesion molecule‐1; BDNF, brain‐derived neurotrophic factor.
Neurological function analysis
Forelimb use asymmetry test revealed significant neurological function differences in the simvastatin and combination therapy groups and no intergroup differences (Figure 3a). The Morris water maze test revealed improved neurological functioning in the combined therapy group (Figure 3b).
Figure 3.

The two therapy groups exhibited significant improvements in neurological function (a). *P < 0.05 for Bonferroni post hoc comparison vs. the control group. The Morris water maze test demonstrated better neurological functioning in the combination therapy group than in the control group (b). *P < 0.05 for the Bonferroni post hoc comparison vs. the combination therapy group.
MRI findings
Hematomas did not change in size across groups (Figure 4a). In an analysis of perihematomal edema, only the combination therapy led to significant reductions in perihematomal edema on day 7 post‐injury (P < 0.05) (Figure 4b). In addition, no significant changes between pre‐ICH and day 3 levels of perihematomal edema volume occurred, though this did significantly decrease from day 3 to day 7 (Figure 4b).
Figure 4.

There were no significant reductions in hematoma size in the control, simvastatin, or combination therapy groups (a). An analysis of perihematomal edema revealed that only the combination therapy group had significant reductions in perihematomal edema on day 7 after injury (b). T2 map image, black arrow indicating hematoma and red arrow indicating perihematomal edema (c). *P < 0.05.
Immunohistochemistry
Immunohistochemistry (IHC) studies revealed greater ICAM‐1 expression in the control group and greater BDNF expression in both therapy groups (Figure 5).
Figure 5.

Increased intracellular adhesion molecule‐1 expression was noted in the control group (no treatment), and greater brain‐derived neurotrophic factor (BDNF) expression was noted in the therapy groups relative to the control group.
Loss of hippocampal CA1 neurons
Both treatment groups had significantly less CA1 neuron loss than the control group. The combination therapy group had significantly less CA1 neuron loss than the simvastatin group (Figure 6).
Figure 6.
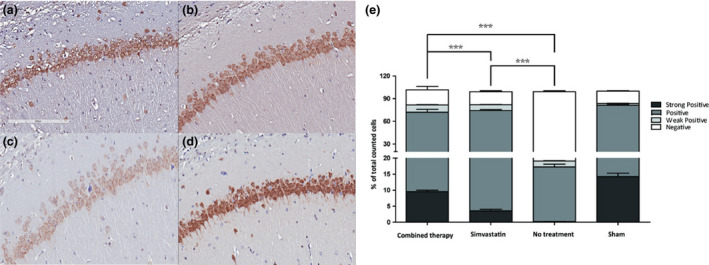
Photomicrographs of immunohistochemical staining of rat hippocampal CA1 in the (a) combination therapy, (b) simvastatin, (c) control, and (d) sham groups using antibodies specific for NeuN. (e) Cell number mean ± SEM (via Aperio software analysis) indicating different staining intensities across groups. The survival neurons with different intensity of NeuN staining were demonstrated in Figure 1. *P < 0.05, **P < 0.01, ***P < 0.005 (Student’s t‐test).
Discussion
Brain injuries after ICH are broadly divided into primary and secondary subtypes. After the sudden rupture of cerebral blood vessels, a hematoma rapidly forms and compression of the surrounding brain tissues occurs, leading to a sharp increase in intracranial pressure and primary brain injury [26]. Secondary brain injury following ICH is mediated by the primary injury (e.g., mass effect, high intracranial pressure, mechanical stress), as well as by the physiological response to the hematoma and the products of hematoma degradation, such as inflammation and cerebral edema [27].
In ICH, inflammation begins immediately after hematoma formation, and increasing evidence has shown that inflammation is a crucial contributor to ICH‐induced secondary brain injury [28]. The inflammatory mechanisms underlying ICH‐induced brain injury are complex and involve multiple signaling pathways. The inflammatory response is an important factor in brain injury after ICH and results in the loss of neurological function. Decreasing or preventing inflammation may be a potential treatment for patients with ICH [26]. Furthermore, the inflammation, thrombin activation, and erythrocyte lysis caused by primary injury may promote the formation of brain edema, which is associated with poor outcomes and more severe injury. Brain edema is a pathological phenomenon in which the brain’s water volume increases and which reflects the degree of brain injury caused by ICH. The mechanism underlying brain edema is complicated and involves vasogenic factors, thrombin formation, erythrocyte lysis, hemoglobin (Hb) toxicity, and inflammatory reactions [29]. To ameliorate the development of inflammatory reaction after ICH might reduce cerebral edema.
Our MRI results revealed significantly reduced cerebral edema after combination therapy. Perihemtomal edema (PHE) develops in response to clot retraction and a hydrostatic pressure changes [30], the mass effect, thrombin formation, erythrocyte lysis, Hb toxicity, complement activation, plasma protein leakage, and blood–brain barrier (BBB) disruption [29]. Inflammation, thrombin activation, and red blood cell lysis further contribute to BBB disruption, resulting in perihematomal edema formation. All of these pathological conditions are also related to the endothelial inflammatory response. Given the results of the MRI examination in the present study, a combination therapy could significantly attenuate the endothelial inflammatory response.
Hematoma size, which determines outcomes in humans and rats, can vary across ICH models with injected blood volume changes or heparin use. In the present study, the volume changes of hematoma between the different groups were not significant. Our MRI analysis revealed that only the combination therapy can reduce PHE on day 7 post‐injury. These finding are compatible with the combination therapy could significantly attenuate the endothelial inflammatory response.
During inflammatory processes, adhesion molecule expression on leukocytes and their ligands on endothelial cells in postcapillary venules increases [31]. Infiltration through the BBB involves leukocyte rolling, adhesion, and transendothelial migration. Given these known mechanisms and a prior study of TBI, we chose ICAM‐1 as a biomarker of the endothelial inflammatory response in the present study [6]. The expression of ICAM‐1 did not differ statistically between the control and therapy groups in the present study. However, given our IHC results, decreased expression of ICAM‐1 occurred in the combination therapy group.
Neurotrophins, endogenous peptides secreted from neuronal and glial cells, are also associated with functional regulation, survival, and the development of individual cells and neuronal networks across the brain. The neurotrophin family of proteins includes nerve growth factor (NGF), BDNF, neurotrophin‐3, and neurotrophin‐4/5, which are classified together based on their structural similarity to NGF, the first neurotrophin discovered [32]. Neurotrophins are able to exert their neuroprotective effect through the transmembrane receptors that they bind to and via the signaling cascades they initiate [33]. For instance, a TBI study found that circulating BDNF is suppressed in TBI and that low BDNF values are associated with poor recovery, suggesting that BDNF deserves further evaluation as a potential biomarker of TBI damage and recovery [21]. In a recent study, BDNF levels were also associated with clinical prognosis in the acute phase of ischemic stroke [34]. Based on these prior reports, we chose BDNF as a biomarker of the experimental ICH model used here. We found that simvastatin and a combination therapy led to significant elevations in BDNF. Given its properties, BDNF is more abundant in circulation than other structural proteins, increasing assay sensitivity. The present study data demonstrated a significant effect of drugs on BDNF in an ICH model. Although circulating BDNF may originate from the hippocampus, cerebral cortex, and basal forebrain [35], it may also derive from other cellular sources, including platelets [36], smooth muscle cells, and vascular endothelial cells [37]. BDNF levels could therefore reflect the degree of vascular endothelium and intracerebral structure destruction.
Neuronal loss in the hippocampal CA1 after ICH may have been due to secondary damage to hippocampal CA1 neurons. Previous studies have demonstrated that secondary brain injury, rather than primary mechanical injury, contributes to some serious complications following ICH [38, 39]. Hematoma formation within the brain activates a coagulation cascade which leads to the production of thrombin, which may induce the release of pro‐inflammatory cytokines [40]. The inflammatory response may therefore exacerbate neuronal impairment. The treatment groups assessed here exhibited less neuron loss and are consistent with attenuated inflammatory responses relative to controls. Another possible mechanism underlying the neuron loss noted in the present study may have been edema formation after ICH. Perihematomal edema further indicates functional and morphological alterations in cerebral capillaries that could result in tissue hypoxia and hippocampal neuron damage [41, 42]. BDNF is the major neurotrophic factor that controls of the functioning of the hippocampus [43], therefore elevated BNDF can decrease neuron loss in the hippocampus in the therapy groups.
The results of the present study demonstrate that simvastatin and combination therapy attenuated the endothelial inflammatory response and perihematomal edema, and improved neurological function in a rat model of ICH. Both treatment groups had significantly less neuron loss than the control group. Notably the combination therapy group had significantly less neuron loss than the simvastatin group.
Before these results can be translated into clinical domains, several aspects require some further understanding. Given the safety concerns associated with the side effect from the high‐dose statin therapy, an additional lipid‐modifying therapy is considered. When added to statins, ezetimibe reduces LDL cholesterol levels by an additional 23‐24%, and side effects such as myotoxicity and/or rhabdomyolysis traditionally caused by statin treatment alone are not noted with combination therapy [38]. Statins also have numerous vasculoprotective properties that lead to overall improvements in endothelial function [44, 45]. Ezetimibe could provide anti‐inflammatory activity of ezetimibe by regulating NF‐κB/MAPK pathway [46]. Such combination therapy could therefore attenuate the endothelial inflammatory response from our results, though differences in simvastatin dose and ezetimibe effect should be examined. Notably, Ezetimibe is absorbed into the circulation and acts locally by inhibiting cholesterol absorption from the small intestine. This drug could therefore provide significant anti‐inflammatory effects by simply administration according to our results. Despite this promise, however, the mechanism(s) underlying ezetimibe efficacy in ICH requires further investigation.
In conclusions, the present study demonstrate that combination therapy may attenuate the endothelial inflammatory response and perihematomal edema in a model of ICH. However, differences between the effects of simvastatin and combination therapy on the endothelial inflammatory response may require further investigation. Furthermore, between the two biomarkers we selected for the evaluation of the endothelial inflammatory response and neurological function after experimental ICH, we found that BDNF was a potential predicting biomarker for ICH.
Funding
This study was supported by E‐DA Hospital Research Grants (EDCHP107003 and EDCHP108005).
Conflict of Interest
The authors declare no conflict of interest.
Authorship Contribution
K‐W Wang: Conceptualization, Methodology, Investigation, Data curation, Manuscript preparation. L‐R Yeh: Methodology, Data curation. K‐Y Liu: Software, Methodology, Data curation, Manuscript preparation. C‐C Chen: Methodology, Software, Data curation. J‐S Chen: Methodology, Investigation, Data curation. H‐K Wang: Methodology, Investigation. H‐J Chen: Conceptualization, Investigation. C‐L Liang: Conceptualization, Methodology, Data curation, Manuscript preparation.
Consent for Publication
No individual person’s data (including any individual details, images, or videos) are cited in the paper.
Wang KW, Liang CL, Yeh LR, et al. Simvastatin–Ezetimibe enhances growth factor expression and attenuates neuron loss in the hippocampus in a model of intracerebral hemorrhage. Fundam Clin Pharmacol. 2021;35(4):634–644. 10.1002/fcp.12635
References
- 1.Cannon J.R., Xi G., Keep R.F.Recent research on changes in genomic regulation and protein expression in intracerebral haemorrhage. Int. J. Stroke (2007) 2 265–269. [DOI] [PubMed] [Google Scholar]
- 2.Chen J., Zhang Z.G., Li Y. et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann. Neurol. (2003) 53 743–751. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L., Zhang Z.G., Ding G.L. et al. Multitargeted effects of statin‐enhanced thrombolytic therapy for stroke with recombinant human tissue‐type plasminogen activator in the rat. Circulation (2005) 112 3486–3494. [DOI] [PubMed] [Google Scholar]
- 4.Lu D., Mahmood A., Qu C., Goussev A., Lu M., Chopp M.Atorvastatin reduction of intracranial hematoma volume in rats subjected to controlled cortical impact. J. Neurosurg. (2004) 101 822–825. [DOI] [PubMed] [Google Scholar]
- 5.Lu D., Qu C., Goussev A. et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J. Neurotrauma (2007) 24 1132–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang K.W., Wang H.K., Chen H.J. et al. Simvastatin combined with antioxidant attenuates the cerebral vascular endothelial inflammatory response in a rat traumatic brain injury. Biomed. Res. Int. (2014) 2014 910260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch J.R., Wang H., McGirt M.J. et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke (2005) 36 2024–2026. [DOI] [PubMed] [Google Scholar]
- 8.Seyfried D., Han Y., Lu D., Chen J., Bydon A., Chopp M.Improvement in neurological outcome after administration of atorvastatin following experimental intracerebral hemorrhage in rats. J. Neurosurg. (2004) 101 104–107. [DOI] [PubMed] [Google Scholar]
- 9.Cannon C.P., Braunwald E., McCabe C.H. et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. (2004) 350 1495–1504. [DOI] [PubMed] [Google Scholar]
- 10.de Lemos J.A., Blazing M.A., Wiviott S.D. et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA (2004) 292 1307–1316. [DOI] [PubMed] [Google Scholar]
- 11.Preiss D., Seshasai S.R., Welsh P. et al. Risk of incident diabetes with intensive dose compared with moderate‐dose statin therapy: a meta‐analysis. JAMA (2011) 305 2556–2564. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg H.N., Elam M.B., Lovato L.C. et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. (2010) 362 1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landray M.J., Haynes R., Hopewell J.C. et al. Effects of extended‐release niacin with laropiprant in high‐risk patients. N. Engl. J. Med. (2014) 371 203–212. [DOI] [PubMed] [Google Scholar]
- 14.Sudhop T., Lütjohann D., Kodal A. et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation (2002) 106 1943–1948. [DOI] [PubMed] [Google Scholar]
- 15.Kosoglou T., Meyer I., Veltri E.P. et al. Pharmacodynamic interaction between the new selective cholesterol absorption inhibitor ezetimibe and simvastatin. Br. J. Clin. Pharmacol. (2002) 54 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krysiak R., Zmuda W., Okopien B.The effect of simvastatin–ezetimibe combination therapy on adipose tissue hormones and systemic inflammation in patients with isolated hypercholesterolemia. Cardiovasc. Ther. (2014) 32 40–46. [DOI] [PubMed] [Google Scholar]
- 17.Reichenbach J.R., Venkatesan R., Yablonskiy D.A., Thompson M.R., Lai S., Haacke E.M.Theory and application of static field inhomogeneity effects in gradient‐echo imaging. J. Magn. Reason. Imaging (1997) 7 266–279. [DOI] [PubMed] [Google Scholar]
- 18.von Kummer R.MRI: the new gold standard for detecting brain hemorrhage? Stroke (2002) 33 1748–1749. [DOI] [PubMed] [Google Scholar]
- 19.Karki K., Knight R.A., Han Y. et al. Simvastatin and atorvastatin improve neurological outcome after experimental intracerebral hemorrhage. Stroke (2009) 40 3384–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight R.A., Han Y., Nagaraja T.N. et al. Temporal MRI assessment of intracerebral hemorrhage in rats. Stroke (2008) 39 2596–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korley F.K., Diaz‐Arrastia R., Wu A.H.B. et al. Circulating brain‐derived neurotrophic factor has diagnostic and prognostic value in traumatic brain injury. J. Neurotrauma (2016) 33 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang T., Chen Q., Li Q. et al. 5‐HT1a activation in PO/AH area induces therapeutic hypothermia in a rat model of intracerebral hemorrhage. Oncotarget. (2017) 26 73613–73626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh K.K., Oh P.C., Sakuma I. et al. Vascular and metabolic effects of ezetimibe combined with simvastatin in patients with hypercholesterolemia. Int. J. Cardiol. (2015) 199 126–131. [DOI] [PubMed] [Google Scholar]
- 24.Barbosa C.P., Ritter A.M., da Silva L.G. et al. Effects of simvastatin, ezetimibe, and their combination on istopathologic alterations caused by adjuvant‐induced arthritis. Inflammation (2014) 37 1035–1043. [DOI] [PubMed] [Google Scholar]
- 25.Hua Y., Schallert T., Keep R.F., Wu J., Hoff J.T., Xi G.Behavioral tests after intracerebral hemorrhage in the rat. Stroke (2002) 33 2478–2484. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y., Wang Y., Wang J., Anne Stetler R., Yang Q.W.Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog. Neurogibol. (2014) 115 25–44. [DOI] [PubMed] [Google Scholar]
- 27.Chen S., Yang Q., Chen G., Zhang J.H.An update on inflammation in the acute phase of intracerebral hemorrhage. Transl. Stroke Res. (2015) 6 4–8. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Wang T., Zhang D. et al. Andrographolide ameliorates intracerebral hemorrhage induced secondary brain injury by inhibiting neuroinflammation induction. Neuropharmacology (2018) 141 305–315. [DOI] [PubMed] [Google Scholar]
- 29.Hoff J.T., Xi G.Brain edema from intracerebral hemorrhage. Acta Neurochir. Suppl. (2003) 86 11–15. [DOI] [PubMed] [Google Scholar]
- 30.Wagner K.R., Xi G., Hua Y. et al. Lobar intracerebral hemorrhage model in pigs: rapid edema development in perihematomal white matter. Stroke (1996) 27 490–497. [DOI] [PubMed] [Google Scholar]
- 31.Muller W.A.Leukocyte‐endothelial cell interactions in the inflammatory response. Lab. Invest. (2002) 82 521–534. [DOI] [PubMed] [Google Scholar]
- 32.Skaper S.D.The neurotrophin family of neurotrophic factors: an overview. Methods Mol. Biol. (2012) 846 1–12. [DOI] [PubMed] [Google Scholar]
- 33.Wurzelmann M., Romeika J., Sun D.Therapeutic potential of brain‐derived neurotrophic factor (BDNF) and a small molecular mimic of BDNF for traumatic brain injury. Neural. Regen. Res. (2017) 12 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mourão A.M., Vicente L.C.C., Abreu M.N.S. et al. Plasma levels of brain‐derived neurotrophic factor are associated with prognosis in the acute phase of ischemic stroke. J. Stroke Cerebrovasc. Dis. (2019) 28 735–740. [DOI] [PubMed] [Google Scholar]
- 35.Yamada K., Nabeshima T.Brain‐derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. (2003) 91 267–270. [DOI] [PubMed] [Google Scholar]
- 36.Fujimura H., Altar C.A., Chen R. et al. Brain‐derived neurotrophic factor is sored in human platelets and released by agonist stimulation. Thromb. Haemost. (2002) 87 728–734. [PubMed] [Google Scholar]
- 37.Lommatzsch M., Braun A., Mannsfeldt A. et al. Abundant production of brain‐derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target‐derived Neurotrophic functions. Am. J. Pathol. (1999) 155 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ducruet A.F., Zacharia B.E., Hickman Z.L. et al. The complement cascade as a therapeutic target in intracerebral hemorrhage. Exp. Neurol. (2009) 219 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiex R., Tsirka S.E.Brain edema after intracerebral hemorrhage: Mechanisms, treatment options, management strategies, and operative indications. Neurosurg. Focus (2007) 22 E6. [DOI] [PubMed] [Google Scholar]
- 40.Hua Y., Wu J., Keep R.F., Nakamura T., Hoff J.T., Xi G.Tumor necrosis factor‐alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery (2006) 58 542–550. [DOI] [PubMed] [Google Scholar]
- 41.Anzabi M., Ardalan M., Iversen N.K., Rafati A.H., Hansen B., Østergaard L.Hippocampal atrophy following subarachnoid hemorrhage correlates with disruption of astrocyte morphology and capillary coverage by AQP4. Front Cell Neurosci. (2018) 12 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Østergaard L., Aamand R., Karabegovic S. et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. (2013) 33 1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erickson K.I., Miller D.L., Roecklein K.A.The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist (2012) 18 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stapleton P.A., Goodwill A.G., James M.E., Brock R.W., Frisbee J.C.Hypercholesterolemia and microvascular dysfunction: interventional strategies. J Inflammation (2010) 7 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearson T.A., Ballantyne C.M., Veltri E. et al. Pooled analyses of effects on C‐reactive protein and low density lipoprotein cholesterol in placebo‐controlled trials of ezetimibe monotherapy or ezetimibe added to baseline statin therapy. Am. J. Cardiol. (2009) 103 369–374. [DOI] [PubMed] [Google Scholar]
- 46.Qin L., Yang Y.B., Yang Y.X. et al. Anti‐inflammatory activity of ezetimibe by regulating NF‐κB/MAPK pathway in THP‐1 macrophages. Pharmacology (2014) 93 69–75. [DOI] [PubMed] [Google Scholar]


