Abstract
Recently, the Klotho protein (Klotho) has received substantial attention as protective factor against cardiovascular complications of chronic kidney disease (CKD). However, the direct effect and mechanism of Klotho on endothelial cells injury are not well‐known. In this study, we incubated human vein umbilical endothelial cells (HUVECs) with uremic toxin indoxyl sulfate (IS) to mimic CKD internal environment and investigated the direct effect of Klotho on the HUVECs injury induced by IS and to explore the mechanism in this process. We found IS inhibited cell viability, increased endoplasmic reticulum stress, and mediated apoptosis of HUVECs. Treatment with Klotho significantly attenuated IS‐induced above effects. Furthermore, Klotho alleviated the IS toxic effect on HUVECs via promoting AMP‐activated protein kinase (AMPK) α1 phosphorylation instead of directly upregulating AMPKα1, which could be partly blocked by AMPK pathway inhibitor‐Compound C. In addition, Klotho also inhibited intercellular adhesion molecule‐1 (ICAM‐1) and vascular cell adhesion molecule‐1 (VCAM‐1) expression induced by IS. Altogether, these results indicated that Klotho can protect HUVECs from IS‐induced injury by alleviating AMPKα1‐mediated endoplasmic reticulum stress.
Keywords: AMP‐activated protein kinase α1, endoplasmic reticulum stress, indoxyl sulfate, Klotho protein, uremic toxin
Short abstract
Indoxyl sulfate (IS) injured human vein umbilical endothelial cells (HUVECs) and increased endoplasmic reticulum stress and mediated apoptosis of HUVECs. Klotho attenuated IS‐induced above effects via promoting AMP‐activated protein kinase (AMPK) α1 phosphorylation instead of directly upregulating AMPKα1.
1. INTRODUCTION
The morbidity and mortality related to cardiovascular disease are much higher in patients with chronic kidney disease (CKD) than in the general population, and these patients are prone to major adverse cardiovascular events (MACE), such as heart failure, myocardial infarction, and cerebrovascular disease (Mathew et al., 2017). Atherosclerotic cardiovascular disease (ASCVD) is more likely to occur in CKD patients and is also one of the important mechanisms of MACE. Atherosclerosis occurs at the early stage of CKD and develops rapidly; this is called “accelerated atherosclerosis.” Injury and dysfunction of endothelial cells are the starting points of atherosclerosis (Cibor et al., 2016) and the most important factors in the progression of atherosclerosis (Dharmashankar & Widlansky, 2010).
Indoxyl sulfate (IS) is a dietary metabolite of tryptophan, which acts as a uremic toxin in kidney‐impaired patients. As renal function declines, IS accumulates in the body. IS cannot be effectively removed by conventional dialysis and brings side effects in the state of uremia. It has been found that IS exhibits vascular toxicity, which can induce oxidative stress, stimulate endothelial particle release, delay wound healing, and damage vascular endothelial cells.
The anti‐aging gene Klotho gene (Kl gene) was originally discovered in studies of aging‐related organ failure. Kl gene overexpression can significantly extend the lives of organisms. The phenotype of Kl gene knockout mice is very similar to the clinical manifestations of CKD, suggesting that CKD might be a state of Kl gene deficiency (Hu, Kuro‐o, & Moe, 2013), and anti‐aging treatment may be a new approach for delaying CKD and the development of its complications (Kooman et al., 2013). Our previous study has shown that supplementation of exogenous Klotho protein (Klotho) protects vascular endothelial cells from uremia serum (Chen et al., 2015). However, the mechanisms remain to be elucidated.
AMP‐activated protein kinase (AMPK) is a signaling molecule that maintains endothelial homeostasis and protects cells from injury and stress, and AMPKα1 is the catalytic isoform expressed in vascular cells and leukocytes (Fisslthaler & Fleming, 2009). Compound C is the inhibitor of AMPK, which inhibits AMPK phosphorylation. The endoplasmic reticulum (ER) is an important organelle in the cytoplasm. Some damage factors disrupt the steady state in the ER and induce endoplasmic reticulum stress (ERS). Excessive ERS, characterized by the elevation of glucose‐regulated protein 78 (GRP78), eventually causes the activation of apoptosis signaling pathway, and CCAAT/enhancer binding protein homologous protein (CHOP) is involved in ERS‐mediated apoptosis as a transcription factor. Atherosclerosis is an inflammatory disease. Intercellular adhesion molecule‐1 (ICAM‐1) and vascular cell adhesion molecule‐1 (VCAM‐1), secreted by activated endothelial cells, may mediate the initial adhesion between endothelial cells and leukocytes and platelets, which play an important role in the development of atherosclerosis.
This study was to investigate the viability and apoptosis of human umbilical vein endothelial cells (HUVECs) in the presence of IS with or without Klotho intervention and also to explore the role of AMPKα1 expression, AMPKα1 phosphorylation, AMPKα1‐mediated ERS, and ICAM‐1 and VCAM‐1 expressions in the toxic effect of IS on HUVECs and the possible protective mechanism of Klotho.
2. MATERIALS AND METHODS
2.1. Reagents and antibodies
HUVECs were purchased from ATCC (Manassas, VA, USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, penicillin, and streptomycin were purchased from Gibco BRL (Grand Island, NY, USA). The cell counting kit 8 (CCK 8) detection kit (Beyotime, Shanghai, China) was used to assess cell viability. The total AMPKα1 (t‐AMPKα1) rabbit antibody, phosphorylated AMPKα1 (p‐AMPKα1) rabbit antibody, and CHOP mouse antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). The AMPK inhibitor Compound C was purchased from Selleck Chemicals (Houston, TX, USA). The GRP78 rat antibody was purchased from Santa Cruz (Dallas, TX, USA). The anti‐cleaved caspase‐3 antibody was purchased from Abcam (Cambridge, MA, USA). The ICAM‐1 rabbit antibody and VCAM‐1 mouse antibody were purchased from Proteintech (Wuhan, Hubei, China). The anti GAPDH antibody was purchased from Wuhan Boster Biological Technology (Wuhan, Hubei, China). The anti‐β‐actin antibody was purchased from Cell Signaling Technology (Danvers, MA, USA). The sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE) gels were purchased from Beyotime Institute of Biotechnology (Shanghai, China). The recombinant Klotho and IS were purchased from PeproTech (Rocky Hill, NJ, USA) and Sigma‐Aldrich (St. Louis, MO, USA), respectively. The Annexin V Fluorescein Propidium Iodide Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA) was used to assess the levels of cell apoptosis using flow cytometry. Hoechst 33342 was purchased from Beyotime Institute of Biotechnology (Shanghai, China). The polymerase chain reaction (PCR) primers were purchased from Ruizheng Biology (Nanjing, China). The AMPKα1 siRNA and nonspecific siRNA were purchased from GenePharma (Shanghai, China).
2.2. Cell culture
The HUVECs were routinely cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C in a humidified atmosphere with 5% CO2. All the experiments were conducted using cells at Passages 5–10.
2.3. CCK‐8 assay
To investigate the inhibitory effect of different concentrations and time points of IS and protective effect of Klotho on viability of HUVECs, cells were seeded in 96‐well plates at a density of 10 × 104 cells/well and cultured at 37°C. After the cells had completely adhered and reached a confluence of 70%, the culture medium was removed and the cells treated with different concentrations of IS (0, 5, 25, and 50 μg/mL) for 48 h or 50 μg/mL IS for different time (0, 12, 24, and 48 h) and 0, 1, 10, and 100 μg/L Klotho preincubated for 1 h and then incubated with 50 μg/mL IS for 48 h. Then, 10 μL CCK‐8 reagent was added, and the cells were cultured at 37°C a further 4 h. Optical density was measured using a microplate reader (OD = 450 nm; Bio Rad Laboratories, Inc., Hercules, CA, USA).
2.4. Small interference RNA transfection
To evaluate the role of AMPKα1 in IS‐induced ERS and the regulation of Klotho, AMPKα1 small interference RNA (siRNA) was synthesized by GenePharma (Shanghai, China). AMPKα1 sense siRNA sequence is 5′‐GCUUGAUGCACACAUGAAUTT‐3′. AMPKα1 antisense siRNA sequence is 5′‐AUUCAUGUGUGCAU CAAGCTT‐3′. As a control (Sicntl), nonspecific siRNA (sense: 5′‐UUCUCCGAACGUGUCACGUTT‐3′, antisense: 5′‐ACGUGACACGU UCGGAGAATT‐3′) was used. Chemically synthesized siRNAs were transfected according to the manufacturer's specifications using Lipofectamine 2000 (Invitrogen, Paisley, UK). Briefly, cells were seeded into six‐well plates and incubated under their normal growth conditions to 70% confluence; 100‐nM siRNA was diluted in 400‐μL culture medium without serum, and 4‐μL Lipofectamine 2000 were added into it. Cells were incubated with the transfection complexes for 20 min at room temperature before mixed into 1.6‐mL fresh culture medium without fetal bovine serum. After 8 h it was replaced by fresh culture medium with 10% fetal bovine serum. Then, the cells were exposed to 50 μg/mL IS or 50 μg/mL IS and 100 μg/L Klotho (preincubated for 1 h) for 48 h. Subsequently, cells were used for assessment.
2.5. Flow cytometry analysis
HUVECs were plated on to six‐well culture plates (10 × 104 cells/well) and cultured at 37°C. After the cells had completely adhered and reached a confluence of 70%, the cells were exposed to 50 μg/mL IS or 50 μg/mL IS and 100 μg/L Klotho (preincubated for 1 h) or 50 μg/mL IS, 100 μg/L Klotho (preincubated for 1 h), and 10 μmol/L AMPK inhibitor Compound C (Selleck Chemicals, Houston, TX, USA) for 48 h. Then, cells were harvested and washed twice in phosphate‐buffered saline. They were suspended and stained using the Annexin V Fluorescein Propidium Iodide Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA). Briefly, cells were suspended with 1× binding buffer at a concentration of 2 × 105 cells per 100 μL. Five microliters of FITC Annexin V and propidium iodide (PI) were added to each solution and incubated for 10 min at room temperature in the dark. Subsequently, we detected the rate of apoptosis by flow cytometry (BD Biosciences, San Jose, CA, USA) at an excitation wavelength of 488 nm and emission wavelength of 530 nm for FITC fluorescence and 610 nm for PI fluorescence. The percentage of viable (PI−, Annexin−), apoptotic (PI−, Annexin+), and necrotic cells (PI+, Annexin+) was evaluated with CellQuest Pro software (BD Biosciences, San Jose, CA, USA).
2.6. Hoechst staining
To detect cell apoptosis and observe the nucleus morphology, HUVECs were plated on to 12‐well culture plates (10 × 104 cells/well) and cultured at 37°C. After the cells had completely adhered and reached a confluence of 70%, cells were grouped as in the flow cytometry analysis experiment section. After 48 h, the cells were stained by Hoechst 33342 (Beyotime Institute of Biotechnology, Shanghai, China). Briefly, cells were washed by phosphate‐buffered saline twice before fixed with 4% paraformaldehyde and then incubated with 500‐μL Hoechst 33342 for 15 min at room temperature in the dark. Subsequently, cells were washed twice with double distilled water, and chromatin condensation was observed under a fluorescence microscope (ZEISS, Jena, Germany).
2.7. Quantitative real‐time PCR analysis
To evaluate the CHOP and GRP78 mRNA expressions, HUVECs were grouped as in the experiment section describing the flow cytometry analysis or small interference RNA transfection experiment. After incubation for 48 h, total RNA was extracted utilizing TRIzol (TaKaRa, Japan). The concentration of the isolated RNA was determined by measurement of the absorbance at 260 nm, and the purity was calculated using A260/A280. The cDNA was synthesized with Prime Script RT Master Mix (TaKaRa, Japan) and used for qPCR with SYBR Premix Ex Taq II (TaKaRa, Japan) on real‐time PCR detection system (Roche Diagnostics, Mannheim, Germany). The relative expression levels of GRP78 and CHOP mRNA were analyzed using the 2 − ΔΔCt method and normalized using GAPDH as a control. The results were presented as fold increase relative to the control. The primers used for qPCR were as follows:
GRP78: Forward 5′‐CATCACGCCGTCCTATGTCG‐3′, Reverse 5′‐CGTCAAAGACCGTGTTCTCG‐3′;
CHOP: Forward 5′‐GGAAACAGAGTGGTCATTCCC‐3′, Reverse 5′‐CTGCTTGAGCCGTTCATTCTC‐3′;
GAPDH: Forward 5′‐AGAAGGCTGGGGCTCATTTG‐3′, Reverse 5′‐AGGGGCCATCCACAGTC TTC‐3′.
2.8. Immunoblotting
To evaluate the CHOP, GRP78, t‐AMPKα1, p‐AMPKα1, ICAM‐1, VCAM‐1, and caspase‐3 protein expressions, HUVECs were grouped as in the experiment section describing the flow cytometry analysis or small interference RNA transfection experiment. After incubation for 48 h, the proteins from the cells were homogenized in lysis buffer and quantified. The proteins (30 μg) from each sample were separated using SDS‐PAGE (the percentage of the spacer gel and separation gel was 8% and 10%, respectively) and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk for 2 h and then incubated overnight at 4°C with the corresponding primary antibodies (CHOP: 1:1000, t‐AMPKα1: 1:1000, p‐AMPKα1: 1:1000, caspase‐3: 1:1000, ICAM‐1: 1:1000, VCAM‐1: 1:1000, and GRP78 1:200). Subsequently, the corresponding secondary antibodies (1:5000) were added, and the signals were developed with an ECL plus Western blotting detection system (Bio Rad Laboratories, Inc., Hercules, CA, USA). The densitometric analysis was performed with Image J software version 1.41 (National Institutes of Health, Bethesda, MD, USA).
2.9. Statistical analysis
Statistical analyses were performed using SPSS 20.0 (SPSS, Inc., Chicago, IL, USA). The values were expressed as the mean ± standard deviation. Multiple comparisons were evaluated using one‐way analysis of variance (ANOVA), and significant differences between two groups were analyzed using the Student Newman Keuls test. P < 0.05 was considered to indicate a statistically significant difference.
3. RESULTS
3.1. IS inhibited the viability of HUVECs in a concentration‐ and time‐dependent manner
Compared with that in the control group, the viability of the HUVECs in the 5, 25, and 50 μg/mL IS groups was decreased, and there were significant differences between the control and 25 and 50 μg/mL IS groups. Compared with that in the control group, the viability of the HUVECs in the 50 μg/mL IS group after 12, 24, and 48 h was decreased, and there were significant differences between the control and 24‐ and 48‐h groups. Klotho alleviated the IS‐induced reductions in HUVECs viability. Compared with that in the 50 μg/mL IS group, the viability of the HUVECs in the 100 μg/L Klotho group increased (Figure 1).
FIGURE 1.
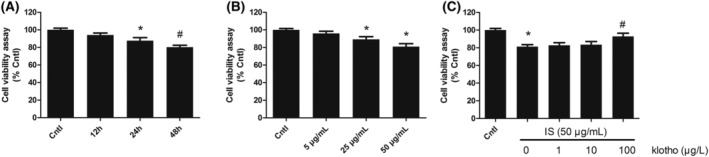
Effects of IS and Klotho on HUVECs viability. Cell viability was detected by CCK‐8 assay. (A) Cells were exposed to 50 μg/mL of IS for 12, 24, and 48 h. (B) Cells were exposed to various concentrations of IS (5, 25, and 50 μg/mL) for 48 h. (C) Cells were pretreated with Klotho (1, 10, and 100 μg/L) for 1 h, then exposed to IS (50 μg/mL) for 48 h. Data were shown as mean ± SD. Statistical differences were expressed as *P < 0.01 versus control; #P < 0.01 versus IS alone. HUVECs, human vein umbilical endothelial cells; IS, indoxyl sulfate
3.2. Klotho inhibited the IS‐stimulated expression of GRP78 and CHOP, and AMPKα1 knockdown blocked this effect of Klotho in HUVECs
Compared with those in the control group, the expression levels of GRP78 and CHOP mRNA and protein in the IS group were markedly increased. Silencing AMPKα1 further increased the expression of GRP78 and CHOP. Klotho treatment decreased the mRNA and protein expression of GRP78 and CHOP. This inhibitory effect of Klotho was blocked by AMPKα1 knockdown (Figure 2).
FIGURE 2.
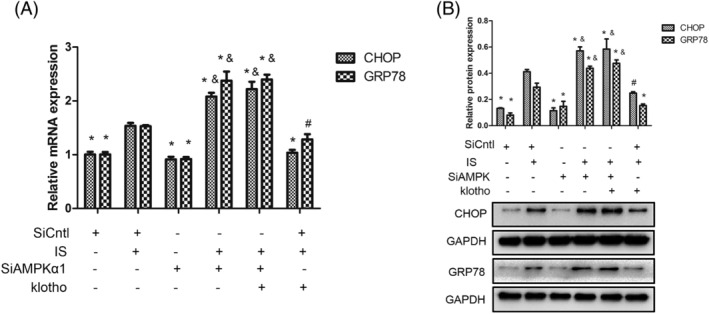
Effects of IS and Klotho on the expression of GRP78 and CHOP in HUVECs. Cells were pretreated with Klotho (100 μg/L) for 1 h, then incubated with IS (50 μg/mL) for further 48 h, followed by transfected with AMPKα1 SiRNA (SiAMPKα1) or scrambled siRNA (SiCntl). (A) Quantitative polymerase chain reaction (qPCR) analysis for GRP78 and CHOP. (B) Western blotting analysis for GRP78 and CHOP. Data were shown as mean ±SD. Statistical differences were expressed as * P < 0.01 versus corresponding IS group; #P < 0.05 versus corresponding IS group; &P < 0.01 versus corresponding IS and Klotho treatment together. HUVECs, human vein umbilical endothelial cells; IS, indoxyl sulfate; AMPKα1, AMP‐activated protein kinase α1; GRP78, glucose‐regulated protein 78; CHOP, CCAAT/enhancer binding protein homologous protein
3.3. Klotho inhibited the IS‐mediated reduction of p‐AMPKα1 in HUVECs
Compared with that in the IS group, the expression of t‐AMPKα1 was not affected by Klotho; however, p‐AMPKα1 was markedly increased. Furthermore, this effect of Klotho was blocked by Compound C. Given that Klotho could not influence the expression of t‐AMPKα1, these results indicated that AMPKα1 was involved in ERS and that the inhibitory effect of Klotho was dependent on the phosphorylation of AMPKα1 rather than on the direct upregulation of AMPKα1 expression (Figure 3A).
FIGURE 3.
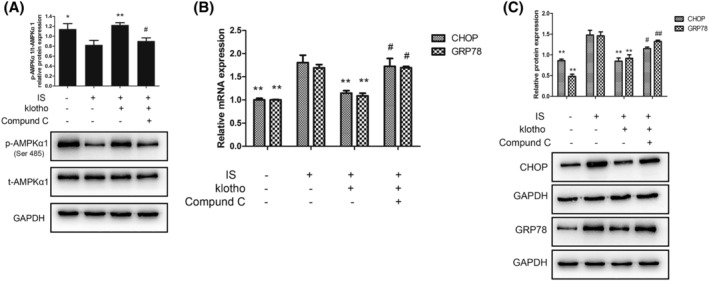
(A) Effects of IS and Klotho on the phosphorylation of AMPKα1. Cells were pretreated with Klotho (100 μg/L) for 1 h, then incubated with IS (50 μg/mL) for further 48 h with or without Compound C (10 μmol/L). (A) Western blotting analysis for p‐AMPKα1 and t‐AMPKα1. Data were shown as mean ± SD. Statistical differences were expressed as * P < 0.05 versus IS alone; ** P < 0.01 versus IS alone; #P < 0.05 versus IS and Klotho treatment together; ##P < 0.01 versus IS and Klotho treatment together. (B,C) Effects of IS and Klotho on the expression of GRP78 and CHOP in HUVECs. Cells were pretreated with Klotho (100 μg/L) for 1 h, then incubated with IS (50 μg/mL) for further 48 h with or without Compound C (10 μmol/L). (B) Quantitative polymerase chain reaction (qPCR) analysis for GRP78 and CHOP. (C) Western blotting analysis for GRP78 and CHOP. Data were shown as mean ± SD. Statistical differences were expressed as *P < 0.05 versus IS alone; **P < 0.01 versus IS alone; #P < 0.05 versus IS and Klotho treatment together; ##P < 0.01 versus IS and Klotho treatment together. HUVECs, human vein umbilical endothelial cells; IS, indoxyl sulfate; t‐AMPKα1, total AMP‐activated protein kinase α1; p‐AMPKα1, phosphorylated AMP‐activated protein kinase α1; GRP78, glucose‐regulated protein 78; CHOP, CCAAT/enhancer binding protein homologous protein
3.4. Klotho inhibited the IS‐induced expression of GRP78 and CHOP, and Compound C blocked this effect of Klotho in HUVECs
Compared with those in the control group, the expression levels of GRP78 and CHOP mRNA and protein in the IS group were markedly upregulated. Klotho treatment reduced the mRNA and protein expression of GRP78 and CHOP. This inhibitory effect of Klotho was blocked by Compound C (Figure 3B,C).
3.5. Klotho inhibited the apoptosis of HUVECs induced by IS
Compared with that in the control group, the apoptosis of the HUVECs in the IS group increased. The apoptotic rates of the IS group and control group were 7.27 ± 0.57% and 1.52 ± 0.12%, respectively, and this difference was statistically significant. The apoptotic rate was reduced when the HUVECs were treated with 100 μg/L Klotho, resulting in an apoptotic rate of 4.67 ± 0.37% (P < 0.01, compared with the IS group). In addition, the anti‐apoptotic effect of Klotho was blocked by the AMPK inhibitor Compound C. In the Compound C group, the apoptotic rate was 6.05 ± 0.12%, which was significantly different from that in the Klotho group (Figure 4A). Furthermore, the expression of caspase‐3 (an important apoptotic protein) increased in the IS group. Klotho decreased the expression of caspase‐3 (Figure 4B). In addition, Hoechst staining to detect the morphological alterations of the HUVECs showed that compared with the control and Klotho treatment, IS treatment led to significant apoptosis with chromatin condensation (Figure 4C).
FIGURE 4.
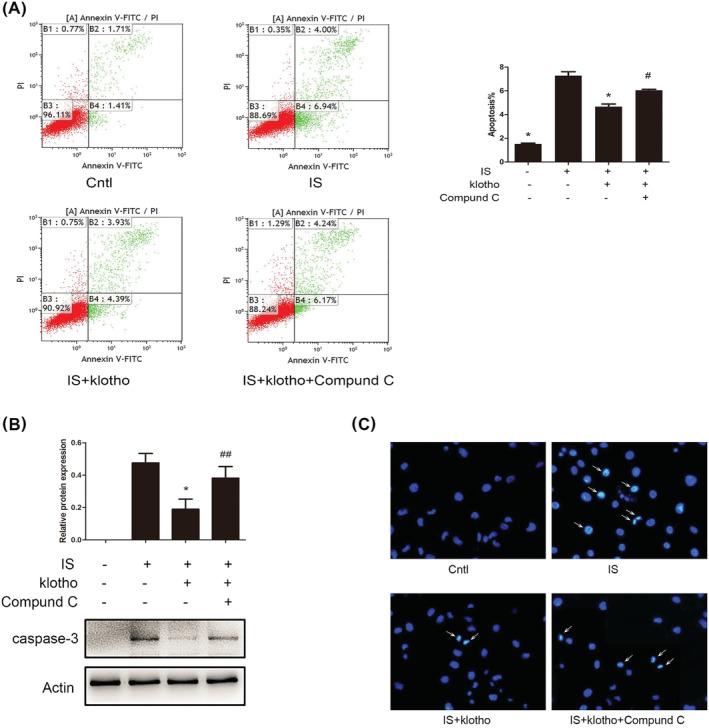
Effects of IS and Klotho on apoptosis in HUVECs. Cells were pretreated with Klotho (100 μg/L) for 1 h, then incubated with IS (50 μg/mL) for further 48 h with or without Compound C (10 μmol/L). (A) Cells apoptosis were determined by flow cytometry. (B) Western blotting analysis for caspase‐3. (C) Hoechst staining (×200). Arrow indicated apoptosis‐induced chromatin condensation. Data were shown as mean ±SD. Statistical differences were expressed as * P < 0.01 versus IS alone; #P < 0.01 versus IS and Klotho treatment together; ##P < 0.05 versus IS and Klotho treatment together. HUVECs, human vein umbilical endothelial cells; IS, indoxyl sulfate
3.6. Klotho inhibited the IS‐induced expression of ICAM‐1 and VCAM‐1, and Compound C blocked this effect of Klotho in HUVECs
Compared with that in the control group, the expression of ICAM‐1 and VCAM‐1 in the IS group was upregulated. Klotho treatment decreased the expression of ICAM‐1 and VCAM‐1. This inhibitory effect of Klotho was blocked by Compound C (Figure 5).
FIGURE 5.
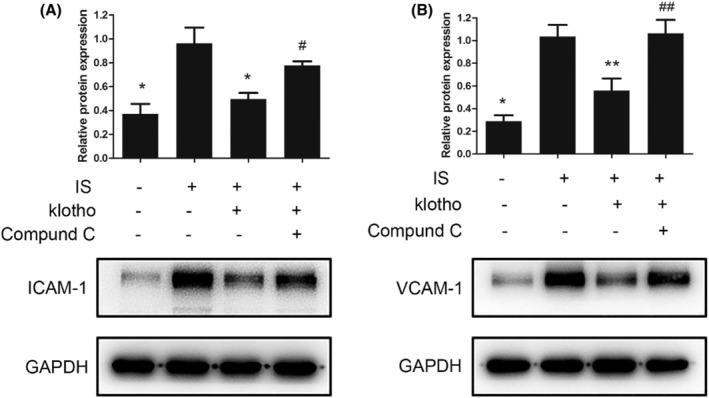
Effects of IS and Klotho on the expression of ICAM‐1 and VCAM‐1 in HUVECs. Cells were pretreated with Klotho (100 μg/L) for 1 h, then incubated with IS (50 μg/mL) for further 48 h with or without Compound C (10 μmol/L). (A) Western blotting analysis for ICAM‐1. (B) Western blotting analysis for VCAM‐1. Data were shown as mean ±SD. Statistical differences were expressed as * P < 0.01 versus IS treatment alone; ** P < 0.05 versus IS alone; #P < 0.05 versus IS and Klotho treatment together. HUVECs, human vein umbilical endothelial cells; IS, indoxyl sulfate; ICAM‐1, intercellular adhesion molecule‐1; VCAM‐1, vascular cell adhesion molecule‐1
4. DISCUSSION
At the end stage of CKD, IS has obvious toxic effects on the cardiovascular system, and its plasma concentrations are nearly 100 times higher than those of the general population (Duranton et al., 2012). IS has cytotoxic effects on renal tubular epithelial cells and mesangial cells, which can promote the development of CKD (Ng et al., 2014). In addition to intrinsic kidney cells, IS also has toxic effects on cells of the cardiovascular system. IS induces myocardial hypertrophy and left ventricular hypertrophy by increasing oxidative stress and activating the p38 or ERK signaling pathway (Yang et al., 2015). IS also induces arrhythmia by regulating myocardial potassium channels (Tang et al., 2015). IS induces the calcification of vascular smooth muscles (Opdebeeck et al., 2019) and accelerates the aging of human aortic smooth muscle cells. In addition, IS also increases the risk of thrombosis after vascular intervention (Chitalia et al., 2013).
Atherosclerosis is one of the most important cardiovascular complications of CKD, and atherosclerotic lesions can occur at the early stage of renal dysfunction (Campean et al., 2005). For uremic patients, atherosclerosis is a major risk factor for long‐term survival in maintenance hemodialysis patients. The pathogenesis of atherosclerosis in CKD patients is complex, and it is related to not only traditional factors, such as hypertension and lipid metabolism disorders, but also chronic inflammatory status, calcium and phosphorus metabolism disorders, oxidative stress, malnutrition, anemia, overload hydration, and coagulation system abnormalities. In addition, the accumulation of metabolites and toxins is closely associated with atherosclerosis. In this study, we found that IS has direct toxic effects on the HUVECs, inhibiting cell viability in a concentration‐ and time‐dependent manner and inducing endothelial cell apoptosis and affecting the expression of adhesion molecules in endothelial cells.
The anti‐aging gene Kl gene was first discovered in 1997. Kl‐deficient mice and CKD subjects have similar phenotypes, suggesting that Kl gene is tightly correlated with the pathogenic mechanisms of CKD (Zou, Wu, He, Ma, & Gao, 2018). The main form of the Klotho is called α‐Klotho. There are two forms of the α‐Klotho: the transmembrane Klotho and the soluble Klotho. The soluble Klotho, which is found in the cerebrospinal fluid, urine, and blood, acts as an endocrine factor and plays an important antioxidative stress, anti‐aging, anti‐apoptosis, and anti‐fibrosis roles and promotes autophagy in distant organs and tissues (Bian, Neyra, Zhan, & Hu, 2015). A large amount of evidence suggests that the Klotho in the circulation system is mainly derived from the kidneys (Hu et al., 2016; Lindberg et al., 2014), and the Klotho levels significantly decrease during CKD in humans and rodents, which represents a state of “pan‐α‐Klotho deficiency” (Hu et al., 2017). The lack of Klotho reduces the tolerance of kidney tissue to injury, increases renal fibrosis, and accelerates the development of CKD, but upregulating Klotho expression can delay the progression of CKD (Kooman et al., 2013). In addition, Klotho also plays an important role in the cardiovascular system. The serum Klotho concentration is negatively correlated with the incidence and severity of coronary artery disease after adjusting for factors such as age, smoking, diabetes, and inflammatory status (Navarro‐González et al., 2014). A recent study by Göçer et al. showed that the serum Klotho concentration is an independent predictor of the occurrence and severity of coronary artery disease (Göçer, Aykan, Kılınç, & Göçer, 2020). Yu et al. found a negative correlation between the serum Klotho concentration and carotid atherosclerosis in maintenance hemodialysis patients (Yu, Kang, Ren, Diao, & Liu, 2018). In addition to being a predictor of cardiovascular disease, Klotho also protects against cardiovascular disease. The recombinant Klotho inhibits the IS‐induced cardiac hypertrophy and ventricular hypertrophy (Yang et al., 2015). Hu et al. found that in mice with CKD, the recombinant Klotho can attenuate cardiac remodeling, improve cardiac function, and delay uremic cardiomyopathy (Hu et al., 2017). The overexpression of Klotho also inhibits TGF‐β‐induced myocardial fibrosis (Liu et al., 2019). Our previous studies have shown that the recombinant Klotho can antagonize the toxic effects of serum from CKD patients with secondary hyperparathyroidism on vascular endothelial cells (Chen, Mao, Yu, et al., 2015) and can alleviate vascular smooth muscle cell calcification induced by β‐glycerophosphoric acid (Chen et al., 2015). Studies on the expression of Klotho in the cardiovascular system are not consistent (Corsetti et al., 2016; Fang et al., 2014; Jimbo et al., 2014; Lindberg et al., 2013; Scialla et al., 2013). Interestingly, even scholars who did not find Klotho expression in the vasculature also believe that soluble Klotho plays a protective role in the cardiovascular system (Mencke et al., 2015). In basic research, the methods to increase Klotho in circulation include transgenic technology, adeno‐associated virus transfection, stimulation of endogenous Klotho production, and direct administration of recombinant Klotho (Chen et al., 2013; Hu et al., 2010). It is almost impossible to use transgenic technology and transfection of adeno‐associated virus directly in humans. It is also not easy to stimulate the production of endogenous Klotho in CKD patients. Direct supplementation with recombinant Klotho may be the most promising method for increasing Klotho expression in humans in the future. In the present study, we found that the recombinant human Klotho protects vascular endothelial cells from the apoptosis induced by IS.
AMPK is a major cellular energy sensor and a master regulator of metabolic homeostasis. A lot of work has confirmed that AMPK is a signaling molecule in endothelial cells that maintains endothelial homeostasis and protects cells from injury and stress (Fisslthaler & Fleming, 2009). AMPK exists as a heterotrimeric complex that is composed of a catalytic subunit (α1 or α2) and two regulatory subunits (β1 or β2 and γ1, γ2, or γ3) in mammals. AMPKα1 is the catalytic isoform expressed in vascular cells and leukocytes (Fisslthaler & Fleming, 2009). Yang et al. found that the specific knockout of AMPKα1 in mouse endothelial cells accelerates the formation of atherosclerosis, and the mechanism is related to reduced glycolysis (Yang et al., 2018). Li et al. found that the upregulation of AMPKα1 reverses the high glucose‐induced decrease in viability and reduces apoptosis and oxidative stress in HUVECs (Li, Li, Wei, & Li, 2016). In addition, AMPKα1 deficiency reduces the lifespan of red blood cells (Wang, Dale, Song, Viollet, & Zou, 2010), suggesting that AMPKα1 may be involved in cellular aging. In this study, we found that Klotho had no significant effect on the total AMPKα1 expression in the presence of IS but promoted the phosphorylation of AMPKα1 and then played a role in protecting endothelial cells from IS‐induced injury.
ER is an important organelle in the cytoplasm and plays an important role in regulating protein synthesis, assembly, and folding. When cells are affected by hypoxia, chemical toxins, and other damage factors, the steady state in the ER is disrupted, and incorrect protein folding or unfolded protein aggregation occurs in the ER; this phenomenon is known as ERS. Early ERS is the adaptive compensatory response to damage stimulation, but excessive ERS damages cells and eventually causes the activation of the apoptotic signaling pathway. GRP78 is an important companion molecule for the ER and the hallmark protein of ERS. The ER is another important organelle involved in apoptosis in addition to mitochondria. Excessive ERS induces apoptosis, and CHOP, which is a transcription factor, plays a key role in ERS‐mediated apoptosis (Tabas & Ron, 2011). ERS plays a vital role in the glomerular podocyte injury induced by advanced glycation end products and in the renal tubular epithelial cell apoptosis induced by aldosterone (Ding, Yang, Zhang, & Gu, 2012). In addition, ESR contributes to the pathogenesis of diabetic nephropathy (Babak, Jun, & Mahmood, 2013). ERS is also involved in the occurrence of atherosclerosis. The deposition of a large number of apoptotic macrophages, accompanied by high GRP78 and CHOP expression in weak fibrous caps, leads to the rupture of plaques in the vascular endothelium of patients with acute coronary syndromes. Furthermore, ERS mediates isoproterenol‐induced mouse cardiomyocyte hypertrophy and apoptosis (Song, Gao, Xiao, Xu, & Si, 2013). In the present study, we found that IS induces ERS in HUVECs and that treatment with the recombinant Klotho alleviates excessive ERS. The expression of GRP78 and CHOP induced by IS is increased, and the inhibitory effect of Klotho is reduced when AMPKα1 is knocked down using siRNA, indicating that AMPKα1 participates in the Klotho‐mediated inhibition of IS‐induced ERS. However, Klotho cannot directly affect the expression of AMPKα1 in the presence of IS but promotes the phosphorylation of AMPKα1, while IS inhibits AMPKα1 phosphorylation. In addition, as AMPK phosphorylation inhibitor, Compound C can block the inhibitory effect of Klotho on ERS and apoptosis. In addition, we also found that IS induced the expression of ICAM‐1 and VCAM‐1, which are important molecules involved in atherosclerosis, and Klotho treatment inhibited their expression, which was blocked by Compound C. It is suggested that Klotho inhibits the expression of IS‐induced adhesion molecules in endothelial cells by inhibiting the phosphorylation of AMPKα1.
5. CONCLUSION
This study shows that uremic toxin IS injures HUVECs via inhibiting AMPKα1 phosphorylation and inducing excessive ERS, and Klotho antagonizes the damage effects of IS (Figure 6). IS may play an important role in the occurrence and development of the vascular endothelial injury complications of CKD, which warrants further work.
FIGURE 6.
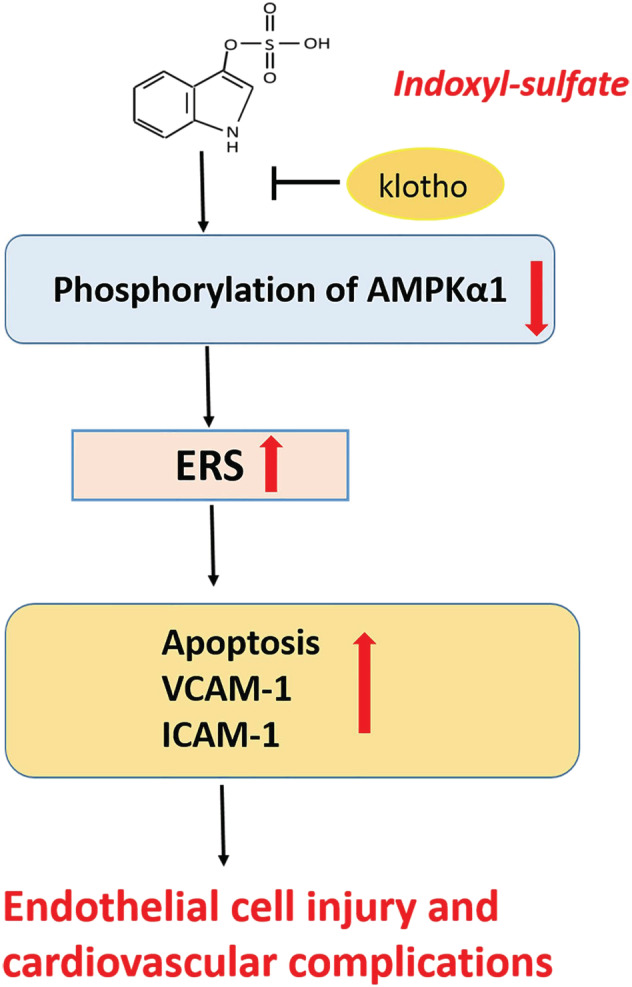
Schematic view of the damage effect of IS on HUVECs. IS induces ERS and its mediated apoptosis and promotes the expression of endothelial adhesion molecules by inhibiting AMPKα1 phosphorylation, ultimately damages HUVECs and causes cardiovascular complications of CKD. IS, indoxyl sulfate; HUVECs, human vein umbilical endothelial cells; ERS, endoplasmic reticulum stress; CKD, chronic kidney disease; ICAM‐1, intercellular adhesion molecule‐1; VCAM‐1,vascular cell adhesion molecule‐1 [Colour figure can be viewed at wileyonlinelibrary.com]
CONFLICT OF INTEREST
All authors declare no competing financial, professional, or personal interests that might have influenced the performance or presentation of this work.
AUTHOR CONTRIBUTIONS
Cheng Chen, Lin Wu, Caidie Xie, and Xiufen Zhao performed the experiments and analyzed the data; Huijuan Mao and Changying Xing edited and revised the manuscript; and all approved the final version of the manuscript.
FUNDING INFORMATION
This work was supported by grants from the National Natural Science Foundation of China (No. 81970639) and the CKD‐MBD Youth Research Fund of Zhongguancun Nephrology Blood Purification Innovation Alliance (No. NBPIA‐2018‐CKDMBD).
Chen C, Wu L, Xie C, Zhao X, Mao H, Xing C. The role of AMP‐activated protein kinase α1‐mediated endoplasmic reticulum stress in alleviating the toxic effect of uremic toxin indoxyl sulfate on vascular endothelial cells by Klotho. J Appl Toxicol. 2021;41:1446–1455. 10.1002/jat.4135
Funding information CKD‐MBD Youth Research Fund of Zhongguancun Nephrology Blood Purification Innovation Alliance, Grant/Award Number: NBPIA‐2018‐CKDMBD; National Natural Science Foundation of China, Grant/Award Number: 81970639
Contributor Information
Huijuan Mao, Email: maohuijuan72@hotmail.com.
Changying Xing, Email: cyxing62@126.com.
REFERENCES
- Babak, B., Jun, Y. L., & Mahmood, M. S. (2013). Endoplasmic reticulum stress response and inflammatory cytokines in type 2 diabetic nephropathy: Role of indoleamine 2,3‐dioxygenase and programmed death‐1. Experimental and Molecular Pathology, 94(2), 343–351. 10.1016/j.yexmp.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Bian, A., Neyra, J. A., Zhan, M., & Hu, M. C. (2015). Klotho, stem cells, and aging. Clinical Interventions in Aging, 10, 1233–1243. 10.2147/CIA.S84978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campean, V., Neureiter, D., Varga, I., Runk, F., Reiman, A., Garlichs, C., … Amann, K. (2005). Atherosclerosis and vascular calcification in chronic renal failure. Kidney and Blood Pressure Research, 28(5–6), 280–289. 10.1159/000090182 [DOI] [PubMed] [Google Scholar]
- Chen, C., Mao, H. J., Yu, X. B., Sun, B., Zeng, M., Zhao, X. F., & Xing, C. Y. (2015). Effect of secondary hyperparathyroidism serum on endothelial cells and intervention with klotho. Molecular Medicine Reports, 12(2), 1983–1990. 10.3892/mmr.2015.3606 [DOI] [PubMed] [Google Scholar]
- Chen, T., Mao, H. J., Chen, C., Wu, L., Wang, N. N., Zhao, X. F., … Xing, C. Y. (2015). The role and mechanism of α‐klotho in the calcification of rat aortic vascular smooth muscle cells. BioMed Research International, 2015, 194362–194367. 10.1155/2015/194362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. H., Kuro‐O, M., Chen, C. H., Sue, Y. M., Chen, Y. C., Wu, H. H., … Cheng, C. Y. (2013). The secreted klotho protein restores phosphate retention and suppresses accelerated aging in klotho mutant mice. European Journal of Pharmacology, 698(1–3), 67–73. 10.1016/j.ejphar.2012.09.032 [DOI] [PubMed] [Google Scholar]
- Chitalia, V. C., Shivanna, S., Martorell, J., Balcells, M., Bosch, I., Kolandaivelu, K., & Edelman, E. R. (2013). Uremic serum and solutes increase post‐vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation, 127(3), 365–376. 10.1161/CIRCULATIONAHA.112.118174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibor, D., Domagala‐Rodacka, R., Rodacki, T., Jurczyszyn, A., Mach, T., & Owczarek, D. (2016). Endothelial dysfunction in inflammatory bowel diseases: Pathogenesis, assessment and implications. World Journal of Gastroenterology, 22(3), 1067–1077. 10.3748/wjg.v22.i3.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsetti, G., Pasini, E., Scarabelli, T. M., Romano, C., Agrawal, P. R., Chen‐Scarabelli, C., … Dioguardi, F. S. (2016). Decreased expression of klotho in cardiac atria biopsy samples from patients at higher risk of atherosclerotic cardiovascular disease. Journal of Geriatric Cardiology, 13(8), 701–711. 10.11909/j.issn.1671-5411.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmashankar, K., & Widlansky, M. E. (2010). Vascular endothelial function and hypertension: Insights and directions. Current Hypertension Reports, 12(6), 448–455. 10.1007/s11906-010-0150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, W., Yang, L., Zhang, M., & Gu, Y. (2012). Reactive oxygen species‐mediated endoplasmic reticulum stress contributes to aldosterone‐induced apoptosis in tubular epithelial cells. Biochemical and Biophysical Research Communications, 418(3), 451–456. 10.1016/j.bbrc.2012.01.037 [DOI] [PubMed] [Google Scholar]
- Duranton, F., Cohen, G., De Smet, R., Rodriguez, M., Jankowski, J., Vanholder, R., … European Uremic Toxin Work Group . (2012). Normal and pathologic concentrations of uremic toxins. Journal of the American Society of Nephrology, 23(7), 1258–1270. 10.1681/ASN.2011121175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, Y., Ginsberg, C., Sugatani, T., Monier‐Faugere, M. C., Malluche, H., & Hruska, K. A. (2014). Early chronic kidney disease‐mineral bone disorder stimulates vascular calcification. Kidney International, 85(1), 142–150. 10.1038/ki.2013.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisslthaler, B., & Fleming, I. (2009). Activation and signaling by the AMP‐activated protein kinase in endothelial cells. Circulation Research, 105(2), 114–127. 10.1161/CIRCRESAHA.109.201590 [DOI] [PubMed] [Google Scholar]
- Göçer, K., Aykan, A. Ç., Kılınç, M., & Göçer, N. S. (2020). Association of serum FGF‐23, klotho, fetuin‐A, osteopontin, osteoprotegerin and hs‐CRP levels with coronary artery disease. Scandinavian Journal of Clinical and Laboratory Investigation, 80(4), 277–281. 10.1080/00365513.2020.1728786 [DOI] [PubMed] [Google Scholar]
- Hu, M. C., Kuro‐o, M., & Moe, O. W. (2013). Klotho and chronic kidney disease. Contributions to Nephrology, 180, 47–63. 10.1159/000346778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, M. C., Shi, M., Gillings, N., Flores, B., Takahashi, M., Kuro‐o, M., & Moe, O. W. (2017). Recombinant α‐klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney International, 91(5), 1104–1114. 10.1016/j.kint.2016.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, M. C., Shi, M., Zhang, J., Addo, T., Cho, H. J., Barker, S. L., … Moe, O. W. (2016). Renal production, uptake, and handling of circulating αKlotho. Journal of the American Society of Nephrology, 27(1), 79–90. 10.1681/ASN.2014101030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, M. C., Shi, M., Zhang, J., Quiñones, H., Kuro‐o, M., & Moe, O. W. (2010). Klotho deficiency is an early biomarker of renal ischemia‐reperfusion injury and its replacement is protective. Kidney International, 78(12), 1240–1251. 10.1038/ki.2010.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimbo, R., Kawakami‐Mori, F., Mu, S., Hirohama, D., Majtan, B., Shimizu, Y., … Shimosawa, T. (2014). Fibroblast growth factor 23 accelerates phosphate‐induced vascular calcification in the absence of klotho deficiency. Kidney International, 85(5), 1103–1111. 10.1038/ki.2013.332 [DOI] [PubMed] [Google Scholar]
- Kooman, J. P., Broers, N. J. H., Usvyat, L., Thijssen, S., Sande, F. M., Cornelis, T., … Kotanko, P. (2013). Out of control: Accelerated aging in uremia. Nephrology, Dialysis, Transplantation, 28(1), 48–54. 10.1093/ndt/gfs451 [DOI] [PubMed] [Google Scholar]
- Li, J., Li, J., Wei, T., & Li, J. (2016). Down‐regulation of MicroRNA‐137 improves high glucose‐induced oxidative stress injury in human umbilical vein endothelial cells by up‐regulation of AMPKα1. Cellular Physiology and Biochemistry, 39(3), 847–859. 10.1159/000447795 [DOI] [PubMed] [Google Scholar]
- Lindberg, K., Amin, R., Moe, O. W., Hu, M. C., Erben, R. G., Wernerson, A. Ö., … Larsson, T. E. (2014). The kidney is the principal organ mediating klotho effects. Journal of the American Society of Nephrology, 25(10), 2169–2175. 10.1681/ASN.201311120.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg, K., Olauson, H., Amin, R., Ponnusamy, A., Goetz, R., Taylor, R. F., … Larsson, T. E. (2013). Arterial klotho expression and FGF23 effects on vascular calcification and function. PLoS ONE, 8(4), e60658. 10.1371/journal.pone.0060658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q., Zhu, L. J., Waaga‐Gasser, A. M., Ding, Y., Cao, M., Jadhav, S. J., … Hsiao, L. L. (2019). The axis of local cardiac endogenous klotho‐TGF‐β1‐Wnt signaling mediates cardiac fibrosis in human. Journal of Molecular and Cellular Cardiology, 136, 113–124. 10.1016/j.yjmcc.2019.09.004 [DOI] [PubMed] [Google Scholar]
- Mathew, R. O., Bangalore, S., Lavelle, M. P., Pellikka, P. A., Sidhu, M. S., Boden, W. E., & Asif, A. (2017). Diagnosis and management of atherosclerotic cardiovascular disease in chronic kidney disease: A review. Kidney International, 91(4), 797–807. 10.1016/j.kint.2016.09.049 [DOI] [PubMed] [Google Scholar]
- Mencke, R., Harms, G., Mirković, K., Struik, J., Ark, J. V., Loon, E. V., … Hillebrands, J. L. (2015). Membrane‐bound klotho is not expressed endogenously in healthy or uraemic human vascular tissue. Cardiovascular Research, 108(2), 220–231. 10.1093/cvr/cvv187 [DOI] [PubMed] [Google Scholar]
- Navarro‐González, J. F., Donate‐Correa, J., Fuentes, M. M., Pérez‐Hernández, H., Martínez‐Sanz, R., & Mora‐Fernández, C. (2014). Reduced klotho is associated with the presence and severity of coronary artery disease. Heart, 100(1), 34–40. 10.1136/heartjnl-2013-304746 [DOI] [PubMed] [Google Scholar]
- Ng, H. Y., Yisireyili, M., Saito, S., Lee, C. T., Adelibieke, Y., Nishijima, F., & Niwa, T. (2014). Indoxyl sulfate downregulates expression of mas receptor via OAT3/AhR/Stat3 pathway in proximal tubular cells. PLoS ONE, 9(3), e91517. 10.1371/journal.pone.0091517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdebeeck, B., Maudsley, S., Azmi, A., Maré, A. D., Leger, W. D., Meijers, B., … Neven, E. (2019). Indoxyl sulfate and p‐Cresyl sulfate promote vascular calcification and associate with glucose intolerance. Journal of the American Society of Nephrology, 30(5), 751–766. 10.1681/ASN.2018060609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialla, J. J., Lau, W. L., Reilly, M. P., Isakova, T., Yang, H. Y., Crouthamel, M. H., … Wolf, M. (2013). Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney International, 83(6), 1159–1168. 10.1038/ki.2013.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S., Gao, P., Xiao, H., Xu, Y., & Si, L. Y. (2013). Klotho suppresses cardiomyocyte apoptosis in mice with stress‐induced cardiac injury via downregulation of endoplasmic reticulum stress. PLoS ONE, 8(12), e82968. 10.1371/journal.pone.0082968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas, I., & Ron, D. (2011). Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nature Cell Biology, 13(3), 184–190. 10.1038/ncb0311-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. H., Wang, C. P., Chung, F. M., Huang, L. L., Yu, T. H., Hung, W. C., … Lai, W. T. (2015). Uremic retention solute indoxyl sulfate level is associated with prolonged QTc interval in early CKD patients. PLoS ONE, 10(3), e0119545. 10.1371/journal.pone.0119545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Dale, G. L., Song, P., Viollet, B., & Zou, M. H. (2010). AMPKalpha1 deletion shortens erythrocyte life span in mice: Role of oxidative stress. Journal of Biological Chemistry, 285(26), 19976–19985. 10.1074/jbc.M110.102467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K., Wang, C., Nie, L., Zhao, X., Gu, J., Guan, X., … Zhao, J. (2015). Klotho protects against indoxyl sulphate‐induced myocardial hypertrophy. Journal of the American Society of Nephrology, 26(10), 2434–2446. 10.1681/ASN.2014060543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Q., Xu, J., Ma, Q., Liu, Z., Sudhahar, V., Cao, Y., & Huo, Y. (2018). PRKAA1/AMPKα1‐driven glycolysis in endothelial cells exposed to disturbed flow protects against atherosclerosis. Nature Communications, 9(1), 4667. 10.1038/s41467-018-07132-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, L., Kang, L., Ren, X. Z., Diao, Z. L., & Liu, W. H. (2018). Circulating α‐klotho levels in hemodialysis patients and their relationship to atherosclerosis. Kidney and Blood Pressure Research, 43(4), 1174–1182. 10.1159/000492245 [DOI] [PubMed] [Google Scholar]
- Zou, D., Wu, W., He, Y., Ma, S., & Gao, J. (2018). The role of klotho in chronic kidney disease. BMC Nephrology, 19(1), 285. 10.1186/s12882-018-1094-z [DOI] [PMC free article] [PubMed] [Google Scholar]


