Abstract
Adenoviruses (AdV), causing fatal disseminated infections in bone marrow transplant (BMT) recipients, are associated not only with hemorrhagic cystitis (HC) but also with hepatitis, conjunctivitis, and viral interstitial pneumonia. The importance of this virus as a cause of disseminated disease, however, has remained underappreciated. AdV infection has been diagnosed primarily through the use of cell culture. The fact that cell culture is insensitive for detecting this virus has hindered recognition of the role that AdV may play in morbidity and mortality in BMT recipients. To emphasize these points, we describe a patient who presented with HC due to AdV serotype 11, genotype c, and died with disseminated infection. In addition to cell culture, this study used a newly developed PCR-based method, capable of detecting all AdV serotypes tested, including different genotypes of serotype 11. The PCR result was positive in all culture-positive samples, including samples of urine, conjunctiva, and bronchoalveolar lavage (BAL). Importantly, the PCR method provided evidence of urinary shedding of AdV in a pretransplant, culture-negative specimen and showed dissemination in a subset of culture-negative specimens, including BAL, blood, and bone marrow samples. The lack of widespread awareness of the fact that localized infections may presage dissemination, and the previous associated lack of rapid, sensitive diagnostic assays, has impaired recognition of AdV infections in patients undergoing BMT. Early detection may contribute to therapy modification and avoidance of unwarranted diagnostic procedures. It may also assist in epidemiologic control of this highly infectious pathogen and lead to a renewed interest in preventive and therapeutic approaches.
Adenovirus (AdV) infections prevalent in immunocompromised populations are a significant cause of morbidity and mortality (19). Between 2 and 18% of patients have been reported to develop significant AdV infections after bone marrow transplantation (BMT) (2, 14, 17). The mortality rate among AdV-infected BMT recipients has been reported to be between 10 and 60% (2, 7, 19). Slow and insensitive culture technologies have made objective diagnosis of disseminated AdV infection problematic, thereby obscuring its importance and range of clinical presentations.
At least 49 distinct serotypes of human AdV, associated with distinct clinical manifestations, are recognized (9). These include respiratory diseases, such as pharyngitis, pneumonia, and a pertussis-like syndrome, as well as keratoconjunctivitis, hemorrhagic cystitis (HC), hepatitis, and gastroenteritis. Although specific serotypes have been associated with the involvement of specific organ systems in an immunocompromised host, localized infections such as HC, gastroenteritis, or pneumonitis may be manifestations of disseminated infection, caused by a single AdV serotype (2, 11, 16).
Because of the increasing prevalence of acquired deficiencies in immune function due to organ transplantation, cancer therapy, or human immunodeficiency virus infection, viral infections have become a major focus of medical attention. In this report, we describe a patient with HC due to AdV type 11c after BMT who developed disseminated infection. Because of current limitations in the conventional cell culture methodology for detection of AdV, we hypothesized that disseminated disease could be better documented by using a newly developed, sensitive PCR assay (5). This case illustrates the importance of recognizing the protean clinical manifestations of AdV infections, the interpretation of viral culture results in BMT recipients, and the potential role of a rapid, sensitive PCR method for early diagnosis of this pathogen. It further illustrates the advantage of the application of this technology to the understanding of the role of AdV in systemic infection.
CASE REPORT
A 20-year-old man underwent autologous BMT for chemotherapy-resistant large-cell lymphoma, B cell type, stage IA. Initial therapy included tumor excision, thymectomy, six cycles of cyclophosphamide, hydroxydaunorubicin, etoposide, and prednisone, and a platinum- and etoposide-based salvage regimen for recurrent disease. Ten days prior to BMT (day −10), his bone marrow was harvested and treated with 4-hydroperoxycyclophosphamide. He received 50 mg of cyclophosphamide/kg of body weight intravenously on days −8, −7, −6, and −5 and 300 cGy each day from day −4 until day −1 (total body irradiation, 1,200 cGy); during this time he developed transient severe nausea, fever, and a nonproductive cough that responded to symptomatic therapy. His fever resolved. Per protocol, during aplasia, he received prophylactic acyclovir, fluconazole, and norfloxacin prophylaxis, all of which was discontinued on day 31, after his absolute neutrophil count returned to more than 500 cells/mm3. A surveillance urine viral culture performed 4 days prior to transplantation was negative after 3 weeks of incubation. On day 3 posttransplantation, he developed fever and mucositis; empiric piperacillin-tazobactam therapy was started. Although the fever and mucositis resolved within 5 days, the antimicrobial regimen was continued. Bacterial and fungal cultures remained negative.
On day 10 his urine was noted to contain trace blood, and bladder irrigation was initiated. On day 11 he developed dysuria and distal penile pain, and by day 12, frank blood clots were noted in his urine. On day 14 his urine culture from day 3 was noted to be positive for AdV. For the remaining 66 days of hospitalization, microscopic hematuria persisted, and gross hematuria sporadically recurred. All subsequent urine cultures remained culture positive for AdV. BK virus was not present on multiple samples tested for that agent by a PCR-based method. On day 20 fever recurred, and amphotericin B was started without effect. The fever persisted for the remaining 60 days of his life. On day 30 his absolute neutrophil count rose above 500 cells/mm3. On day 32 his temperature rose to 40.1°C, and for the first time, he suffered pulmonary impairment and required mechanical ventilation. High-dose steroid therapy was initiated on day 33; on day 34, a bronchoalveolar-lavage (BAL) and an open-lung biopsy were performed. The biopsy revealed diffuse alveolar damage with hyaline membranes and no signs of inflammation. The result of immunohistologic staining performed on the lung biopsy by using an AdV hexon protein monoclonal antibody was considered negative, although background staining interfered with the interpretation. Cultures from these specimens remained negative for bacterial, fungal, and viral pathogens.
On days 35 to 38 his serum transaminase levels rose from the normal range and remained between 82 and 365 IU/liter (for aspartate aminotransferase) and between 112 and 433 IU/liter (for alanine aminotransferase), 2 to 10 times the upper limit of normal, for the remainder of his course; total bilirubin levels ranged from 2 to 10 mg/dl. The ratio of aspartate aminotransferase to alanine aminotransferase during this 35-day period was approximately 1:1.4. Assays for acute infection with hepatitis viruses B and C were negative, as were a blood culture for viral pathogens on day 46 and a bone marrow culture on day 53. The patient remained febrile and ventilator dependent, and on day 49 antibiotics and steroids were discontinued. On day 51 vancomycin and imipenem were begun empirically, without effect. He was noted to have bilateral conjunctivitis, and on day 52, specimens of conjunctiva and BAL were obtained, both of which grew AdV. High-dose steroids were restarted, and his temperature decreased somewhat; however, his respiratory status failed to respond. On day 71 ribavirin therapy was tried; however, his respiratory status continued to deteriorate, he developed multi-organ-system failure, and on day 80, he died. Postmortem examination was declined.
MATERIALS AND METHODS
Culture method.
Viral culture was performed by standard methods (5). Clinical samples in viral transport medium were inoculated within 90 min of procurement into multiple cell lines, including MRC-5, three primary monkey kidney lines (African green, cynomolgus, and rhesus), and also human neonatal kidney lines (if AdV was suspected). The cultures were examined for cytopathic effect for 3 to 4 weeks, depending on the specimen source. The presence of AdV was confirmed by indirect immunofluorescence (Bartels Inc., Issaquah, Wash.). AdV isolates were submitted to the State of Maryland Department of Health and Mental Hygiene virology laboratories for serotyping and to Adriana E. Kajon, University of Georgia, Athens, for genome typing by restriction analysis with endonucleases BamHI and SmaI (12).
PCR.
PCR primers for AdV detection were selected based on sequences in the hexon gene, a part of the AdV genome that is highly conserved among the different serotypes. A PCR-based assay method initially developed to detect AdV from conjunctival samples (18) was modified to apply to different specimen types (5). Unlike some previously described primer sequences used for detection of AdV from clinical samples (1), these primers detected all types of AdV tested, including different genotypes of serotype 11 (5).
Clinical samples in viral transport medium (VTM) were stored at −70°C after culture was performed. Urine, BAL, and conjunctival swab samples in VTM were retrospectively tested by PCR without prior extraction of nucleic acids. Whole-blood, serum, plasma, and bone marrow samples were preprocessed to remove inhibitors by using the QIAamp Blood kit (Qiagen Inc., Valencia, Calif.). Two microliters of urine, BAL, or conjunctival-swab samples, or 10 μl of DNA extracted from blood or bone marrow, was used in each PCR (5). Each run included negative controls (distilled water and normal urine) and a positive control (purified DNA from AdV serotype 2). The presence of a 139-bp band on an ethidium bromide-stained gel was considered a positive result.
RESULTS
Diagnostic tests.
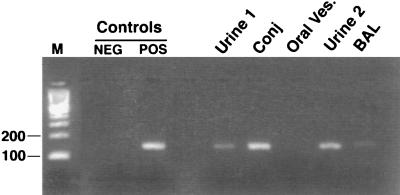
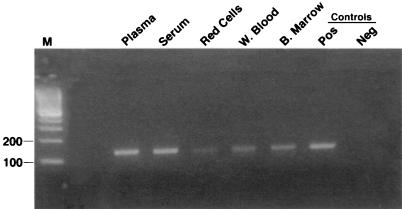
The results of culture and PCR for AdV are summarized in Table 1. The pretransplantation surveillance urine sample, negative for AdV by culture, was positive by PCR (Fig. 1, urine 1). All subsequent urine samples were positive for AdV by both culture and PCR. BAL samples taken on days 34 and 52 after transplantation were PCR positive, while only the day-52 sample was positive by culture. Cytopathic effect in positive cultures required as many as 21 days of incubation for the positive BAL sample and some urine samples, while PCR results were available within 24 h of testing. The lung biopsy sample obtained on day 34 remained negative by both culture and PCR. Blood, plasma, and serum obtained on day 70 were PCR positive, confirming bloodstream dissemination (Fig. 2), although a blood sample obtained on day 53 remained negative by culture. On day 52, the conjunctival-swab sample was positive for AdV by culture and PCR, while a swab obtained from a perioral vesicle was negative by both methods (Fig. 1). PCR-positive results were obtained on multiple runs on different days, and all negative controls tested concurrently remained negative. Urine specimens donated by 23 healthy volunteers also remained negative by PCR (5).
TABLE 1.
Results of AdV culture and the PCR-based method from clinical samples and correlation with clinical statusa
| Sample | Method | Detection of AdV in sample taken on the following day before or after BMT:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −4 | 3 | 10 | 17 | 25 | 34 | 38 | 45 | 52–53 | 59 | 66 | 73 | ||
| Urine | Culture (daysb) | − | + (10) | + (3) | + (17) | + (7) | + (9) | + (10) | + (8) | + (21) | + (8) | + (3) | + (3) |
| PCR | + | + | + | + | + | + | + | + | + | + | + | + | |
| BAL | Culture (days) | − | + (21) | ||||||||||
| PCR | + | + | |||||||||||
| Lung biopsy | Culture (days) | − | |||||||||||
| PCR | − | ||||||||||||
| Conjunctiva | Culture (days) | + (3) | |||||||||||
| PCR | + | ||||||||||||
| Blood | Culture (days) | − | NDc | ||||||||||
| PCR | ND | + | |||||||||||
| Bone marrow | Culture (days) | − | |||||||||||
| PCR | + | ||||||||||||
HC was noted on day 12, respiratory distress on day 32, and conjunctivitis on day 51. These conditions persisted until death on day 80.
Days of culture before cytopathic effect was detected.
ND, not done.
FIG. 1.
Detection of AdV in samples from different body sites by the PCR-based method. Lanes: M, molecular size marker; NEG, negative control (water); POS, positive control (purified DNA from AdV serotype 2); Urine 1, urine sample obtained on day −4; Conj, conjunctival-swab sample; Oral Ves., perioral-vesicle swab; Urine 2, urine sample obtained on day 45; BAL, BAL sample obtained on day 34.
FIG. 2.
Detection of AdV in blood by the PCR-based method. Plasma, serum, erythrocyte (Red Cells), and whole-blood (W. Blood) samples were obtained on day 73; bone marrow (B. Marrow) samples were obtained on day 52. The negative (Neg) control was water, and the positive (Pos) control was purified DNA from AdV serotype 2. M, molecular size marker.
Viral isolates recovered from urine (day 10), conjunctiva (day 52), and BAL (day 52) were serotyped; all were identified as serotype 11. AdV isolates from urine and conjunctiva that were analyzed by restriction with endonucleases BamHI and SmaI were identified as belonging to genome type 11c.
DISCUSSION
Patients with AdV infections may present with diverse manifestations, including urinary, conjunctival, respiratory, hepatic, and/or systemic symptoms and signs (8, 19). Infections occur in both immunocompetent and immunocompromised individuals, affecting different organ systems as a function of virus type and the patient’s underlying pathology. This case helps emphasize the difficulties encountered in such patients in recognizing the presence of disseminated AdV infection, and in placing that virus into proper perspective, when one is assessing the patient’s disease state and clinical course. In this patient, virus was detected in the urine while the patient was asymptomatic, e.g., 14 days prior to the development of hematuria and HC; the cystitis preceded evidence of dissemination. This patient clearly developed disseminated disease. He had a consistent clinical presentation, including his pulmonary and hepatic picture, and lacked evidence of alternative pathology, such as advanced graft versus host disease, or alternative microbial pathogens, sought through extensive serologic, culture, immunologic, and/or molecular testing. He failed extensive therapeutic approaches, directed against a broad range of alternative entities. All of these features, combined with the detection of AdV in multiple samples of urine, blood, BAL, conjunctiva, and bone marrow, lead to the conclusion that the explanation for his disease was severe, progressive, disseminated AdV.
As also noted here, culture-based diagnosis can require days or weeks (8); the sensitivity can be inadequate or unknown, especially when transport to the laboratory causes processing delays. Antibodies to AdV, present in most healthy individuals, and passively transferred to the immunocompromised by transfusion of blood products, may neutralize the virus, preventing positive cultures, without necessarily impeding molecular detection technologies. Detection by reading a biopsy specimen by light microscopy, with or without the use of immunodiagnostics, has also proven to be insensitive. These problems emphasize the need for a more rapid and sensitive diagnostic approach.
The newly developed PCR method for detecting AdV nucleic acids in clinical samples that was applied in this case gives an analytic sensitivity as low as 0.2 PFU/ml (5). Although urine often contains inhibitors of taq polymerase, in this case, 2-μl aliquots of urine did not inhibit amplification and detection of the AdV DNA. In subsequent work, in order to avoid inhibition, we introduced preparative steps to remove inhibitors from urine samples (5). The primers used in this assay, unlike many reported previously, have been proven to be capable of detecting different genomes of AdV type 11, one of the types most commonly found in HC in BMT recipients, and the type causing disease in this patient. That this assay is more sensitive than culture was borne out in this case. Samples positive by PCR but initially negative by culture (both urine and BAL specimens) were positive by both methods in subsequent samplings, as the disease progressed. Further, identification of AdV DNA by PCR in blood, bone marrow, and BAL that had been previously reported culture negative provided documentation of systemic involvement, then recognized as consistent with the patient’s clinical picture.
This case also illustrates that direct tissue diagnosis, though desirable, may not be possible, due to the risk of hemorrhage by the time the diagnosis has been considered. Only a single, small transbronchial biopsy specimen was obtainable in a bronchoscopy performed on day 32, early in the course of the patient’s disseminated disease, and at a time when his BAL culture remained negative. Diagnoses must be addressed and managed in such patients based on integration of clinical presentations and course, coupled with the best obtainable diagnostic information. In this case, respiratory cultures did not become positive until a second BAL sample was taken on day 52. The detection of AdV by PCR from the culture-negative BAL specimen taken on day 32 provided the initial support for the diagnosis of pulmonary AdV. Dissemination was also documented by positive PCR results on culture-negative blood and bone marrow, information unobtainable through other means. Of particular interest in this regard is the fact that the PCR-based method showed that the patient had shed AdV even in advance of his transplant, at a time when he was asymptomatic. It suggests that disease in this patient was due to reactivation of latent virus rather than acquisition of a new strain. While AdV serotype 11 is a frequent isolate from BMT recipients (2, 7, 15), the original environmental source and mode of transmission in most patients is not understood, since isolates of this serotype are infrequently detected in community surveys (15). Serotype 11 AdV is known to have considerable genetic variability (12). Some genome type 11 viruses have been shown to have distinct tropisms for the respiratory tract (AdV 11a) or urinary tract (AdV 11p) (10). The AdV isolated from this patient, genotype 11c, is most often isolated from urine (12); however, in the present case, it was also present systemically. The patient had received an autologous transplant. In one study of AdV in transplant recipients, one investigator found that none of 3 culture-positive autologous recipients had disease, while 13 of 39 allogeneic patients developed severe systemic disease (6). The reason for dissemination in this case was not clear.
The importance of establishing the diagnosis is highlighted by this case. Prior to establishing the presence of AdV, the patient had received multiple diagnostic procedures, and had been placed on steroid therapy, in an attempt to address his downhill course. Earlier suspicion and testing would have modified his care. In addition, it would have permitted the institution of strict isolation, protecting other immunocompromised patients from the risk of AdV spread from his eye infection or respiratory secretions.
Increased recognition of AdV infection would have reduced the cost and complications of therapy directed at chemotherapy-associated HC and of therapy directed towards other infectious agents. It would also have decreased unnecessary diagnostic procedures and permitted the institution of infection control measures to avoid the risk of epidemic spread of this disseminated respiratory pathogen. It could also have modified the use of immunosuppressive therapy when the diagnosis was unclear. Currently, no specific proven efficacious therapeutic agent against AdV exists; however, some reports suggest efficacy for ribavirin or prophylactic intravenous immunoglobulin administration (6, 13). As better therapeutic options for AdV infections are developed, rapid and sensitive diagnostic tools such as PCR will be required to better define the complete spectrum of disease and incidence of these infections. Moreover, because of the potential for significant morbidity or fatal infection and because BMT is an increasingly important therapeutic approach, studies using sensitive methods for diagnosis are needed to assess the role of AdV as a pathogen in immunocompromised patients.
Aspects of this case that suggest avenues for future investigation include studies of the apparently broad tissue tropism of the patient’s AdV, characterization of the molecular epidemiology of the pathogen, the influence of genomic variability on virulence, and the early clinical signs in such a patient. It is clear also that additional patients must be prospectively monitored in order to develop an understanding of the significance of pretransplant recovery of AdV DNA as a predictor of subsequent life-threatening, disseminated disease.
ACKNOWLEDGMENTS
This study was supported by the Educational Fund of the Division of Medical Microbiology, Department of Pathology, The Johns Hopkins Medical Institution.
We thank Michael Forman for technical advice; Adriana Kajon for genome typing; and William Merz, Gary Ketner, and Richard Jones for scientific advice.
REFERENCES
- 1.Allard A, Girones R, Juto P, Wadell G. Polymerase chain reaction for detection of adenoviruses in stool samples. J Clin Microbiol. 1990;28:2659–2667. doi: 10.1128/jcm.28.12.2659-2667.1990. . (Erratum, 29:2683, 1991.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambinder R F, Burns W, Forman M, Charache P, Arthur R, Beschorner W, Santos G, Saral R. Hemorrhagic cystitis associated with adenovirus infection in bone marrow transplantation. Arch Intern Med. 1986;146:1400–1401. [PubMed] [Google Scholar]
- 3.Azzi A, Fanci R, Bosi A, Ciappi S, Zakrzewska K, de Santis R, Laszlo D, Guidi S, Saccardi R, Vannucchi A M. Monitoring of polyomavirus BK viruria in bone marrow transplantation patients by DNA hybridization assay and by polymerase chain reaction: an approach to assess the relationship between BK viruria and hemorrhagic cystitis. Bone Marrow Transplant. 1994;14:235–240. [PubMed] [Google Scholar]
- 4.de Jong P J, Valderrama G, Spigland I, Horwitz M S. Adenovirus isolates from urine of patients with acquired immunodeficiency syndrome. Lancet. 1983;ii:1293–1296. doi: 10.1016/s0140-6736(83)92411-x. [DOI] [PubMed] [Google Scholar]
- 5.Echavarria M, Forman M, Ticehurst J, Dumler J S, Charache P. A PCR method for detection of adenovirus in urine of normal and HIV-infected individuals. J Clin Microbiol. 1998;36:3323–3326. doi: 10.1128/jcm.36.11.3323-3326.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flomenberg P, Babbitt J, Drobyski W R, Ash R C, Carrigan D R, Sedmak G V, McAuliffe T, Camitta B, Horowitz M M, Bunin N. Increasing incidence of adenovirus disease in bone marrow transplant recipients. J Infect Dis. 1994;169:775–781. doi: 10.1093/infdis/169.4.775. [DOI] [PubMed] [Google Scholar]
- 7.Hierholzer J C. Adenoviruses in the immunocompromised host. Clin Microbiol Rev. 1992;5:262–274. doi: 10.1128/cmr.5.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hierholzer J C. Adenoviruses. In: Lennette E H, Lennette D A, Lennette E T, editors. Diagnostic procedure for viral, rickettsial and chlamydial infections. Washington, D.C: American Public Health Association; 1995. pp. 169–188. [Google Scholar]
- 9.Horwitz M S. Adenoviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2149–2171. [Google Scholar]
- 10.Kidd A H, Jonsson M, Garwicz D, Kajon A E, Wermenbol A G, Verweij M W, De Jong J C. Rapid subgenus identification of human adenovirus isolates by a general PCR. J Clin Microbiol. 1996;34:622–627. doi: 10.1128/jcm.34.3.622-627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landry M L, Fong C K, Neddermann K, Solomon L, Hsiung G D. Disseminated adenovirus infection in an immunocompromised host. Pitfalls in diagnosis. Am J Med. 1987;83:555–559. doi: 10.1016/0002-9343(87)90770-4. [DOI] [PubMed] [Google Scholar]
- 12.Li Q G, Hambraeus J, Wadell G. Genetic relationship between thirteen genome types of adenovirus 11, 34, and 35 with different tropisms. Intervirology. 1991;32:338–350. doi: 10.1159/000150218. [DOI] [PubMed] [Google Scholar]
- 13.Murphy G F, Wood D P J, McRoberts J W, Henslee-Downey P J. Adenovirus-associated hemorrhagic cystitis treated with intravenous ribavirin. J Urol. 1993;149:565–566. doi: 10.1016/s0022-5347(17)36149-9. [DOI] [PubMed] [Google Scholar]
- 14.Sencer S F, Haake R J, Weisdorf D J. Hemorrhagic cystitis after bone marrow transplantation. Risk factors and complications. Transplantation. 1993;56:875–879. doi: 10.1097/00007890-199310000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Shields A F, Hackman R C, Fife K H, Corey L, Meyers J D. Adenovirus infections in patients undergoing bone-marrow transplantation. N Engl J Med. 1985;312:529–533. doi: 10.1056/NEJM198502283120901. [DOI] [PubMed] [Google Scholar]
- 16.Siegal F P, Dikman S H, Arayata R B, Bottone E J. Fatal disseminated adenovirus 11 pneumonia in an agammaglobulinemic patient. Am J Med. 1981;71:1062–1067. doi: 10.1016/0002-9343(81)90343-0. [DOI] [PubMed] [Google Scholar]
- 17.Wasserman R, August C S, Plotkin S A. Viral infections in pediatric bone marrow transplant patients. Pediatr Infect Dis J. 1988;7:109–115. doi: 10.1097/00006454-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Yeo A C, Cooper R J, Morris D J, Storey C C. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. Development of a multiplex polymerase chain reaction for detection of adenovirus, herpes simplex and Chlamydia trachomatis DNA in eye swabs, abstr. C-416; p. 192. [Google Scholar]
- 19.Zahradnik J M, Spencer M J, Porter D D. Adenovirus infection in the immunocompromised patient. Am J Med. 1980;68:725–732. doi: 10.1016/0002-9343(80)90262-4. [DOI] [PubMed] [Google Scholar]