Abstract
Background
Many observational studies have found a direct association between adverse in utero, perinatal and postnatal exposures and offspring’s depression. These findings are consistent with the ‘developmental origins of disease hypothesis’. But no review has comprehensively summarized the roles of these exposures. This review aims to systematically scrutinize the strength of associations between individual prenatal, perinatal, and postnatal exposures and subsequent depression in offspring.
Methods
We conducted a systematic review and meta‐analysis to synthesize the literature from the EMBASE, HealthStar, PsychoInfo, and Medline databases since their inception to September 1, 2019. English language articles on population‐based prospective cohort studies examining the associations between in utero, perinatal, and postnatal exposures and offspring’s depression were searched. Random‐effects models were used to calculate pooled estimates, and heterogeneity and sensitivity tests were conducted to explore potential confounders in the relationships of depression and early‐life factors. Qualitative analysis was also conducted.
Results
Sixty‐four prospective cohort studies with 28 exposures studied in the relationships to offspring’s depression met inclusion criteria. The meta‐analysis found 12 prenatal, perinatal, and postnatal characteristics were associated with an increased risk of depression in offspring: low birth weight, premature birth, small gestational age, maternal education, socioeconomic status, having younger parents (<20 years), having older parents (≥35 years), maternal smoking, paternal smoking, maternal stress, maternal anxiety, and prenatal depression. Heterogeneity and sensitivity tests supported the findings. By and large, study characteristics had no effects on conclusions. Qualitative analyses generally supported the findings of meta‐analysis and reported on additional risk factors.
Conclusions
This review provides a robust and comprehensive overview of the lasting psychopathological effects of in utero, perinatal, and postnatal exposures. The findings highlight the need for clinical and public health interventions focusing on the identified risk factors. Large prospective cohort studies are warranted to investigate the combined effects of multiple co‐existing early‐life exposures.
Keywords: Depression, in utero exposures, perinatal exposures, risk factors, children
Introduction
Major depression is a public health problem internationally, especially in high‐ and upper‐middle‐income countries (Rehm & Shield, 2019). It is estimated that major depression was one of the five leading causes (out of 328) of years lived with disability (YLDs) in 195 countries and territories from 1990 to 2016, contributing 34.1 million years to the total YLDs (Vos et al., 2015). Accumulating evidence shows adverse conditions in pregnancy and early life are associated with later negative neuropsychiatric outcomes (Hazel, Hammen, Brennan, & Najman, 2008; Newman et al., 2016; Strüber, Strüber, & Roth, 2014). Adverse in utero and perinatal exposures were associated with mental health status in adolescence and adulthood. The association between adverse in utero and perinatal exposures and mental health outcomes in later adulthood could involve alternations in cellular aging (Entringer et al., 2011). The alternations of cellular aging could then trigger epigenetic modifications and increase the susceptibility of mental disorders among affected individuals (Vaiserman & Koliada, 2017). Although there is evidence that adverse in utero and perinatal exposures are associated with subsequent health outcomes in adolescence and adulthood, the underlying mechanisms of the ‘biological embedding’ of early‐life experiences are not sufficiently understood. Empirical studies on fetal programming effects have shown that maternal stress or undernutrition leads to fetal growth retardation, which compromises in fetal development and growth linking with early onset of adult diseases (Calkins & Devaskar, 2011). The developmental origins of health and disease (DOHaD) model highlights the roles for in utero, perinatal, and postnatal factors in the development of major depression during the life course (O'Donnell & Meaney, 2017). However, as mental disorders are complex diseases involved multiple risk factors, the causal conclusions between the in utero and perinatal exposures and adolescent and adult mental health status warrant further investigation.
The following mechanisms have been proposed to understand the associations between prenatal and perinatal factors and subsequent psychopathology, including (a) intergenerational transmission of maternal stress (Hentges, Graham, Plamondon, Tough, & Madigan, 2019), (b) low birth weight has been associated with impaired executive function, educational achievements, and an elevated risk of major depression (Aizer & Currie, 2014), (c) antenatal maternal emotional wellbeing has been closely linked with offspring’s neurodevelopmental outcomes (Glover, 2014; Pearson et al., 2013, 2013), (d) glucocorticoids have been shown to mediate the association between maternal adversities and offspring development (Desplats, 2015; Meaney, Szyf, & Seckl, 2007), and (e) the integration of genetic and prenatal environmental factors on DNA methylation is seen to have enduring influences on normal development and health (Czamara et al., 2019).
While an increasing number of observational studies has found that several prenatal, perinatal, and postnatal factors increased the risk of depression across the life span, most of these studies were cross‐sectional leaving them open to the criticism that cross‐sectional designs increase the likelihood of contributing spurious associations between these factors and depression (Betts, Williams, Najman, & Alati, 2015; Fryer, McGee, Matt, Riley, & Mattson, 2007; Owens & Hinshaw, 2013; Quarini et al., 2016; Richardson et al., 2016; Whelan, Leibenluft, Stringaris, & Barker, 2015). There is a lack of systematic evidence that comprehensively summarizes the factors during early life that contribute to the development of depression. A critical review of these in utero, perinatal, and postnatal conditions not only stimulates possible etiological explanations of the reported associations in depression but also suggests directions for care and prevention services to provide adequate and appropriate support to those with identified risk factors. We are not aware of any systematic review of in utero, perinatal, and postnatal factors on depression that comprehensively synthesizes those attributes associated with the risk of depression. This present review aims to objectively evaluate the role of each early‐life exposure (occurring after conception, before or after birth (up to 1 year old), or characteristics of the perinatal environment) in major depression or depressive symptoms across the life span. The exposures were grouped into biological, psychological, and sociological factors based on the biopsychosocial model proposed by Engel (1977) which provides a fundamental framework to systematically consider the roles of biological, psychological, and sociological factors in the process of disease and health. This model endorsed a holistic approach, linking normality to abnormality through biological and psychosocial mechanisms (Frazier, 2020). Findings of this review could add new knowledge to the DOHaD hypothesis by clearly showing the factors that are robustly associated with depression and draw attentions to these identified factors noting their importance and the necessity for high quality maternal and child care to address them.
Methods
Search strategy
This systematic review and meta‐analysis are guided by the Preferred Reporting Items for Systematic Reviews (PRISMA) and Meta‐analysis of Observational Studies in Epidemiology (MOOSE) checklists (Table S1) (Moher, Liberati, Tetzlaff, Altman, & Prisma Group, 2009; Stroup et al., 2000). The protocol of this study was registered in PROSPERO, number CRD42019147074. We searched for prospective longitudinal studies on in utero, perinatal/postnatal exposures. These exposures included birth weight, baby’s length at birth, birth order, gestational age (GA), small gestational age (SGA), socioeconomic status at birth, maternal education, teen parents, parental age greater than 35 years of age, single mother, maternal stress, maternal anxiety, maternal pre‐ and postnatal depression, exposure to maternal drinking, maternal smoking and paternal smoking, and exposure to bisphenol A, fetal antiepileptic drug, selective serotonin reuptake inhibitors, antibiotics, organic pollutants, elemental carbon attributable to traffic (ECAT), marijuana, cocaine, famine, maternal infection, and maternal chronic disorders. We first conducted a computerized search for bibliographic databases, including EMBASE, HealthStar, PsychoInfo, and Medline from their inception until September 1, 2019 for published articles on the above‐mentioned maternal and child conditions and major depression. The detailed search strategies for each database are fully described in Appendix S1. We then applied a snowball technique to search the reference lists of all relevant studies for further potential studies. In addition, we manually searched other resources to maximize the relevant studies. The reference lists of selected articles, review articles on relevant topic, and the gray literature were all screened. Only articles published in English were included.
Eligibility criteria
Articles that met the following criteria were included in this systematic review: (a) population: had a clear diagnosis for major depression or depressive symptoms, according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) and its updates, International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD‐10) or other generally accepted criteria; (b) phenomenon of interest: provided the information on in utero, perinatal, or early‐life exposures; (c) comparison: had a comparison group(s) without the exposure(s); (d) outcome: provided statistical indicators to examine the impact of in utero and perinatal factors on major depression across the life span; and (e) study design: were longitudinal cohort studies, including population‐based cohort studies and registry‐based studies.
Selection of the studies
Two researchers (YS and XM) independently screened and selected the retrieved articles by titles and abstracts, followed by full texts. An article was discarded if both reviewers agreed that the article did not meet the inclusion criteria. Inconsistencies in interpretation were resolved through group discussions among the authors. Endnote and RefWorks were used as bibliographic software.
Data extraction, synthesis, and quality assessment
Data on first author(s), year of publication, study site, sample size, age of offspring at enrollment, length of follow‐up, type of depression, prenatal, perinatal, and postnatal factors, measures of major depression or depressive symptoms, other covariates, and major results were extracted independently. If there were multiple reports from a single cohort, only the record with more complete information was extracted. If there were any discrepancies in reports, we contacted the study authors for clarification. Any disagreements were solved by group discussions. For those articles which did not have the data to calculate the pooled estimates, we provided a narrative description instead and kept the results for qualitative analysis. And if several studies were from the same cohort study with the same exposures, the one with the longest follow‐up time was chosen for analysis.
The reviewers endeavored to contact the original authors of the studies with missing information to gather complete information. We offered open‐ended questions to prevent the skewness of responses. The methodological quality of the included studies was assessed by the Newcastle–Ottawa Scale (NOS) for assessing the quality of cohort studies (Wells, 2001). The NOS rating is composed of eight items covering three domains: selection of study groups, comparability of case and comparison groups, and ascertainment of outcome or exposure. The total score of the NOS ranges between 0 and 9. The scores of good quality studies ranged from 7 to 9, studies scoring 6 were judged fair, and those scoring <5 were considered poor.
This review included both quantitative and qualitative analyses for the selected articles. For the quantitative analysis only, original studies with the necessary data to calculate an odds ratio were included, whereas the qualitative synthesis included the rest of the studies for which odd ratios could not be calculated or could not be synthesized by meta‐analysis due to high heterogeneity. These later studies had diverse categorizations of variables, or statistical indicators that could not be translated into odds ratios. Or they were included in the qualitative analysis because of the limited number of available studies on a given risk factor. A total of 64 articles were included in this review. Of the included studies, 29 reported only quantitative results, 31 reported only qualitatively analyzed results, and four reported both quantitative and qualitative results. As a result, 33 studies were used in the meta‐analysis and 35 studies were qualitatively synthesized. We report results of both the quantitative and qualitative syntheses based on the studied exposure.
Meta‐analysis
DerSimonian and Laird I2 statistics were used to test heterogeneity across studies. We used both funnel plots and Egger/Begg tests to examine publication bias. Compared with funnel plots, the Egger test provides a more objective way to estimate the reliability of the results. We then applied the trim and fill method to explore potential publication bias. Based on the results of heterogeneity tests, random‐effects model was used to calculate pooled estimates, which is more conservative compared to fixed‐effects model. Meta‐regression analysis was conducted to identify possible explanations for the heterogeneity. Sensitivity analysis was also performed to explore the effects of the potential confounders. We did subgroup analysis for different prenatal and perinatal/postnatal factors. Analyses were conducted using RevMan version 5.3 and Stata version 15.0.
Results
We started with a total of 9,418 titles from the four bibliographic databases. A total of 4,293 duplicates were then removed. A total of 1,392 articles were identified as eligible studies for full‐text review with 1,328 being subsequently excluded for detailed reasons. After applying the inclusion criteria, 64 studies remained for this systematic review and meta‐analysis. A flowchart of the study selection process is presented in Figure 1.
Figure 1.
A summary of study selection process [Colour figure can be viewed at zslpublications.onlinelibrary.wiley.com.]
Table S2 presents a summary of the characteristics of 64 studies reviewed (Alati et al., 2007; Barker, Copeland, Maughan, Jaffee, & Uher, 2012; Betts, Salom, Williams, Najman, & Alati, 2015; Biederman, Martelon, Woodworth, Spencer, & Faraone, 2017; Buizer‐Voskamp et al., 2011; Butler, 2014; Chiu et al., 2019; Cohen et al., 2013; Colman, Ataullahjan, Naicker, & Van Lieshout, 2012; Elmasry, Goodwin, Terry, & Tehranifar, 2014; Fan & Eaton, 2001; Fergusson, Woodward, & Horwood, 1998; Gale & Martyn, 2004; Geoffroy, Gunnell, Clark, & Power, 2018; Gray, Day, Leech, & Richardson, 2005; Harley et al., 2013; Herva et al., 2008; Johnson, O'Reilly, Ni, Wolke, & Marlow, 2019; Joinson, Kounali, & Lewis, 2017; Kiff et al., 2012; Kingsbury et al., 2016; Leung, Leung, & Schooling, 2015; Levine, 2014; Levy‐Shiff et al., 1994; Li et al., 2018; Liu et al., 2011; Loret de Mola et al.., 2015; Malm et al., 2016; Meier et al., 2017; Menezes et al., 2013; Murphy et al., 2017; Nomura, Brooks‐Gunn, Davey, Ham, & Fifer, 2007; Nomura, Wickramaratne, et al., 2007; O'Connor & Kasari, 2000; Pawlby, Hay, Sharp, Waters, & O'Keane, 2009; Pearson et al., 2013, 2013; Pereira et al., 2012; Perera et al., 2016; Perquier, Lasfargues, Mesrine, Clavel‐Chapelon, & Fagherazzi, 2014; Phillips, Hammen, Brennan, Najman, & Bor, 2005; Pitzer, Jennen‐Steinmetz, Esser, Schmidt, & Laucht, 2011; Plant, Pariante, Sharp, & Pawlby, 2015; Power et al., 2007; Ramchandani et al., 2008; Raposa, Hammen, Brennan, & Najman, 2014; Richardson, Goldschmidt, Larkby, & Day, 2013; Saigal, Pinelli, Hoult, Kim, & Boyle, 2003; Slykerman et al., 2015, 2017; Sonon, Richardson, Cornelius, Kim, & Day, 2016; Strom et al., 2014; Taka‐Eilola Nee Riekki, Veijola, Murray, Koskela, & Maki, 2019; Taylor et al., 2017; Tearne et al., 2016; Tracy, Salo, Slopen, Udo, & Appleton, 2019; Tuovinen et al., 2014; Van Lieshout & Boylan, 2010; Wang, Leung, Lam, & Schooling, 2015; Westrupp, Northam, Doyle, Callanan, & Anderson, 2011; Yamasaki et al., 2019; Yolton et al., 2019; Zohsel et al., 2017). Of the 64 studies, 18 studies were conducted in the United States, 13 in United Kingdom, 6 in Australia, 3 in Canada, 4 in Finland, 1 in France, 4 in China, 2 in Denmark, 2 in Germany, 4 in Brazil, 1 in Japan, 3 in New Zealand, 1 in Netherlands, 1 in Norway, and 1 in Israeli. The NOS score of study quality for the included studies ranged from 4 to 9 with the median score of 6.9. This review includes mostly good quality studies. Thirty‐eight of the studies were judged to be good quality studies, and 25 were rated as being of fair quality with one study rated as poor. Details of study quality can be found in Table S3. Of the studies reporting quantitative results, 17 were judged as good quality studies, 16 were judged as being of fair quality. Of the studies reporting qualitatively synthesized results, 21 were judged to be good quality, nine were judged fair, and one was poor quality.
Table 1 provides a concise summary of the meta‐analysis’s findings. Of the 28 early‐life exposures studied, a total of 12 prenatal and perinatal/postnatal factors were significantly associated with offspring’s depression, namely low birth weight, premature birth, SGA, maternal education, socioeconomic position, having younger parents (<20 years old), having older parents (≥35 years), maternal smoking, paternal smoking, maternal stress, maternal anxiety, and prenatal depression. Forest plots of the studied individual and pooled odds ratios are presented in Figure 2. Heterogeneity tests for most of the studied variables were not significant (p > .05) with exceptions being socioeconomic status (SES), maternal smoking, maternal prenatal depression, low birth weight, and GA. Since high heterogeneity was found for the pooled estimates for some risk factors, we used meta‐regression analyses to explore if age onset of depression (≤17 and >18 year of old) could explain the heterogeneity across the selected studies. The univariate meta‐regression showed that age of onset had a significant impact on the heterogeneity across studies. Table S4 displays the results of the meta‐regression models.
Table 1.
A summary table of the meta‐analysis findings
| Factors | Number of studies | Odds Ratio | 95%CI | Find significant association |
|---|---|---|---|---|
| Biological factors | ||||
| Low birth weight (<2,500 g) | 10 | 1.44 | 1.17, 1.76 | Yes |
| Gestational age (Premature children < 37 weeks) | 7 | 1.38 | 1.00, 1.90 | Yes |
| Small gestational age | 3 | 1.46 | 1.11, 1.94 | Yes |
| Birth order | 5 | 1.08 | 0.91, 1.29 | No |
| Teen parents (<20 years old) | 9 | 1.46 | 1.22, 1.74 | Yes |
| Parents' age >35 years | 6 | 1.13 | 1.07, 1.20 | Yes |
| Psychological factors | ||||
| Maternal stress | 4 | 1.12 | 1.04, 1.22 | Yes |
| Maternal anxiety | 2 | 1.09 | 1.02, 1.17 | Yes |
| Maternal prenatal depression | 8 | 1.36 | 1.07, 1.72 | Yes |
| Maternal postnatal depression | 3 | 1.52 | 0.92, 2.50 | No |
| Sociological factors | ||||
| Maternal education (<9 years of study) | 9 | 1.37 | 1.19, 1.57 | Yes |
| Socioeconomic status (Low) | 6 | 1.29 | 1.10, 1.52 | Yes |
| Single mother | 2 | 1.20 | 0.64, 2.25 | No |
| Maternal smoking | 13 | 1.45 | 1.28, 1.63 | Yes |
| Paternal smoking | 4 | 1.28 | 1.11, 1.48 | Yes |
| Maternal drinking | 2 | 1.09 | 0.96, 1.24 | No |
Figure 2.
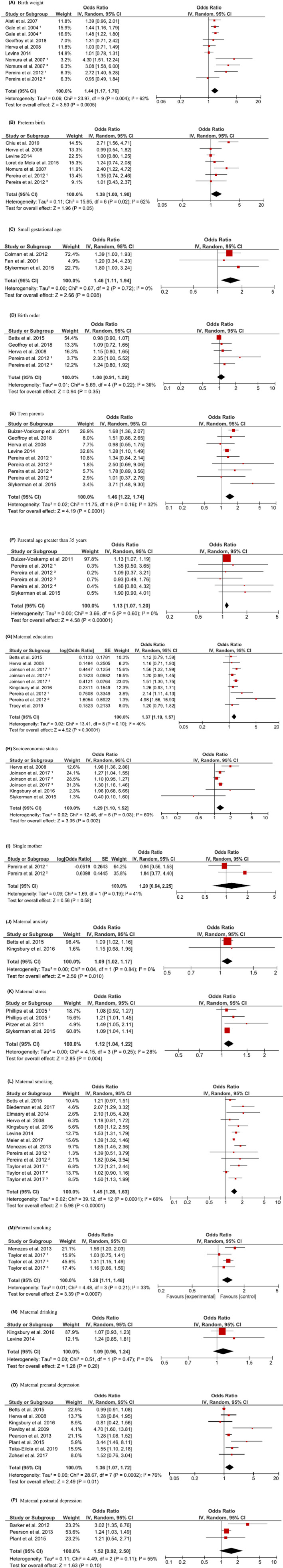
Pooled ORs for the association between prenatal, perinatal, and postnatal factors and depression risk. Gale1 is the study among females, Gale2 is the study among males; Nomura1 is the New York State Psychiatric Institute/Columbia University study, Nomura2 is the National Collaborative Perinatal Project (NCPP) study; Pereira1 study compares depression in children 7–9 years with maternal factors, Pereira2 study compares depression in children 10–11 years with maternal factors, Pereira3 study compares depression in children 7–9 years with paternal factors, Pereira4 study compares depression in children 10–11 years with paternal factors; Joinson1 study compares depression in children less than 12 years old, Joinson2 study compares depression in children at 12–16 years old, Joinson3 study compares depression in children at 12–17 years old; Phillips1 is the study on prenatal stress, Phillips2 is the study on prenatal stress; Taylor1 is the ALSPAC study, Taylor2 is the HUNT study, and Taylor3 is the Pelotas 1982 study [Colour figure can be viewed at zslpublications.onlinelibrary.wiley.com.]
Because some exposures only had one publication in the selected studies, we then applied qualitative approach to synthesize these exposures instead. Additionally, studies with either diverse categorization or statistical indicators that could not be converted into odds ratios were also summarized as qualitatively analyzed studies. Noteworthy, although some studies presented the findings with odds ratios, their categorizations of exposures could not be harmonized thus these studies were described qualitatively. Detailed results from these qualitatively analyzed studies are reported in Table S5. These qualitatively analyzed studies are also noted by the # symbol in Table S2. Although there are some overlapped risk factors between qualitatively and quantitatively analyzed studies, there are some risk factors that are unique to the qualitatively analyzed studies. All qualitatively analyzed results are summarized in Table 2. We grouped in utero, perinatal and postnatal exposures into biological, psychological, and sociological factors following the biopsychosocial model of mental illness.
Table 2.
A summary of the qualitative analyzed findings
| Factors | Number of studies | Results | Major findings |
|---|---|---|---|
| Biological factors | |||
| Low birth weighta | 7 | Negative (4) Positive (3) | Three studies reported an association between low birth weight and depression in offspring, while four studies reported null association. |
| Gestational agea | 4 |
Negative (3) Positive (1) |
One study reported an association between preterm birth and depression in offspring, while three studies reported null association. |
| Small gestational agea | 1 | Positive | One study reported an association between small gestational age and depression in offspring in adolescence. |
| Baby’s length at Birth | 1 | Negative | One study reported null association between birth length and depression in offspring. |
| Teen parentsa | 4 |
Positive (3) Negative (1) |
Three studies reported an association between having teen parents and depression in offspring, while one study reported null association. |
| Parents' age >35 yearsa | 1 | Positive | One study reported an association between having elderly parents and depression in male offspring. |
| Maternal infections | 2 | Mixed | One study reported an association between maternal infections and depression in offspring, while one study reported null association. |
| Chronic diseases | 2 | Positive | Two studies reported that maternal hypertensive disorder and diabetes were associated with an elevated risk of depression in offspring, respectively. |
| Bisphenol A | 2 | Positive | Two studies reported an association between Bisphenol A and depression in offspring. |
| Fetal antiepileptic drug | 1 | Negative | One study reported null association between fetal antiepileptic drug and depression. |
| SSRI | 1 | Positive | One study reported an association between SSRI and depression in offspring. |
| Antibiotics | 1 | Positive | One study reported an association between antibiotics use and depression in offspring. |
| Organic pollutants | 1 | Negative | One study reported null association between fetal exposure to persistent organic pollutants and depression. |
| ECAT | 1 | Positive | One study reported an association between prenatal ECAT exposure and depression in offspring. |
| Marijuana exposure | 2 | Positive | Two studies reported an association between maternal marijuana exposure and depression in offspring. |
| Cocaine exposure | 1 | Negative | One study reported null association between cocaine exposure and depression. |
| Psychological factors | |||
| Maternal stressa | 2 | Mixed | One study reported an association between maternal stress and depression in offspring, while one study reported null association. |
| Maternal prenatal depressiona | 2 | Mixed | One study reported an association between maternal prenatal depression and depression in offspring, while one study reported null association. |
| Paternal postnatal depression | 1 | Negative | One study reported null association between paternal postnatal depression and depression in offspring. |
| Sociological factors | |||
| Socioeconomic statusa | 2 | Mixed | One study reported an association between low socioeconomic status and depression in offspring, while one study reported null association. |
| Maternal smokinga | 4 |
Negative (3) Positive (1) |
One study reported an association between maternal smoking and depression in offspring, while three studies reported null association. |
| Maternal drinking | 3 |
Positive (2) Negative (1) |
Two studies reported an association between maternal drinking and depression in offspring, while one study reported null association. |
| Famine | 1 | Positive | One study reported null association between famine in utero and depression in offspring. |
ECAT, Elemental carbon attributable to traffic; SSRI, Selective serotonin reuptake inhibitors.
Risk factors found to be significantly associated with offspring’s depression in the meta‐analysis.
Biological factors
Low birth weight
Ten studies were included in the meta‐analysis (Alati et al., 2007; Gale & Martyn, 2004; Geoffroy et al., 2018; Herva et al., 2008; Levine, 2014; Nomura, Brooks‐Gunn, et al., 2007; Nomura, Wickramaratne, et al., 2007; Pereira et al., 2012). The findings indicated that low birth weight (<2,500 g) was associated with an elevated risk of depression, with a pooled OR of 1.44 (95%CI, 1.17–1.76, k = 10, N = 27,574). There was a high degree of heterogeneity between studies (I 2 = 62%, p < .01). Visual assessment of a funnel plot, and Egger as well as Begg test provided no evidence of obvious publication bias.
However, the findings in the qualitative analysis were mixed (k = 7). Three of seven studies reported an association between low birth weight and depression (Levy‐Shiff et al., 1994; Loret de Mola et al., 2015; Van Lieshout & Boylan, 2010). In contrast, two studies found no association between birth weight and major depression (OR = 0.91; 95%CI, 0.82–1.01; OR = 1.15; 95%CI, 0.96–1.36) (Betts, Salom, et al., 2015; Perquier et al., 2014). Two additional studies, one on very low birth weight (<1,500 g) and the other on extremely low birth weight (<1,000 g) did not find any association between low birth weight and depression (Saigal et al., 2003; Westrupp et al., 2011) (Details could be found in Table S5).
Gestational age
In the meta‐analysis of seven studies (Chiu et al., 2019; Herva et al., 2008; Levine, 2014; Loret de Mola et al., 2015; Nomura, Brooks‐Gunn, et al., 2007; Pereira et al., 2012), premature children (<37 weeks) were found at an elevated risk of depression (OR = 1.38; 95%CI, 1.00–1.90, k = 7, N = 128,001) and there was a high degree of heterogeneity between studies (I 2 = 62%, p = .02). No obvious publication bias was observed from visual assessment of a funnel plot or Egger/Begg test.
In contrast, the findings of qualitatively analyzed studies (k = 4) on the relationship between gestational age and depression were generally negative. One study reported that gestational age was linked with a higher risk of offspring’s depression (r = .82, p < .001) (Levy‐Shiff et al., 1994), whereas three studies found no statistical evidence of such association including one study on extremely preterm children (<26 weeks) (Cohen et al., 2013; Johnson et al., 2019; Wang et al., 2015).
Small gestational age at birth
The meta‐analysis of three studies suggested that being small for gestational age at birth was more likely to lead to major depression (OR = 1.46; 95%CI, 1.11–1.94, k = 3, N = 6,165) (Colman et al., 2012; Fan & Eaton, 2001; Slykerman et al., 2015). There was no evidence of heterogeneity between studies (I 2 = 0%, p = .72). Visual inspection of a funnel plot, and Egger as well as Begg test provided no evidence of obvious publication bias.
In the qualitative analysis, one study found small gestational age was associated with an increased risk of depression onset only in adolescence but not in childhood (tadolescence = 2.70, p < .01; tchildhood = 0.15, p < .88) (Van Lieshout & Boylan, 2010). It supported our significant findings of a pooled OR of 1.46.
Birth order
The meta‐analysis of five studies found that there was no statistical association between birth order and depression (OR = 1.08; 95%CI, 0.91–1.29, k = 5, N = 21,246), when comparing individuals who were second born or later to those who were first born individuals (Betts, Salom, et al., 2015; Geoffroy et al., 2018; Herva et al., 2008; Pereira et al., 2012). There was no evidence of heterogeneity between studies (I 2 = 30%, p = .22). The visual inspection of the funnel plot and Begg test did not indicate any evidence of publication bias. However, the Egger test found significant publication bias (p = .031).
Baby's length at birth
One qualitative study reported null association between baby’s birth length and depression (Perquier et al., 2014). There were no comparable meta‐analysis findings.
Age of parents
There were nine studies exploring the association between having teen parents (<20 years old) and depression in offspring in the meta‐analysis (Buizer‐Voskamp et al., 2011; Geoffroy et al., 2018; Herva et al., 2008; Levine, 2014; Pereira et al., 2012; Slykerman et al., 2015). The findings showed that having teen parents elevated the risk of offspring’s depression (OR = 1.46; 95% CI, 1.22–1.74, k = 9, N = 93,850) and there was no evidence of heterogeneity between studies (I 2 = 32%, p = .16).
Likewise, in the meta‐analysis of six studies (Buizer‐Voskamp et al., 2011; Pereira et al., 2012; Slykerman et al., 2015), we found that offspring who had parents 35 years of age and older also reported a higher risk of depression (OR = 1.13; 95% CI, 1.07–1.20, k = 6, N = 73,208). There was no evidence of heterogeneity between studies (I 2 = 0%, p = .60). No obvious publication bias was observed from the visual assessment of a funnel plot nor Egger/Begg test for both of the exposures.
A total of four qualitatively analyzed studies explored the relationship between having teen parents and the risk of depression in offspring, with 3 of 4 studies reported a positive association (Kiff et al., 2012; Slykerman et al., 2015; Tracy et al., 2019). One study reported non‐significant findings (Tearne et al., 2016).
Additionally, the only qualitatively analyzed study reported that parental age greater than 35 years increased the risk of depression for girls but not for boys (Tearne et al., 2016).
Physical health problems
There was no meta‐analysis on the exposure of physical health problems due to the limited number of eligible studies.
Qualitatively analyzed findings (k = 4) on maternal infections were inconsistent, including one study reporting an elevated risk of depression among offspring (Murphy et al., 2017), and non‐significant findings in another study (Betts, Salom, et al., 2015). Two studies reported that maternal hypertensive disorder and diabetes were associated with an elevated risk of depression among offspring, respectively (Tuovinen et al., 2014; Yamasaki et al., 2019).
Chemical, and drug exposures
There is only qualitative synthesis available for the chemical and drug exposures. We did not perform a meta‐analysis for these factors because of limitations in available studies and high heterogeneity in the measurement of these exposures. A total of eight chemical or drug exposures have been studied to explore their effects on offspring’s depression, including bisphenol A (BPA), fetal antiepileptic drug, selective serotonin reuptake inhibitors, antibiotics, organic pollutants, elemental carbon attributable to traffic (ECAT), marijuana exposure, and cocaine exposure. Prenatal BPA exposure was consistently found to be associated with an elevated risk of depressive symptoms and depression in boys (Harley et al., 2013; Perera et al., 2016). In terms of drugs, prenatal SSRI exposure (Malm et al., 2016), antibiotics use (Slykerman et al., 2017), prenatal or early‐life exposure to elemental carbon attributable to traffic (ECAT) (Yolton et al., 2019), and maternal marijuana exposure (Gray et al., 2005; Sonon et al., 2016) were associated with an elevated risk of depression. There were no statistical associations between offspring's depression and exposure to fetal antiepileptic drug (Cohen et al., 2013), persistent organic pollutants (Strom et al., 2014), and maternal cocaine use (Richardson et al., 2013).
Psychological factors
Maternal stress
The meta‐analysis of four studies found that: (1) maternal stress was associated with offspring’s depression (OR = 1.12; 95% CI, 1.04–1.22, k = 4, N = 2,582) (Phillips et al., 2005; Pitzer et al., 2011; Slykerman et al., 2015). There was no evidence of heterogeneity between studies (I 2 = 28%, p = .25); (2) prenatal maternal stress (two studies) was associated with the increased risk for depression with the pooled odds ratio of 1.09 (95%CI, 1.04–1.14, k = 2, N = 1,157), and there was no evidence of heterogeneity between studies (I 2 = 0%, p = .91); and (3) maternal postnatal stress (two studies) was similarly reported to elevate the risk of depression among offspring (OR = 1.27; 95%CI, 1.07–1.51, k = 2, N = 1,425). No evidence of heterogeneity between studies was found (I 2 = 7%, p = .30). Visual inspection of funnel plots, and Egger as well as Begg tests provided no evidence of obvious publication bias.
The qualitative analyzed findings (k = 2) on the relationship between maternal stress and offspring’s depression risk are mixed, with one study finding a positive relationship whereas another did not (Gray et al., 2005; Murphy et al., 2017).
Maternal anxiety
The meta‐analysis of two studies found that maternal anxiety increased the risk of depression (OR = 1.09; 95% CI, 1.02–1.17, k = 2, N = 13,127) and there was no evidence of heterogeneity between studies (I 2 = 0%, p = .84) (Betts, Salom, et al., 2015; Kingsbury et al., 2016). There was no evidence of obvious publication bias based on the visual assessment of a funnel plot and the Egger/Begg test.
Maternal prenatal depression
In the quantitative analyses of eight studies, maternal prenatal depression increased the risk of depression among offspring (OR = 1.36; 95% CI 1.07–1.72, k = 8, N = 36,985) (Betts, Salom, et al., 2015; Herva et al., 2008; Kingsbury et al., 2016; Pawlby et al., 2009; Pearson et al., 2013, 2013; Plant et al., 2015; Taka‐Eilola Nee Riekki et al., 2019; Zohsel et al., 2017). There was a statistically significant heterogeneity (I 2 = 76%, p < .01). The visual inspection of the funnel plot and Begg test did not indicate any evidence of publication bias. However, the result of Egger test found significant publication bias (p = .032).
In the two qualitatively analyzed studies (k = 2), maternal prenatal depression was significantly associated with depressive symptoms in one study (Gray et al., 2005), but not in another (Raposa et al., 2014).
Parental postnatal depression
Our meta‐analysis of three studies found no statistically significant association between maternal postnatal depression and offspring’s depression (OR = 1.52; 95%CI, 0.92–2.50, k = 3, N = 10,379) (Barker et al., 2012; Pearson et al., 2013, 2013; Plant et al., 2015). There was no evidence of heterogeneity between studies (I 2 = 55%, p = 0.11). Visual inspection of a funnel plot, and Egger as well as Begg test provided no evidence of obvious publication bias.
In the qualitative study, one study reported a null association between paternal postnatal depression and depression in their offspring (Ramchandani et al., 2008), which is consistent with other findings in this systematic review.
Sociological factors
Education
Our meta‐analysis of nine studies found that low maternal education (<9 years of study) was associated with the elevated risk of depression (OR = 1.37; 95% CI, 1.19–1.57, k = 9, N = 41,768) (Betts, Salom, et al., 2015; Herva et al., 2008; Joinson et al., 2017; Kingsbury et al., 2016; Pereira et al., 2012; Tracy et al., 2019). There was no evidence of heterogeneity between studies (I 2 = 40%, p = .10). No evidence of obvious publication bias was observed based on the visual assessment of a funnel plot and the Egger/Begg test.
SES
Six studies were included in the meta‐analysis investigating the relationship between SES and depression in offspring (Herva et al., 2008; Joinson et al., 2017; Kingsbury et al., 2016; Slykerman et al., 2015). The results suggested that low SES was associated with a higher risk of depression (OR = 1.29; 95% CI, 1.10–1.52, k = 6, N = 28,710). There was a statistically significant heterogeneity (I 2 = 60%, p = .03). No obvious publication bias was found from visual inspection of a funnel plot, and Egger as well as Begg test.
Two other qualitatively analyzed studies (k = 2) had mixed findings, with one study reported a significant relationship between low SES and an increased risk of depression and the other found no association (Butler, 2014; Power et al., 2007).
Having a single mother
The meta‐analysis including two studies found that having a single mother did not increase a child’s risk of depression (OR = 1.20; 95% CI, 0.64–2.25, k = 2, N = 1,444) (Pereira et al., 2012). There was no evidence of heterogeneity between studies (I 2 = 41%, p = .19). No obvious publication bias was observed from visual inspection of a funnel plot, and Egger as well as Begg test.
Parental smoking
There were 13 studies exploring the association between maternal smoking and depression risk in offspring (Betts, Salom, et al., 2015; Biederman et al., 2017; Elmasry et al., 2014; Herva et al., 2008; Kingsbury et al., 2016; Levine, 2014; Meier et al., 2017; Menezes et al., 2013; Pereira et al., 2012; Taylor et al., 2017). Maternal smoking was associated with the increased risk of depression in the meta‐analysis (OR = 1.45; 95%CI: 1.28–1.63, k = 13, N = 823,532). There was a statistically significant heterogeneity (I 2 = 69%, p < .01).
Likewise, paternal smoking was also associated with the increased risk of depression in the meta‐analysis of four studies (OR = 1.28; 95%CI: 1.11–1.48, k = 4, N = 26,237) (Menezes et al., 2013; Taylor et al., 2017). There was no evidence of heterogeneity between studies (I 2 = 33%, p = .21). Visual assessment of funnel plots, and Egger as well as Begg tests provided no evidence of obvious publication bias for maternal smoking exposure and paternal smoking exposure.
In contrast, the qualitatively analyzed studies (k = 4) were largely unsupportive of a relationship between maternal smoking and offspring depression. One study reported significant associations across all trimesters (r 1st trimester = .07, p < .10; r 2nd trimester = .07, p < .10; r 3rd trimester = .06, p < .10) (Gray et al., 2005), but three other studies found no association (Fergusson et al., 1998; Leung et al., 2015; Liu et al., 2011).
Maternal drinking
The meta‐analysis did not find a statistically significant association between mothers’ drinking habits and depression among two studies (OR = 1.09; 95%CI, 0.96–1.24, k = 2, N = 13,976) (Kingsbury et al., 2016; Levine, 2014), and there was no evidence of heterogeneity between studies (I 2 = 0%, p = .47). No obvious publication bias was observed from visual assessment of a funnel plot nor Egger/Begg test.
Qualitatively analyzed studies (k = 3) suggest a potential link between maternal drinking and offspring depression, with two qualitatively analyzed studies reporting that exposure to maternal drinking increased the risk of depression and depressive symptoms in offspring (Gray et al., 2005; O'Connor & Kasari, 2000); however, another study reported no association between maternal drinking (defined as >1 drink per day) and offspring depression (Betts, Salom, et al., 2015).
Famine
One Chinese study found that exposure to famine in utero increased the risk of depression among offspring (Li et al., 2018).
For the meta‐analysis
Publication bias
We used Begg and Egger tests to explore publication bias. Most of the studied variables did not have publication bias. The shapes of the funnel plots did not indicate any evidence of obvious asymmetry (see Figure S1). Although Begg test found no significant publication bias for all the exposures, the results of Egger test suggested that two variables (birth order and maternal prenatal depression) had publication bias (Table S6). The trim and fill method was then used to recalculate the pooled results for these two variables. The adjusted pooled effect of birth order exhibited a similar trend (OR = 0.99; 95% CI 0.84–1.18) with three potential studies were filled. For prenatal depression, the adjusted pooled effect became non‐significant (OR = 1.21; 95% CI 0.90–1.55) after two additional studies were added.
Sensitivity analysis
Each study was excluded at one time to test the stability of the pooled results. We found that findings of three variables (SGA, parental age greater than 35 years, and paternal smoking) changed when we removed one study at a time. The rest of the results remained unchanged (see Figure S2). Subgroup analyses were also utilized for prenatal, perinatal, and postnatal exposures. Subgroup analyses supported that the effects of prenatal, perinatal, and postnatal exposures on depression all remained significant (see Figures S3a and S3b).
Discussion
This review provides the first comprehensive overview of prenatal, perinatal, and postnatal exposures related to offspring’s depression. These early‐life exposures cover biological, psychological, and sociological characteristics that are pertinent for depression. A total of 28 prenatal, perinatal, and postnatal exposures had been reported on in the 64 prospective cohort studies, we provide both quantitative and qualitative syntheses. For the quantitative synthesis, our meta‐analysis identified that a total of 12 prenatal and perinatal/postnatal factors were associated with an increased risk of depression. These factors were low birth weight, premature birth, small gestational age, having teen birth parents (<20 years old), having birth parents older than 35 years, maternal education, SES, maternal smoking, paternal smoking, maternal stress, maternal anxiety, and maternal prenatal depression. The qualitative syntheses identified a total of 23 factors (some overlapped with quantitative analysis) and were generally supportive of the relationships between some of the risk factors identified in the meta‐analysis and offspring’s depression. The qualitative analysis also provided additional information on other risk factors that may influence offspring’s depression. Together these studies provided solid evidence for a developmental origin of depression hypothesis.
For the biological factors that are studied in this review, our findings are generally consistent with previous reviews on specific biological factors. For example, a previous systematic review of 14 studies reported a positive association between low birth weight and depression, but not for premature birth and SGA (Loret de Mola, de França, Quevedo Lde, & Horta, 2014). They noted that conservative analytical approaches and publication bias might confound the relationships between premature birth, SGA, and depression. Our findings on low birth weight and depression are consistent with the literature. Another meta‐analysis also reported a small but significant effect of low birth weight on depression (Wojcik, Lee, Colman, Hardy, & Hotopf, 2013). These associations of birth outcome measures (low birth weight, SGA, and premature birth) can be potentially explained by the glucocorticoid programming of the hypothalamic–pituitary–adrenal axis (HPAA) (Seckl & Meaney, 2004). Preterm and SGA births were linked with altered HPAA activity in an early acquired and persisting neurophysiological vulnerability (Patton, Coffey, Carlin, Olsson, & Morley, 2004), which then becomes involved in the development of depression due to an increased sensitivity to adversity (Räikkönen et al., 2008). Low birth weight may be related to an elevated level of circulating glucocorticoids. Other hormonal systems like growth hormone – insulin‐like growth factor system, can synergize HPAA to regulate the fetal growth and brain development and may contribute to the pathogenesis of depression (Russo, Gluckman, Feldman, & Werther, 2005). These birth measures are also related to the activity of placental enzymes, which regulate maternal‐fetal glucocorticoid and serotonin transfer. These enzymes are known as key modulators of fetal programming in adult mental disorders (Bonnin & Levitt, 2011). Neurological damage caused by preterm births, such as cerebellar hemorrhagic injury, has been related to internalizing behavioral problems (Limperopoulos et al., 2007). We found no association between birth order and subsequent depression. The literature on birth order and depression is mixed (Carballo et al., 2013; Hardt, Weyer, Dragan, & Laubach, 2017). The relationship between birth order and depression needs to consider the presence of different family sizes, spacing between offspring, the underlying perceptions adolescents had of their older sibling, or support levels within the sibling dyads (Finan, Ohannessian, & Gordon, 2018).
There has been an increasing interest in the relationship between parental age and psychiatric disorders (Chudal et al., 2014; D'Onofrio et al., 2014). We found both younger parents (<20 years) and older parents (>35 years) were more likely to have depressed children. This is consistent with prior reviews on autism and psychotic disorders (Lopez‐Castroman et al., 2010; Sandin et al., 2012). Younger parents are more likely to be challenged by unplanned parenting, stress, marital discord, lack of parenting experience, and economic circumstances, which in turn affect the trajectory of normal development of their children (Kessler et al., 2010). Older parents may be also unprepared for the relationship and life changes brought by the advent of a child.
Our review found that the studied psychosocial factors (maternal stress, anxiety, and prenatal depression) were related to depression in offspring. Depression, stress, and anxiety during pregnancy can trigger increased levels of maternal glucocorticoids, which have been considered as the key connection linking maternal adversity to the fetus (McGowan & Matthews, 2018; Stroud et al., 2016). Evidence has also shown that maternal glucocorticoids increase the risk of children psychopathology (Buss, Davis, et al., 2012). Also, depression is somewhat heritable (Agrawal, Jacobson, Gardner, Prescott, & Kendler, 2004). Maternal stress and prenatal anxiety may be associated with less optimal development trajectory for offspring (Pesonen, Räikkönen, Strandberg, & Järvenpää, 2005). Genetic susceptibilities combine with early‐life environmental adversities can posit a threat to a normal growth and development.
Empirical psychological and social studies have stressed the importance of the timing of exposures, that is, the period of development in which the infant may be more susceptible to the effects of these exposures (Michel & Tyler, 2005; Sanger, Iles, Andrew, & Ramchandani, 2015). For example, studies have shown that the activation of the maternal stress response especially during the second and third trimesters, including glucocorticoid signaling and the immune response, could damage offspring’s brain development (Wang, Kloth, & Badura, 2014). Maternal depression in the early postnatal months has been found to be closely related to child development (Rigato, Stets, Bonneville‐Roussy, & Holmboe, 2020). Maternal depression may not have a single sensitive period but instead has a cascading influence and cumulative impact across the whole pregnancy period and into early childhood (Bagner, Pettit, Lewinsohn, & Seeley, 2010).
Although prenatal depression was seen as the strongest predictor of postnatal depression and may persist into the postnatal period (Heron, O'Connor, Evans, Golding, & Glover, 2004), in this present review we also observed that maternal prenatal depression was an independent risk factor for offspring’s depression and the contributing role of maternal postnatal depression in offspring’s depression was diminished. Therefore, we could propose that the effect of prenatal depression in offspring’s depression is independent from the effect of postnatal maternal depression in offspring’s depression. Some supporting evidence has been found which demonstrated that there is generally no effect of change in maternal depression from the prenatal to postnatal period with the effect of postnatal depression limited to mothers with lower education (Pearson et al., 2013, 2013). These findings suggest that prenatal and postnatal maternal depression may affect offspring via different pathways. Prenatal depression might exert a detrimental effect on utero through biological mechanisms (Srinivasan, Pearson, Johnson, Lewis, & Lewis, 2020). A great deal of physiological changes of depressed mothers during pregnancy posit an adverse intrauterine environment which can alter fetal developmental programming (Wen et al., 2017). In contrast, postnatal depression impacts offspring possibly through environmental factors such as parental discipline and social engagement which predict increased vulnerability to psychopathology in offspring (Murray, 2009). Depressed mothers have been found to be less sensitive in responding to and interacting with their children, and more likely to have unpredictable parenting styles (Milgrom, Westley, & Gemmill, 2004). Therefore, it is suggested there is no direct effect of postnatal depression on offspring, but its effect is possibly mediated through the pathways of the home environment (Pearson et al., 2013, 2013).
For sociological characteristics, lower SES and lower maternal education were associated with an increased risk in subsequent depression in children. Consistent with our findings, a previous systematic review of 55 studies reported that children from socioeconomically disadvantaged families were at a higher risk of mental health problems. This meta‐analysis also found that low parental education was the strongest risk factor for mental disorders among children and adolescents (Reiss, 2013). Children from low SES families, especially for those with parents of having lower levels of education, have limited access to the material, psychological, and social resources that model important contributions to the development of positive mental health (McLaughlin et al., 2011).
Maternal smoking and paternal smoking were associated with an increased risk of offspring’s depression in the current meta‐analysis. One of the explanations is the ‘fetal programming hypothesis’, which hypothesizes that the developmental origin of psychiatric disorders might be a result of prenatal exposure to adverse intrauterine conditions such as infection, pollution, smoking, and other substance use, altering the body’s organs and systems (Buss, Entringer, & Wadhwa, 2012). Stroud and his colleague highlighted an important role of placental serotonin signaling in modulating prenatal programming of the fetal HPA axis by maternal adversity (Stroud et al., 2016). More importantly, we observed a slightly stronger maternal‐offspring association compared with paternal‐offspring association for smoking exposures during pregnancy. We anticipate that there is a causal intrauterine effect between smoking and depression in offspring rather than a link due to genetic and shared environmental confounders. If the genetic and shared environmental confounders accounted for this association, the magnitude of the association between maternal and paternal smoking and depression in offspring would be similar (Langley, Heron, Smith, & Thapar, 2012; Richmond, Al‐Amin, Smith, & Relton, 2014). The possible explanations to account for the differential effects of maternal and/or paternal smoking on the fetus are the unique maternal utero effects come through causal pathways affecting fetal programming (Taylor et al., 2017). Future studies (e.g., family‐based sibling control studies), which partially account for genetic and environmental confounders, are needed to reveal the nature of the relationship between parental smoking and offspring’s depression.
This review provides the first comprehensive overview of prenatal, perinatal, and postnatal factors associated with offspring’s subsequent depression. This review used a comprehensive search and followed the recommended guidelines for reporting of results and methodological quality assessment to ensure the findings objectively summarize the literature on the DOHaD in depression. This review only included prospective cohort studies, good quality studies, studies with large sample sizes, with relatively long and adequate follow‐up periods, with reliable measurements of exposures and outcomes to deliver robust evidence on developmental origins of depression.
However, several potential limitations should be noted. First, 2 of the 16 factors in the meta‐analysis (birth order and prenatal depression) have publication bias. The pooled estimate of prenatal depression became nonsignificant after taking two additional studies (as suggested by trim and fill tests) into account. Interpretation of the relationship between prenatal depression and offspring’s depression warrants attention. Second, we observed high heterogeneity for a few exposures and the limited number of studies in some exposures precluded the detailed subgroup analyses. Also, even though 33 studies were included in the overall meta‐analysis, for some exposures there were generally fewer than six studies included, thus limiting the statistical power to detect heterogeneity and to assess potential effect modification caused by study characteristics. Third, although we used subgroup analysis, sensitivity analysis, and meta‐regression analysis to explore potential confounders involved in the relationship between prenatal, perinatal, and postnatal factors and depression, there are still other unknown and unobserved confounders that might also be involved in this relationship. Both measured and unmeasured familial confounders can influence the parent–child relationship. Kingsbury et al. (2016) reported that neither maternal drinking nor maternal prenatal depression was associated with offspring depression after controlling for sex, ethnicity, maternal teen status, maternal education, maternal history of depression, prenatal smoking, or alcohol use, prenatal depression or anxiety, high maternal stress at 8 months postpartum, and maternal depression at 8 years postpartum. A Swedish study found that there was no clear evidence to support the association between maternal smoking during pregnancy and offspring depression in a sibling‐controlled analysis with the same family context and genetic predisposition (Taylor et al., 2017), though there are limitations in using sibling controls. Fourth, meta‐analysis cannot take all studied covariates from original studies into account because of variations in what were measured across the included studies. We used sensitivity and subgroup analysis instead to explore potential confounders that might be involved in the relationship between offspring’s depression and prenatal, perinatal, and postnatal exposures.
Conclusion
This systematic review provides a robust and comprehensive overview of prenatal, perinatal, and postnatal factors associated with the risk of offspring’s depression. Within the framework of the biopsychosocial model, a total of 12 prenatal and perinatal/postnatal factors among 28 studied factors were significantly associated with offspring’s depression in the meta‐analysis. The findings of the review highlight the need for clinical and public health interventions to focus on these identified risk factors. Large prospective cohort studies are warranted to investigate the combined effects of co‐existing early‐life exposures.
Supporting information
Figure S1. Funnel plot for the analysis of in utero and perinatal factors and depression risk.
Figure S2. Sensitivity analyses for the association between in utero and perinatal factors and depression risk.
Figure S3a. Subgroup meta‐analysis of the relationship between prenatal factors and offspring’s depression.
Figure S3b. Subgroup meta‐analysis of the relationship between perinatal/postnatal factors and offspring’s depression.
Table S1. MOOSE checklist for meta‐analyses of observational studies.
Table S2. Characteristics of included studies on in utero, perinatal and postnatal exposures, and depression risk.
Table S3. A summary of the Newcastle–Ottawa Scale (NOS) quality assessment for selected studies.
Table S4. Meta‐regression analyses.
Table S5. Detailed findings from qualitative studies.
Table S6. Publication bias for the included studies.
Appendix S1. Search strategies.
Acknowledgements
Y.S. would like to thank a PhD graduate scholarship from the China Scholarship Council. X.M. also acknowledges the support of the Canadian Institute of Health Research (PJT‐148845) and the Canada First Excellence Research Fund provided to McGill University for Healthy Brains for Healthy Lives, and a career scholar award from the Fonds de recherche du Québec‐Sante, Canada. The authors have declared that they have no competing or potential conflicts of interest.
Key points.
This systematic review provides the first overview that comprehensively synthesizes the effects of prenatal, perinatal, and postnatal attributes on offspring’s depression.
Low birth weight, premature birth, small gestational age, maternal education, socioeconomic status, having young parents (<20 years), having older parents (≥35 years), maternal smoking, paternal smoking, maternal stress, maternal anxiety, and maternal prenatal depression significantly increased the risk of depression among offspring.
The qualitative analysis generally provides support for the meta‐analysis findings and adds information on a range of additional risk factors influencing depression in offspring.
Findings of this review highlight the need for clinical and public health interventions to focus on these identified risk factors. Large prospective cohort studies are warranted to investigate the combined effect of co‐existing early‐life exposures.
Conflict of interest statement: No conflicts declared.
Contributor Information
Carl D'Arcy, Email: carl.darcy@usask.ca.
Xiangfei Meng, Email: xiangfei.meng@mcgill.ca.
References
References
- Agrawal, A., Jacobson, K.C., Gardner, C.O., Prescott, C.A., & Kendler, K.S. (2004). A population based twin study of sex differences in depressive symptoms. Twin Research, 7, 176–181. [DOI] [PubMed] [Google Scholar]
- Aizer, A., & Currie, J. (2014). The intergenerational transmission of inequality: maternal disadvantage and health at birth. Science, 344, 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagner, D.M., Pettit, J.W., Lewinsohn, P.M., & Seeley, J.R. (2010). Effect of maternal depression on child behavior: a sensitive period? Journal of the American Academy of Child and Adolescent Psychiatry, 49, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts, K.S., Williams, G.M., Najman, J.M., & Alati, R. (2015). The relationship between maternal depressive, anxious, and stress symptoms during pregnancy and adult offspring behavioral and emotional problems. Depression and Anxiety, 32, 82–90. [DOI] [PubMed] [Google Scholar]
- Bonnin, A., & Levitt, P. (2011). Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience, 197, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, C., Davis, E.P., Shahbaba, B., Pruessner, J.C., Head, K., & Sandman, C.A. (2012). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences of the United States of America, 109, E1312–E1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, C., Entringer, S., & Wadhwa, P.D. (2012). Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Science Signaling, 5, pt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins, K., & Devaskar, S.U. (2011). Fetal origins of adult disease. Current Problems in Pediatric and Adolescent Health Care, 41, 158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo, J.J., García‐Nieto, R., Álvarez‐García, R., Caro‐Cañizares, I., López‐Castromán, J., Muñoz‐Lorenzo, L., … & Baca‐García, E. (2013). Sibship size, birth order, family structure and childhood mental disorders. Social Psychiatry and Psychiatric Epidemiology, 48, 1327–1333. [DOI] [PubMed] [Google Scholar]
- Chudal, R., Gissler, M., Sucksdorff, D., Lehti, V., Suominen, A., Hinkka‐Yli‐Salomäki, S., … & Sourander, A. (2014). Parental age and the risk of bipolar disorders. Bipolar Disorders, 16, 624–632. [DOI] [PubMed] [Google Scholar]
- Czamara, D., Eraslan, G., Page, C.M., Lahti, J., Lahti‐Pulkkinen, M., Hämäläinen, E., … & Binder, E.B. (2019). Integrated analysis of environmental and genetic influences on cord blood DNA methylation in new‐borns. Nature Communications, 10, 2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio, B.M., Rickert, M.E., Frans, E., Kuja‐Halkola, R., Almqvist, C., Sjölander, A., … & Lichtenstein, P. (2014). Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry, 71, 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats, P.A. (2015). Perinatal programming of neurodevelopment: epigenetic mechanisms and the prenatal shaping of the brain. Advances in Neurobiology, 10, 335–361. [DOI] [PubMed] [Google Scholar]
- Engel, G.L. (1977). The need for a new medical model: a challenge for biomedicine. Science, 196, 129–136. [DOI] [PubMed] [Google Scholar]
- Entringer, S., Epel, E.S., Kumsta, R., Lin, J., Hellhammer, D.H., Blackburn, E.H., … & Wadhwa, P.D. (2011). Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proceedings of the National Academy of Sciences of the United States of America, 108, E513–E518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan, L.J., Ohannessian, C.M., & Gordon, M.S. (2018). Trajectories of depressive symptoms from adolescence to emerging adulthood: The influence of parents, peers, and siblings. Developmental Psychology, 54, 1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier, L.D. (2020). The past, present, and future of the biopsychosocial model: A review of The Biopsychosocial Model of Health and Disease: New philosophical and scientific developments by Derek Bolton and Grant Gillett. New Ideas in Psychology, 57, 100755. [Google Scholar]
- Fryer, S.L., McGee, C.L., Matt, G.E., Riley, E.P., & Mattson, S.N. (2007). Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics, 119, e733–e741. [DOI] [PubMed] [Google Scholar]
- Glover, V. (2014). Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Practice & Research: Clinical Obstetrics & Gynaecology, 28, 25–35. [DOI] [PubMed] [Google Scholar]
- Hardt, J., Weyer, L., Dragan, M., & Laubach, W. (2017). Anxiety and depression as an effect of birth order or being an only child: Results of an internet survey in Poland and Germany. Insights on the Depression and Anxiety, 8, 2018. [Google Scholar]
- Hazel, N.A., Hammen, C., Brennan, P.A., & Najman, J. (2008). Early childhood adversity and adolescent depression: The mediating role of continued stress. Psychological Medicine, 38, 581–589. [DOI] [PubMed] [Google Scholar]
- Hentges, R.F., Graham, S.A., Plamondon, A., Tough, S., & Madigan, S. (2019). A developmental cascade from prenatal stress to child internalizing and externalizing problems. Journal of Pediatric Psychology, 44, 1057–1067. [DOI] [PubMed] [Google Scholar]
- Heron, J., O'Connor, T.G., Evans, J., Golding, J., Glover, V., & Team, A. S. (2004). The course of anxiety and depression through pregnancy and the postpartum in a community sample. Journal of Affective Disorders, 80, 65–73. [DOI] [PubMed] [Google Scholar]
- Kessler, R.C., McLaughlin, K.A., Green, J.G., Gruber, M.J., Sampson, N.A., Zaslavsky, A.M., … & Williams, D.R. (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. British Journal of Psychiatry, 197, 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury, M., Weeks, M., MacKinnon, N., Evans, J., Mahedy, L., Dykxhoorn, J., & Colman, I. (2016). Stressful life events during pregnancy and offspring depression: Evidence from a prospective cohort study. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 709–716. [DOI] [PubMed] [Google Scholar]
- Langley, K., Heron, J., Smith, G.D., & Thapar, A. (2012). Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: Testing for intrauterine effects. American Journal of Epidemiology, 176, 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos, C., Bassan, H., Gauvreau, K., Robertson, R.L., Sullivan, N.R., Benson, C.B., … & duPlessis, A.J. (2007). Does cerebellar injury in premature infants contribute to the high prevalence of long‐term cognitive, learning, and behavioral disability in survivors? Pediatrics, 120, 584–593. [DOI] [PubMed] [Google Scholar]
- Lopez‐Castroman, J., Gómez, D.D., Belloso, J.J.C., Fernandez‐Navarro, P., Perez‐Rodriguez, M.M., Villamor, I.B., … & Baca‐Garcia, E. (2010). Differences in maternal and paternal age between schizophrenia and other psychiatric disorders. Schizophrenia Research, 116, 184–190. [DOI] [PubMed] [Google Scholar]
- Loret de Mola, C., de França, G.V., Quevedo Lde, A., & Horta, B.L. (2014). Low birth weight, preterm birth and small for gestational age association with adult depression: systematic review and meta‐analysis. British Journal of Psychiatry, 205, 340–347. [DOI] [PubMed] [Google Scholar]
- McGowan, P.O., & Matthews, S.G. (2018). Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology, 159, 69–82. [DOI] [PubMed] [Google Scholar]
- McLaughlin, K.A., Breslau, J., Green, J.G., Lakoma, M.D., Sampson, N.A., Zaslavsky, A.M., & Kessler, R.C. (2011). Childhood socio‐economic status and the onset, persistence, and severity of DSM‐IV mental disorders in a US national sample. Social Science & Medicine, 73, 1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney, M.J., Szyf, M., & Seckl, J.R. (2007). Epigenetic mechanisms of perinatal programming of hypothalamic‐pituitary‐adrenal function and health. Trends in Molecular Medicine, 13, 269–277. [DOI] [PubMed] [Google Scholar]
- Michel, G.F., & Tyler, A.N. (2005). Critical period: A history of the transition from questions of when, to what, to how. Developmental Psychobiology, 46, 156–162. [DOI] [PubMed] [Google Scholar]
- Milgrom, J., Westley, D.T., & Gemmill, A.W. (2004). The mediating role of maternal responsiveness in some longer term effects of postnatal depression on infant development. Infant Behavior and Development, 27, 443–454. [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D.G., & Prisma Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Med, 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, L. (2009). The development of children of postnatally depressed mothers: Evidence from the Cambridge longitudinal study. Psychoanalytic Psychotherapy, 23, 185–199. [Google Scholar]
- Newman, L., Judd, F., Olsson, C.A., Castle, D., Bousman, C., Sheehan, P., … & Everall, I. (2016). Early origins of mental disorder ‐ risk factors in the perinatal and infant period. BMC Psychiatry, 16, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, K.J., & Meaney, M.J. (2017). Fetal origins of mental health: The developmental origins of health and disease hypothesis. American Journal of Psychiatry, 174, 319–328. [DOI] [PubMed] [Google Scholar]
- Owens, E.B., & Hinshaw, S.P. (2013). Perinatal problems and psychiatric comorbidity among children with ADHD. Journal of Clinical Child and Adolescent Psychology, 42, 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, G.C., Coffey, C., Carlin, J.B., Olsson, C.A., & Morley, R. (2004). Prematurity at birth and adolescent depressive disorder. The British Journal of Psychiatry, 184, 446–447. [DOI] [PubMed] [Google Scholar]
- Pearson, R.M., Evans, J., Kounali, D., Lewis, G., Heron, J., Ramchandani, P.G., … & Stein, A. (2013). Maternal depression during pregnancy and the postnatal period: Risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry, 70, 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen, A.‐K., Räikkönen, K., Strandberg, T.E., & Järvenpää, A.L. (2005). Continuity of maternal stress from the pre‐ to the postnatal period: Associations with infant's positive, negative and overall temperamental reactivity. Infant Behavior & Development, 28, 36–47. [Google Scholar]
- Quarini, C., Pearson, R.M., Stein, A., Ramchandani, P.G., Lewis, G., & Evans, J. (2016). Are female children more vulnerable to the long‐term effects of maternal depression during pregnancy? Journal of Affective Disorders, 189, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räikkönen, K., Pesonen, A.‐K., Heinonen, K., Kajantie, E., Hovi, P., Järvenpää, A.‐L., … & Andersson, S. (2008). Depression in young adults with very low birth weight: The Helsinki study of very low‐birth‐weight adults. Archives of General Psychiatry, 65, 290–296. [DOI] [PubMed] [Google Scholar]
- Rehm, J., & Shield, K.D. (2019). Global burden of disease and the impact of mental and addictive disorders. Current Psychiatry Reports, 21, 10. [DOI] [PubMed] [Google Scholar]
- Reiss, F. (2013). Socioeconomic inequalities and mental health problems in children and adolescents: A systematic review. Social Science & Medicine, 90, 24–31. [DOI] [PubMed] [Google Scholar]
- Richardson, M.A., Grant‐Knight, W., Beeghly, M., Rose‐Jacobs, R., Chen, C.A., Appugliese, D.P., … & Frank, D.A. (2016). Psychological distress among school‐aged children with and without intrauterine cocaine exposure: Perinatal versus contextual effects. Journal of Abnormal Child Psychology, 44, 547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond, R.C., Al‐Amin, A., Smith, G.D., & Relton, C.L. (2014). Approaches for drawing causal inferences from epidemiological birth cohorts: A review. Early Human Development, 90, 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigato, S., Stets, M., Bonneville‐Roussy, A., & Holmboe, K. (2020). Impact of maternal depressive symptoms on the development of infant temperament: Cascading effects during the first year of life. Social Development, 1–19. 10.1111/sode.12448 [DOI] [Google Scholar]
- Russo, V.C., Gluckman, P.D., Feldman, E.L., & Werther, G.A. (2005). The insulin‐like growth factor system and its pleiotropic functions in brain. Endocrine Reviews, 26, 916–943. [DOI] [PubMed] [Google Scholar]
- Sandin, S., Hultman, C.M., Kolevzon, A., Gross, R., MacCabe, J.H., & Reichenberg, A. (2012). Advancing maternal age is associated with increasing risk for autism: A review and meta‐analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 477–486. [DOI] [PubMed] [Google Scholar]
- Sanger, C., Iles, J.E., Andrew, C.S., & Ramchandani, P.G. (2015). Associations between postnatal maternal depression and psychological outcomes in adolescent offspring: A systematic review. Archives of Women's Mental Health, 18, 147–162. [DOI] [PubMed] [Google Scholar]
- Seckl, J.R., & Meaney, M.J. (2004). Glucocorticoid programming. Annals of the New York Academy of Sciences, 1032, 63–84. [DOI] [PubMed] [Google Scholar]
- Srinivasan, R., Pearson, R.M., Johnson, S., Lewis, G., & Lewis, G. (2020). Maternal perinatal depressive symptoms and offspring psychotic experiences at 18 years of age: A longitudinal study. The Lancet Psychiatry, 7, 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud, L.R., Papandonatos, G.D., Parade, S.H., Salisbury, A.L., Phipps, M.G., Lester, B.M., … & Marsit, C.J. (2016). Prenatal major depressive disorder, placenta glucocorticoid and serotonergic signaling, and infant cortisol response. Psychosomatic Medicine, 78, 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup, D.F., Berlin, J.A., Morton, S.C., Olkin, I., Williamson, G.D., Rennie, D., … & Thacker, S.B. (2000). Meta‐analysis of observational studies in epidemiology: A proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA, 283, 2008–2012. [DOI] [PubMed] [Google Scholar]
- Strüber, N., Strüber, D., & Roth, G. (2014). Impact of early adversity on glucocorticoid regulation and later mental disorders. Neuroscience & Biobehavioral Reviews, 38, 17–37. [DOI] [PubMed] [Google Scholar]
- Taylor, A.E., Carslake, D., de Mola, C.L., Rydell, M., Nilsen, T.I.L., Bjørngaard, J.H., … & Munafò, M.R. (2017). Maternal smoking in pregnancy and offspring depression: A cross cohort and negative control study. Scientific Reports, 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiserman, A.M., & Koliada, A.K. (2017). Early‐life adversity and long‐term neurobehavioral outcomes: Epigenome as a bridge? Human Genomics, 11, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos, T., Barber, R.M., Bell, B., Bertozzi‐Villa, A., Biryukov, S., Bolliger, I., … & Murray, C.J.L. (2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet, 386, 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S.‐S.‐H., Kloth, A.D., & Badura, A. (2014). The cerebellum, sensitive periods, and autism. Neuron, 83, 518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, G. (2001). The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta‐analyses. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford
- Wen, D., Poh, J., Ni, S., Chong, Y., Chen, H., Kwek, K., … & Meaney, M. (2017). Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Translational Psychiatry, 7, e1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan, Y.M., Leibenluft, E., Stringaris, A., & Barker, E.D. (2015). Pathways from maternal depressive symptoms to adolescent depressive symptoms: The unique contribution of irritability symptoms. Journal of Child Psychology and Psychiatry, 56, 1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik, W., Lee, W., Colman, I., Hardy, R., & Hotopf, M. (2013). Foetal origins of depression? A systematic review and meta‐analysis of low birth weight and later depression. Psychological Medicine, 43, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Systematic review and meta‐analysis references
- Alati, R., Lawlor, D.A., Mamun, A.A., Williams, G.M., Najman, J.M., O'Callaghan, M., & Bor, W. (2007). Is there a fetal origin of depression? Evidence from the Mater University Study of Pregnancy and its outcomes. American Journal of Epidemiology, 165, 575–582. [DOI] [PubMed] [Google Scholar]
- Barker, E.D., Copeland, W., Maughan, B., Jaffee, S.R., & Uher, R. (2012). Relative impact of maternal depression and associated risk factors on offspring psychopathology. British Journal of Psychiatry, 200, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts, K.S., Salom, C.L., Williams, G.M., Najman, J.M., & Alati, R. (2015). Associations between self‐reported symptoms of prenatal maternal infection and post‐traumatic stress disorder in offspring: evidence from a prospective birth cohort study. Journal of Affective Disorders, 175, 241–247. [DOI] [PubMed] [Google Scholar]
- Biederman, J., Martelon, M., Woodworth, K.Y., Spencer, T.J., & Faraone, S.V. (2017). Is maternal smoking during pregnancy a risk factor for cigarette smoking in offspring? A longitudinal controlled study of ADHD children grown up. Journal of Attention Disorders, 21, 975–985. [DOI] [PubMed] [Google Scholar]
- Buizer‐Voskamp, J.E., Laan, W., Staal, W.G., Hennekam, E.A., Aukes, M.F., Termorshuizen, F., … & Ophoff, R.A. (2011). Paternal age and psychiatric disorders: Findings from a Dutch population registry. Schizophrenia Research, 129, 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, A.C. (2014). Poverty and adolescent depressive symptoms. American Journal of Orthopsychiatry, 84, 82–94. [DOI] [PubMed] [Google Scholar]
- Chiu, T.F., Yu, T.M., Chuang, Y.W., Sun, K.T., Li, C.Y., Su, Y.C., & Kao, C.H. (2019). Sequential risk of depression in children born prematurely: A nationwide population‐ based analysis. Journal of Affective Disorders, 243, 42–47. [DOI] [PubMed] [Google Scholar]
- Cohen, M.J., Meador, K.J., Browning, N., May, R., Baker, G.A., Clayton‐Smith, J., … & Loring, D.W. (2013). Fetal antiepileptic drug exposure: Adaptive and emotional/behavioral functioning at age 6years. Epilepsy & Behavior, 29, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman, I., Ataullahjan, A., Naicker, K., & Van Lieshout, R.J. (2012). Birth weight, stress, and symptoms of depression in adolescence: Evidence of fetal programming in a national Canadian cohort. The Canadian Journal of Psychiatry, 57, 422–428. [DOI] [PubMed] [Google Scholar]
- Elmasry, H., Goodwin, R.D., Terry, M.B., & Tehranifar, P. (2014). Early life exposure to cigarette smoke and depressive symptoms among women in midlife. Nicotine & Tobacco Research, 16, 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, A.P., & Eaton, W.W. (2001). Longitudinal study assessing the joint effects of socio‐economic status and birth risks on adult emotional and nervous conditions. The British Journal of Psychiatry, 40, s78–s83. [DOI] [PubMed] [Google Scholar]
- Fergusson, D.M., Woodward, L.J., & Horwood, L.J. (1998). Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of General Psychiatry, 55, 721–727. [DOI] [PubMed] [Google Scholar]
- Gale, C.R., & Martyn, C.N. (2004). Birth weight and later risk of depression in a national birth cohort. The British Journal of Psychiatry, 184, 28–33. [DOI] [PubMed] [Google Scholar]
- Geoffroy, M.C., Gunnell, D., Clark, C., & Power, C. (2018). Are early‐life antecedents of suicide mortality associated with psychiatric disorders and suicidal ideation in midlife? Acta Psychiatrica Scandinavica, 137, 116–124. [DOI] [PubMed] [Google Scholar]
- Gray, K.A., Day, N.L., Leech, S., & Richardson, G.A. (2005). Prenatal marijuana exposure: Effect on child depressive symptoms at ten years of age. Neurotoxicology and Teratology, 27, 439–448. [DOI] [PubMed] [Google Scholar]
- Harley, K.G., Gunier, R.B., Kogut, K., Johnson, C., Bradman, A., Calafat, A.M., & Eskenazi, B. (2013). Prenatal and early childhood bisphenol A concentrations and behavior in school‐aged children. Environmental Research, 126, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herva, A., Pouta, A., Hakko, H., Laksy, K., Joukamaa, M., & Veijola, J. (2008). Birth measures and depression at age 31 years: The Northern Finland 1966 Birth Cohort Study. Psychiatry Research, 160, 263–270. [DOI] [PubMed] [Google Scholar]
- Johnson, S., O'Reilly, H., Ni, Y., Wolke, D., & Marlow, N. (2019). Psychiatric symptoms and disorders in extremely preterm young adults at 19 years of age and longitudinal findings from middle childhood. Journal of the American Academy of Child and Adolescent Psychiatry, 58, 820–826.e6. [DOI] [PubMed] [Google Scholar]
- Joinson, C., Kounali, D., & Lewis, G. (2017). Family socioeconomic position in early life and onset of depressive symptoms and depression: a prospective cohort study. Social Psychiatry and Psychiatric Epidemiology, 52, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiff, C.J., Cortes, R., Lengua, L., Kosterman, R., Hawkins, J.D., & Mason, W.A. (2012). Effects of timing of adversity on adolescent and young adult adjustment. Journal of Research on Adolescence, 22, 284–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury, M., Weeks, M., MacKinnon, N., Evans, J., Mahedy, L., Dykxhoorn, J., & Colman, I. (2016). Stressful life events during pregnancy and offspring depression: Evidence from a Prospective Cohort Study. Child & Adolescent Psychiatry, 55, 709–716.e2. [DOI] [PubMed] [Google Scholar]
- Leung, C.Y., Leung, G.M., & Schooling, C.M. (2015). Early second‐hand smoke exposure and child and adolescent mental health: evidence from Hong Kong's ‘Children of 1997’ birth cohort. Addiction, 110, 1811–1824. [DOI] [PubMed] [Google Scholar]
- Levine, S.Z. (2014). Low birth‐weight and risk for major depression: a community‐based longitudinal study. Psychiatry Research, 215, 618–623. [DOI] [PubMed] [Google Scholar]
- Levy‐Shiff, R., Einat, G., Har‐Even, D., Mogilner, M., Mogilner, S., Lerman, M., & Krikler, R. (1994). Emotional and behavioral adjustment in children born prematurely. Journal of Clinical Child Psychology, 23, 323–333. [Google Scholar]
- Li, C., Miles, T., Shen, L., Shen, Y.E., Liu, T., Zhang, M., … & Huang, C. (2018). Early‐life exposure to severe famine and subsequent risk of depressive symptoms in late adulthood: The China Health and Retirement Longitudinal Study. The British Journal of Psychiatry, 213, 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T., Gatsonis, C.A., Baylin, A., Kubzansky, L.D., Loucks, E.B., & Buka, S.L. (2011). Maternal smoking during pregnancy and anger temperament among adult offspring. Journal of Psychiatric Research, 45, 1648–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loret de Mola, C., Quevedo, L.A., Pinheiro, R.T., Gonçalves, H., Gigante, D.P., Motta, J.V.S. … & Horta, B.L. (2015). The effect of fetal and childhood growth over depression in early adulthood in a Southern Brazilian Birth Cohort. PLoS One, 10, e0140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm, H., Brown, A.S., Gissler, M., Gyllenberg, D., Hinkka‐Yli‐Salomäki, S., McKeague, I.W., … & Sourander, A. (2016). Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: A National Register‐Based Study. Journal of the American Academy of Child & Adolescent Psychiatry, 55, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier, S.M., Plessen, K.J., Verhulst, F., Mors, O., Mortensen, P.B., Pedersen, C.B., & Agerbo, E. (2017). Familial confounding of the association between maternal smoking during pregnancy and internalizing disorders in offspring. Psychological Medicine, 47, 1417–1426. [DOI] [PubMed] [Google Scholar]
- Menezes, A.M.B., Murray, J., László, M., Wehrmeister, F.C., Hallal, P.C., Gonçalves, H., … & Barros, F.C. (2013). Happiness and depression in adolescence after maternal smoking during pregnancy: Birth cohort study. PLoS One, 8, e80370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S.K., Fineberg, A.M., Maxwell, S.D., Alloy, L.B., Zimmermann, L., Krigbaum, N.Y., … & Ellman, L.M. (2017). Maternal infection and stress during pregnancy and depressive symptoms in adolescent offspring. Psychiatry Research, 257, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura, Y., Brooks‐Gunn, J., Davey, C., Ham, J., & Fifer, W.P. (2007). The role of perinatal problems in risk of co‐morbid psychiatric and medical disorders in adulthood. Psychological Medicine, 37, 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura, Y., Wickramaratne, P.J., Pilowsky, D.J., Newcorn, J.H., Bruder‐Costello, B., Davey, C., … & Weissman, M.M. (2007). Low birth weight and risk of affective disorders and selected medical illness in offspring at high and low risk for depression. Comprehensive Psychiatry, 48, 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, M.J., & Kasari, C. (2000). Prenatal alcohol exposure and depressive features in children. Alcoholism‐Clinical and Experimental Research, 24, 1084–1092. [PubMed] [Google Scholar]
- Pawlby, S., Hay, D.F., Sharp, D., Waters, C.S., & O'Keane, V. (2009). Antenatal depression predicts depression in adolescent offspring: Prospective longitudinal community‐based study. Journal of Affective Disorders, 113, 236–243. [DOI] [PubMed] [Google Scholar]
- Pearson, R.M., Evans, J., Kounali, D., Lewis, G., Heron, J., Ramchandani, P.G., … & Stein, A. (2013). Maternal depression during pregnancy and the postnatal period: Risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry, 70, 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, T.S., Silva, A.A., Alves, M.T., Simões, V.M., Batista, R.F., Rodriguez, J.D., … & Bettiol, H. (2012). Perinatal and early life factors associated with symptoms of depression in Brazilian children. BMC Public Health, 12, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, F., Nolte, E.L., Wang, Y.A., Margolis, A.E., Calafat, A.M., Wang, S., … & Herbstman, J. (2016). Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environmental Research, 151, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perquier, F., Lasfargues, A., Mesrine, S., Clavel‐Chapelon, F., & Fagherazzi, G. (2014). Body‐size throughout life and risk of depression in postmenopausal women: Findings from the E3N cohort. Obesity, 22, 1926–1934. [DOI] [PubMed] [Google Scholar]
- Phillips, N.K., Hammen, C.L., Brennan, P.A., Najman, J.M., & Bor, W. (2005). Early adversity and the prospective prediction of depressive and anxiety disorders in adolescents. Journal of Abnormal Child Psychology, 33, 13–24. [DOI] [PubMed] [Google Scholar]
- Pitzer, M., Jennen‐Steinmetz, C., Esser, G., Schmidt, M.H., & Laucht, M. (2011). Prediction of preadolescent depressive symptoms from child temperament, maternal distress, and gender: Results of a prospective, longitudinal study. Developmental & Behavioral Pediatrics, 32, 18–26. [DOI] [PubMed] [Google Scholar]
- Plant, D.T., Pariante, C.M., Sharp, D., & Pawlby, S. (2015). Maternal depression during pregnancy and offspring depression in adulthood: Role of child maltreatment. The British Journal of Psychiatry, 207, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, C., Atherton, K., Strachan, D.P., Shepherd, P., Fuller, E., Davis, A., … & Stansfeld, S. (2007). Life‐course influences on health in British adults: Effects of socio‐economic position in childhood and adulthood. International Journal of Epidemiology, 36, 532–539. [DOI] [PubMed] [Google Scholar]
- Ramchandani, P.G., Stein, A., O'Connor, T.G., Heron, J., Murray, L., & Evans, J. (2008). Depression in men in the postnatal period and later child psychopathology: A population cohort study. Journal of the American Academy of Child & Adolescent Psychiatry, 47, 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposa, E., Hammen, C., Brennan, P., & Najman, J. (2014). The long‐term effects of maternal depression: Early childhood physical health as a pathway to offspring depression. Journal of Adolescent Health, 54, 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, G.A., Goldschmidt, L., Larkby, C., & Day, N.L. (2013). Effects of prenatal cocaine exposure on child behavior and growth at 10 years of age. Neurotoxicology and Teratology, 40, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal, S., Pinelli, J., Hoult, L., Kim, M.M., & Boyle, M. (2003). Psychopathology and social competencies of adolescents who were extremely low birth weight. Pediatrics, 111, 969–975. [DOI] [PubMed] [Google Scholar]
- Slykerman, R.F., Thompson, J., Waldie, K., Murphy, R., Wall, C., & Mitchell, E.A. (2015). Maternal stress during pregnancy is associated with moderate to severe depression in 11‐year‐old children. Acta Paediatrica, 104, 68–74. [DOI] [PubMed] [Google Scholar]
- Slykerman, R.F., Thompson, J., Waldie, K.E., Murphy, R., Wall, C., & Mitchell, E.A. (2017). Antibiotics in the first year of life and subsequent neurocognitive outcomes. Acta Paediatrica, 106, 87–94. [DOI] [PubMed] [Google Scholar]
- Sonon, K., Richardson, G.A., Cornelius, J., Kim, K.H., & Day, N.L. (2016). Developmental pathways from prenatal marijuana exposure to Cannabis Use Disorder in young adulthood. Neurotoxicology and Teratology, 58, 46–52. [DOI] [PubMed] [Google Scholar]
- Strom, M., Hansen, S., Olsen, S.F., Haug, L.S., Rantakokko, P., Kiviranta, H., & Halldorsson, T.I. (2014). Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes–a prospective study with long‐term follow‐up. Environment International, 68, 41–48. [DOI] [PubMed] [Google Scholar]
- Taka‐Eilola (nèe Riekki), T., Veijola, J., Murray, G.K., Koskela, J., & Mäki, P. (2019). Severe mood disorders and schizophrenia in the adult offspring of antenatally depressed mothers in the Northern Finland 1966 Birth Cohort: Relationship to parental severe mental disorder. Journal of Affective Disorders, 249, 63–72. [DOI] [PubMed] [Google Scholar]
- Taylor, A.E., Carslake, D., de Mola, C.L., Rydell, M., Nilsen, T.I.L., Bjørngaard, J.H., … & Munafò, M.R. (2017). Maternal smoking in pregnancy and offspring depression: A cross cohort and negative control study. Scientific Reports, 7, 12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tearne, J.E., Robinson, M., Jacoby, P., Allen, K.L., Cunningham, N.K., Li, J., & McLean, N.J. (2016). Older maternal age is associated with depression, anxiety, and stress symptoms in young adult female offspring. Journal of Abnormal Psychology, 125, 1–10. [DOI] [PubMed] [Google Scholar]
- Tracy, M., Salo, M., Slopen, N., Udo, T., & Appleton, A.A. (2019). Trajectories of childhood adversity and the risk of depression in young adulthood: Results from the Avon Longitudinal Study of Parents and Children. Depression and Anxiety, 36, 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuovinen, S., Aalto‐Viljakainen, T., Eriksson, J.G., Kajantie, E., Lahti, J., Pesonen, A.‐K., … & Räikkönen, K. (2014). Maternal hypertensive disorders during pregnancy: Adaptive functioning and psychiatric and psychological problems of the older offspring. BJOG: An International Journal of Obstetrics & Gynaecology, 121, 1482–1491. [DOI] [PubMed] [Google Scholar]
- Van Lieshout, R.J., & Boylan, K. (2010). Increased depressive symptoms in female but not male adolescents born at low birth weight in the offspring of a national cohort. The Canadian Journal of Psychiatry, 55, 422–430. [DOI] [PubMed] [Google Scholar]
- Wang, H., Leung, G.M., Lam, H.S., & Schooling, C.M. (2015). Gestational age and adolescent mental health: Evidence from Hong Kong's 'Children of 1997' birth cohort. Archives of Disease in Childhood, 100, 856–862. [DOI] [PubMed] [Google Scholar]
- Westrupp, E.M., Northam, E., Doyle, L.W., Callanan, C., & Anderson, P.J. (2011). Adult psychiatric outcomes of very low birth weight survivors. Australian & New Zealand Journal of Psychiatry, 45, 1069–1077. [DOI] [PubMed] [Google Scholar]
- Yamasaki, S., Ando, S., Richards, M., Hatch, S.L., Koike, S., Fujikawa, S., … & Nishida, A. (2019). Maternal diabetes in early pregnancy, and psychotic experiences and depressive symptoms in 10‐year‐old offspring: A population‐based birth cohort study. Schizophrenia Research, 206, 52–57. [DOI] [PubMed] [Google Scholar]
- Yolton, K., Khoury, J.C., Burkle, J., LeMasters, G., Cecil, K., & Ryan, P. (2019). lifetime exposure to traffic‐related air pollution and symptoms of depression and anxiety at age 12 years. Environmental Research, 173, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohsel, K., Holz, N.E., Hohm, E., Schmidt, M.H., Esser, G., Brandeis, D., … & Laucht, M. (2017). Fewer self‐reported depressive symptoms in young adults exposed to maternal depressed mood during pregnancy. Journal of Affective Disorders, 209, 155–162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Funnel plot for the analysis of in utero and perinatal factors and depression risk.
Figure S2. Sensitivity analyses for the association between in utero and perinatal factors and depression risk.
Figure S3a. Subgroup meta‐analysis of the relationship between prenatal factors and offspring’s depression.
Figure S3b. Subgroup meta‐analysis of the relationship between perinatal/postnatal factors and offspring’s depression.
Table S1. MOOSE checklist for meta‐analyses of observational studies.
Table S2. Characteristics of included studies on in utero, perinatal and postnatal exposures, and depression risk.
Table S3. A summary of the Newcastle–Ottawa Scale (NOS) quality assessment for selected studies.
Table S4. Meta‐regression analyses.
Table S5. Detailed findings from qualitative studies.
Table S6. Publication bias for the included studies.
Appendix S1. Search strategies.


