Abstract
Aim
Angiotensin receptor blockers (ARBs) reduce vascular complications in diabetes independently of blood pressure. Experimental studies suggested that ARBs may restore the detoxifying enzyme glyoxalase 1, thereby lowering dicarbonyls such as methylglyoxal. Human data on the effects of ARBs on plasma dicarbonyl levels are lacking. We investigated, in individuals with type 2 diabetes, whether irbesartan lowered plasma levels of the dicarbonyls methylglyoxal, glyoxal, 3‐deoxyglucosone and their derived advanced glycation end products (AGEs), and increased d‐lactate, reflecting greater methylglyoxal flux.
Methods
We analysed a subset of the Irbesartan in Patients with T2D and Microalbuminuria (IRMA2) study. We measured plasma dicarbonyls methylglyoxal, glyoxal and 3‐deoxyglucosone, free AGEs and d‐lactate using ultra‐performance liquid chromatography tandem mass‐spectrometry (UPLC‐MS/MS) in the treatment arm receiving 300 mg irbesartan (n = 121) and a placebo group (n = 101) at baseline and after 1 and 2 years. Effect of treatment was analysed with repeated measurements ANOVA.
Results
There was a slight, but significant difference in baseline median methylglyoxal levels [placebo 1119 (907–1509) nmol/l vs. irbesartan 300 mg 1053 (820–1427) nmol/l], but no significant changes were observed in any of the plasma dicarbonyls over time in either group and there was no effect of irbesartan treatment on plasma free AGEs or d‐lactate levels at either 1 or 2 years.
Conclusion
Irbesartan treatment does not change plasma levels of the dicarbonyls methylglyoxal, glyoxal and 3‐deoxyglucosone, free AGEs or d‐lactate in type 2 diabetes. This indicates that increased dicarbonyls in type 2 diabetes are not targetable by ARBs, and other approaches to lower systemic dicarbonyls are needed in type 2 diabetes. (Clinical Trial Registry No: #NCT00317915).
What's new?
The angiotensin receptor blocker irbesartan reduces progression of diabetic kidney disease independently of blood pressure in individuals with type 2 diabetes.
Methylglyoxal is a major driver of diabetic kidney disease and may be lowered by angiotensin receptor blockers.
Irbesartan (300 mg) did not lower plasma levels of methylglyoxal, or any of the additional glycation markers measured in this study.
This indicates that increased dicarbonyls in type 2 diabetes are not targetable by irbesartan, and other approaches to lower systemic dicarbonyls are needed in type 2 diabetes.
1. INTRODUCTION
Type 2 diabetes is associated with the development of cardiovascular disease (CVD), chronic kidney disease (CKD) and is a main cause of end‐stage kidney disease (ESKD). The renin–angiotensin–aldosterone system plays an important role in the development of CVD and CKD in type 2 diabetes.1, 2 Inhibition of the renin–angiotensin–aldosterone system with ARBs reduces the incidence of CKD and CVD in type 2 diabetes.3, 4 However, the mechanisms through which ARBs attenuate renal and cardiovascular risk remain incompletely understood. This was highlighted by the seminal original Irbesartan in T2D With Microalbuminuria 2 (IRMA2) investigation, in which the ARB irbesartan reduced progression of diabetic CKD, independently of blood pressure.4 Therefore, research into the mechanisms of action of ARBs is still needed to maximize the benefit of this vital class of medications for the prevention of diabetic complications. This is highly relevant because the incidence of CVD and CKD in type 2 diabetes remains high and many individuals still progress towards ESKD despite optimal treatment with ARBs.5
A potential underlying mechanism of the protective effects of ARBs is a reduction in the accumulation of methylglyoxal, a highly reactive glucose metabolite and the major precursor in the rapid formation of advanced glycation end products (AGEs).6 Methylglyoxal levels are increased in type 2 diabetes,7 and higher plasma methylglyoxal levels were associated with CVD, albuminuria and a decline in eGFR in type 2 diabetes.8, 9, 10 Methylglyoxal is detoxified by the glyoxalase system to d‐lactate, with glyoxalase 1 as the rate‐limiting enzyme.11 Glyoxalase 1 expression is lower in kidneys affected by CKD.12 In line with this, glyoxalase 1 overexpression attenuated albuminuria in diabetic rats,13 while glyoxalase 1 knockdown caused a CKD‐like phenotype even in normoglycaemic mice.14 These findings imply that glyoxalase 1 is a major protective factor against CKD. Interestingly, it has been shown in an experimental study that the ARB candesartan attenuates the formation of methylglyoxal levels through enhanced glyoxalase 1 expression.15 These observations led to the hypothesis that the health benefits of ARBs are due, at least in part, to increased expression of glyoxalase 1 and a decrease in methylglyoxal levels. However, human studies about the effect of ARBs on systemic methylglyoxal levels are lacking. To explore whether irbesartan lowers dicarbonyl stress, we have now investigated plasma levels of methylglyoxal and two other major dicarbonyls, glyoxal and 3‐deoxyglucosone, as well as d‐lactate and free levels of the major dicarbonyl‐derived AGEs Nε‐carboxymethyllysine (CML), Nε‐carboxyethyllysine (CEL) and methylglyoxal‐derived hydroimidazolone (MG‐H1) in the IRMA2 sub‐study with a follow‐up period of 2 years.
2. METHODS
2.1. Ethical approval
The study protocol was in accordance with the Declaration of Helsinki and approved by the institutional review board at each centre. All participants gave written informed consent.
2.2. Statistical analyses
Variables with skewed distribution (plasma dicarbonyls) were ln‐transformed prior to further analyses. Changes in plasma dicarbonyl levels over the 2‐year follow‐up time were examined according to the intention‐to‐treat principle and with the use of repeated measurement ANOVA. As a sensitivity analysis, we stratified our data set on the median baseline HbA1c value of the current data set to investigate whether the effect of irbesartan differed by glycaemic control. For this analyses 19 individuals were initially excluded because of missing baseline HbA1c values. When we added these individuals to the above median HbA1c group, reasoning that individuals with missing values on average have poorer control, the results did not change. All statistical analyses were performed using SPSS version 23.
2.3. Study design and participants
The IRMA 2 study was a 2‐year multicentre, double‐blind, parallel randomized controlled trial in individuals with type 2 diabetes and microalbuminuria in which the main goal was to compare the effects of irbesartan (150 or 300 mg once daily) vs. placebo, in addition to conventional anti‐hypertensive treatment, on the development of overt nephropathy.4 Briefly, we enrolled individuals with type 2 diabetes (diagnosed according to the WHO criteria) and hypertension (defined as mean SBP >135 mmHg and/or mean DBP >85 mmHg in two of three consecutive measurements 1 week apart) and persistent microalbuminuria (defined as an albumin excretion rate of 20–200 mg/min in two of three consecutive overnight urine samples) and a serum creatinine concentration of 133 µmol/l for men and 97 µmol/l for women. A total of 590 (186 women) individuals, aged 30–70 years, were included and randomly assigned to receive 150 mg irbesartan once daily (n = 195), 300 mg irbesartan once daily (n = 194) or matching placebo (n = 201) (Figure S1). Results regarding the primary end point, and other secondary end points such as markers of inflammation and endothelial dysfunction, have been reported previously.4 The aim of this study was to evaluate the effect of irbesartan treatment on glycation, using several state‐of‐the art panels to quantify plasma levels of the plasma dicarbonyls methylglyoxal, glyoxal and 3‐deoxyglucosone, the free AGE levels of CML, CEL, MG‐H1 and d‐lactate. Samples for the assessment of these markers at baseline and at 1‐ and 2‐year follow‐up were available from 121 participants in the treatment arm receiving 300 mg irbesartan and from 101 participants receiving placebo. In the initial analyses of this study, no significant effects of 150 mg irbesartan treatment vs. placebo were found on the development of nephropathy, creatinine clearance or blood pressure. The 150 mg irbesartan samples were not included in the current analyses because they are no longer available as they unfortunately had been discarded by mistake in the past. Individuals included in the current analyses had similar baseline characteristics to the original investigation (data not shown).4
2.4. Biochemical measurements
HbA1c was measured by ion‐exchange high‐performance liquid chromatography. Serum creatinine concentration was originally measured by the Jaffe reaction with the use of a Hoffmann LaRoche kit and creatinine clearance (in ml min−1 1.73 m−2 body surface area) was estimated using the Cockcroft–Gault equation. These variables were evaluated at the time of randomization, 2 and 4 weeks after randomization and at 3, 6, 12, 18 and 22–24 months.
2.5. Measurement of plasma dicarbonyls
Plasma samples were stored at −80C° until analyses. Plasma dicarbonyls were measured with ultra‐performance liquid chromatography tandem mass‐spectrometry (UPLC‐MS/MS).16 In brief, EDTA plasma samples (25 μl) were mixed with 75 μl d 8‐O‐phenylenediamine (oPD; 10 mg oPD in 10 ml 1.6 mol/l perchloric acid) in an Eppendorf cup. After an overnight (20 h) reaction at room temperature and shielded from light, 10 μl of internal standard solution was added. Samples were mixed and subsequently centrifuged for 20 min at 21 000 g at a temperature of 4°C; 10 μl was injected for UPLC/MSMS analysis. The interassay variations for methylglyoxal, glyoxal and 3‐deoxyglucosone were 4.3%, 5.1% and 2.2%, respectively.16
Plasma d‐lactate levels were measured with UPLC‐MS/MS.17 Some 25 μl of plasma was derivatized with diacetyl‐l‐tartaric anhydride and separated on a C18‐reversed phase column. d‐Lactate inter‐ and intra‐assay variations were between 2% and 9%.
Plasma free MG‐H1 levels were measured with UPLC‐MS/MS.18 Some 50 µl plasma was used, samples were derivatized with butanolic hydrochloric acid and subsequently detected in multiple reaction monitoring mode using a Xevo TQ MS (Waters, Milford, MA, USA). Quantification of free MG‐H1 was performed by calculating the ratio of each unlabelled peak area to the corresponding internal standard peak area. In plasma, the intra‐ and interassay variations of protein‐bound CML, CEL and MG‐H1 were between 4.8% and 18.8%, and for free CML, CEL and MG‐H1 were between 2.8% and 7.1%.
3. RESULTS
Baseline characteristics of the current subset of the IRMA2 trial are shown in Table 1. An in‐depth description of the complete study has been provided elsewhere.4
TABLE 1.
Baseline characteristics of the study participants
| Placebo (n = 101) | 300 mg Irbesartan (n = 121) | |
|---|---|---|
| Sex (% women) | 32.1 | 30.8 |
| Age (years) | 57.7 ± 9.3 | 57.3 ± 7.8 |
| Known duration of diabetes (years) | 7.0 (3.0–14.5) | 7.0 (4.0–12.0) |
| HbA1c (mmol/mol) | 53 ± 18 | 52 ± 19 |
| HbA1c (%) | 7.0 ± 1.6 | 6.9 ± 1.7 |
| BMI (kg/m2) | 30.4 ± 4.3 | 29.9 ± 4.3 |
| Total cholesterol (mmol/l) | 5.7 ± 1.1 | 5.8 ± 1.1 |
| Current smoking (%) | 19.3 | 14.6 |
| Systolic blood pressure (mmHg) | 153.1 ± 14.3 | 154.5 ± 13.2 |
| Diastolic blood pressure (mmHg) | 89.8 ± 8.4 | 91.9 ± 9.7 |
| Creatinine clearance (ml min−1 1.73 m−2) | 110.0 (89.5–130.5) | 106.0 (92.5–124.3) |
| Urinary albumin excretion (mg per 24 h) | 51.0 (33.0–82.0) | 51.0 (35.0–87.0) |
Data are given as mean ± sd, median (IQR) or percentages as appropriate and stratified according to treatment group (300 mg irbesartan or placebo).
3.1. Effects of 300 mg irbesartan treatment on plasma levels of methylglyoxal, glyoxal and 3‐deoxyglucosone
Plasma methylglyoxal levels were significantly lower at baseline in the group randomized to 300 mg irbesartan (Table 2 and Figure 1). Plasma levels of methylglyoxal, glyoxal and 3‐deoxyglucosone did not change significantly over the 1‐ and 2‐year follow‐up time in either group, and did not differ between groups (Table 2 and Figure 1). These results did not change when we adjusted the repeated measurements in ANOVA for sex, age, HbA1c, duration of diabetes and BMI (data not shown).
TABLE 2.
Plasma dicarbonyls, d‐lactate and free advanced glycation end product levels at baseline and at 1‐ and 2‐year follow‐up
| Baseline | Year 1 | Year 2 | ||||
|---|---|---|---|---|---|---|
| Placebo | 300 mg Irbesartan | Placebo | 300 mg Irbesartan | Placebo | 300 mg Irbesartan | |
| Methylglyoxal (nmol/l) | 1119 (907–1590) | 1053 (821–1448) | 1131 (873–1488) | 1147 (824–1539) | 1078 (876–1448) | 1142 (839–1565) |
| Glyoxal (nmol/l) | 6079 (4967–7164) | 6003 (5153–6939) | 6067 (5108–7959) | 6304 (5230–7642) | 6036 (4928–7758) | 6238 (5169–7332) |
| 3‐Deoxyglucosone (nmol/l) | 5499 (4446–6555) | 5211 (4204–6491) | 5582 (4548–6846) | 5311 (4304–6305) | 5319 (4279–7111) | 5657 (4184–6573) |
| d‐Lactate (mmol/l) | 13.8 (9.2–22.3) | 14.0 (9.9–20.3) | 14.8 (10.0–19.4) | 15.2 (10.6–21.2) | 15.4 (10.3–21.2) | 14.9 (10.8–22.4) |
| Free CML (nmol/l) | 116.8 (98.4–158.1) | 107.0 (84.6–134.9) | 116.5 (92.5–154.8) | 111.9 (92.6–134.5) | 125.2 (98.8–155.3) | 121.1 (96.5–154.7) |
| Free CEL (nmol/l) | 63.3 (49.1–81.1) | 52.6 (42.4–70.4) | 63.4 (43.4–80.7) | 58.1 (47.6–77.6) | 66.2 (51.0–84.7) | 63.5 (50.2–82.9) |
| Free MG‐H1 (nmol/l) | 198.0 (138.7–284.7) | 175.9 (114.7–265.0) | 198.0 (138.7–284.7) | 175.9 (114.7–265.0 | 201.2 (137.4–298.5) | 198.6 (127.3–285.2) |
Data are expressed as median (IQR) and stratified according to treatment group (300 mg irbesartan or placebo).
Abbreviations: CEL, Nε‐carboxyethyllysine; CML, Nε‐carboxymethyllysine; MG‐H1, methylglyoxal‐derived hydroimidazolone.
FIGURE 1.
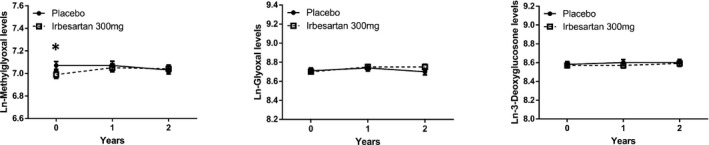
Two‐way plots of plasma levels of Ln‐transformed methylglyoxal, glyoxal and 3‐deoxyglucosone at baseline, and 1‐ and 2‐year follow‐up for the irbesartan (IRB) 300 mg group and the placebo (PL) group. Data are presented as mean ± se. *P < 0.05 vs. placebo. Differences between groups and over time were tested with repeated measures ANOVA, after Ln‐transformation of the outcome variables
3.2. Effects of 300 mg irbesartan treatment on plasma levels of d‐lactate
Plasma levels of d‐lactate did not change significantly in either group after 1‐ and 2‐year follow‐up time and no difference was found between groups (Table 2 and Figure 2).
FIGURE 2.
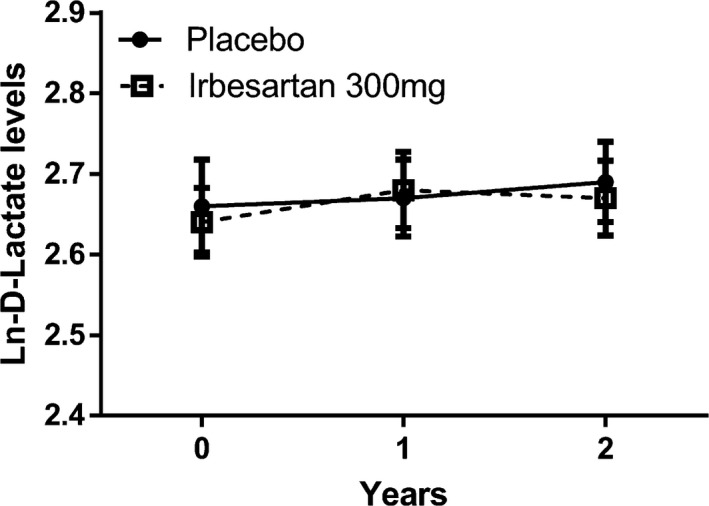
Two‐way plot of plasma levels of d‐lactate at baseline, and 1‐ and 2‐year follow‐up for the irbesartan (IRB) 300 mg group and the placebo (PL) group. Data are presented as mean ± se. *P < 0.05 vs. placebo. Differences between groups and over time were tested with repeated measures ANOVA after Ln‐transformation of the outcome variable
3.3. Effects of 300 mg irbesartan treatment on plasma levels of free CML, CEL and MG‐H1
We next analysed levels of the free plasma AGEs CML, CEL and MG‐H1 (Table 2 and Figure 3). At baseline, prior to treatment, plasma CML, CEL and MG‐H1 appeared to be higher in the group randomised to placebo treatment, and this was statistically significant for CML and CEL (Table 2 and Figure 3). However, at 1‐ and 2‐year follow‐up we observed no differences between the placebo or irbesartan groups.
FIGURE 3.
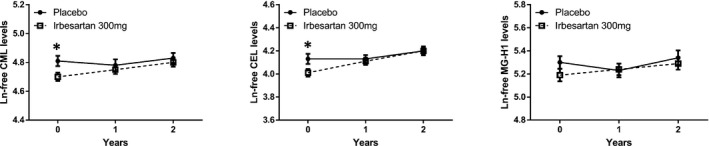
Two‐way plots of plasma levels of free Nε‐carboxymethyllysine (CML), Nε‐carboxyethyllysine (CEL) and methylglyoxal‐derived hydroimidazolone (MG‐H1) at baseline, and 1‐ and 2‐year follow‐up for the irbesartan (IRB) 300 mg group and the placebo (PL) group. Data are presented as mean ± se. *P < 0.05 vs. placebo. Differences between groups and over time were tested with repeated measures ANOVA after Ln‐transformation of the outcome variable
4. DISCUSSION
The current analyses of the IRMA2 study showed that irbesartan at a concentration of 300 mg vs. placebo does not lower plasma levels of methylglyoxal or the other dicarbonyls glyoxal and 3‐deoxyglucosone, d‐lactate or the free plasma AGEs CML, CEL and MG‐H1.
These results suggest that ARBs, or at least irbesartan, does not act as a potent agent to lower systemic dicarbonyl stress as reflected by changes in plasma dicarbonyl, d‐lactate or free AGE levels, although the effectiveness of irbesartan has been demonstrated by its ability to reduce progression of albuminuria in the original trial. Because plasma dicarbonyl levels are not lower than in previous investigations we performed,8, 9, 19 it seems unlikely that there was insufficient dicarbonyl stress in the current study to effectively detect a reduction by irbesartan. Our current finding is in line with prior analyses from our group in the IRMA2 subset, in which we found that the plasma protein‐bound AGEs CML and CEL were not affected by irbesartan.20 The current study expands upon this finding by direct measurements of dicarbonyls, as well as including free plasma AGEs and d‐lactate as a potential marker of methylglyoxal detoxification by glyoxalase 1.
The IRMA2 study was performed in the clinical era prior to the newer glucose‐lowering drugs such as sodium–glucose reuptake inhibitors (SGLT2i) and glucagon‐like peptide 1 (GLP1) analogues. This is actually an advantage in terms of specifying the effects of ARBs on plasma dicarbonyls, because we can exclude an interaction of these newer compounds with plasma dicarbonyls, which could have masked the effects of irbesartan on plasma dicarbonyl levels. Adjustment for HbA1c did not change our results, which makes it less likely that changes in glucose and thus dicarbonyls, covered the effect of ARBs on dicarbonyls.
Previous experimental studies have mainly focused on in‐depth analyses of the influence of ARBs on methylglyoxal and glyoxalase expression in retinal and renal tissues15, 21, 22 and it has been demonstrated that ARBs modulate methylglyoxal levels in specific microvascular beds.15 This is likely achieved by blocking angiotensin II signalling and related down‐regulation of glyoxalase 1. In this study, however, we did not find an effect of irbesartan on plasma levels of methylglyoxal. Because we do not have data about tissue methylglyoxal levels, we cannot rule out the possibility that there is an effect of irbesartan on methylglyoxal levels in specific tissues that are not reflected by changes in plasma glycation levels. This was indeed suggested by a rodent study that showed decreased CML levels in diabetic kidneys by the ARB valsartan, while overall plasma fluorescence as a reflection of glycation was not altered.22 Therefore, we cannot disprove earlier pre‐clinical studies based on our current investigation. Additionally, it might be that the beneficial effects of ARBs are restricted to experimental models of diabetes.
This study also has the limitation that we have only data about plasma levels of dicarbonyls and not urinary levels; we cannot exclude an effect of ARBs on urinary dicarbonyls. In fact, it has been demonstrated that irbesartan significantly decreased urinary excretion of the dicarbonyls‐derived AGEs MG‐H1 and glyoxal‐derived hydroimidasolone (G‐H1). As urinary free MG‐H1 and G‐H1 are thought to originate mainly from proteolysis of methylglyoxal and glyoxal‐modified proteins,23 these changes may indicate decreased dicarbonyl stress in renal tissues with irbesartan. This is in accordance with the effect of angiotensin blockade in regulation of glyoxalase 1 in vitro.15 However, because of the potent effect of glucose‐lowering treatment on plasma methylglyoxal levels in type 2 diabetes,19, 24 it is unlikely that ARBs constitute a main mitigating treatment to reduce dicarbonyl stress in diabetes.
4.1. Conclusion
In conclusion, irbesartan did not lower plasma dicarbonyls and it is therefore less likely that ARBs attenuate renal and cardiovascular risk through attenuation of dicarbonyl stress. In addition, there is still an unmet need for compounds to effectively lower systemic dicarbonyl stress in order to reduce the burden of CKD and CVD in diabetes. It is not known whether more recent reno‐protective agents like SGLT2 inhibitors and GLP1 receptor agonists have an effect on dicarbonyl stress. SGLT2 inhibitors in particular have demonstrated renal benefits and although initially introduced for their glucose‐lowering effect, this seems not to explain the renal and cardiovascular benefits.
COMPETING INTERESTS
FP reports having received research grants from AstraZeneca, Novo Nordisk and Novartis and lecture fees from Novartis, Eli Lilly, MSD, AstraZeneca, Sanofi, Novo Nordisk and Boehringer Ingelheim and having served as a consultant for Astra Zeneca, Bayer, Amgen, Novo Nordisk and MSD. PR has received consultancy and/or speaking fees (to his institution) from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, Sanofi Aventis, and Vifor. Research grants from AbbVie, AstraZeneca and Novo Nordisk.
AUTHOR CONTRIBUTIONS
MP and NMJH analysed the data and wrote the manuscript. JLJMS measured dicarbonyls, wrote and edited the manuscript. MvW measured dicarbonyls and edited the manuscript. FP, MvG, PR, HHP and CDAS edited the manuscript. CGS is the principal investigator of the study, wrote and edited the manuscript.
Supporting information
ACKNOWLEDGEMENTS
N.M.J.H. is supported by a Dr E. Dekker grant by the Dutch Heart Foundation (2017T039) and a junior post‐doc grant from the Dutch Diabetes Foundation (2017.85.005).
Funding information
N.M.J.H. is supported by a Dr E. Dekker grant by the Dutch Heart Foundation (2017T039) and a junior post‐doc grant from the Dutch Diabetes Foundation (2017.85.005).
REFERENCES
- 1.Volpe M, Savoia C, De Paolis P, Ostrowska B, Tarasi D, Rubattu S. The renin–angiotensin system as a risk factor and therapeutic target for cardiovascular and renal disease. J Am Soc Nephrol. 2002;13(Suppl 3):S173‐S178. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz‐Ortega M, Lorenzo O, Rupérez M, et al. Role of the renin–angiotensin system in vascular diseases: expanding the field. Hypertension. 2001;38:1382‐1387. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Cooper ME, De Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861‐869. [DOI] [PubMed] [Google Scholar]
- 4.Parving HH, Lehnert H, Bröchner‐Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870‐878. [DOI] [PubMed] [Google Scholar]
- 5.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295‐2306. [DOI] [PubMed] [Google Scholar]
- 6.Schalkwijk C, Stehouwer CD. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications and other age‐related diseases. Physiol Rev. 2020;100:407‐461. [DOI] [PubMed] [Google Scholar]
- 7.Maessen DE, Brouwers O, Gaens KH, et al. Delayed intervention with pyridoxamine improves metabolic function and prevents adipose tissue inflammation and insulin resistance in high‐fat diet‐induced obese mice. Diabetes. 2016;65:956‐966. [DOI] [PubMed] [Google Scholar]
- 8.Hanssen NMJ, Scheijen JLJM, Jorsal A, et al. Higher plasma methylglyoxal levels are associated with incident cardiovascular disease in individuals with type 1 diabetes: a 12‐year follow‐up study. Diabetes. 2017;66:2278‐2283. [DOI] [PubMed] [Google Scholar]
- 9.Hanssen NMJ, Westerink J, Scheijen JLJM, Van Der Graaf Y, Stehouwer CDA, Schalkwijk CG. Higher plasma methylglyoxal levels are associated with incident cardiovascular disease and mortality in individuals with type 2 diabetes. Diabetes Care. 2018;41:1689‐1695. [DOI] [PubMed] [Google Scholar]
- 10.Jensen TM, Vistisen D, Fleming T, et al. Methylglyoxal is associated with changes in kidney function among individuals with screen‐detected type 2 diabetes mellitus. Diabet Med. 2016;33:1625‐1631. [DOI] [PubMed] [Google Scholar]
- 11.Hanssen NMJ, Stehouwer CDA, Schalkwijk CG. Methylglyoxal and glyoxalase I in atherosclerosis. Biochem Soc Trans. 2014;42:443‐449. [DOI] [PubMed] [Google Scholar]
- 12.Qi W, Keenan HA, Li Q, et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med. 2017;23:753‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwers O, Niessen PMG, Miyata T, et al. Glyoxalase‐1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia. 2014;57:224‐235. [DOI] [PubMed] [Google Scholar]
- 14.Giacco F, Du X, D’Agati VD, et al. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes. 2014;63:291‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AG, Tan G, Binger KJ, et al. Candesartan attenuates diabetic retinal vascular pathology by restoring glyoxalase‐I function. Diabetes. 2010;59:3208‐3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheijen JLJM, Schalkwijk CG. Quantification of glyoxal, methylglyoxal and 3‐deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry: evaluation of blood specimen. Clin Chem Lab Med. 2014;52:85‐91. [DOI] [PubMed] [Google Scholar]
- 17.Scheijen JLJM, Hanssen NMJ, Van De Waarenburg MPH, Jonkers DMAE, Stehouwer CDA, Schalkwijk CG. l(+) and d(−) lactate are increased in plasma and urine samples of type 2 diabetes as measured by a simultaneous quantification of l(+) and d(−) lactate by reversed‐phase liquid chromatography tandem mass spectrometry. Exp Diabetes Res. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheijen JLJM, Hanssen NMJ, van Greevenbroek MM, et al. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: the CODAM study. Clin Nutr. 2018;37:919‐925. [DOI] [PubMed] [Google Scholar]
- 19.Maessen DE, Hanssen NM, Scheijen JL, et al. Post‐glucose load plasma α‐dicarbonyl concentrations are increased in individuals with impaired glucose metabolism and type 2 diabetes: the CODAM study. Diabetes Care. 2015;38:913‐920. [DOI] [PubMed] [Google Scholar]
- 20.Engelen L, Persson F, Ferreira I, et al. Irbesartan treatment does not influence plasma levels of the advanced glycation end products N ε(1‐carboxymethyl)lysine and N ε(1‐carboxyethyl)lysine in patients with type 2 diabetes and microalbuminuria. A randomized controlled trial. Nephrol Dial Transplant. 2011;26:3573‐3577. [DOI] [PubMed] [Google Scholar]
- 21.Dhar I, Dhar A, Wu L, Desai KM. Methylglyoxal, a reactive glucose metabolite, increases renin angiotensin aldosterone and blood pressure in male Sprague‐Dawley rats. Am J Hypertens. 2014;27:308‐316. [DOI] [PubMed] [Google Scholar]
- 22.Forbes JM, Thomas MC, Thorpe SR, Alderson NL, Cooper ME. The effects of valsartan on the accumulation of circulating and renal advanced glycation end products in experimental diabetes. Kidney Int. 2004;66(Suppl. 92):S105‐S107. [DOI] [PubMed] [Google Scholar]
- 23.Karachalias N, Babaei‐Jadidi R, Rabbani N, Thornalley PJ. Increased protein damage in renal glomeruli, retina, nerve, plasma and urine and its prevention by thiamine and benfotiamine therapy in a rat model of diabetes. Diabetologia. 2010;53:1506‐1516. [DOI] [PubMed] [Google Scholar]
- 24.Beisswenger PJ, Howell SK, Touchette AD, Lal S, Szwergold BS. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes. 1999;48:198‐202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


