Abstract
Background
Attention‐deficit/hyperactivity disorder (ADHD) is a highly heritable neurodevelopmental disorder sharing genetic risk factors with other common psychiatric disorders. However, intergenerational recurrence patterns of ADHD from parents to sons and daughters are not known. We aimed to examine the risk of ADHD in offspring of parents with ADHD and parents with other psychiatric disorders by parental and offspring sex, using parents without the specific disorders as comparison.
Methods
In a generation study linking data from several population‐based registries, all Norwegians born 1967–2011 (n = 2,486,088; Medical Birth Registry of Norway) and their parents were followed to 2015. To estimate intergenerational recurrence risk, we calculated prevalence differences (PD) and the relative risk (RR) of ADHD in offspring by parental ADHD, bipolar disorder (BD), schizophrenia spectrum disorder (SCZ), major depression (MDD), all by parental and offspring sex.
Results
The absolute prevalence of ADHD in offspring of parents with ADHD was very high, especially in sons of two affected parents (41.5% and 25.1% in sons and daughters, respectively), and far higher than in offspring of parents with BD, SCZ or MDD. Intergenerational recurrence risks were higher for maternal than paternal ADHD (RRmaternal 8.4, 95% confidence interval (CI) 8.2–8.6 vs. RRpaternal 6.2, 6.0–6.4) and this was also true on the absolute scale (PDmaternal 21.1% (20.5–21.7) vs. PDpaternal 14.8% (14.3–15.4)). RRs were higher in daughters, while PDs higher in sons. Parental SCZ, BD and MDD were associated with an approximately doubled risk of offspring ADHD compared to parents without the respective disorders, and estimates did not differ significantly between daughters and sons.
Conclusions
The intergenerational recurrence risks of ADHD were high and higher from mothers with ADHD than fathers with ADHD. Other parental psychiatric disorders also conferred increased risk of offspring ADHD, but far lower, indicating a sex‐ and diagnosis‐specific intergenerational recurrence risk in parents with ADHD.
Keywords: Attention‐deficit/hyperactivity disorder, intergenerational recurrence risk, parent‐of‐origin, epidemiology, sex differences
Introduction
Attention‐deficit/hyperactivity disorder (ADHD) is a highly heritable neurodevelopmental disorder with polygenic and environmental origins (Faraone & Larsson, 2019; Pettersson, Lichtenstein, Lichtenstein, Larsson, Song, & Polderman, 2019), and often comorbid with other psychiatric disorders (Chen et al., 2018; Solberg et al., 2018). Genetic studies indicate that different genetic mechanisms contribute to the risk of ADHD through rare and common genetic variants, gene × environment interactions, as well as by parent‐of‐origin effects (POE) (Faraone & Larsson, 2019; Zayats, Johansson, & Haavik, 2015). The term POE refers to maternal and paternal genotypes not contributing equally to the development of a phenotype in their offspring, thus differentially influencing the heritability of ADHD (Zayats et al., 2015). Several genetic mechanisms could account for such differences in intergenerational recurrence effects from mothers and fathers to offspring; for example, genomic imprinting, effects of the maternal genome on the intrauterine environment, mitochondrial genome and sex chromosomes (Khramtsova, Davis, & Stranger, 2019). Further, shared genetic (Doherty & Owen, 2014; Ruderfer et al., 2014; The Brainstorm Consortium, 2018), and environmental factors (Pettersson, Larsson, D'Onofrio, Almqvist, & Lichtenstein, 2019) between ADHD and other psychiatric disorders may also contribute to the risk of ADHD in offspring when parents have other psychiatric disorders (Rasic, Hajek, Alda, & Uher, 2014).
In addition to the direct effect of transmitted alleles from parents, nontransmitted alleles can act through their impact on the pre‐ and postnatal environment. This includes ‘genetic nurturing effects’, that is, environmental effects with a genetic component (Kong et al., 2018). Even if both parents contribute to genetic nurture, maternal and paternal effects can differ for different outcomes. Thus, Kong and colleagues observed a stronger maternal than paternal nurturing effect on health aspects of the offspring, while both parents contributed similarly on educational attainment (Kong et al., 2018).
It has been shown that in ~50% of families where one child had been diagnosed with ADHD, at least one parent had ADHD (Smalley et al., 2000; Takeda et al., 2010). Another clinical study showed that children received higher ratings of ADHD if mothers had a history of ADHD than if fathers had a history of ADHD (Goos, Ezzatian, & Schachar, 2007), whereas a family study showed that both mothers’ and fathers’ ADHD equally increased the risk of ADHD in offspring (Thissen, Rommelse, Altink, Oosterlaan, & Buitelaar, 2014). We have not found any studies investigating the role of offspring sex in intergenerational ADHD recurrence risk. Further, the mentioned studies are small and originate from a time when parents were not likely to be diagnosed with ADHD as adults, and none of the studies have been population‐based, thus vulnerable to bias related to missing information. Moreover, relatively little is known about the associations between other parental psychiatric disorders and offspring ADHD.
Therefore, our overall aim was to examine intergenerational recurrence risk of ADHD, and risk of ADHD in offspring of parents with other psychiatric disorders within an epidemiological framework, including an evaluation of parent‐of‐origin effects and the role of offspring sex. Such data can provide new information about ADHD transmission, increase etiological understanding, and provide risk estimates that could prove helpful in diagnosis and clinical management of ADHD.
Methods and measures
The registries
We linked multiple nationwide, population‐based registries in Norway, all with compulsory notification: The Medical Birth Registry of Norway (MBRN), established in 1967 (Irgens, 2000), the Norwegian Prescription Database (NorPD) established in 2004 (Furu et al., 2010), the Norwegian Patient Registry (NPR) with linkable data from 2008 (Nesvag et al., 2017), and the National Educational Database (NUDB) from Statistics Norway (Steingrimsdottir et al., 2012), (Appendix S1 for details). A unique national identification number made it possible to link individual records from the registries. The study was approved by the Regional Committees for Medical Research Ethics in Norway (2011/2272), and reported according to guidelines suggested by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Initiative (von Elm et al., 2007).
Inclusions/exclusions and exposure definitions
We included individuals registered as births in the MBRN during 1967–2011 and alive at record linkage in 2015. We used biological sex as assigned at birth. Individuals (both parents and offspring) who were dispensed ADHD medication (methylphenidate, racemic amphetamine, dexamphetamine or atomoxetine) at any time during 2004–2015 (NorPD), or had received a diagnosis of ADHD or hyperkinetic disorder (International Classification of Diseases (ICD‐10) code F90) in the NPR during 2008–2015 were defined as ‘having ADHD’. Individuals prescribed central stimulants for narcolepsy were excluded from the ADHD definition (Appendix S2 for details). We defined exposure groups according to whether the mother and/or the father had ADHD and assessed the risk of offspring ADHD in these groups. We excluded individuals without information on fathers (n = 19,264; 0.8%), of which 455 (2.4%) had mothers with ADHD, however, we analyzed mother–offspring ADHD recurrence risk for these mothers in a sensitivity analysis.
Likewise, we assessed the risk of ADHD in offspring of parents registered with an ICD‐10 code of either bipolar disorder (BD; F30–F31), schizophrenia spectrum disorder (SCZ; F20–F29), or major depressive disorder (MDD; F32–F33). We defined exposure groups based on the parental disorder as described for ADHD, however, for these analyses parents with ADHD were excluded. For all the exposure groups, including parents with ADHD, parents without the specific disorder served as the reference group.
Outcomes
In all analyses, the outcome was defined as offspring ADHD. We analyzed the prevalence of ADHD in sons and daughters born to parents in all the described exposure groups and evaluated differences in prevalence of ADHD between offspring of parents with and without the various disorders. We use the term ‘intergenerational recurrence risk’ as the relative risk (RR) of ADHD in offspring by parental ADHD. We assessed the recurrence risk for offspring born 1967–2011 (offspring age range 4–48 years, mothers 14–98 years, and fathers 15–100 years at record linkage in 2015). In these analyses, we adjusted for offspring year of birth only (5‐year groups from 1967 to 2011, with 1967–1973 as reference period).
Statistical analyses
To evaluate offspring ADHD by parental ADHD and other psychiatric disorders, both prevalence rates, prevalence differences (PD) and relative risks (RR) were calculated using predicted absolute prevalence from a Poisson regression model with adjustment for year of birth (5‐year groups; Zou, 2004). To account for correlation between siblings, we used mother’s identification number as a cluster variable in the analyses. We also calculated the parent–offspring recurrence of ADHD: (a) confined to first offspring born 1967–2011, and (b) for a narrower follow‐up period, confined to offspring born 1981–2011 whose parents were born 1967–1997, as sensitivity analyses.
We examined intergenerational recurrence risks within an epidemiological framework. Therefore, we chose epidemiological measures of associations (Khoury, Beaty, & Cohen, 1993). However, as a supplementary analysis, we also calculated tetrachoric correlations (mother–offspring and father–offspring) as a quantification of the genetic and environmental (familial) contributions from mother/father to offspring.
Two‐sided tests with a significance threshold of p < .05 were used in all analyses. Analyses were carried out with SPSS Statistics 23 (IBM Corp. Released, 2013) and STATA intercooled version 14 (StataCorp, 2015. StataCorp LP) from October 3, 2018, to October 1, 2020.
Results
We included a total of 2,486,088 offspring born 1967–2011 and alive in 2015. A total of 79,719 offspring (37.6% females) with ADHD were identified. Among these, 20,032 (0.8%) offspring with maternal ADHD only, 16,952 (0.7%) offspring with paternal ADHD only, 1,545 (0.06%) offspring where both parents had ADHD, and 2,447,559 (98.5%) offspring with neither paternal nor maternal ADHD were identified (offspring age range 4–48 years at record linkage in 2015). Mothers and fathers diagnosed with ADHD were younger at first childbirth than mothers without ADHD (Table S1). The prevalence of diagnosed ADHD in our data was 2.4% in adults (born 1967–1997), and 3.3% in children and adolescents (born 1998–2011), with male:female ratio 1.5 for adult offspring and 2.5 for child offspring.
When using absolute measures, we observed a very high adjusted prevalence of ADHD in offspring of parents with ADHD (17.8%–33.8%, Figure 1), and especially in sons to affected parents. When both parents had ADHD, we found that 41.5% of sons and 25.1% of daughters were diagnosed with ADHD (Table 1). In contrast, adjusted prevalence of offspring ADHD was considerably lower when parents had BD, SCZ or MDD, although higher than in families with unaffected parents (Figure 1; Table S5). Also, the prevalence of offspring ADHD was more similar across these parental disorders.
Figure 1.
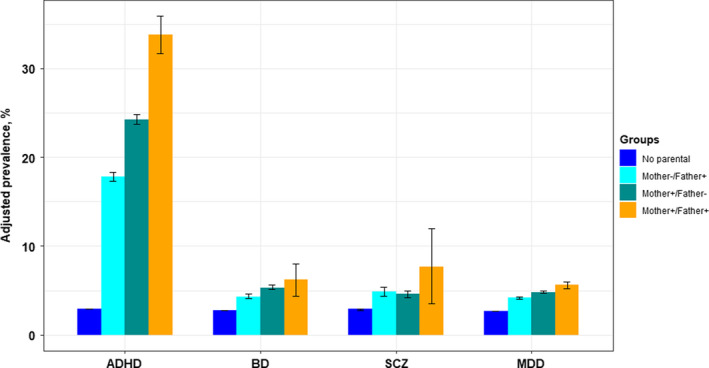
Adjusted prevalence (%) of ADHD in offspring of parents with ADHD, schizophrenia spectrum disorder, bipolar disorder, and major depressive disorder, in three parental exposure groups. Offspring born 1967–2011, the Medical Birth Registry of Norway. ADHD, attention‐deficit/hyperactivity disorder; BD, bipolar disorder; SCZ, schizophrenia spectrum disorder; MDD, major depressive disorder
Table 1.
Parent–offspring recurrence of ADHD in offspring (mothers and fathers to offspring born 1967–2011, the Medical Birth Registry of Norway)
| Mothers and fathers with ADHD +/ or without ADHD‐ | ||||
|---|---|---|---|---|
| Mother− / Father− | Mother−/Father+ | Mother+/Father− | Mother+/Father+ | |
| Total couple of parents No. (%) | 2,447,559 (98.5) | 16,952 (0.7) | 20,032 (0.8) | 1,545 (0.06) |
| Crude prevalences No. (%) | ||||
| ADHD offspring | 70,191 (2.9) | 3,371 (19.9) | 5,612 (28.0) | 545 (35.3) |
| ADHD daughter | 25,460 (2.1) | 1,171 (14.3) | 2,173 (23.1) | 209 (28.1) |
| ADHD son | 44,731 (3.6) | 2,200 (25.1) | 3,439 (32.3) | 336 (42.0) |
| Adjusted prevalencesa (%,95% CI) | ||||
| ADHD offspring | 2.9 (2.9–2.9) | 17.8 (17.3–18.3) | 24.2 (23.7–24.8) | 33.8 (31.7–35.9) |
| ADHD daughter | 2.2 (2.1–2.2) | 13.3 (12.9–13.7) | 18.0 (17.5–18.4) | 25.1 (23.6–26.7) |
| ADHD son | 3.6 (3.5–3.6) | 21.9 (21.3–22.6) | 29.7 (29.0–30.3) | 41.5 (39.0–44.1) |
| Prevalence differencea (%,95% CI) | ||||
| ADHD offspring | 0 (ref) | 14.8 (14.3–15.4) | 21.1 (20.5–21.7) | 30.7 (28.4–32.9) |
| ADHD daughter | 0 (ref) | 11.1 (10.7–11.6) | 15.8 (15.4–16.3) | 23.0 (21.3–24.7) |
| ADHD son | 0 (ref) | 18.4 (17.7–19.1) | 26.1 (25.4–26.8) | 37.9 (35.2–40.7) |
| Relative riska (95% CI) | ||||
| ADHD offspring | 1.0 (ref) | 6.2 (6.0–6.4) | 8.4 (8.2–8.6) | 11.7 (11.0–12.5) |
| ADHD daughter | 1.0 (ref) | 6.7 (6.4–7.1) | 10.4 (10.0–10.8) | 15.2 (13.7–16.9) |
| ADHD son | 1.0 (ref) | 5.9 (5.7–6.1) | 7.4 (7.2–7.6) | 10.1 (9.4–10.9) |
Adjusted for year of birth (5‐year groups, from 1967 to 2011, with 1967–1973 as the reference period), CI = confidence interval.
When evaluating the differences in prevalence of offspring ADHD in families with parental ADHD compared to unaffected parents, we found larger prevalence differences (PDs) when only the mother had ADHD than when only the father had ADHD (PDmothers 21.1% (20.5–21.7); PDfathers 14.8% (14.3–15.4); Table 1). Also, the PDs were larger for sons than for daughters in all three exposure groups.
When we compared the intergenerational recurrence risks of ADHD from mothers and fathers on the relative scale, we again found that maternal ADHD showed stronger associations with offspring ADHD than paternal ADHD (RRmaternal 8.4; 95% confidence interval (CI) 8.2–8.6 vs. RRpaternal = 6.2; 6.0–6.4). The highest intergenerational recurrence risk was found when both parents had ADHD (RRboth = 11.7; 11.0–12.5; Table 1). In contrast to what was found on the absolute scale where PDs were larger in sons than daughters, parent–offspring RR was larger in daughters than in sons both for maternal and paternal ADHD. Maternal associations were highest both in daughters and sons. The corresponding results for first‐born offspring only, thus excluding correlated/dependent data, and for offspring with younger parents, were in line with the overall results (Tables S2–S4).
Adjusted prevalence of offspring ADHD was considerably lower when parents had BD, SCZ or MDD, although higher than in families unaffected by any of these disorders (Figure 1; Table S5). Also, the prevalence of offspring ADHD was more similar across these other parental psychiatric disorders. Further, on the absolute scale, maternal BD and MDD seemed to confer a higher risk than their paternal counterparts, while for SCZ, associations did not differ by parental sex. However, PDs for offspring ADHD were largest when both parents were affected. All PDs were larger for sons than daughters regardless of affected parent (Table S5).
Parental BD, SCZ and MDD were also associated with increased risk of offspring ADHD on the relative scale, with RRs of ~2.0 (Figure 2; Table S5). Relative risks were highest when both parents had a psychiatric disorder (RRs 2.1–2.7, Table S5). In contrast to the findings in families with parental ADHD, RRs of offspring ADHD did not differ significantly between daughters and sons in families where parents had BD, SCZ or MDD, for example, RRdaughter 1.5 (1.4–1.7) and RRson 1.6 (1.5–1.7) with paternal BD, and RRson 1.9 (1.8–2.0) and RRdaughter 1.9 (1.8–2.1) with maternal BD (Table S5).
Figure 2.
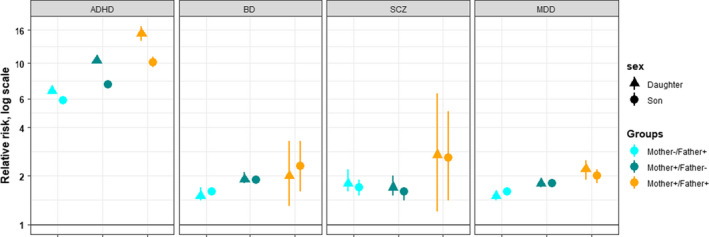
Relative risk of ADHD in sons and daughters of parents with ADHD, bipolar disorder, schizophrenia spectrum disorder, and major depressive disorder. ADHD, attention‐deficit/hyperactivity disorder; BD, bipolar disorder; SCZ, schizophrenia spectrum disorder; MDD, major depressive disorder. For parents with BD, SCZ and MDD, the ADHD diagnosis were excluded
In an additional sensitivity analysis, where we calculated the intergenerational recurrence risk of ADHD in offspring where information on fathers was missing, the RRs from mothers to sons and daughters were 3.4 (95% CI, 2.7–4.3) and 5.7 (95% CI, 4.5–7.4), respectively, slightly lower but with similar pattern as in the previous analyses. Finally, we also calculated the tetrachoric correlations as a quantification of the familial contribution of ADHD from mother and father to the child. The results showed a stronger mother–offspring (~0.5) than father–offspring (~0.4) correlation, and this was true independent of the other parent’s ADHD. The familial contribution from parents with other psychiatric disorders were significantly lower (~0.1), and almost similar from mothers and fathers (Table S6).
Discussion
In this first nationwide population‐based study of parent–offspring recurrence risk of ADHD, we found very high prevalence rates of ADHD in offspring of parents with ADHD, especially in sons of two affected parents. We found stronger maternal‐ than paternal‐offspring associations both when using relative (RRs) and absolute (PDs) effect measures, regardless of offspring sex. However, on the relative scale, with an overall 6–12‐fold increased risk of ADHD in offspring, the largest estimate was for maternal ADHD to daughters, while on the absolute scale, the largest estimate was for maternal ADHD to sons. Independent of using relative or absolute effect measures, the strongest intergenerational associations were found when both parents had ADHD. Prevalence rates were also slightly higher in offspring of parents with BD, SCZ or MDD than in offspring of unaffected parents, but far lower than when parents had ADHD. In families where parents had BD, SCZ or MDD, we found an approximately doubled risk of offspring ADHD compared to families with unaffected parents, and the relative risk estimates did not differ between daughters and sons. Our epidemiological findings indicate an additional diagnostic‐specific increase in risk related to parental ADHD, which was confirmed also by analyses of genetic contribution.
The prevalence of ADHD diagnosis in offspring of parents diagnosed with ADHD was very high, for example, a boy born to a woman diagnosed with ADHD and a father without ADHD has an approximately one in three chance of receiving an ADHD diagnosis himself. Further, in the group of offspring where both parents had ADHD, 41.5% of sons and 25.1% of daughters had been diagnosed with ADHD. The high prevalence rates of ADHD, especially in sons, could indicate that clinicians are specifically aware of ADHD in offspring of parents with ADHD. However, this awareness may be directed toward boys mainly, typically more often referred and diagnosed in childhood compared to girls, and a potential underdiagnosing of girls is possible. Girls often show a different ADHD symptom profile than boys, with more inattentive ADHD symptoms and less externalizing behavior probably leading to this referral bias (Rucklidge, 2010).
Although the prevalence of offspring ADHD was slightly higher in families where parents had BD, SCZ or MDD than in families with unaffected parents, they were considerably lower than when parents had ADHD. This may partially be explained by clinicians being more aware of a possible ADHD diagnosis in offspring when parents have ADHD, but also by the large focus in society on ADHD among teachers and parents who are the main source of referrals in childhood (Rucklidge, 2010).
Associations between parental ADHD and offspring ADHD evaluated by RRs, were strong (Smalley et al., 2000; Takeda et al., 2010). Our finding of a stronger intergenerational recurrence risk from maternal than paternal ADHD to offspring has previously been reported in at least two clinical studies (Faraone et al., 1995; Goos et al., 2007). Several genetic and/or environmental mechanisms might account for the relatively stronger influence of mothers compared to fathers, like POE (Gustavson et al., 2017; Harold et al., 2013; Khramtsova et al., 2019; Zayats et al., 2015). A ‘genetic nurturing effect’ could also play a role and affect the phenotype of the parent (Kong et al., 2018), and as such indirectly influence the offspring differentially through parentally mediated environmental effects. Martin and colleagues found no differences in the increase of polygenic burden in females with ADHD compared to males with ADHD (Martin et al., 2018). However, in register‐based data they observed that siblings to female individuals with ADHD were at higher risk of ADHD than siblings to affected males, in line with the putative female protective effect: This implies that females require greater exposure to genetic and environmental factors associated with ADHD to be diagnosed (Jacquemont et al., 2014; Taylor et al., 2016). An interpretation of some of the results is that the girls who do receive an ADHD diagnosis are more severely affected than boys, which could explain why parental ADHD is a stronger risk factor for ADHD diagnosed in girls. The larger familial contribution to offspring from mothers than fathers with ADHD, ~0.5 and ~0.4, respectively, supports this interpretation.
It is also possible that the stronger maternal‐offspring estimates can be explained by sex differences in health‐seeking behavior, with men seeking both somatic and mental health services less than women (Harris et al., 2016; Smith, Braunack‐Mayer, & Wittert, 2006; Thompson et al., 2016). Thus, ADHD in women may be detected when they seek health care for psychiatric symptoms, or when their children are diagnosed with ADHD, where mothers may recognize similar symptoms in themselves (Williamson & Johnston, 2015). As ADHD in women is less common, mothers may be especially attuned to noticing these difficulties in their offspring, and this may influence on the stronger recurrence risk of ADHD from mothers.
In our study, offspring of parents with BD, SCZ or MDD showed a more general, unspecific, and approximately doubled risk of ADHD, compared to the higher diagnosis‐specific RR in parents with ADHD themselves, which ranged from 6 to 12. This is supported by previous studies (Gottesman, Laursen, Bertelsen, & Mortensen, 2010; Rasic et al., 2014). Shared common genetic and environmental factors between ADHD and other psychiatric disorders may contribute to the elevated risk of ADHD in offspring of parents with BD, SCZ or MDD (Pettersson, D'Onofrio, & Lichtenstein, 2019; The Brainstorm Consortium, 2018). In the Brainstorm Consortium study, Antilla and colleagues found a high degree of genetic correlation among many of the psychiatric disorders studied, including ADHD, BD, SCZ and MDD. The familial contribution from parents with other psychiatric disorders were significantly lower than from parents with ADHD. In total, this could explain the increased risk of offspring ADHD also in families where parents have other major psychiatric disorders, however, our findings indicate an additional diagnosis‐specific increase in risk related to parental ADHD.
Strengths and limitations
To our knowledge, this is the largest population‐based study on parent–offspring recurrence risk of ADHD conducted so far. It is also the first study to evaluate the influence of both parental and offspring sex. The nationwide, population‐based registries with complete generational data have compulsory notification, thus reducing selection bias. Our data was also large enough to allow the analysis of intergenerational recurrence risk in families where only the mother, only the father or both parents had ADHD, BD, SCZ or MDD.
In Norway, diagnostic assessment and pharmacological treatment of ADHD is always based on evaluation by specialist physicians or psychologists. We chose to identify individuals with ADHD either by a dispensed prescription of ADHD medication (from the NorPD) or by an ADHD diagnosis (from the NPR) in line with previous Scandinavian register‐based research (Kendler, Ohlsson, Sundquist, & Sundquist, 2016; Larsson, Chang, D'Onofrio, & Lichtenstein, 2014; Skoglund, Chen, Franck, Lichtenstein, & Larsson, 2015), including a validation study (Larsson et al., 2013).
Limitations include the fact that our ADHD diagnoses were based on data from 2004–2015 (NorPD) and 2008–2015 (NPR). Since the NorPD was established in 2004, individuals diagnosed and treated for ADHD exclusively before 2004, will be undetected. Further, some individuals with ADHD will not receive medication because of contraindications or other causes (e.g., substance use or cardiovascular disorders); these patients are identified in the NPR, but from 2008 only. During 2008–2015, a total of 9,346 (23.3%) adults were registered with an ADHD diagnosis in the NPR without receiving medication. There may be more men with ADHD not receiving ADHD medication due to contraindications, and this may affect the sex differences, especially in the period 2004–2008, when our cases are based on dispensed ADHD medication only. Further, a Norwegian report showed that ~20% of children were not prescribed ADHD medication when diagnosed with ADHD (2008–2015; Ørstavik et al., 2016). Therefore, the prevalence estimates in our study are likely somewhat lower than the true prevalence, perhaps mostly for individuals with less severe ADHD symptoms. We did not use time‐to‐event methods (e.g., Cox‐regression), given that the register follow‐up time is relatively short (2004–2015) compared to the birth years of the study population (1967–2011) and their parents.
However, most of the individuals who received a diagnosis in adult age have likely suffered from the disorder since early childhood. Unfortunately, we had no information about emigration or deaths. In the sample of younger parents to offspring born 1999–2011, the mortality was 0.6% for fathers and 0.2% for mothers. To control for the effect of parental death, we calculated the risk of ADHD in offspring from younger parents, parents to children born 1999–2011. Here, we found almost similar risk estimates and a similar pattern as in the main analyses (Table S2). In addition, one could argue that nonbiological fathers could influence the stronger effect from mothers, especially related to ADHD, however, this is found to be rare (Larmuseau et al., 2017; Larmuseau, Matthijs, & Wenseleers, 2016). Further, by 2015, we identified only 15 children born in 2011 diagnosed with ADHD, which means that some, but very few were diagnosed with ADHD at the age of 4. However, since we wanted to include all children diagnosed with ADHD, even at this young age, these were also included, in line with recent guidelines (Loe, Kakar, & Sanders, 2020).
In conclusion, and in line with the known heritability of ADHD, we have shown that parental ADHD has a strong, diagnosis‐specific association with offspring ADHD. Moreover, a stronger mother–offspring than father–offspring intergenerational recurrence risk of ADHD could support a number of etiological factors, including stronger maternal genetic effects, a stronger effect of maternal nontransmitted alleles, the female protective effect, and differences in health‐seeking behavior. Clinicians working in child‐ and adolescent as well as adult psychiatry should be aware of these very high recurrence rates of ADHD in sons to affected parents and evaluate possible diagnostic bias and underdiagnosing of daughters. Early recognition is important to prevent the risk of increased morbidity associated with ADHD for both males and females. Further research is needed to understand underlying environmental and genetic causes of these observed sex differences in intergenerational recurrence risk and prevalence of ADHD in affected families.
Supporting information
Appendix S1. Description of the registries.
Appendix S2. Narcolepsy.
Table S1. Sample characteristics of mothers and fathers with and without ADHD in the study population including offspring born 1967–2011 and followed to 2015. Norway, (N = 2,486,088).
Table S2. Parent–offspring recurrence of ADHD in offspring (mothers and fathers to offspring born 1999–2011, the Medical Birth Registry of Norway).
Table S3. Parent–offspring recurrence of ADHD confined to first offspring, born 1967–2011. The Medical Birth Registry of Norway.
Table S4. Parent–offspring recurrence of ADHD confined to first offspring born 1981–2011, mothers and fathers born 1967–1996. The Medical Birth Registry of Norway.
Table S5. Parental psychopathology as risk factors of offspring ADHD (mothers and fathers to offspring born 1967–2011, the Medical Birth Registry of Norway).a,b
Table S6. The familial contributions for ADHD in offspring from mothers and fathers with ADHD and other psychiatric disorders, mothers and fathers born 1967–1996. The Medical Birth Registry of Norway.
Acknowledgements
This study was supported by Stiftelsen Kristian Gerhard Jebsen (SKGJ‐MED‐002), University of Bergen and EU Horizon 2020 under grant agreement 667302 (CoCA). B.S.S. and K.K. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. Due to Norwegian ethical and legal restrictions, the data underlying this study cannot be made freely available. Data from the Medical Birth Registry of Norway and the Norwegian Prescription Database, both at the Norwegian Institute of Public Health, and the Norwegian Patient Registry, at the Health Directorate, are available for researchers upon request, after approval from the Regional Committees for Medical and Health Research Ethics. Information about the data, the registries and how to apply for data can be found at the following website URL: https://helsedata.no/. J.H. has served as a speaker for Eli‐Lilly, HB Pharma, Biocodex, Takeda and Shire. The remaining authors have declared that they have no competing or potential conflicts of interest.
Key points.
ADHD is a highly heritable neurodevelopmental disorder; but the intergenerational recurrence risk patterns of ADHD from parents to sons and daughters have not been studied.
The present population‐based intergenerational study demonstrates a stronger recurrence risk of ADHD from mothers than from fathers, and in both cases, strongest to daughters.
Other parental psychiatric disorders also confer increased risk of offspring ADHD, with parental sex‐specific risk patterns, but with no differences between daughters and sons.
Clinicians working in child‐ and adolescent as well as adult psychiatry should be aware of the very high prevalence of ADHD in sons to affected parents and evaluate possible diagnostic bias and underdiagnosing of daughters.
Conflict of interest statement: See Acknowledgements for full disclosures.
References
- Chen, Q.I., Hartman, C.A., Haavik, J., Harro, J., Klungsøyr, K., Hegvik, T.‐A., … & Larsson, H. (2018). Common psychiatric and metabolic comorbidity of adult attention‐deficit/hyperactivity disorder: A population‐based cross‐sectional study. PLoS One, 13, e0204516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, J.L., & Owen, M.J. (2014). Genomic insights into the overlap between psychiatric disorders: implications for research and clinical practice. Genome Medicine, 6, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone, S.V., Biederman, J., Chen, W.J., Milberger, S., Warburton, R., & Tsuang, M.T. (1995). Genetic heterogeneity in attention‐deficit hyperactivity disorder (ADHD): gender, psychiatric comorbidity, and maternal ADHD. Journal of Abnormal Psychology, 104, 334–345. [DOI] [PubMed] [Google Scholar]
- Faraone, S.V., & Larsson, H. (2019). Genetics of attention deficit hyperactivity disorder. Molecular Psychiatry, 24, 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furu, K., Wettermark, B., Andersen, M., Martikainen, J.E., Almarsdottir, A.B., & Sorensen, H.T. (2010). The Nordic countries as a cohort for pharmacoepidemiological research. Basic & Clinical Pharmacology & Toxicology, 106, 86–94. [DOI] [PubMed] [Google Scholar]
- Goos, L.M., Ezzatian, P., & Schachar, R. (2007). Parent‐of‐origin effects in attention‐deficit hyperactivity disorder. Psychiatry Research, 149, 1–9. [DOI] [PubMed] [Google Scholar]
- Gottesman, I.I., Laursen, T.M., Bertelsen, A., & Mortensen, P.B. (2010). Severe mental disorders in offspring with 2 psychiatrically ill parents. Archives of General Psychiatry, 67, 252–257. [DOI] [PubMed] [Google Scholar]
- Gustavson, K., Ystrom, E., Stoltenberg, C., Susser, E., Surén, P., Magnus, P., … & Reichborn‐Kjennerud, T. (2017). Smoking in pregnancy and child ADHD. Pediatrics, 139, e20162509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold, G.T., Leve, L.D., Barrett, D., Elam, K., Neiderhiser, J.M., Natsuaki, M.N., … & Thapar, A. (2013). Biological and rearing mother influences on child ADHD symptoms: revisiting the developmental interface between nature and nurture. Journal of Child Psychology and Psychiatry, 54, 1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, M.G., Baxter, A.J., Reavley, N., Diminic, S., Pirkis, J., & Whiteford, H.A. (2016). Gender‐related patterns and determinants of recent help‐seeking for past‐year affective, anxiety and substance use disorders: Findings from a national epidemiological survey. Epidemiology and Psychiatric Sciences, 25, 548–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. Released . (2013). IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. [Google Scholar]
- Irgens, L.M. (2000). The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstetricia et Gynecologica Scandinavica, 79, 435–439. [PubMed] [Google Scholar]
- Jacquemont, S., Coe, B., Hersch, M., Duyzend, M., Krumm, N., Bergmann, S., … & Eichler, E. (2014). A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. American Journal of Human Genetics, 94, 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler, K.S., Ohlsson, H., Sundquist, K., & Sundquist, J. (2016). Cross‐generational transmission from drug abuse in parents to attention‐deficit/hyperactivity disorder in children. Psychological Medicine, 46, 1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury, M.J., Beaty, T.H., & Cohen, B.H. (1993). Fundamentals of genetic epidemiology. New York, NY: Oxford University Press. [Google Scholar]
- Khramtsova, E.A., Davis, L.K., & Stranger, B.E. (2019). The role of sex in the genomics of human complex traits. Nature Reviews Genetics, 20, 173–190. [DOI] [PubMed] [Google Scholar]
- Kong, A., Thorleifsson, G., Frigge, M.L., Vilhjalmsson, B.J., Young, A.I., Thorgeirsson, T.E., … & Stefansson, K. (2018). The nature of nurture: Effects of parental genotypes. Science, 359, 424–428. [DOI] [PubMed] [Google Scholar]
- Larmuseau, M.H.D., Claerhout, S., Gruyters, L., Nivelle, K., Vandenbosch, M., Peeters, A., … & Decorte, R. (2017). Genetic‐genealogy approach reveals low rate of extrapair paternity in historical Dutch populations. American Journal of Human Biology, 29, e23046. [DOI] [PubMed] [Google Scholar]
- Larmuseau, M.H.D., Matthijs, K., & Wenseleers, T. (2016). Cuckolded fathers rare in human populations. Trends in Ecology & Evolution, 31, 327–329. [DOI] [PubMed] [Google Scholar]
- Larsson, H., Chang, Z., D'Onofrio, B.M., & Lichtenstein, P. (2014). The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychological Medicine, 44, 2223–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, H., Ryden, E., Boman, M., Langstrom, N., Lichtenstein, P., & Landen, M. (2013). Risk of bipolar disorder and schizophrenia in relatives of people with attention‐deficit hyperactivity disorder. British Journal of Psychiatry, 203, 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe, I.M., Kakar, P.A., & Sanders, L.M. (2020). Diagnosis, evaluation, and treatment of attention‐deficit/hyperactivity disorder. JAMA Pediatrics. 10.1001/jamapediatrics.2020.2218 [DOI] [PubMed] [Google Scholar]
- Martin, J., Walters, R.K., Demontis, D., Mattheisen, M., Lee, S.H., Robinson, E., … & Werge, T. (2018). A genetic investigation of sex bias in the prevalence of attention‐deficit/hyperactivity disorder. Biological Psychiatry, 83, 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvåg, R., Jönsson, E.G., Bakken, I.J., Knudsen, G.P., Bjella, T.D., Reichborn‐Kjennerud, T., … & Andreassen, O.A. (2017). The quality of severe mental disorder diagnoses in a national health registry as compared to research diagnoses based on structured interview. BMC Psychiatry, 17, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørstavik, R., Gustavson, K., Rohrer‐Baumgartner, N., Biele, G., Furu, K., Karlstad, Ø., … & Aase, H. (2016). ADHD i Norge ‐ en statusrapport.
- Pettersson, E., D'Onofrio, B., & Lichtenstein, P. (2019). Exploring the association of sex differences and exposure to maternal smoking with low fetal growth‐reply. JAMA Psychiatry, 76, 767. [DOI] [PubMed] [Google Scholar]
- Pettersson, E., Larsson, H., D'Onofrio, B., Almqvist, C., & Lichtenstein, P. (2019). Association of fetal growth with general and specific mental health conditions. JAMA Psychiatry, 76, 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson, E., Lichtenstein, P., Larsson, H., Song, J., Attention Deficit/Hyperactivity Disorder Working Group of the iPSYCH‐Broad‐PGC Consortium , Autism Spectrum Disorder Working Group of the iPSYCH‐Broad‐PGC Consortium , … & Polderman, T.J.C. (2019). Genetic influences on eight psychiatric disorders based on family data of 4 408 646 full and half‐siblings, and genetic data of 333 748 cases and controls. Psychological Medicine, 49, 1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasic, D., Hajek, T., Alda, M., & Uher, R. (2014). Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: A meta‐analysis of family high‐risk studies. Schizophrenia Bulletin, 40, 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucklidge, J.J. (2010). Gender differences in attention‐deficit/hyperactivity disorder. Psychiatric Clinics of North America, 33, 357–373. [DOI] [PubMed] [Google Scholar]
- Ruderfer, D.M., Fanous, A.H., Ripke, S., McQuillin, A., Amdur, R.L., Schizophrenia Working Group of the Psychiatric Genomics Consortium , … & Kendler, K.S. (2014). Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Molecular Psychiatry, 19, 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund, C., Chen, Q., Franck, J., Lichtenstein, P., & Larsson, H. (2015). Attention‐deficit/hyperactivity disorder and risk for substance use disorders in relatives. Biological Psychiatry, 77, 880–886. [DOI] [PubMed] [Google Scholar]
- Smalley, S.L., McGough, J.J., Del'Homme, M., NewDelman, J., Gordon, E., Kim, T., … & McCracken, J.T. (2000). Familial clustering of symptoms and disruptive behaviors in multiplex families with attention‐deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 1135–1143. [DOI] [PubMed] [Google Scholar]
- Smith, J.A., Braunack‐Mayer, A., & Wittert, G. (2006). What do we know about men's help‐seeking and health service use? Medical Journal of Australia, 184, 81–83. [DOI] [PubMed] [Google Scholar]
- Solberg, B.S., Halmoy, A., Engeland, A., Igland, J., Haavik, J., & Klungsoyr, K. (2018). Gender differences in psychiatric comorbidity: a population‐based study of 40 000 adults with attention deficit hyperactivity disorder. Acta Psychiatrica Scandinavica, 137, 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . (2015). Stata statistical software: Release 14. College Station, TX: StataCorp LP. [Google Scholar]
- Steingrímsdóttir, Ó.A., Næss, Ø., Moe, J.O., Grøholt, E.‐K., Thelle, D.S., Strand, B.H., & Bævre, K. (2012). Trends in life expectancy by education in Norway 1961–2009. European Journal of Epidemiology, 27, 163–171. [DOI] [PubMed] [Google Scholar]
- Takeda, T., Stotesbery, K., Power, T., Ambrosini, P.J., Berrettini, W., Hakonarson, H., & Elia, J. (2010). Parental ADHD status and its association with proband ADHD subtype and severity. Journal of Pediatrics, 157, 995–1000 e1001. [DOI] [PubMed] [Google Scholar]
- Taylor, M.J., Lichtenstein, P., Larsson, H., Anckarsater, H., Greven, C.U., & Ronald, A. (2016). Is there a female protective effect against attention‐deficit/hyperactivity disorder? Evidence from two representative twin samples. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 504–512.e502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Brainstorm Consortium . (2018). Analysis of shared heritability in common disorders of the brain. Science, 360, eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thissen, A.J., Rommelse, N.N., Altink, M.E., Oosterlaan, J., & Buitelaar, J.K. (2014). Parent‐of‐origin effects in ADHD: distinct influences of paternal and maternal ADHD on neuropsychological functioning in offspring. Journal of Attention Disorders, 18, 521–531. [DOI] [PubMed] [Google Scholar]
- Thompson, A.E., Anisimowicz, Y., Miedema, B., Hogg, W., Wodchis, W.P., & Aubrey‐Bassler, K. (2016). The influence of gender and other patient characteristics on health care‐seeking behaviour: a QUALICOPC study. BMC Family Practice, 17, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm, E., Altman, D.G., Egger, M., Pocock, S.J., Gotzsche, P.C., Vandenbroucke, J.P., & STROBE Initiative . (2007). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet, 370, 1453–1457. [DOI] [PubMed] [Google Scholar]
- Williamson, D., & Johnston, C. (2015). Gender differences in adults with attention‐deficit/hyperactivity disorder: A narrative review. Clinical Psychology Review, 40, 15–27. [DOI] [PubMed] [Google Scholar]
- Zayats, T., Johansson, S., & Haavik, J. (2015). Expanding the toolbox of ADHD genetics. How can we make sense of parent of origin effects in ADHD and related behavioral phenotypes? Behavioral and Brain Functions, 11, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, G. (2004). A modified poisson regression approach to prospective studies with binary data. American Journal of Epidemiology, 159, 702–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Description of the registries.
Appendix S2. Narcolepsy.
Table S1. Sample characteristics of mothers and fathers with and without ADHD in the study population including offspring born 1967–2011 and followed to 2015. Norway, (N = 2,486,088).
Table S2. Parent–offspring recurrence of ADHD in offspring (mothers and fathers to offspring born 1999–2011, the Medical Birth Registry of Norway).
Table S3. Parent–offspring recurrence of ADHD confined to first offspring, born 1967–2011. The Medical Birth Registry of Norway.
Table S4. Parent–offspring recurrence of ADHD confined to first offspring born 1981–2011, mothers and fathers born 1967–1996. The Medical Birth Registry of Norway.
Table S5. Parental psychopathology as risk factors of offspring ADHD (mothers and fathers to offspring born 1967–2011, the Medical Birth Registry of Norway).a,b
Table S6. The familial contributions for ADHD in offspring from mothers and fathers with ADHD and other psychiatric disorders, mothers and fathers born 1967–1996. The Medical Birth Registry of Norway.


