Abstract
Objective
Ophthalmic postherpetic neuralgia (PHN) is the final stage of herpes zoster (HZ) ophthalmicus and a severe refractory neuropathic pain, thus there is no curative treatment that could alleviate pain and reduce the incidence of ophthalmic PHN now. The purpose of this study is to evaluate therapeutic efficacy of short‐term peripheral nerve stimulation (PNS) for elder patients with HZ ophthalmicus.
Materials and Methods
We performed a retrospective study from March 2015 to August 2019 in our pain department. All the HZ ophthalmicus patients underwent supraorbital nerve short‐term PNS were included. The patients' data, including numeric rating scale (NRS), 36‐Item short form health survey (SF‐36), and analgesic consumptions, were retrospectively analyzed. Severe side effects also were recorded.
Results
A total of 68 patients were enrolled in this study. The NRS scores were significantly decreased at different time points after short‐term PNS compared to baseline (p < 0.001). The SF‐36 scores, including general health, social function, emotional role, mental health, bodily pain, physical functioning, physical role, and vitality, were significantly improved at different time points after treatment (p < 0.001). The average dosages of tramadol and pregabalin administered (mg/d) were both significantly reduced compared to baseline (p < 0.001). There was no bleeding, infection, pain increase, and other side effects after treatment.
Conclusions
Short‐term PNS is an effective and safe therapeutic alternative for elder patients with HZ ophthalmicus and could reduce the incidence of ophthalmic PHN.
Keywords: 36‐item Short Form health survey, herpes zoster ophthalmicus, neuropathic pain, numeric rating scale, peripheral nerve stimulation
INTRODUCTION
Herpes zoster (HZ) ophthalmicus occurs when the latent herpes zoster virus are reactivated and spread from the ganglion of gasser to the ophthalmic nerve (1, 2). It has been estimated that the incidence of HZ is 4–5 per 1000 patients, from which 10–20% cases are HZ ophthalmicus (3). The symptoms can be severe pain and a periocular cutaneous rash limited to the periorbital region (4).
Postherpetic neuralgia (PHN) can be defined as neuropathic pain persisting more than six months after scar tissue formation (5). Older age is a vital factor associated with higher risk to develop to PHN (6, 7). PHN is a refractory chronic pain syndrome with a complex cause and pathogenesis which is unclear by far, however, there was no effective treatments to cure it (8, 9). Hence the prevention from HZ ophthalmicus to ophthalmic PHN in elder patients is an essential therapy strategy.
Peripheral nerve stimulation (PNS) refers to implant a lead near the nerve to stimulate it directly (10, 11). This technique has been used to treat intractable various pain, such as occipital neuralgia, cervicogenic headache, PHN, intractable cancer pain, and so on (12, 13, 14). Long‐term PNS is one of the better treatments for ophthalmic PHN (15), but is it necessary for HZ ophthalmicus? In this study, we would investigate the efficacy of short‐term PNS for elder HZ ophthalmicus patients in order to prevent its development to ophthalmic PHN.
MATERIALS AND METHODS
Study Patients
This retrospective study was approved by the human research ethics committee of the First Affiliated Hospital of China Medical University (No: 2019‐323‐015, Chairman: Prof. Jian Kang, March 23, 2019). This study was be carried out according to the regulations of ethics committee strictly. We reviewed the inpatient records of patients who received short‐term PNS treatment at the Department of Pain Clinic from March 2015 to August 2019.
Inclusion and Exclusion Criteria
Patients were included in the study according to the following criteria: 1) 65 years old or elder patients and HZ ophthalmicus history was no longer than 90 days, 2) numeric rating scale (NRS) ≥ 5, 3) patients treated with short‐term PNS. PNS treatment inclusion criteria included: severe sharp, burning, and so on; pain within the distribution area of HZ; typical neuropathic pain: spontaneous pain, hyperalgesia, and hypersensitivity; not coagulation dysfunction; no infection within the distribution area of HZ; ability to tolerate PNS treatment; not consciousness disorder.
Patients were excluded for the following reasons: 1) treated with other interventional therapy; 2) absence of 180 days follow‐up data.
A total of 196 HZ ophthalmicus patients were initially examined. Among them, 107 patients met the initial inclusion criteria. However, 39 patients were excluded due to exclusion criteria (17 patients treated with other interventional therapy; 22 patients were dropped out within 180 days). Hence, 68 patients were finally included in this study (Fig. 1).
Figure 1.
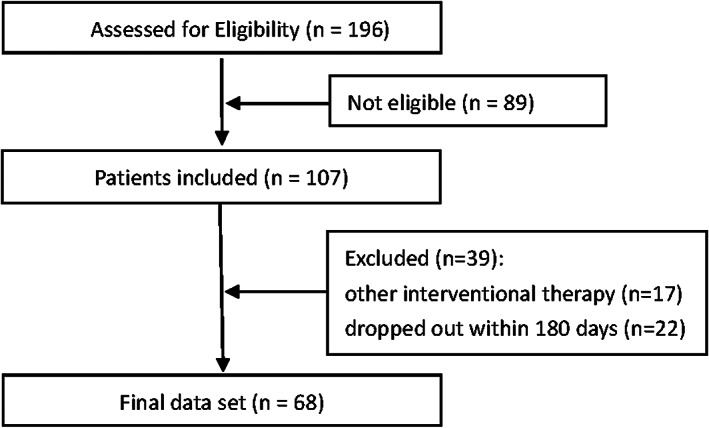
Patient flow diagram.
Description of Interventions
Patients were placed supine position on the treatment bed with no pillow. After sterilization and local anesthesia with 0.5% lidocaine, a modified Tuohy needle was perpendicular punctured though skin 20 mm above the lateral canthus. Then a 1 × 8 eight‐contact percutaneous lead (Model: 18366901, St. Jude Medical, St. Paul, MN, USA) was implanted though puncture needle at an appropriate physiologic and anatomic position under the guidance of C‐arm (Fig. 2). A successful stimulation was defined as “pleasant paresthesia covering at least 50 percent of the painful area” (16).
Figure 2.
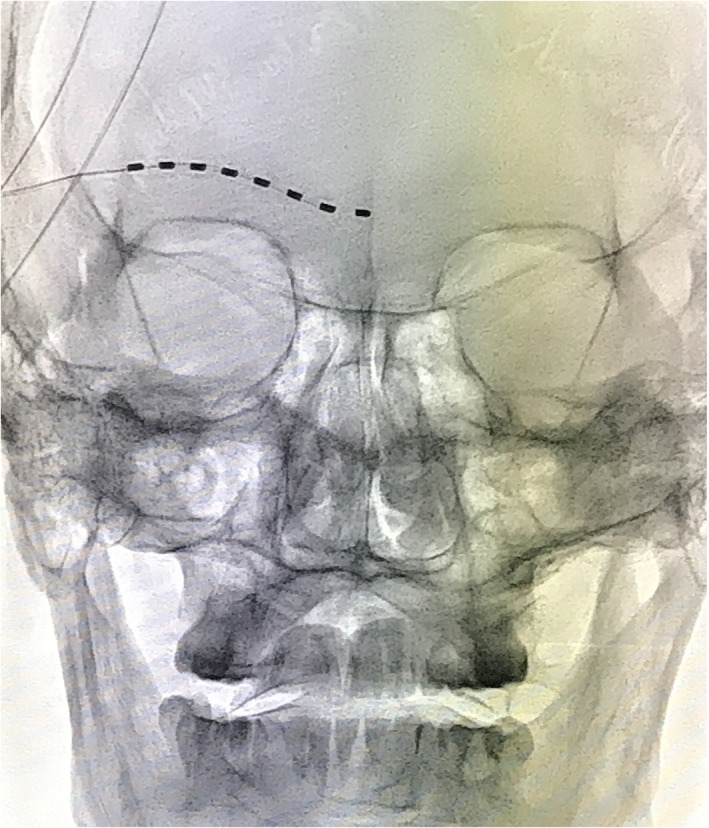
X‐ray image of an electrode lead punctured. [Color figure can be viewed at wileyonlinelibrary.com]
All patient received only one lead electrode and were successfully implanted the lead. The stimulation was at the following settings: tonic mode (only tonic mode could be used in Chinese mainland), constant current amplitude of 4–10 mA, pulse width of 240–500 μs, frequency of 40 Hz, and contact polarity of 2 −, 7 +. In order to make patients sleep well at night, this program was shut down during 10 pm–6 am. The patient could receive PNS treatment in home if he got well analgesic effect with steady stimulation after two days in‐hospital observation. Patients got a short‐term PNS treatment for 7–14 days according to the degree of pain relief.
Drug Administration
All patients could be administered analgesic agents, including tramadol and pregabalin for pain control if they were suffered severe pain. The two drugs could be combined medication. The dosage was increased or reduced according to the alteration of pain severity.
Outcome Measures
Numeric Rating Scale
The NRS scores before treatment and on days 3, 7, 14, 30, 90, and 180 after treatment were collected.
SF‐36
The Chinese version SF‐36 scores, including general health, social function, emotional role, mental health, bodily pain, physical functioning, physical role and vitality, before treatment and on days 30, 90, and 180 after treatment also were collected.
Average Dosages of Analgesic Agents
Tramadol and pregabalin could be combined medication if patients suffered severe pain. The average daily dosages (mg/d) were collected before treatment and on days 30, 90, and 180 after treatment.
Side Effects
Any side effects, including bleeding at the puncture site, infection, increased pain, and other adverse reactions, were collected.
Statistical Analysis
Numeric variables are expressed as mean ± SD values and the number of observations. Categorical variables are described using the number of frequencies and percentages. The statistical analysis was performed using Microsoft Excel and SPSS version 18.0 software (SPSS, Inc., Chicago, IL, USA). The differences in NRS and SF‐36 scores were analyzed by paired t‐test. The variations in the consumption of analgesics were assessed using chi‐squared test and Fisher's exact test. A p < 0.05 was considered to be statistically significant. Safety analyses were conducted for the incidence of side effects.
RESULTS
Demographics
Sixty‐eight patients were finally enrolled in this study. Demographic characteristics of the patients were summarized in Table 1. The mean age was 73.26 ± 9.26 years old (ranging from 65 to 93 years old), the mean duration of pain starting from the rash onset was 59.98 ± 21.50 days (ranging from 15 to 90 days), and the mean duration of stimulation was 10.77 ± 2.45 days.
Table 1.
Demographic Characteristics of Patients (Mean ± SD).
| Patients | Study group (n = 68) |
|---|---|
| Age (years) | 73.26 ± 9.26 |
| Female/male, n | 37/31 |
| Weight (kg) | 65.46 ± 10.23 |
| Disease duration (days) | 59.98 ± 21.50 |
| Average pain scores | 7.33 ± 1.87 |
| Stimulation duration (days) | 10.77 ± 2.45 |
| Develop to PHN (n) | 4 |
Numeric Rating Scale
Patients obtained a significant pain relief on three days after treatment (3.39 ± 1.45) compared to baseline (7.33 ± 1.87) and the NRS scores declined gradually during the all the follow‐up period (p < 0.001; Fig. 3). At the 180 days follow‐up point, a total of 4 patients (5.8%, 4/68) were converted to ophthalmic PHN (NRS ≥ 3), including 2 patients (2/68, 2.9%) suffered severe pain (NRS ≥ 6). On the other hand, 64 patients (94.2%, 64/68) could obtain excellent pain relief (NRS ≤ 2).
Figure 3.
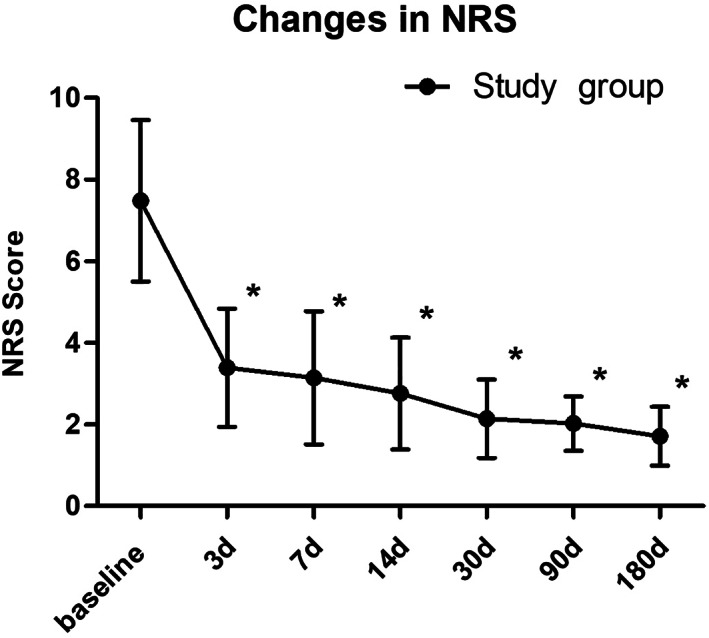
Significantly decreased mean NRS scores after short‐term PNS. *p < 0.001.
SF‐36
General Health
General health scores increased significantly on 30 days after treatment compared to baseline and could rise gradually during all the follow‐up period (p < 0.001) (Fig. 4a).
Figure 4.
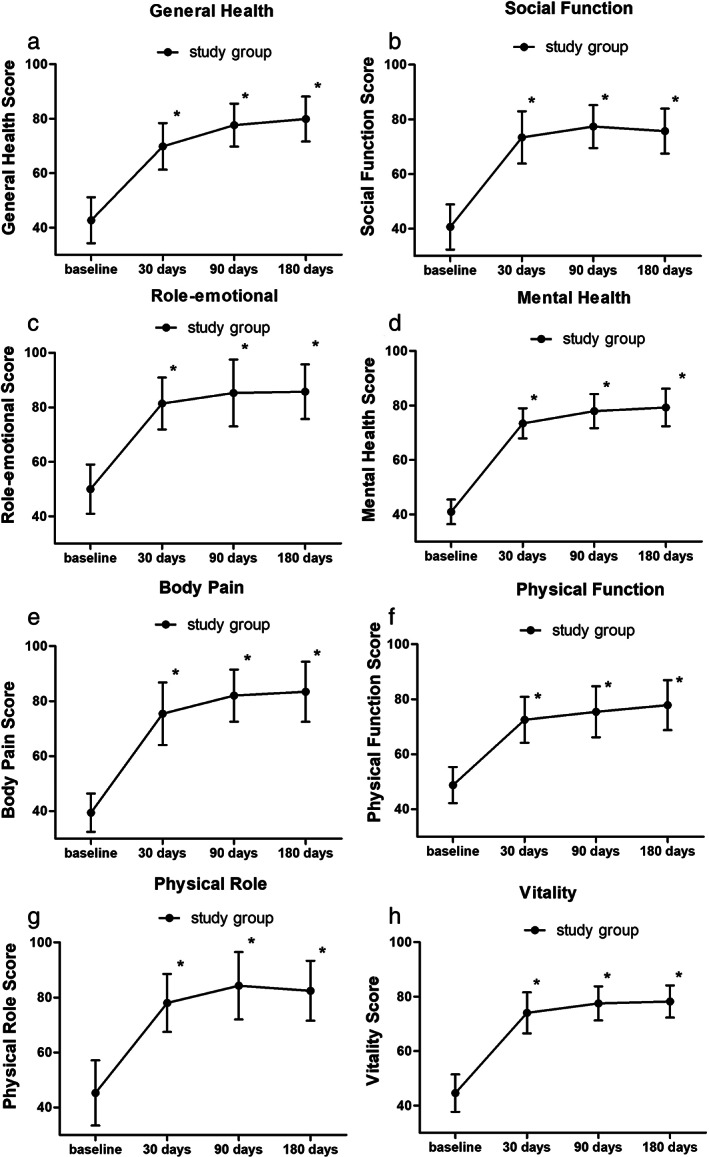
Significantly improved mean SF‐36 scores after short‐term PNS. *p < 0.001.
Social Function
Social function scores increased significantly on 30 days after treatment compared to baseline and could maintain during the all the follow‐up period (p < 0.001) (Fig. 4b).
Emotional Role
Emotional role scores increased significantly on 30 days after treatment compared to baseline and could maintain during the all the follow‐up period (p < 0.001) (Fig. 4c).
Mental Health
Mental health scores increased significantly on 30 days after treatment compared to baseline and could rise gradually during the all the follow‐up period (p < 0.001) (Fig. 4d).
Body Pain
Body pain scores increased significantly on 30 days after treatment compared to baseline and could rise gradually during the all the follow‐up period (p < 0.001) (Fig. 4e).
Physical Function
Body pain scores increased significantly on 30 days after treatment compared to baseline and could rise gradually during the all the follow‐up period (p < 0.001) (Fig. 4f).
Physical Role
Physical role scores increased significantly on 30 days after treatment compared to baseline and could maintain during the all the follow‐up period (p < 0.001) (Fig. 4g).
Vitality
Vitality scores increased significantly on 30 days after treatment compared to baseline and could maintain during the all the follow‐up period (p < 0.001) (Fig. 4h).
Analgesic Agents
The daily dosages of tramadol (p < 0.001) and pregabalin (p < 0.001) were both significantly reduced on each time point after PNS treatment compared to baseline (Fig. 5).
Figure 5.
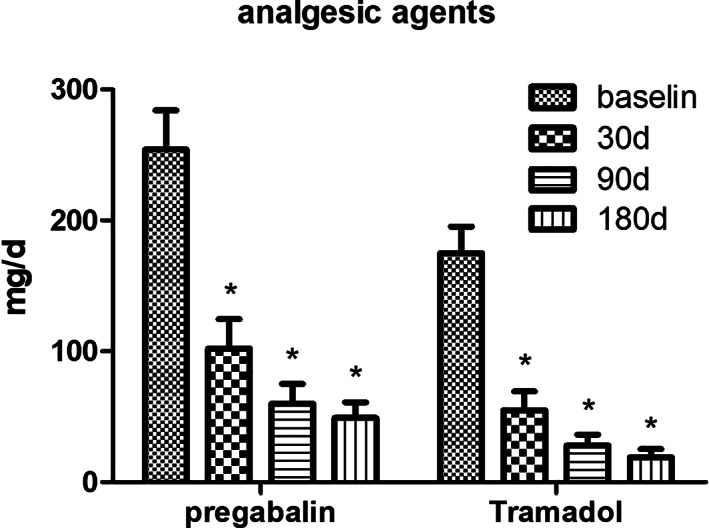
Significantly decreased mean dosages of pregabalin and tramadol after short‐term PNS. *p < 0.001.
Side Effects
There was no patient suffered bleeding at the puncture site, infection, increased pain, or other side effects after treatment. No patient was withdrawn from the treatment due to adverse reactions.
DISCUSSION
Short‐term PNS on supraorbital nerve not only effectively relieved pain, but also significantly reduced the average dosage of analgesic agents for elder patients with HZ ophthalmicus. The results also revealed that the treatment could significantly improve SF‐36 scores, which reflect the overall quality of life. This therapeutic strategy could reduce the incidence of ophthalmic PHN in elder HZ ophthalmicus patients.
As age is a risk factor in the incidence of PHN, 12.4% of elderly HZ patients (≥65 years) could convert to PHN without effective treatment (17). Because ophthalmic PHN pain can be very severe, even disturbing sleep and disabling patients, it should be prevented as well as possible, especially for older patients (18). Our previous articles (19) has shown that high‐voltage, long‐duration pulsed radiofrequency on gasserian ganglion is an effective for acute/subacute zoster‐related trigeminal neuralgia. Because primary sensory neuron of ophthalmic nerve is localized deeply in the gasserian ganglion, it may cause serious complications if puncturing deeply though oval foramen.
PNS has been used in the treatment of several chronic refractory pain conditions including pain due to peripheral nerve dysfunctions, complex regional pain syndrome, and cranial neuralgias (20, 21, 22).The supraorbital nerve is a branch of the ophthalmic nerve, which extends from the supraorbital notch or foramen to the subcutaneous area and distributes in the frontal skin (23). PNS on supraorbital nerve is a simple and safe operation. All the patients in this study completed implantation of the electrodes within one hour and there was no serious side effect after treatment.
In our study, 64 patients (94.2%) could obtain excellent pain relief and were satisfied with therapeutic effect. This result illustrated that the permanent implantation of PNS was unnecessary for these patients. They could save the massive medical funds and avoid damages of further surgery. The incidence in our study of converted to ophthalmic PHN (4 patients, 5.8%) was significantly decreased compared to Weitzman's result (12.4%) (17). Although PNS was applied to one branch of ophthalmic nerve (supraorbital nerve) in our study, dramatic relief of pain could be observed in all the distribution area of ophthalmic nerve. It is likely to be due to PNS could decrease hyperexcitability and long‐term potentiation of neurons (24). The average dosages of tramadol and pregabalin also were significantly reduced compared to baseline. Patients in the study also were experienced significantly improved quality of life.
However, there were several limitations in this study, which should be addressed in future trial. First, this was a retrospective study and patients were recruited from a single center, and the sample size was small. Second, the patients were only followed for 180 days after treatment. Future study should be a RCT trial with larger sample and longer follow‐up. However, the current findings revealed that short‐term PNS was an effective pain relief method for elder HZ ophthalmicus patients.
CONCLUSIONS
Short‐term PNS on supraorbital nerve can obviously relieve the pain for elder HZ ophthalmicus patients, thus improving the quality of life with less analgesic drugs and reducing the incidence of ophthalmic PHN.
Authorship Statement
Cheng‐fu Wan was responsible for the clinical experimental design, clinical trial practice, statistical analysis of data, and writing of the manuscript. Tao Song was responsible for the clinical experimental design and manuscript review. Both authors approved the final version of the manuscript.
COMMENT
This article presents observational data of short‐term peripheral nerve stimulation (PNS) efficacy in elderly patients series of herpes zoster ophthalmicus. This group of authors from China present their clinical experience of the application peripheral nerve stimulation (PNS) as a temporary, surgically invasive modality for treatment to prevent the transformation of herpes zoster ophthalmicus to postherpetic neuralgia. Data from 68 patients with severe pain (numerical rating scale more than 5) and without any interventional therapy was obtained in retrospective fashion with 180 days of follow up trial of temporary implantation and stimulation of the peripheral part of the supraorbital branch of the trigeminal nerve. The results of this study are interesting in several aspects:
This short‐term PNS trial showed significant efficacy rate for control of facial pain due to HZ ophthalmicus, which was achieved on day 3 after the procedure. 94.2% of study subjects obtained pain reduction to NRS less then 2 which was maintained over 180 days follow up period with reduction of daily doses of pain medication and improvement in index score of general health.
The data of this study support the evidence that the trial of trigeminal nerve PNS is a safe procedure: no bleeding at the puncture site, infection, or increase of pain was recorded. The authors provided data of a 5.8% conversion rate from HZ ophthalmicus to PHN which is twice less than the literature data shows for the natural history of ophthalmic PHN. Taking into account that approximately 30% of patients with PHN experienced facial pain that lasted more than a year and could last up to 10 years short‐term invasive trial of PNS could significantly decrease the cost of medical management. However, an important limitation of this study is the absence of a control group and even historical control data. Therefore, before recommending these methods to clinical practice, data of this study should be confirmed by a multicenter controlled clinical trial.
Serge Rasskazoff, MD
Belleville, IL USA
Acknowledgements
We would like to thank all the patients, doctors, and nurses involved in the current study.
Wan, C., Song, T. 2021. Short‐Term Peripheral Nerve Stimulation Relieve Pain for Elder Herpes Zoster Ophthalmicus Patients: A Retrospective Study. Neuromodulation 2021;24: 1121–1126.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: This study was financially supported by a grant from Liaoning Natural Science Foundation (No. 20180530063).
Conflict of Interest: The authors reported no conflict of interest.
REFERENCES
- 1.Moniuszko ASM, Zajkowska A, Garkowski A, Czupryna P, Pancewicz S, Zajkowska J. Blindness resulting from orbital complications of ophthalmic zoster. Postepy Dermatol Alergol 2015;32:396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Mello Vitor B, Foureaux ECM, Porto FBO. Herpes zoster optic neuritis. Int Ophthalmol 2011;31:233–236. [DOI] [PubMed] [Google Scholar]
- 3.Vrcek I, Choudhury E, Durairaj V. Herpes zoster ophthalmicus: a review for the internist. Am J Med 2017;130:21–26. [DOI] [PubMed] [Google Scholar]
- 4.Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2008;115:S3–S12. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y, Li H, Hong T, Zhao R, Yao P, Zhao G. Efficacy and safety of computed tomography‐guided pulsed radiofrequency modulation of thoracic dorsal root ganglion on herpes zoster neuralgia. Neuromodulation 2019;22:108–114. [DOI] [PubMed] [Google Scholar]
- 6.Forbes HJ, Thomas SL, Smeeth L et al. A systematic review and meta‐analysis of risk factors for postherpetic neuralgia. Pain 2016;157:30–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai K, Rampakakis E, Tsai TF et al. Predictors of postherpetic neuralgia in patients with herpes zoster: a pooled analysis of prospective cohort studies from north and Latin America and Asia. Int J Infect Dis 2015;34:126–131. [DOI] [PubMed] [Google Scholar]
- 8.Schlereth T, Heiland A, Breimhorst M et al. Association between pain, central sensitization and anxiety in postherpetic neuralgi. Eur J Pain 2015;19:193–201. [DOI] [PubMed] [Google Scholar]
- 9.Johnson RW, Rice ASC. Clinical practice. Postherpetic neuralgia. N Engl J Med 2014;371:1526–1533. [DOI] [PubMed] [Google Scholar]
- 10.Meyer‐Frießem CH, Wiegand T, Eitner L et al. Effects of spinal cord and peripheral nerve stimulation reflected in sensory profiles and endogenous pain modulation. Clin J Pain 2019;35:111–120. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Ashkenazi A, Smith JW, Jen N, Deer TR, Zhou C. Long‐term clinical outcome of peripheral nerve stimulation for chronic headache and complication prevention. Anesth Pain Med 2016;6:e35983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnstone CS, Sundaraj R. Occipital nerve stimulation for the treatment of occipital neuralgia‐eight case studies. Neuromodulation 2006;9:41–47. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigo‐Royo MDAJ, Quero J, Lorente MC, Acin P, Azcona J. Peripheral neurostimulation in the management of cervicogenic headache: four case reports. Neuromodulation 2005;8:241–248. [DOI] [PubMed] [Google Scholar]
- 14.Palmisani S, Al‐Kaisy A, Arcioni R, Smith T, Negro A, Lambru G. A six year retrospective review of occipital nerve stimulation practice controversies and challenges of an emerging technique for treating refractory headache syndromes. J Headache Pain 2013;14:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurklinsky S, Palmer SC, Arroliga MJ, Ghazi SM. Neuromodulation in postherpetic neuralgia: case reports and review of the literature. Pain Med 2018;19:1237–1244. [DOI] [PubMed] [Google Scholar]
- 16.Song JJPA, Bell R. Present and potential use of spinal cord stimulation to control chronic pain. Pain Physician 2014;17(3):235–246. [PubMed] [Google Scholar]
- 17.Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of Herpes Zoster and its complications. J Infect 2013;67:463–469. [DOI] [PubMed] [Google Scholar]
- 18.Leev S. Herpes zoster and postherpeticneural gain the elderly. GeriatrNurs 2000;21:132–135. [Google Scholar]
- 19.Wan C, Dong DS, Song T. High‐voltage, long‐duration pulsed radiofrequency on Gasserian ganglion improves acute/subacute zoster‐related trigeminal neuralgia: a randomized, double‐blinded, controlled trial. Pain Physician 2019;22:361–368. [PubMed] [Google Scholar]
- 20.Goroszeniuk T, David P, Shetty A, Eldabe S, O'Keeffe D, Racz G. Percutaneous peripheral neuromodulation lead insertion using a novel stimulating coudé needle. Neuromodulation 2014;17:506–509. [DOI] [PubMed] [Google Scholar]
- 21.Slavin KV, Colpan ME, Munawar N, Wess C, Nersesyan H. Trigeminal and occipital peripheral nerve stimulation for craniofacial pain: a single‐institution experience and review of the literature. Neurosurg Focus 2006;21:E5. [DOI] [PubMed] [Google Scholar]
- 22.Nayak R, Banik RK. Current innovations in peripheral nerve stimulation. Pain Res Treat 2018;2018:9091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vukovic Cvetkovic V, Jensen RH. Neurostimulation for the treatment of chronic migraine and cluster headache. Acta Neurol Scand 2019;139:4–17. [DOI] [PubMed] [Google Scholar]
- 24.Chakravarthy KV, Xing F, Bruno K et al. A review of spinal and peripheral neuromodulation and neuroinflammation: lessons learned thus far and future prospects of biotype development. Neuromodulation 2019;22:235–243. [DOI] [PubMed] [Google Scholar]


