Abstract
INTRODUCTION:
There are limited data on the incidence, predictors, and time to future liver abnormalities in patients with intrahepatic cholestasis of pregnancy (ICP).
METHODS:
Single-center retrospective study of pregnant women with and without ICP who delivered from 2005 to 2009 evaluating incidence and time to future liver abnormalities. Women returning for care with liver function tests at a minimum of 6 months postpartum were included. Liver disease diagnoses and liver functions test abnormalities were compared. Time to development of alanine aminotransferase (ALT) >25 U/L, alkaline phosphatase (ALP) >140 U/L, and diagnosis of liver disease (through imaging or clinical evaluation) were compared between women with and without ICP using Kaplan-Meier methods and Cox regression models.
RESULTS:
A total of 255 women with ICP and 131 age-matched control subjects with delivery during the same period were identified. Subjects in both groups were similar in follow-up time, age at pregnancy, prepregnancy body mass index, and ethnicity (≥75% were Hispanic in both groups). On univariate analyses, ICP was associated with increased incidence of ALT >25 U/L P < 0.01 ALP >140 U/L (P < 0.01) and liver disease (P = 0.03). Adjusting for metabolic factors, ICP diagnosis was associated with risk of future liver abnormalities: postpartum ALT >25 U/L (hazard ratio [HR] 1.9, P < 0.01), ALP >140 U/L (HR 3.4, P < 0.01), and liver disease (HR 1.5, P = 0.05).
DISCUSSION:
In our cohort of urban women, ICP diagnosis predicted risk of future liver disease and abnormal liver tests. Women with pregnancies complicated by ICP may benefit from surveillance for postpartum liver abnormalities.
INTRODUCTION
Intrahepatic cholestasis of pregnancy (ICP) is the most common pregnancy-specific liver disease, typically occurring in the second and third trimesters of pregnancy (1). In the United States, ICP occurs in 0.25%–0.32% of pregnancies, but is more common in certain ethnic groups, particularly among Hispanics, Scandinavians, and Southeast Asians (2,3). The highest reported prevalence in the United States is 5.6% in a primarily Hispanic community in Los Angeles (4). ICP has been associated with adverse fetal outcomes including spontaneous preterm birth, meconium staining of the amniotic fluid, and stillbirth (5). A 2019 systematic review and meta-analysis by Ovadia and Williamson (6) found ICP patients had greater risks of spontaneous preterm birth and iatrogenic preterm birth particularly at bile acid levels ≥100 μmol/L.
Although the exact etiology of ICP is unknown, it is believed to be caused by the combination of hormonal, environmental, and genetic factors (2). Several studies have identified risk factors for the condition, including advanced maternal age (AMA), multiple gestations, and assisted reproductive pregnancy (7–9). In addition, women with hepatitis C (HCV) have higher rates of ICP and those who develop ICP tend to have earlier onset of ICP-related symptoms (2,10).
Given the potentially life-threatening complications to the fetus and the fact that symptoms of the condition typically resolve within 48 hours of delivery or with therapeutic management, mostresearch on ICP hasfocused on obstetricalmanagement and fetal outcomes (11,12). For the mother, ICP was previously believed to be a pregnancy-specific condition with preexisting risk factors but no troubling long-term sequelae. However, several European cohort studies have shown greater risk of adverse hepatic and systemic health outcomes in ICP patients—biliary tree cancer, liver cancer, gallstones, cirrhosis, immune-mediated diseases (thyroid disease, diabetes mellitus, psoriasis, and Crohn’s disease), cardiovascular disease, and breast cancer (10–16).
In the United States, there are limited data on long-term liver morbidity among patients with ICP. Using data from a cohort of women in a hospital with increased prevalence of ICP (2.5%) (11), we investigated incidence, predictors, and time to occurrence of future liver abnormalities (see Visual Abstract, Supplementary Digital Content 1, http://links.lww.com/AJG/B827).
PATIENTS AND METHODS
We conducted a single-center retrospective cohort study of women with and without ICP to determine risk of future liver abnormalities.
Study setting
We reviewed the records of pregnant women with and without ICP who delivered from 2005 to 2009 at Elmhurst Hospital (EHC), a public community hospital in New York City (NYC) which serves predominantly Hispanic patients (Hispanic—54%, White—6%, Black—7%, Asian—16%, and other—17%) (17) with a high prevalence of ICP (2.5% of all deliveries) (11). EHC provides care for more Medicaid and uninsured patients than any hospital in NYC. Sixty-six percent of inpatient hospitalizations, 78% of emergency department visits, and 84% of clinic visits are for Medicaid or insured patients compared with approximately 42%, 61%, and 71%, respectively, in other NYC public hospitals (18). The Department of Obstetrics and Gynecology at EHC maintains a database of all pregnancies complicated by ICP from 2005 to present. To the best of our knowledge, this is the largest US database of patients with ICP (n = 871).
Study population
Diagnosis of ICP was verified for patients based on presence of newly developed pruritus in pregnancy and abnormal laboratory results (bile acid level >10 μmol/L; while best clinical practice suggests bile acids taken in a fasting state, obstetricians typically order serum bile acid levels when there is clinical suspicion of ICP even if nonfasting). All women were tested for hepatitis C virus and human immunodeficiency virus as part of routine prenatal testing, and some women were tested for HCV (48 ICP and 8 non-ICP). We identified 131 non-ICP comparators who delivered during the study period.
Postpartum follow-up variables included total number of postpartum liver tests (LTs), highest alanine aminotransferase (ALT), aspartate aminotransferase, alkaline phosphatase (ALP) and gamma-glutamyl transferase levels, subsequent ICP diagnosis, liver toxic medication use, cholecystectomy, metabolic syndrome diagnoses (hyperlipidemia, diabetes, and hypertension), liver imaging results, and liver disease diagnosis.
Inclusion/exclusion criteria
We evaluated all ICP patients who delivered during the study period. Each woman was studied as a single case and women with multiple pregnancies during the period were only included once, using data from the first pregnancy. To analyze the long-term effects of ICP (as opposed to persistently elevated LT elevation after pregnancy), women returning for care with liver function tests at a minimum of 6 months postpartum were included in analyses. Women lost to follow-up or women who did not have LTs were excluded. Women with documented history of chronic liver disease before pregnancy and women noted to have liver function tests performed onlyas part of routine surveillance when on medications known to cause elevated LTs, including isoniazid, statins, synthroid, methimazole, and HAART therapy, were excluded. Charts were reviewed for other causes of pregnancy-related liver diseases (haemolysis, elevated liver enzymes, low platelet count, acute fatty liver of pregnancy, biliary duct diseases, and hyperemesis gravidarum) in current and previous pregnancies. Patients with previous pregnancy-specific liver disease were included, while ones with these conditions in the pregnancy being studied were excluded from analyses.
Cases of ICP were identified through the EHC’s obstetrics and gynecology department internal database. Non-ICP comparators were healthy pregnancies obtained through hospital’s January 1, 2005–December 31, 2009 birth records. A total of 19,449 non-ICP deliveries were recorded over this period. Comparators were not suspected of having ICP in pregnancy (and therefore did not have bile acid laboratory tests ordered), returned to care ≥6 months after delivery, and had LTs during the study period.
An age-matched design with a case-comparison group ratio of 2:1 was used because of feasibility issues regarding identification and data collection for non-ICP women. Cases were matched to a group of women without ICP based on age (year of birth ± 1 year). To detect an association at the 5% significance level with 80% power (based on an estimated 10% and 25% prevalence of liver disease in the non-ICP and ICP populations), a sample size of 255 cases was required to detect a di?erence in liver abnormalities between groups.
Clinical outcomes
The primary outcome was diagnosis of liver conditions including viral hepatitis, autoimmune hepatitis, primary biliary cholangitis (PBC), primary sclerosing cholangitis, and nonalcoholic fatty liver disease (NAFLD) as ascertained by medical chart review of clinical documentation as well as imaging for signs of liver abnormalities including fatty infiltration and increased echogenicity of the liver, steatosis, cirrhosis, or evidence of portal hypertension from review of abdominal ultrasound, computed tomography, or magnetic resonance imaging reports.
Secondary outcome was incident of liver disease or abnormal liver function tests ≥6 months after ICP delivery. Liver function abnormalities were determined by the following criteria: ALT >25 U/L (upper limit of normal, ULN), ALT >50 U/L, ALP >140 U/L (ULN), and ALP >280 U/L. We examined the time to development of 3 outcomes: (i) ALT >25 U/L; (ii) ALP >140 U/L; and (iii) diagnosis of liver disease (through imaging or clinical evaluation).
Statistical analyses
The Student t test and χ2 tests were used to compare baseline characteristics, liver function test abnormalities, and liver imaging data between ICP patients and controls. Kaplan-Meier curves were fitted for development of (i) ALT >25 U/L, (ii) ALP >140 U/L, or (iii) diagnosis of liver disease. Differences in outcomes were determined using the log-rank test. Cox proportional hazards models were used to estimate predictors of future liver disease, ALT, and ALP elevation among the entire study cohort as well as in ICP patients. Subjects not experiencing an event were censored at their most recent follow-up date. Multivariable models were fit adjusted for ICP diagnosis in current pregnancy, ethnicity, diabetes status, body mass index (BMI), and maternal age. All analyses were performed using the Statistical Analysis System statistical software package, version 9.4 (SAS Institute, Cary, NC).
RESULTS
We identified 475 deliveries complicated by ICP from 2005 to 2009. Two hundred fifty-five of 475 (53.7%) returned to care during the follow-up period and served as thecases in our analytic cohort (Figure 1). ICP women who returned for care were statistically similar to the 220 ICP women who did not return for care or qualify for inclusion in key metrics: age at delivery (P = 0.81), BMI (P = 0.08), parity (P = 0.63), gestational diabetes (GDM) (P = 0.78), and preeclampsia (P = 0.42). Among ICP and non-ICP women, both groups had a median of 4 laboratory tests with ALT performed ≥6 months postpartum (interquartile range [IQR] 2–6 for comparators and 2–8 for ICP women). Median follow-up time was 7.8 years in the ICP group (IQR 5.5–10.7) and 9.7 years in the comparison group (IQR 5.8–11.3). Eighty-nine percent of the ICP patients and 79% of the comparison were identified as Hispanic (P = 0.01) (Table 1). Median age at time of pregnancy, median baseline BMI, and incidence of GDM were similar between groups (Table 1). Higher rates of preeclampsia (9.8 vs 2.3%, P < 0.01) was seen in the ICP group. Approximately 7.1% of ICP patients had a history of previous ICP, compared with none in the comparison group. Among ICP women, those with and without a history of previous ICP (18 vs 237) were similar in ethnicity, age at delivery, and rates of adverse pregnancy outcomes in current pregnancy. Significant differences were seen in BMI and parity (see Supplementary Table 1, Supplementary Digital Content 2, http://links.lww.com/AJG/B826).
Figure 1.
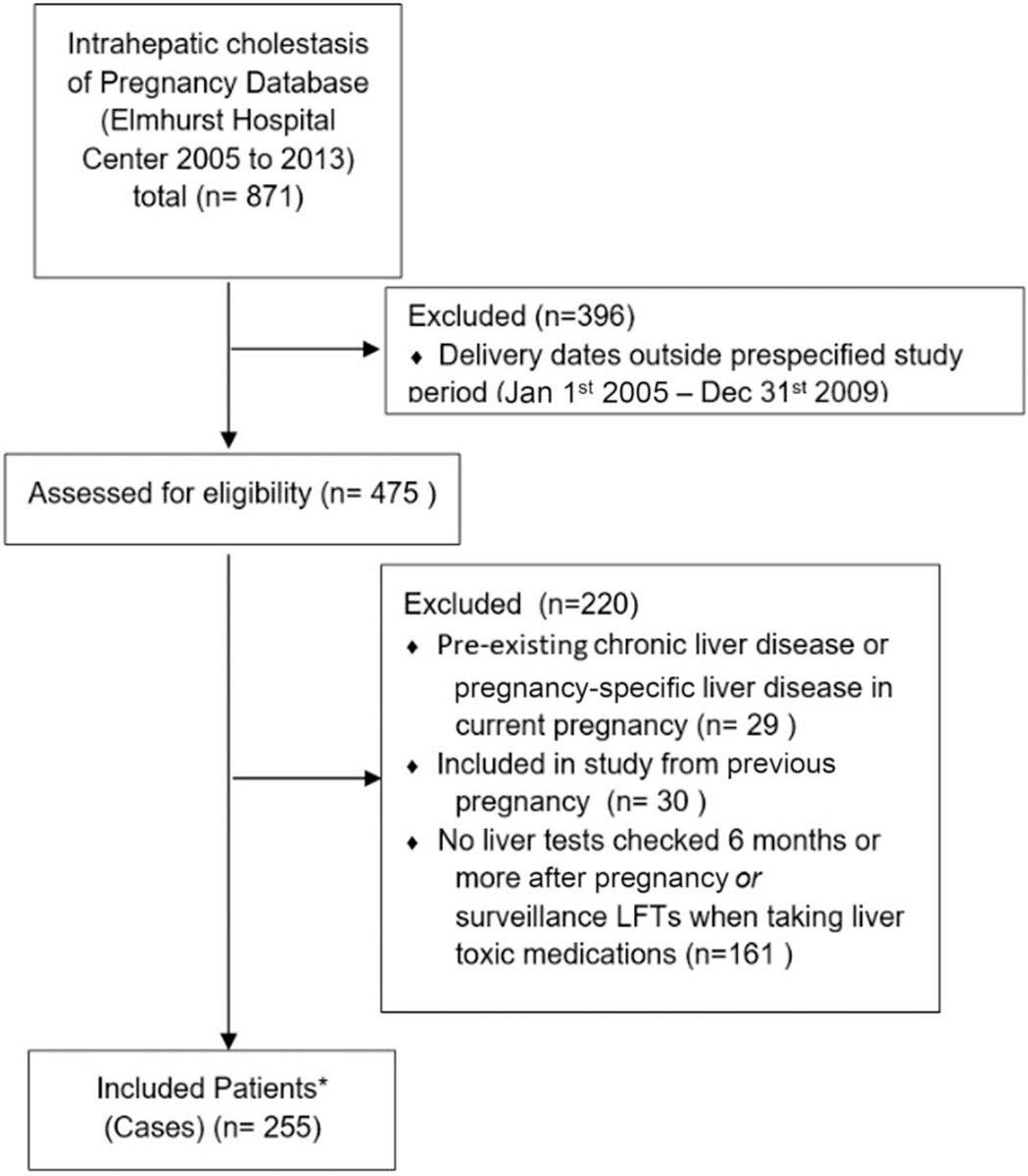
CONSORT flow diagram of the study participants. LFT, liver function test. *Controls were selected from delivery birth records from the year 2005–2009. Control patients were never suspected of having ICP or diagnosed with ICP and were age matched (+/− 1 year).
Table 1.
Baseline characteristics of ICP cases versus comparators
| Variable | ICP (n = 255) | Control (n = 131) | P |
|---|---|---|---|
| Demographics | |||
| Median age, yr (IQR) | 26 (23–31) | 28 (23–31) | 0.732 |
| Ethnicity | |||
| Hispanic, n (%) | 227 (89) | 104 (79) | 0.012 |
| Non-Hispanic, n (%) | 28(11) | 27 (21) | |
| Pregnancy history | |||
| Nulliparous, n (%) | 84 (32.9) | 35 (27) | 0.21 |
| Median parity (IQR) | 1 (0–2) | 1 (0–2) | 0.24 |
| Previous ICP, n (%) | 18(7.1) | 0(0) | <0.01 |
| ICP in subsequent pregnancy, n (%) | 85 (23.9) | 0(0) | <0.01 |
| ICP in previous and subsequent pregnancy, n (%) | 6 (2.4) | 0(0) | <0.01 |
| Multiple gestations in this pregnancy, n (%) | 5 (2.0) | 1 (0.76) | 0.26 |
| Metabolic risk factors | |||
| Median BMI, kg/m2 (IQR) | 25.8 (22.9–28.0) | 25.8 (23.1–28.9) | 0.16 |
| Preeclampsia, n (%) | 25 (9.8) | 3 (2.3) | <0.01 |
| Pregestational diabetes, n (%) | 1 (0.39) | 4(3) | 0.04 |
| Gestational diabetes, n (%) | 31 (12.2) | 23 (18) | 0.15 |
| BA levels | |||
| Highest median BA concentration (IQR) | 32.8 (20.5–66) | n/a | |
| BA >40 μmol/L, n (%) | 106 (42) | n/a | |
| BA >50 μmol/L, n (%) | 81 (32) | n/a | |
| BA >100 μmol/L, n (%) | 29 (11) | n/a | |
| Highest median ALT level(IQR) | 48 (27–92) | 18 (14–26) | <0.01 |
| Highest median AST level(IQR) | 40 (29–61) | 22 (18–28) | <0.01 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BA, bile acid; BMI, body mass index; ICP, intrahepatic cholestasis of pregnancy; IQR, interquartile range; n/a: not available in medical charts.
Risk of elevated ALT
A higher number of ICP patients had ALT >25 U/L at least once ≥6 months postpartum (75 vs 48%, P < 0.01), with a significantly higher median maximum ALT level (46.0 vs 25.5 U/L, P = 0.03) (Table 2). A significantly greater number of ICP patients also had ALT >50 U/L (twice the ULN) during the study period (48 vs 21%, P < 0.01).
Table 2.
Outcomes by group
| Variable | ICP (n = 255) | Control (n = 131) | P |
|---|---|---|---|
| ALT >25 U/L, n (%) | 191 (75) | 63 (48) | <0.01 |
| ALT >50 U/L, n (%) | 121 (48) | 27 (21) | <0.01 |
| ALP >140 U/L, n (%) | 125 (49) | 23 (18) | <0.01 |
| ALP >280 U/L, n (%) | 42 (16) | 1 (0.8) | <0.01 |
| Postpartum liver imaging, n (%) | 110 (43) | 40 (31) | 0.02 |
| Median ALT level (IQR) | 46 (25–104) | 25.5 (18–46) | 0.03 |
| Median AST level (IQR) | 34 (24–66) | 24 (20–36) | 0.03 |
| Median ALP level (IQR) | 134 (82–246) | 86 (66–118) | <0.01 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; ICP, intrahepatic cholestasis of pregnancy; IQR, interquartile range.
Time to ALT >25 U/L was significantly different between the ICP and non-ICP comparison group (P < 0.01) (Figure 2a). Among ICP and non-ICP women with ALT >25 U/L, median time to ALT >25 U/L was 3.8 and 9.2 years, respectively. Adjusting for metabolic risk factors (including ICP diagnosis in current pregnancy, ethnicity, diabetes status, BMI, and maternal age), ICP diagnosis and Hispanic ethnicity were associated with increased incidence of postpartum ALT >25 U/L (hazard ratio [HR] 1.9, P < 0.01 and HR 1.6, P = 0.03) (Table 3).
Figure 2.
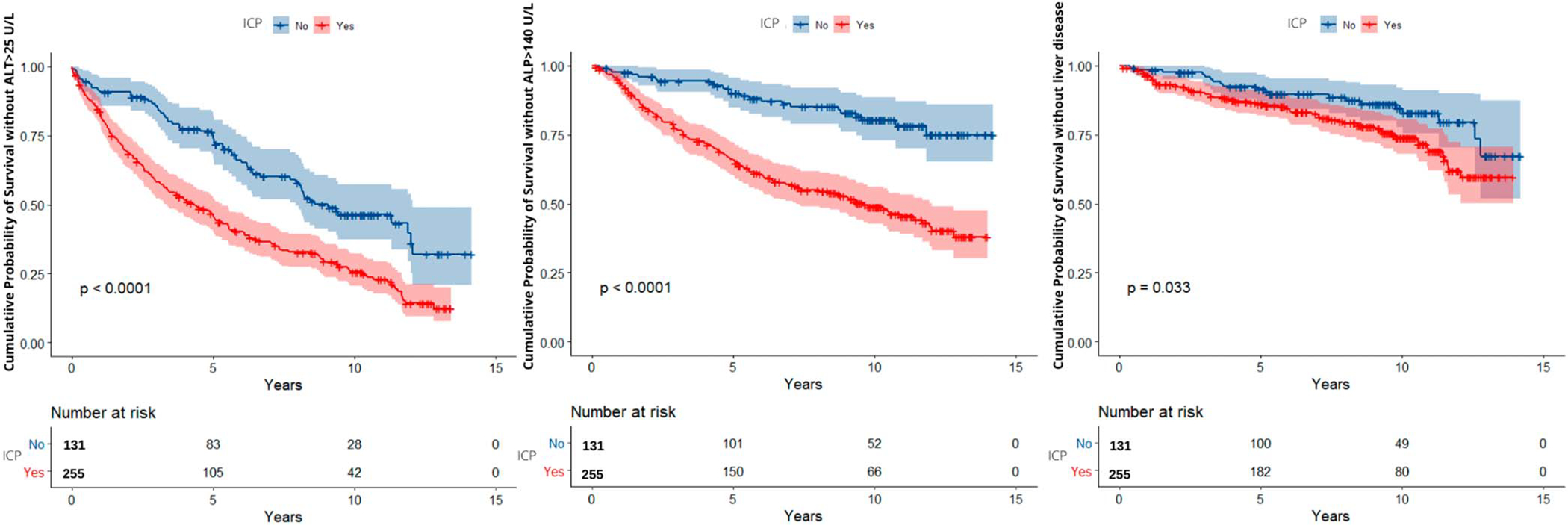
(a) Kaplan-Meier curve for alanine aminotransferase (ALT) level >25 U/L postpartum based on ICP diagnosis. (b) Kaplan-Meier curve for alkaline phosphatase (ALP) level >140 U/L postpartum based on ICP diagnosis. (c) Kaplan-Meier curve for liver disease postpartum based on ICP diagnosis. ICP, intrahepatic cholestasis of pregnancy.
Table 3.
Predictors of time to occurrence of liver disease and time to abnormal ALT for entire study cohort (ICP and control patients)
| Variables | Time to liver disease |
Time to ALT >25 U/L |
Time to ALP >140 U/L |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Hispanic | 2.0 | 0.80–4.8 | 0.14 | 1.6 | 1.1–2.5 | 0.03 | 2.5 | 1.3–5.0 | <0.01 |
| AMA (≥35 yr) | 1.8 | 0.99–3.2 | 0.05 | 1.2 | 0.82–1.8 | 0.36 | 0.71 | 0.40–1.3 | 0.26 |
| GDM diagnosis in current pregnancy | 1.2 | 0.62–2.2 | 0.62 | 1.2 | 0.86–1.8 | 0.25 | 0.98 | 0.58–1.7 | 0.93 |
| Overweight or higher (BMI >25 kg/m2) | 2.0 | 1.2–3.3 | <0.01 | 1.2 | 0.94–1.6 | 0.14 | 0.77 | 0.55–1.1 | 0.12 |
| ICP diagnosis in current pregnancy | 1.5 | 1.03–2.9 | 0.05 | 1.9 | 1.5–2.6 | <0.01 | 3.4 | 2.2–5.4 | <0.01 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, advanced maternal age; BMI, body mass index; CI, confidence interval; GDM, gestational diabetes; HR, hazard ratio; ICP, intrahepatic cholestasis of pregnancy.
Risk of elevated ALP
Highest median ALP in the ICP group was 134 U/L (IQR 82–246) and 86 U/L (IQR 66–118) in the non-ICP group. One hundred twenty-five (48%) ICP patients and 23 (18%) non-ICP patients had postpartum ALP >140 U/L P < 0.01 (Table 2). Forty-two (16%) ICP patients and 1 (0.8%) non-ICP patient had ALP levels >280 U/L (twice ULN; P < 0.0001) (Table 2).
Time to ALP >140 U/L was significantly different between ICP and non-ICP groups P < 0.01 (Figure 2b). Median time to highest ALP was 4.0 years and 9.7 years in ICP and non-ICP groups, respectively. Adjusting for metabolic risk factors (including ICP diagnosis in current pregnancy, ethnicity, diabetes status, BMI, and maternal age), ICP diagnosis and Hispanic ethnicity was associated with increased incidence of postpartum elevated ALP (HR 3.4, P < 0.01 and HR 2.5, P < 0.01) (Table 3).
ICP and risk of later liver disease diagnosis
Sixty (24%) ICP patients and 20 (15%) non-ICP women were diagnosed with liver disease, the most common disease diagnosis being NAFLD with ICP patients having more postpartum liver imaging (Tables 2 and 4). No patients with PBC were identified.
Table 4.
Liver disease as identified by chart review and liver imaging
| Condition | ICP (n = 255) | Control (n = 131) |
|---|---|---|
| Otherchronic nonalcoholic liver disease (specified in clinicaldocumentation or seen on liver imaging), n (%) | 52 (20) | 15(11) |
| Chronic liver disease unspecified, n (%) | 6 (2.4) | 5 (4.6) |
| Hepatitis C or other hepatitis, n (%) | 2 (0.7) | 0(0) |
| No liver condition specified in patient chart, n (%) | 195 (76) | 111 (85) |
| Totalwith liver disease diagnosed, n (%) | 60 (24) | 20 (15) |
Time to liver disease diagnosis was significantly different between ICP and non-ICP groups (P = 0.03) (Figure 2c). For ICP women with liver disease, median time to liver disease diagnosis was 13.1 years. Median time to liver disease for non-ICP subjects could not be estimated because of the relatively small number of controls achieving this outcome during the study period. Adjusting for metabolic factors listed above, ICP was associated with a 1.5 times increased incidence ofliver disease (P = 0.05) and BMI >25 kg/m2 was associated with 2.0 times increased incidence of liver disease (P = 0.006) (Table 3).
Predictors of future liver disease, abnormal ALT, and abnormal ALP in ICP cohort
In univariable analysis of predictors of liver disease, AMA (P = 0.02) and BMI >25 kg/m2 (P < 0.01) were sensitive in predicting future liver disease. ALT >50 U/L (P = 0.56) and ALT >25 U/L (P = 0.74) during pregnancy, Hispanic identity (P = 0.08), GDM (P = 0.55), and bile acid >50 μmol/L (P = 0.10) were not predictive of future liver disease. Predictors of ALT >25 U/L postpartum include Hispanic identity (P = 0.04) and ALT >50 U/L in pregnancy (P < 0.01). ALT >25 U/L during pregnancy (P = 0.11), AMA (P = 0.57), GDM (P = 0.41), BMI >25 kg/m2 (P = 0.53), and bile acid >50 μmol/L (P = 0.63) were not predictive of future ALT elevation. Hispanic identity (P = 0.02) and bile acid >50 μmol/L (P = 0.03) were predictive of future ALP elevation. ALT >50 U/L (P = 0.05), ALT >25 U/L (P = 0.21) in pregnancy, GDM (P = 0.74), AMA (P = 0.09), and BMI >25 kg/m2 (P = 0.82) were not predictive of future ALP elevation (Table 5).
Table 5.
Univariate predictors of time to occurrence of liver diseasea and time to abnormal ALT and ALP for women with ICP
| Covariates | Time to liver disease |
Time to ALT >25 U/L |
Time to ALP >140 U/L |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Hispanic | 1.2 | 0.85–14.2 | 0.08 | 1.7 | 1.0–2.9 | 0.04 | 2.4 | 1.1–5.2 | 0.02 |
| AMA (≥35 yr) | 2.2 | 1.5–4.1 | 0.02 | 1.1 | 0.73–1.8 | 0.57 | 0.6 | 0.28–1.1 | 0.09 |
| GDM | 1.3 | 0.60–2.6 | 0.55 | 0.82 | 0.51–1.3 | 0.41 | 0.74 | 0.40–1.4 | 0.74 |
| Overweightor higher (BMI >25 kg/m2) | 2.4 | 1.4–4.3 | <0.01 | 1.1 | 083–1.4 | 0.53 | 1.2 | 0.57–1.2 | 0.82 |
| Bile acid levels >50 μmol/L | 0.61 | 0.34–1.1 | 0.10 | 1.1 | 0.80–1.4 | 0.63 | 1.5 | 1.0–2.1 | 0.03 |
| ALT >50 U/L in pregnancy | 0.86 | 0.52–1.4 | 0.56 | 1.3 | 1.0–1.8 | 0.009 | 1.3 | 0.92–1.9 | 0.13 |
| ALT >25 U/L in pregnancy | 1.1 | 0.55–2.3 | 0.74 | 1.4 | 0.92–2.1 | 0.11 | 1.4 | 0.82–2.4 | 0.21 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, advanced maternal age; BMI, body mass index; CI, confidence interval; GDM, gestational diabetes; HR, hazard ratio; ICP, intrahepatic cholestasis of pregnancy.
Liver disease defined by chart review and imaging findings.
In multivariable analysis adjusting for metabolic factors above, BMI >25 kg/m2 in an ICP pregnancy was associated with increased incidence of liver disease diagnosis (HR 2.1, confidence interval [CI] 1.1–3.7, P = 0.02), ALT >50 U/L in pregnancy was associated with increased incidence of ALT >25 U/L postpartum (HR 1.5, CI 1.1–2.0, P = 0.01), and Hispanic identity was associated with increased incidence of ALP >140 U/L postpartum (HR 2.3, CI 1.1–5.0, P = 0.03) (Table 6).
Table 6.
Multivariate predictors of time to occurrence of liver diseasea and time to abnormal ALT and ALP for women with ICP
| Covariates | Time to liver disease |
Time to ALT >25 U/L |
Time to ALP >140 U/L |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Hispanic | 2.7 | 0.66–11.4 | 0.16 | 1.6 | 0.92–2.7 | 0.10 | 2.3 | 1.1–5.0 | 0.03 |
| AMA (≥35 yr) | 1.7 | 0.91–3.3 | 0.09 | 1.1 | 0.74–1.8 | 0.54 | 0.3 | 0.31–1.2 | 0.15 |
| GDM | 1.0 | 0.46–2.1 | 0.98 | 0.78 | 0.49–1.2 | 0.32 | 0.44 | 0.44–1.5 | 0.55 |
| Overweightor higher (BMI >25 kg/m2) | 2.1 | 1.1–3.7 | 0.02 | 1.0 | 0.75–1.3 | 0.98 | 0.54 | 0.54–1.1 | 0.15 |
| Bile acid levels >50 μmol/L | 0.61 | 0.33–1.1 | 0.12 | 0.95 | 0.69–1.3 | 0.77 | 1.3 | 0.91–1.9 | 0.15 |
| ALT >50 U/L in pregnancy | 1.3 | 0.74–2.2 | 0.38 | 1.5 | 1.1–2.1 | 0.01 | 1.4 | 0.94–2.1 | 0.10 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, advanced maternal age; BMI, body mass index; CI, confidence interval; GDM, gestational diabetes; HR, hazard ratio; ICP, intrahepatic cholestasis of pregnancy.
Liver disease defined by chart review and imaging findings.
DISCUSSION
To the best of our knowledge, this is the largest clinically based study in the United States evaluating risk of future liver dysfunction among ICP patients. Compared with women without ICP during pregnancy, ICP patients were at higher risk of future liver abnormalities, highlighting potential underlying liver disease not previously diagnosed, with more rapid development of these outcomes compared to women without ICP during pregnancy. The higher incidence of abnormal liver enzymes compared with liver disease diagnosis suggests possible underdiagnosis of future liver disease in ICP patients, especially, given that diagnosis of NAFLD—the most common liver disease diagnosis among ICP women—was incidentally diagnosed on imaging in 29/52 (55%) patients in our cohort with NAFLD. As such, many more women who may not have had radiologic imaging could have had NAFLD and/or other liver diseases. No patient in our cohort developed PBC, liver malignancy, or cirrhosis, although this related to our patient demographics and the relatively short period of our study (the longest follow-up period was 14 years, from 2005 to 2019). Our findings echo results in predominantly European cohort studies which suggest significant long-term health consequences in ICP patients and demonstrate ICP is not a condition limited to pregnancy (but rather associated with future consequences). A 2015 Swedish study of 125,000 pregnancies found a 2.5-fold increased risk of biliary tree cancer and 3.5-fold increased risk of liver cancer among ICP patients (10,13). When controlling for HCV, ICP patients remained at 2.5-fold increased risk of later liver malignancy (10,13). ICP patients have been reported to have 3–5-fold increased rates of subsequent hepatobiliary diseases, including gallstones and cirrhosis (19). ICP puts women at increased risk of immune-mediated diseases including thyroid disease (30% higher than controls), diabetes mellitus (47%), psoriasis (27%), and Crohn’s disease (55%), and at slightly higher risk of cardiovascular disease if they were also diagnosed with preeclampsia during pregnancy (10,13). Smaller Finnish studies (n = 571 and n = 575) found deaths from gastrointestinal diseases are overrepresented among ICP women and increased rates of later hepatobiliary disease, breast cancer, and hypothyroidism (14–16).
An increased susceptibility to ICP in women with certain genetic variants in biliary transporters and receptors has been demonstrated, some of which also play a role in the development of future liver disease. Gene variants associated with more severe forms of ICP include ABCB4, which encodes the multidrug resistance protein 3 (5,12,20–24). Deficiency in multidrug resistance protein 3 (a transporter involved in translocating phospholipids into bile) leads to biliary duct injury and cholestasis (25). Other variants include mutations in the ABCB11 gene, which encodes the major bile efflux system in hepatocytes—bile salt export pump (BSEP). In a European study, ABCB11 variants accounted for 1% of ICP cases and is believed to reduce the folding efficiency of BSEP (21). Nongenetic pregnancy-related factors including increased sex steroids likely impact expressivity of ICP in genetically susceptible individuals (23). Estrogen and progesterone metabolites, which peak in the third trimester of pregnancy, may inhibit BSEP, leading to increased maternal bile acid (26,27). Mutations in more than one of these genes have been associated with severe and early-onset ICP and have been linked to liver cancer, fibrosis, and cirrhosis (28–31). Although no genetic testing was available on our patient population, shared common mutations in ICP and liver disease may explain increased prevalence in liver abnormalities.
Our findings show a high prevalence of NAFLD in Hispanic patients (32–34). In addition to having the highest rates of metabolic syndrome and other risk factors for NAFLD, Hispanics have a genetic predisposition to NAFLD (32), in the patatin-like phospholipase domain containing 3 (PNPLA3)-I148M genetic variant (33,34). Given that Hispanics are known to be more susceptible to NAFLD and have increased prevalence of ICP, this study is important in taking a closer look at this unique population. It should be noted that since the case and non-ICP groups were balanced for metabolic risk factors (BMI and pre-GDM), the higher risk of NAFLD in ICP patients was not due to higher prevalence of preexisting metabolic conditions, although there was a higher percentage of Hispanics in the ICP group compared with controls.
Our study has limitations. Although a rare condition, this is a relatively small sample of ICP patients. However, ICP is typically undercaptured in larger administrative and claims-based databases, making an electronic health record–based approach the most feasible in identifying a cohort of ICP patients with adequate follow-up. In addition, misclassification may have occurred since outcomes were ascertained clinically. Clinicians typically order bile acid tests for patients with high clinical suspicion of ICP, and therefore, patients in our non-ICP comparison group may have had ICP but were not diagnosed. We only included women in our study who had follow-up and had LTs checked—thus, the prevalence of liver disease in both groups may be overestimated given selection bias, although this would have biased our comparison toward null. The relatively short duration of follow-up limited our ability to assess the longer term burden of liver disease. The lack of prepregnancy baseline laboratories for our cohort is another limitation. As is common in pregnancy-related research, most women did not have LTs checked before pregnancy (they only presented to care during pregnancy, as opposed to for preconception counseling). In addition, given that these women were asked about their alcohol use when they presented in pregnancy (potentially biasing their answers), alcohol use and therefore alcoholic liver disease is likely underdocumented. Moreover, given the high prevalence of NAFLD, we wished to explore the effects of postpregnancy weight gain/BMI on risk of future liver abnormalities. However, our data did not allow for utilization of postpartum BMI in estimation of development of liver abnormalities since postpartum BMI was not available for all patients. Finally, some women may have gone to other institutions for future care; hence, their follow-up dataare not fully captured since we only had access to EHC data. Despite these limitations, our study includes one of the largest cohorts of ICP patients in the United States because of the increased prevalence of ICP at the community hospital where this research was conducted and is among the first to directly assess future liver disease risk through detailed review.
Future directions should include further engagement of ICP patients for genetic and laboratory testing that may clarify the natural history of ICP. However, although we do not have genetic data, we have data on ethnicity and the well-established association of genetics liver disease and ICP in Hispanics which matches our overall findings.
Overall, this study demonstrates ICP puts women at risk of later liver abnormalities. Although this type of study does not prove causation of future liver disease, it highlights how ICP may unmask undiagnosed liver disease ormay be an important risk factor for later liver disease. Currently, there are no guidelines for postpartum follow-up care for patients with ICP. However, it is clear that patients with ICP should be monitored during the postpartum period to evaluate for long-term development of liver disease, and further research is needed to identify predictors of future liver abnormalities in this population, so that high-risk women can be linked to appropriate care.
Supplementary Material
Study Highlights.
WHAT IS KNOWN
Intrahepatic cholestasis of pregnancy (ICP) is the most common pregnancy-specific liver disease.
There are limited data regarding risk of future liver disease or liver abnormalities among women with ICP.
WHAT IS NEW HERE
ICP seems to be associated with future liver abnormalities.
ICP women developed liver disease earlier than women without ICP.
Footnotes
CONFLICTS OF INTEREST
Potential competing interests: None to report.
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/B826, http://links.lww.com/AJG/B827
REFERENCES
- 1.Tran TT, Ahn J, Reau NS. ACG clinical guideline: Liver disease and pregnancy. Am J Gastroenterol 2016;111(2):176–94; quiz 196. [DOI] [PubMed] [Google Scholar]
- 2.Menżyk T, Bator M, Derra A, et al. The role of metabolic disorders in the pathogenesis of intrahepatic cholestasis of pregnancy. Clin Exp Hepatol 2018;4(4):217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bull LN, Hu D, Shah S, et al. Intrahepatic cholestasis of pregnancy (ICP) in U.S. Latinas and Chileans: Clinical features, ancestry analysis, and admixture mapping. PLoS One 2015;10(6):e0131211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee RH, Goodwin TM, Greenspoon J, et al. The prevalence of intrahepatic cholestasis of pregnancy in a primarily Latina Los Angeles population. J Perinatol 2006;26(9):527–32. [DOI] [PubMed] [Google Scholar]
- 5.Geenes V, Chappell LC, Seed PT, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: A prospective population-based case-control study. Hepatology 2014;59(4):1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ovadia C, Williamson C. Intrahepatic cholestasis of pregnancy: Recent advances. Clin Dermatol 2016;34(3):327–34. [DOI] [PubMed] [Google Scholar]
- 7.Jie Z, Yiling D, Ling Y. Association of assisted reproductive technology with adverse pregnancy outcomes. Iran J Reprod Med 2015;13(3):169–80. [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez MC, Reyes H, Arrese M, et al. Intrahepatic cholestasis of pregnancy in twin pregnancies. J Hepatol 1989;9(1):84–90. [DOI] [PubMed] [Google Scholar]
- 9.Heinonen S, Kirkinen P. Pregnancy outcome with intrahepatic cholestasis. Obstet Gynecol 1999;94(2):189–93. [DOI] [PubMed] [Google Scholar]
- 10.Marschall HU, Wikström Shemer E, Ludvigsson JF, et al. Intrahepatic cholestasis of pregnancy and associated hepatobiliary disease: A population-based cohort study. Hepatology 2013;58(4):1385–91. [DOI] [PubMed] [Google Scholar]
- 11.Kohari KS, Carroll R, Capogna S, et al. Outcome after implementation of a modern management strategy for intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med 2017;30(11):1342–6. [DOI] [PubMed] [Google Scholar]
- 12.Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol 2014;124(1):120–33. [DOI] [PubMed] [Google Scholar]
- 13.Wikström Shemer EA, Stephansson O, Thuresson M, et al. Intrahepatic cholestasis of pregnancy and cancer, immune-mediated and cardiovascular diseases: A population-based cohort study. J Hepatol 2015; 63(2):456–61. [DOI] [PubMed] [Google Scholar]
- 14.Turunen K, Mölsä A, Helander K, et al. Health history after intrahepatic cholestasis of pregnancy. Acta Obstet Gynecol Scand 2012;91(6):679–85. [DOI] [PubMed] [Google Scholar]
- 15.Hämäläinen ST, Turunen K, Mattila KJ, et al. Intrahepatic cholestasis of pregnancy and comorbidity: A 44-year follow-up study. Acta Obstet Gynecol Scand 2012;98(12):1534–9. [DOI] [PubMed] [Google Scholar]
- 16.Hämäläinen ST, Turunen K, Mattila KJ, et al. Intrahepatic cholestasis of pregnancy and associated causes of death: A cohort study with follow-up of 27–46 years. BMC Womens Health 2018;18(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz M, Allen M, Rocha I. 2019 Community Health Needs Assessment. (https://www.nychealthandhospitals.org/elmhurst/community-health-needs-assessment-report/2019). Published online 2019. Accessed April 26, 2020.
- 18.Henry V. 2013 Community Health Needs Assessment and Implementation Strategy. (https://www.nychealthandhospitals.org/wp-content/uploads/2016/07/chna-elmhurst-2013.pdf). Published online 2019. Accessed October 9, 2020.
- 19.Ropponen A, Sund R, Riikonen S, et al. Intrahepatic cholestasis of pregnancy as an indicator of liver and biliary diseases: A population-based study. Hepatology 2006;43(4):723–8. [DOI] [PubMed] [Google Scholar]
- 20.Pauli-Magnus C, Lang T, Meier Y, et al. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics 2004;14(2):91–102. [DOI] [PubMed] [Google Scholar]
- 21.Dixon PH, van Mil SWC, Chambers J, et al. Contributionof variant alleles of ABCB11 to susceptibility to intrahepatic cholestasis of pregnancy. Gut 2009;58(4):537–44. [DOI] [PubMed] [Google Scholar]
- 22.Van Mil SWC, Milona A, Dixon PH, et al. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology 2007;133(2):507–16. [DOI] [PubMed] [Google Scholar]
- 23.Jacquemin E, Cresteil D, Manouvrier S, et al. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet 1999;353(9148):210–1. [DOI] [PubMed] [Google Scholar]
- 24.Wasmuth HE, Glantz A, Keppeler H, et al. Intrahepatic cholestasis of pregnancy: The severe form is associated with common variants of the hepatobiliary phospholipid transporter ABCB4 gene. Gut 2007;56(2): 265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polin RA, Abman SH, Rowitch DH, et al. Fetal and Neonatal Physiology. Elsevier, North York, ON, 2017. [Google Scholar]
- 26.Meier Y, Zodan T, Lang C, et al. Increased susceptibility for intrahepatic cholestasis of pregnancy and contraceptive-induced cholestasis in carriers of the 1331T>C polymorphism in the bile salt export pump. World J Gastroenterol 2008;14(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stieger B, Fattinger K, Madon J, et al. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (BSEP) of rat liver. Gastroenterology 2000;118(2):422–30. [DOI] [PubMed] [Google Scholar]
- 28.Keitel V, Vogt C, Häussinger D, et al. Combined mutations of canalicular transporter proteins cause severe intrahepatic cholestasis of pregnancy. Gastroenterology 2006;131(2):624–9. [DOI] [PubMed] [Google Scholar]
- 29.Zimmer V, Krawczyk M, Mahler M, et al. Severe hepatocellular dysfunction in obstetric cholestasis related to combined genetic variation in hepatobiliary transporters. Clin Exp Obstet Gynecol 2012;39(1):32–5. [PubMed] [Google Scholar]
- 30.Dröge C, Bonus M, Baumann U, et al. Sequencing of FIC1, BSEP and MDR3 in a large cohort of patients with cholestasis revealed a high number of different genetic variants. J Hepatol 2017;67(6):1253–64. [DOI] [PubMed] [Google Scholar]
- 31.Reichert MC, Lammert F. ABCB4 gene aberrations in human liver disease: An evolving spectrum. Semin Liver Dis 2018;38(4): 299–307. [DOI] [PubMed] [Google Scholar]
- 32.Heiss G, Snyder ML, Teng Y, et al. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: The Hispanic Community Health Study/Study of Latinos. Diabetes Care 2014; 37(8):2391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saab S, Manne V, Nieto J, et al. Nonalcoholic fatty liver disease in Latinos. Clin Gastroenterol Hepatol 2016;14(1):5–12; quiz e9–10. [DOI] [PubMed] [Google Scholar]
- 34.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011;53(6):1883–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


