Abstract
BACKGROUND
The exclusion of pregnant women from initial COVID-19 messenger RNA vaccine trials raised hesitancy regarding the benefits of vaccination for pregnant women, hence little is known about vaccines’ efficacy in this population.
OBJECTIVE
To determine the maternal-neonatal transplacental transfer of SARS-CoV-2 antibodies among vaccinated parturient women. A control group of COVID-19-recovered patients was included to compare the immunoglobulin G levels between vaccinated and recovered patients.
STUDY DESIGN
This is a prospective cohort study conducted in a single tertiary medical center in Israel between February and March 2021; parturient women vaccinated with the BNT162b2 messenger RNA vaccine during pregnancy were included and compared with COVID-19-recovered parturient women. SARS-CoV-2 immunoglobulin G antibodies were measured in maternal and cord sera, dried blood spot samples taken from newborns, and breast milk samples. The primary aim was to determine whether neonatal cord and dried blood spot samples were positive for SARS-CoV-2 antibodies and to evaluate the transfer ratio, defined as cord blood immunoglobulin G divided by maternal immunoglobulin G levels.
RESULTS
The study included 64 vaccinated parturient women and 11 parturient women who had COVID-19 during pregnancy. All maternal blood sera samples and 98.3% of the cord blood sera samples were positive for SARS-Cov-2 immunoglobulin G with median concentrations of 26.1 (interquartile range, 22.0–39.7) and 20.2 (interquartile range, 12.7–29.0), respectively. Similarly, 96.4% of neonatal blood spot samples and all breast milk samples were positive for SARS-CoV-2 immunoglobulin G with median concentrations of 11.0 (interquartile range, 7.2–12.8) and 4.9 (interquartile range, 3.8–6.0), respectively. There was a significant positive correlation between maternal serum levels of SARS-CoV-2 immunoglobulin G and cord blood (r=0.483; P=.0001), neonatal blood spot (r=0.515; P=.004), and breast milk levels (r=0.396; P=.005) of SARS-CoV-2 immunoglobulin G. The median placental transfer ratio of SARS-COV-2 immunoglobulin G was 0.77. Comparison of vaccinated and recovered COVID-19 patients revealed significantly higher SARS-CoV-2 immunoglobulin G levels in maternal serum and cord blood among vaccinated women (P<.0001).
CONCLUSION
Our study demonstrated the efficient transfer of SARS-CoV-2 immunoglobulin G across the placenta in women, vaccinated with the BNT162b2 messenger RNA vaccine during pregnancy, to their neonates, with a positive correlation between maternal serum and cord blood antibody concentrations. In addition to maternal protection against COVID-19, the vaccine may also provide neonatal humoral immunity.
Key words: antibodies transfer, COVID-19 vaccine, neonatal immunity, pregnancy, SARS-CoV-2 antibodies
AJOG MFM at a Glance.
Why was this study conducted?
Pregnant women were excluded from initial trials of SARS-CoV-2 vaccines, hence little is known about their efficacy in this population.
It is known that a maternal-neonatal transfer of antibodies occurs both transplacentally and via lactation.
We sought to determine the maternal-neonatal transfer of SARS-CoV-2 immunoglobulin (Ig) G antibodies among vaccinated parturient women.
Key findings
SARS-CoV-2 IgG antibodies were detected in cord blood, newborn dried blood spot, and breast milk samples.
Neonatal and breast milk antibody levels were positively correlated with maternal serum antibody levels. Higher levels of cord blood antibodies were detected in vaccinated women than in COVID-19-recovered women.
What does this add to what is known?
High rates of maternal-neonatal SARS-CoV-2 antibodies transfer occur in vaccinated pregnant women.
In addition to maternal protection against COVID-19, the SARS-CoV-2 vaccine may also provide neonatal humoral immunity.
Introduction
Accumulating evidence indicates that pregnant women are more likely to experience COVID-19 complications than nonpregnant women.1 The novel BNT162b2 messenger RNA (mRNA) vaccine against SARS-CoV-2 was shown to be effective both in a randomized trial and in a nationwide mass vaccination setting, yet pregnant and lactating women were excluded from all clinical trials.2 , 3 Vaccination recommendations during pregnancy differ greatly around the globe. In view of the risks associated with COVID-19 in pregnancy, which seem to outweigh the potential side effects of the BNT162b2 mRNA vaccine, the Israeli Ministry of Health has issued an official recommendation for vaccinating pregnant women.4, 5, 6, 7, 8
Transplacental passage of maternal-derived SARS-CoV-2 antibodies in seropositive women infected with COVID-19 has been described, showing that cord blood immunoglobulin G (IgG) antibody concentrations were directly associated with maternal antibody concentrations and with time elapsed from maternal infection to delivery.9, 10, 11, 12 In addition to the maternal protection against COVID-19 following vaccination, the potential benefit of transplacental and lactational passage of antibodies is of immense importance because it may provide infants with protection against COVID-19 at a phase in which their humoral response is still inefficient.13, 14, 15, 16, 17, 18, 19 Such findings may provide important additional insight for deciding whether to vaccinate pregnant women.
Our aim was to assess the transfer of maternal antibodies in infants born to women vaccinated with the BNT162b2 mRNA vaccine during pregnancy by measuring SARS-CoV-2 antibodies in maternal and cord sera, infant blood spot samples, and breast milk during postpartum hospitalization.
Materials and Methods
This was a prospective cohort study of parturient women vaccinated with the BNT162b2 mRNA vaccine during pregnancy and COVID-19-recovered parturient women admitted for delivery at a single tertiary medical center in February and March 2021. Inclusion criteria included pregnant women aged ≥18 years who had received 2 dosages of the BNT162b2 mRNA COVID-19 vaccine at least 14 days before delivery. COVID-19-recovered women with previous documented positive polymerase chain reaction (PCR) tests for SARS-CoV-2 served as a control group; patients were defined as recovered if they were asymptomatic for 3 days or more after 10 days had elapsed from the initial positive PCR test. Documentation of a positive PCR test during the acute phase of infection and a signed form by the Ministry of Health stating that the patients were defined as recovered was required. Eligible women were offered to participate on admittance to the delivery room or operating room in cases of elective cesarean delivery. Data regarding general medical history and pregnancy outcomes were collected.
Maternal blood samples were obtained on admittance, and cord blood samples were taken immediately postpartum. Serum samples were centrifuged at 4000 g for 4 minutes at room temperature. The samples were tested for SARS-CoV-2 receptor-binding domain (RBD) IgG using the access SARS-CoV-2 IgG assay (Beckman Coulter, CA) according to the manufacturer's instructions, and those with a sample/cutoff ratio of ≥1.1 were considered positive. Dried blood spot (DBS) samples were obtained from the infants on Guthrie cards 1 day after delivery. Breast milk samples were collected during postpartum hospitalization. DBS and breast milk samples were tested for SARS-CoV-2 IgG with an RBD enzyme-linked immunosorbent assay as previously demonstrated,20 , 21 with the following modifications: breast milk samples were diluted in the ratio 1:2 for IgG. For DBS samples, 4 mm punches were taken from a single Guthrie card and antibodies were extracted by incubation with 250 μL of 1% skim milk in phosphate-buffered saline with 0.05% Tween for 90 minutes. One hundred microliters of extracted material were taken for IgG testing and the transfer ratio was calculated as cord blood SARS-CoV-2 IgG divided by maternal IgG concentration. The transfer ratio, defined as median maternal serum IgG levels divided by cord blood median IgG levels, was calculated.
This study was approved by the Sheba Medical Center Institutional Review Board (approval number 8142-21).
Descriptive statistics were used to assess the demographic and clinical characteristics of the participants and are presented as mean ± standard deviation or median and interquartile ranges (IQR), as appropriate. SARS-CoV-2 antibody levels were analyzed, and the correlation between maternal concentrations and cord, neonatal, and breast milk concentrations was assessed.
Results
The study included 64 parturient women vaccinated with 2 doses of the BNT162b2 mRNA vaccine and 11 parturient women who had COVID-19 during pregnancy. The demographic and clinical characteristics are presented in Table 1 . The mean time interval between the second vaccination and delivery was 21.7 (±11.0) days for vaccinated women, and 92.5 (±75.8) days between a positive PCR test for SARS-CoV-2 infection and delivery in recovered COVID-19 patients (P<.0001). All maternal blood sera samples and 98.3% of the cord blood sera samples were positive for SARS-CoV-2 IgG with median concentrations of 26.1 (IQR, 22.0–39.7) and 20.2 (IQR, 12.7–29.0), respectively. Similarly, 96.4% of the neonatal blood spot samples (n=55) and all breast milk samples (n=30) were positive for SARS-CoV-2 IgG with median concentrations of 11.0 (IQR, 7.2–12.8) and 4.9 (IQR, 3.8–6.0), respectively (Table 2 ).
Table 1.
Cohort demographic characteristics of parturient women following BNT162b2 messenger RNA vaccine vs COVID-19 recovered women
| Characteristic | BNT162b2 mRNA vaccinated women (n=64) | COVID-19 recovered women (n=11) | P value | |
|---|---|---|---|---|
| Maternal age (y) | 33.8 (±5.8) | 32.7 (±5.7) | .56 | |
| BMI | 28.2 (±4.9) | 31.6 (±4.6) | .06 | |
| Type I diabetes mellitus | 1 (1.6) | 1 (9.1) | .27 | |
| Pregestational hypertension | 1 (1.6) | 0 | .99 | |
| Other medical conditions | Hypercoagulation | 3 (4.7) | 0 (0) | .99 |
| Autoimmune disease | 5 (7.8) | 2 (18.2) | .58 | |
| Smoking | 4 (6.3) | 1 (9.1) | .99 | |
| Medications | Levothyroxine or PTU | 3 (4.7) | 1 (9.1) | .47 |
| Aspirin and/or LMWH | 5 (7.8) | 0 (0) | .59 | |
| Prednisone | 3 (4.7) | 0 (0) | .99 | |
| Insulin | 1 (1.6) | 2 (18.2) | .054 | |
| Gestational diabetes mellitus | 4 (6.3) | 2 (18.2) | .21 | |
| Gestational hypertensive disease | 2 (3.1) | 0 (0) | .72 | |
| Gestational age at vaccination—second dosage or positive SARS-CoV-2 PCR (wk) | 33.5 (±3.2) | 27.2 (±11) | .07 | |
| Gestational age at delivery (wk) | 38.7 (±1.3) | 39.0 (±1.2) | .46 | |
| Vaginal delivery | 43 (64) | 8 (72.7) | .99 | |
| Newborn gender-male | 28 (43.9) | 6 (54.5) | .53 | |
| Neonatal birthweight (g) | 3187.2 (±484.3) | 3470.0 (±427.9) | .06 | |
| Second vaccine or positive SARS-CoV-2 PCR to sampling interval (d) | 21.7 (±11.0) | 92.5 (±75.8) | <.0001 | |
| Multiple gestation | 2 (3.1) | 0 (0) | .72 | |
Data are presented as number (percentage) or mean (±standard deviation), where appropriate.
Autoimmune disease comprised hypothyroidism and ulcerative colitis. Gestational hypertensive disease comprised pregnancy induced hypertension and preeclampsia toxemia.
BMI, body mass index; LMWH, low-molecular weight heparin; mRNA, messenger RNA; PCR, polymerase chain reaction; PTU, propylthiouracil.
Nir. Maternal-neonatal SARS-CoV-2 immunoglobulin G antibodies transfer following vaccination of pregnant women. Am J Obstet Gynecol MFM 2021.
Table 2.
Serology for COVID-19–specific antibodies
| Samples | BNT162b2 mRNA vaccinated women (n=64) | COVID-19 recovered women (n=11) | P value |
|---|---|---|---|
| Maternal serum IgG | 26.1 (22.0–39.7) | 2.6 (0.9–3.5) | <.0001 |
| Neonatal cord blood | 20.2 (12.7–29.0) | 3.27 (0.5–4.6) | <.0001 |
| DBSa | 11.0 (7.2–12.8) | N/A | |
| Breastmilk IgGb | 4.9 (3.8–6.0) | N/A |
Data are presented as median (interquartile range).
DBS, dried blood spot; IgG, immunoglobulin G; mRNA, messenger RNA; N/A, not applicable.
Neonatal DBS samples (n=55);
Breastmilk samples (n=30).
Nir. Maternal-neonatal SARS-CoV-2 immunoglobulin G antibodies transfer following vaccination of pregnant women. Am J Obstet Gynecol MFM 2021.
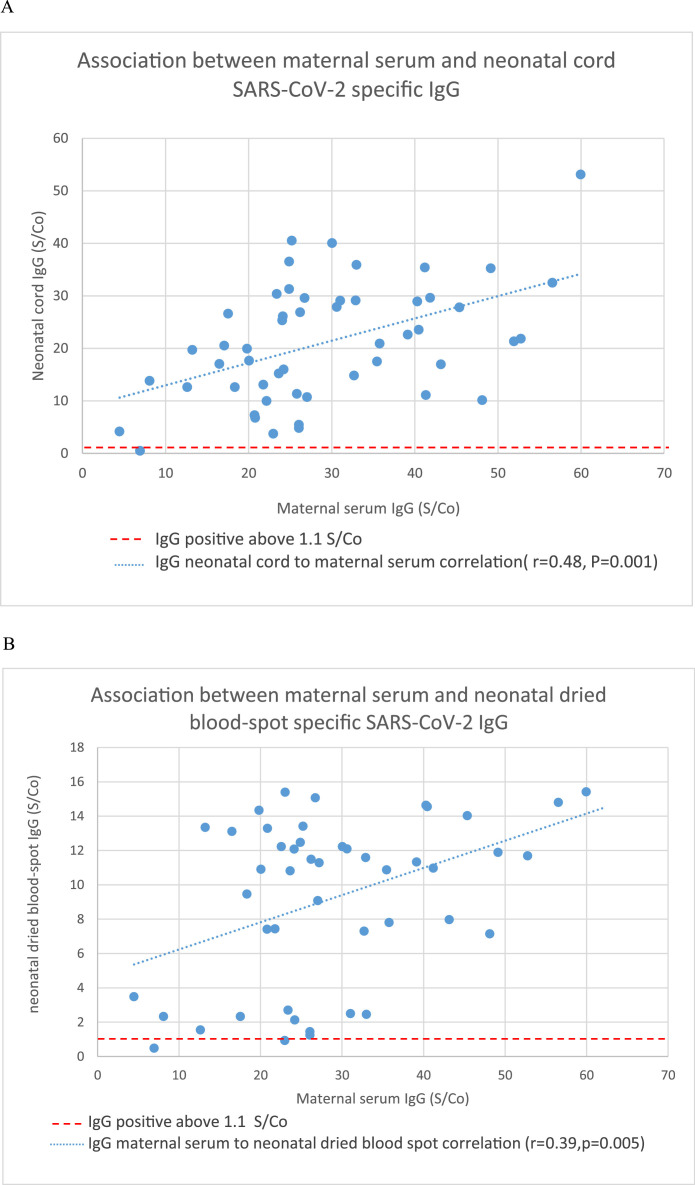
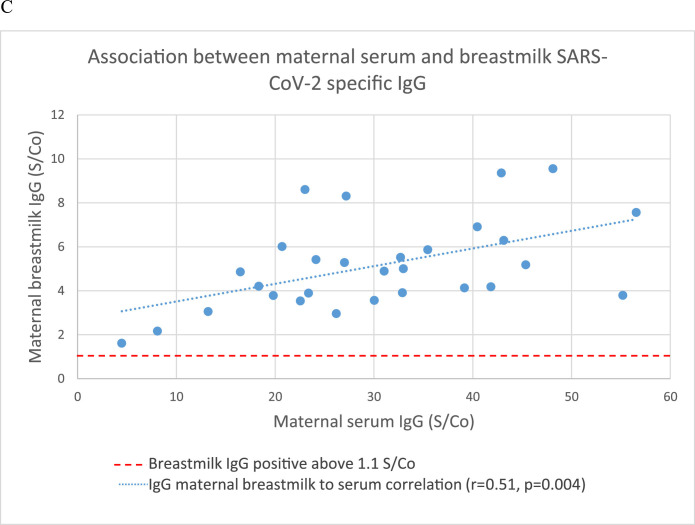
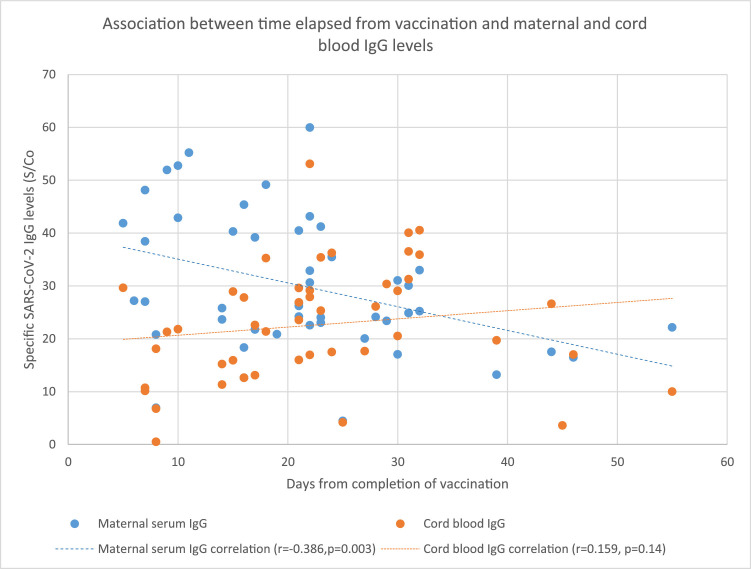
There was a significant positive correlation between maternal serum levels of SARS-CoV-2 IgG and cord blood (r=0.483; P=.0001), neonatal blood spot (r=0.515; P=.004), and breast milk levels (r=0.396; P=.005) of SARS-CoV-2 IgG (Figure 1 A–C). The median placental transfer ratio of SARS-CoV-2 IgG was 0.77. We observed a significant negative correlation between the time elapsed since the second dosage of the vaccine and maternal serum IgG levels (r=−0.386; P=.003), but the correlation with cord blood IgG levels was not as clear (r=0.159; P=.14) (Figure 2 ). Comparison between vaccinated and recovered COVID-19 patients revealed significantly higher SARS-CoV-2 IgG levels in maternal and cord blood samples in vaccinated women (P<.0001) (Table 2).
Figure 1.
Association between maternal and neonatal SARS-CoV-2 IgG
Association between maternal serum and (A) neonatal cord SARS-CoV-2 specific IgG; (B) neonatal dried blood spot specific SARS-CoV-2 IgG; (C) breastmilk SARS-CoV-2 specific IgG.
IgG, immunoglobulin G.
Nir. Maternal-neonatal SARS-CoV-2 immunoglobulin G antibodies transfer following vaccination of pregnant women. Am J Obstet Gynecol MFM 2021.
Figure 2.
Association between time elapsed from vaccination and maternal and cord blood IgG levels
IgG, immunoglobulin G.
Nir. Maternal-neonatal SARS-CoV-2 immunoglobulin G antibodies transfer following vaccination of pregnant women. Am J Obstet Gynecol MFM 2021.
Comment
Principal findings
Our study demonstrated the efficient transfer of SARS-CoV-2 IgG across the placenta in women vaccinated with the BNT162b2 mRNA vaccine during pregnancy to their neonates, with a positive correlation between the maternal serum and cord blood antibody concentrations. Neonatal blood spot and breast milk samples of vaccinated parturient women were also positive for SARS-CoV-2 IgG. Vaccine-induced maternal serum and cord blood antibody titers were higher than those found in recovered COVID-19 patients.
The presence of neonatal SARS-CoV-2 antibodies after maternal vaccination indicates that in addition to maternal protection against COVID-19, the BNT162b2 mRNA vaccine may also provide neonatal immunity while humoral response is still inefficient. Such findings may provide important additional insight for deciding whether to vaccinate pregnant women, especially given the increased maternal morbidity and mortality associated with COVID-19 in pregnancy.
Results in the context of what is known
In a previous study on parturient women who had COVID-19 during pregnancy, maternal SARS-CoV-2 IgG antibodies were transferred across the placenta in 87% of the patients. In addition, cord blood antibody levels were correlated with maternal antibody concentrations and the duration between onset of infection and delivery.9 In our study, cord and neonatal blood spot levels of antibodies were significantly higher in infants of vaccinated parturient women than in those of women recovered from COVID-19. In line with our results, a recent study by Gray et al22 also showed a greater response in vaccinated women than in recovered patients. Yet, it is unclear whether vaccination confers better neonatal immunity than maternal infection with SARS-CoV-2 during pregnancy. Of note, the time elapsed between disease or vaccination and delivery was significantly longer among the COVID-19-recovered patients, which could also explain the difference we found in cord and neonatal blood antibody levels between the 2 groups.
The optimal timing of maternal vaccination to achieve maximal protection of the newborn is still unknown. Mithal et al23 recently showed that the antibody transfer ratio seems to increase with latency from vaccination, suggesting that earlier vaccination may produce greater infant immunity. Studies on other vaccinations found that placental transfer ratios increased when the time between maternal infection and delivery was longer.24 Other vaccinations in pregnancy such as tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) immunization are given between 27 and 36 weeks of gestation according to the American College of Obstetricians and Gynecologists and the US Centers for Disease Control and Prevention recommendations.25 However, some studies have indicated that the Tdap vaccine might be more effective when administered during the second trimester of pregnancy.26 In the current study, all participants were vaccinated in the third trimester at a mean gestational age of 33.5 weeks, but no correlation between time from vaccination and cord blood antibody levels was demonstrated. However, a recent study including 20 parturient women who received the BNT162b2 mRNA COVID-19 vaccine found cord blood antibody concentrations to be correlated with time since vaccination.27 The placental transfer ratio reported in this study was 0.34, which is lower than the ratio of 0.77 found in our study. This difference may be attributed to the longer interval between vaccination and delivery in our cohort (21.7 vs 11 days). Because transplacental transfer begins at around 17 weeks of gestation, increasing exponentially as gestation advances, maternal vaccination in the early second trimester might be optimal for neonatal protection.28 Data derived from other vaccine studies indicate that transplacentally acquired antibodies usually decline by the second month of life, and the protective efficacy is expected to be reduced at the age of 6 to 12 months.
Based on our study, we cannot estimate the duration of the potential protection against COVID-19 among infants. However, we demonstrated SARS-CoV-2 IgG in breast milk samples of lactating women after delivery, which may further enhance neonatal immunity. Similarly, Gray et al showed the presence of vaccine-generated antibodies in breast milk samples of 31 lactating women who received the COVID-19 mRNA vaccine.22 The role and extent to which antibodies transferred through breast milk can protect breastfed infants is still unresolved.
Clinical and research implications
The results of this study show that in addition to maternal protection against COVID-19 during pregnancy, the novel BNT162b2 mRNA vaccine may also potentially provide protection to newborns during the sensitive period in which their humoral protection is ineffective. Further research is needed to reinforce public health policy regarding vaccination during pregnancy. Despite the reassuring findings of the current study, further research is also needed to determine several unanswered questions. First, for how long does the potential humoral protection from COVID-19 lasts, and how clinically effective is this protection during infancy. Second, the optimal timing of vaccination during pregnancy with regard to neonatal protection remains unresolved, and additional large-scale research is required.
Strengths and limitations
This is a large study assessing maternal-neonatal transfer of antibodies following COVID-19 vaccination of pregnant women. The strengths of our study include: the prospective assessment of several different markers of maternal-neonatal transfer of antibodies in a short period of time; sample collection in close temporal proximity to the date of vaccination; and the inclusion of a relatively large cohort of vaccinated parturient women, an important group which was not previously included in clinical trials evaluating the effectiveness of the BNT162b2 mRNA vaccine. Moreover, a comparison group of COVID-19-recovered parturient women was also included using the same methods. This study has several limitations: first, lack of long-term follow-up and serial sample collection. Second, because all women were vaccinated during a short window of the third trimester (33.5±3 weeks), we cannot determine the optimal time for vaccination. Given that the vaccine was a new treatment when this study was conducted, women vaccinated during the first or second trimester were not included because they did not deliver by the time when the study was completed. Furthermore, this study did not prove the clinical efficacy of maternal vaccination in protecting infants from COVID-19. Neither the degree of neonatal protection against SARS-CoV-2 nor the duration of such potential protection can be assessed from this study alone, and further large-scale, long-term research is required to elucidate this issue.
Conclusions
We demonstrated that parturient women fully vaccinated with 2 dosages of the BNT162b2 mRNA vaccine before delivery transfer SARS-CoV-2 IgG antibodies to their infants, with evidence of antibodies in cord blood, neonatal blood spot samples, and breast milk samples. These data show an additional benefit of the novel BNT162b2 mRNA vaccine in potentially providing protection to newborns during the sensitive period in which their humoral protection is ineffective. Further research is needed to determine the optimal time for vaccination during pregnancy with regard to newborn immunity, and to establish whether transplacentally transferred SARS-CoV-2 antibodies provide clinically effective and long-lasting infant protection.
Footnotes
The authors report no conflict of interest.
This study was funded by the Ferring COVID-19 Investigational Grant.
Cite this article as: Nir O, Schwartz A, Toussia-Cohen S, et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am J Obstet Gynecol MFM 2021;XX:x.ex–x.ex.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ajogmf.2021.100492.
Appendix. Supplementary materials
References
- 1.Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zipursky JS, Greenberg RA, Maxwell C, Bogler T. Pregnancy, breastfeeding and the SARS-CoV-2 vaccine: an ethics-based framework for shared decision-making. CMAJ. 2021;193:E312–E314. doi: 10.1503/cmaj.202833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists. COVID-19 vaccination considerations for obstetrics-gynecologic care. 2020. Available at:https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19. Accessed October 1, 2021.
- 6.Royal College of Obstetricians and Gynaecologists. COVID-19 vaccines, pregnancy and breastfeeding. 2021. Available at:https://www.rcog.org.uk/en/guidelines-research-services/coronavirus-covid-19-pregnancy-and-womens-health/covid-19-vaccines-and-pregnancy/covid-19-vaccines-pregnancy-and-breastfeeding/. Accessed August 20, 2021.
- 7.Israel Society of Obstetrics and Gynecology. COVID 19 Vaccination. 2020. Available at:https://govextra.gov.il/media/30093/pregnancy-covid19-vaccine.pdf. Accessed December 20, 2020.
- 8.Poliquin V, Castillo E, Boucoiran I, et al. SOGC Statement on COVID-19 vaccination in pregnancy. 2021. Available at:https://sogc.org/common/Uploaded%20files/Latest%20News/SOGC_Statement_COVID-19_Vaccination_in_Pregnancy.pdf. Accessed May 25, 2021.
- 9.Flannery DD, Gouma S, Dhudasia MB, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021;175:594–600. doi: 10.1001/jamapediatrics.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van de Perre P. Transfer of antibody via mother's milk. Vaccine. 2003;21:3374–3376. doi: 10.1016/s0264-410x(03)00336-0. [DOI] [PubMed] [Google Scholar]
- 14.Post AL, Li SH, Berry M, et al. Efficiency of placental transfer of vaccine-elicited antibodies relative to prenatal Tdap vaccination status. Vaccine. 2020;38:4869–4876. doi: 10.1016/j.vaccine.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz FM, Patel SM, Jackson LA, et al. Safety and immunogenicity of three seasonal inactivated influenza vaccines among pregnant women and antibody persistence in their infants. Vaccine. 2020;38:5355–5363. doi: 10.1016/j.vaccine.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fouda GG, Martinez DR, Swamy GK, Permar SR. The Impact of IgG transplacental transfer on early life immunity. Immunohorizons. 2018;2:14–25. doi: 10.4049/immunohorizons.1700057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kachikis A, Englund JA. Maternal immunization: optimizing protection for the mother and infant. J Infect. 2016;72(Suppl):S83–S90. doi: 10.1016/j.jinf.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Hanson LA. Breastfeeding provides passive and likely long-lasting active immunity. Ann Allergy Asthma Immunol. 1998;81:523–533. doi: 10.1016/S1081-1206(10)62704-4. [DOI] [PubMed] [Google Scholar]
- 19.Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3:442–474. doi: 10.3390/nu3040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Indenbaum V, Koren R, Katz-Likvornik S, et al. Testing IgG antibodies against the RBD of SARS-CoV-2 is sufficient and necessary for COVID-19 diagnosis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oved K, Olmer L, Shemer-Avni Y, et al. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225 doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mithal LB, Otero S, Shanes ED, Goldstein JA, Miller ES. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obstet Gynecol. 2021;225:192–194. doi: 10.1016/j.ajog.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426–439. doi: 10.1056/NEJMoa1908380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Committee Opinion No. 718: update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:e153–e157. doi: 10.1097/AOG.0000000000002301. [DOI] [PubMed] [Google Scholar]
- 26.Eberhardt CS, Blanchard-Rohner G, Lemaître B, et al. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis. 2016;62:829–836. doi: 10.1093/cid/ciw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rottenstreich A, Zarbiv G, Oiknine-Djian E, Zigron R, Wolf DG, Porat S. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab266. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz FM. Can we protect pregnant women and young infants from COVID-19 through maternal immunization? JAMA Pediatr. 2021;175:561–562. doi: 10.1001/jamapediatrics.2021.0043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.