Abstract
Background
A potentially important aspect of the humoral immune response to Covid-19 is avidity, the overall binding strength between antibody and antigen. As low avidity is associated with a risk of re- infection in several viral infections, avidity might be of value to predict risk for reinfection with covid-19.
Objectives
The purpose of this study was to describe the maturation of IgG avidity and the antibody-levels over time in patients with PCR-confirmed non-severe covid-19.
Study design
Prospective longitudinal cohort study including patients with RT-PCR confirmed covid-19. Blood samples were drawn 1, 3 and 6 months after infection. Antibody levels and IgG-avidity were analysed.
Results
The majority had detectable s- and n-antibodies (88,1%, 89,1%, N = 75). The level of total n-antibodies significantly increased from 1 to 3 months (median value 28,3 vs 39,3 s/co, p<0.001) and significantly decreased from 3 to 6 months (median value 39,3 vs 17,1 s/co, p<0.001). A significant decrease in the IgG anti-spike levels (median value 37,6, 24,1 and 18,2 RU/ml, p<0.001) as well as a significant increase in the IgG-avidity index (median values 51,6, 66,0 and 71,0%, p<0.001) were seen from 1 to 3 to 6 months.
Conclusion
We found a significant ongoing increase in avidity maturation after Covid-19 whilst the levels of antibodies were declining, suggesting a possible aspect of long-term immunity.
Keywords: Covid-19, Avidity, Antibody, SARS-CoV-2, Anti-spike, Anti-nucleocapsid
1. Background
In March 2020, WHO declared Covid-19 to be pandemic. Since then, at least 179 million people have been affected and over 3,8 million people have died [1]. Treatment options are limited to supportive care, including high doses of oxygen, although corticosteroids and tocilizumab have been shown to have some effect [2,3]. Health care systems have been overrun with Covid-19 cases, health-care workers have had a high risk of infection and lockdown measures have had significant socioeconomic effects [4], [5], [6]. Recently, worldwide vaccination programs have commenced in order to control the spread of the virus. All aspects of immunity, both following Covid-19 infections and/or vaccination, are of great interest for the future management of this pandemic.
A clinical infection with SARS-CoV-2 often presents with mild symptoms such as fever, fatigue and dry cough with the majority undergoing a non-severe illness [7], [8], [9]. SARS-CoV-2 infection usually stimulates the humoral immune system to produce antibodies against spike-glycoprotein (S) and nucleocapsid protein (N), most often within three weeks after infection [10]. The humoral response can be assessed not only by the quantity, persistence and the neutralizing capacity of the antibodies but also by the avidity of the antibodies. Avidity is a measure of the overall strength of the binding between antibody and antigen; the functional affinity [11]. Typically, the avidity is low during the initial response to a viral infection but increases over time [12,13]. For some viral infections, for example cytomegalovirus, avidity measurement can be utilised to distinguish between current or past infection and generation of high avidity IgG is required to develop long-lasting immunity [11,14,15].
In several types of viral infection, low avidity of IgG antibodies is associated with increased risk of repeated infection [16,17]. Eidge et al. have shown that declining levels of antibodies against seasonal coronavirus are associated with a high probability of a repeated infection after 12 months [18]. For that reason, avidity, in addition to antibody levels, may be of value to predict immunity and hence risk of reinfection with covid-19, both after infection and after vaccination. Studies have also shown high degree of variability in the kinetics of IgM- and IgG-antibodies in Covid-19 [10,19]. Therefore, testing for avidity may also have a role to differentiate between acute or past Covid-19 in some patients [20].
To date, only a few studies of adequate size have reported avidity in context of Covid-19 [21,22], with most of the available research only including smaller cohorts with no or few serial patient samples [23], [24], [25], [26], [27].
2. Objectives
In this study, we explore the maturation of IgG avidity and the antibody-levels over time in patients with PCR-confirmed non-severe covid-19.
3. Study design
3.1. Participants
The study is a prospective longitudinal study conducted in a regional hospital (Hallands Hospital) in Sweden. Patients with a positive Covid-19 reverse transcription polymerase chain reaction (RT-PCR) test during late June - August 2020 were identified by the laboratory notification system or by the regional surveillance system and invited to join the study within one day after the positive PCR. In addition to a positive PCR-test inclusion criteria were being more than 15 years old, being resident in the county of Halland (one of 21 regions in Sweden), not being hospitalized by the time of diagnosis and having an available mobile phone number. Invitations to the study, as well as all further contact with the participants, were communicated by mobile phone text messages, using a digital system approved by the regional health-care authorities for health-care-information (Entermedic, Entergate AB). Exclusion criteria were lack of consent to participate and absence of serial sampling (less than two samples). The study was approved by the Swedish Ethical Review Authority, approval number Drn: 2020–02,691 and 2021–00,355.
3.2. Procedures
After inclusion, a self-report questionnaire about medical history, medications and experienced symptoms was sent by mobile phone text messages. Thereafter, all participants received self-report questionnaires on experienced symptoms once per week.
Blood samples were drawn at one, three and six months after diagnosis. Samples were stored in a biobank at −20°.
3.3. Analysis
3.3.1. RT-PCR
Samples from nasopharynx were analysed with an in-house RT-PCR according to Corman et al., with VIASURE SARS-CoV-2 S gene Real Time Detection Kit (CerTest Biotec) on BD Max™ System or with Xpert Xpress SARS-CoV-2 on Cepheid's GeneXpert® according to manufacturer´s instructions [28].
3.3.2. SARS-CoV-2 antibody detection
SARS-CoV-2 antibodies against N-protein (total Ig) were measured using Elecsys Anti-SARS-CoV-2 on Roche Cobas e801 (Roche Diagnostics). Results are reported as cut-off index (signal sample/cut-off, s/co) with values >1 considered as positive. Serum samples from 2016 (n = 100) were used as negative controls.
SARS-CoV-2 antibodies against S-protein (IgG) were measured using Anti-SARS-CoV-2 QuantiVac ELISA (Euroimmun) manually. Results are reported in RU/ml and according to manufacturer ≥11 RU/ml is considered as positive, ≥8 - <11 borderline and <8 is considered negative. For the purpose of this study, we considered results >8 RU/ml as cut-off for detectable antibodies. Serum samples from 2016 (n = 53) were used as negative controls.
3.3.3. Avidity ELISA
Samples with detectable spike-antibodies were analysed using Anti-SARS-CoV-2 QuantiVac ELISA (IgG) (Euroimmun) according to the manufacturer`s instructions but integrating a soaking step in urea containing wash buffert. Samples were added in duplicate to the antigen coated wells. After the first incubation step, 300 µl 4 M urea containing wash buffert was added to one well and wash buffert without urea to the other well serving as reference. After 10 min, wash steps as well as the rest of the procedure was carried out according to the manufacturer´s instruction. The avidity index was described as ODurea/ODreference and multiplied by 100 to be expressed as percentage. Specimens generating IgG-values above the linear range of the assay were diluted and reanalysed. The concentration of 4 M urea was chosen after initial optimization experiments with 4 pairs of samples (collected 2 weeks and 4 weeks after onset of symptoms) and used throughout the study.
From each assay run in the study, two samples were reanalysed, serving as a positive control. These samples showed a mean deviation from one experiment to the other of 6,6%.
3.4. Statistics
All analyses were performed in SPSS statistic 27. Wilcoxon Signed Rank test was used to analyze differences over time and Mann-Whitney were used to analyze differences between groups. p<0.05 was considered significant. For descriptive analysis median values were used.
4. Results
During the time for inclusion, approximately 800 cases of PCR-confirmed Covid-19 were registered in the county of Halland and invitations to the study were sent to 203 individuals. In total, 75 participants were included. For cohort characteristics see table 1 .
Table 1.
Cohort characteristics.
| Table 1.Cohort characteristics | n (%) |
|---|---|
| Age, median (range) | 50 (19–76) |
| Men (mean age 49, median age 54) |
26 (35%) |
| Women (mean age 46, median age 48) |
49 (65%) |
| Healthcare workers | 17 (23%) |
| Long term symptoms (> 2months) | 12 (16%) |
| Comorbidities: | 21 (28%) |
| Obesitas (BMI >30) | 12 (16%) |
| Hypertension | 8 (11%) |
| Heart disease | 1 (1%) |
| Lung disease (COPD or Asthma) | 3 (4%) |
| Gastrointestinal disease | 2 (3%) |
| Reumatic disorder | 3 (4%) |
| Immunosuppressive treatment | 1 (1%) |
| Psychiatric disorder | 3 (4%) |
| Neurological disease | 1 (1%) |
| Chronic headache/migraine | 5 (7%) |
| Chronic pain | 3 (4%) |
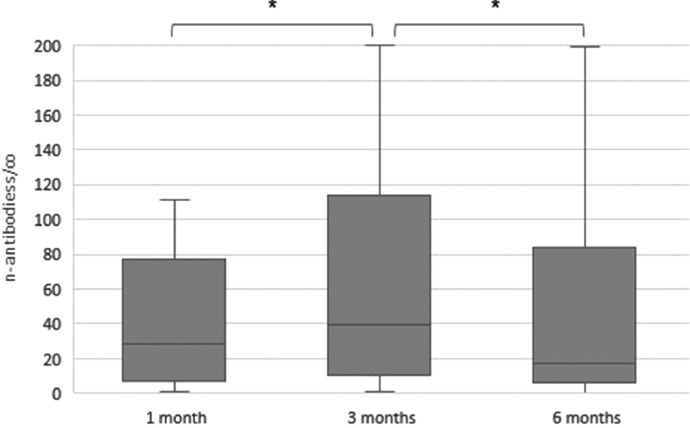
Antibodies against n-protein were detected in 85,7%, 89,1% and 88,7% at 1, 3 and 6 months, respectively (n = 60/70, 66/74 and 63/71, respectively). The level of n-antibodies significantly increased from 1 to 3 months (median value 28,3 vs 39,3 s/co, p<0.001) and significantly decreased from 3 to 6 months (median value 39,3 vs 17,1 s/co, p<0.001), see Fig. 1 . No significant differences in antibody levels were seen between health care workers vs non-health care workers (p>0.05) nor between individuals with long-term symptoms of covid-19 and those without long-term symptoms (p>0.05). Significant differences were seen between men and women at 3 and 6 months (median value at 3 months 99,0 vs 23,1 s/co, p = 0.025, at 6 months 55,3 vs 11,4 s/co, p = 0.018), see table 2 . 10/70 (14%) were seronegative after 1 month and one patient had a seroconversion at 3 months with the rest remaining negative.
Fig. 1.
Levels of n-antibodies (s/co), time after positive PCR. *p<0.001.
Table 2.
Median value (range) of antibody levels and avidity index, time after positive PCR. When not listed p= >0.05.
| Table 2. Median value (range) of antibody levels and avidity index, time after positive PCR | 1 month | 3 months | 6 months |
|---|---|---|---|
| Nucleocapsid antibodies (s/co) | n = 61 | n = 66 | n = 64 |
| Men | 41,9 (1,5–106,0) | 99,0 (2,7–200,0) * | 55,3 (1,4–199,0) * |
| Women | 21,1 (0,9–111,0) | 23,1 (2,2–167) | 11,4 (0,5–195) |
| Health care workers | 18,6 (0,9–97,6) | 27,2 (2,2–167) | 9,5 (1,1–182) |
| Non-health care workers | 33,3 (1,5–111,0) | 54,1 (1,4–200,0) | 26,1 (0,5–199) |
| Longterm symptoms | 15,3 (3,3–45,3) | 22,8 (4,2–118,0) | 7,4 (1,2–117,0) |
| Non-longterm symptoms | 37,4 (0,9–111,0) | 55,2 (1,4–200,0) | 30,9 (0,5–199,0) |
| *p = 0.025 | *p = 0.018 | ||
| Spike antibodies (RU/ml) | n = 52 | n = 52 | n = 49 |
| Men | 67,0 (9,7–449,7) | 31,7 (5,7–244,5) | 14,7 (2,2–129) |
| Women | 30,5 (11,2–216,6) | 21,3 (4,4–138,3) | 18,7 (3,1–119,5) |
| Health care workers | 41,7 (9,7–185,4) | 21,7 (5,7–94,2) | 24,9 (3,7–73,6) |
| Non-health care workers | 37,6 (11,2–449,7) | 26,2 (4,4–244,5) | 14,8 (2,2–129,0) |
| Longterm symptoms | 18,1 (11,2–449,7) | 11,0 (6,8–244,5) | 9,2 (3,1–129,0) |
| Non-longterm symptoms | 48,8 (9,7–392,3) | 29,3 (4,4–141,8) | 18,7 (2,2–119,5) |
| Avidity index of spike antibodies (%) | n = 52 | n = 52 | n = 49 |
| Men | 50,6 (35,9–67,1) | 61,4 (40,6–73,3) * | 67,5 (43,0–84,8) |
| Women | 53,9 (28,3–74,3) | 66,7 (41,4–88,7) | 74,8 (38,0–94,0) |
| Health care workers | 50,6 (42,6–74,3) | 64,5 (45,2–88,7) | 77,7 (46,2–94,0) |
| Non-health care workers | 52,2 (28,3–69,0) | 66,0 (40,6–81,8) | 70,2 (38,0–94,0) |
| Longterm symptoms | 54,9 (50,1–62,3) | 66,2 (49,2–73,6) | 74,1 (66,6–82,1) |
| Non-longterm symptoms | 50,5 (28,3–74,3) | 64,1 (40,6–88,7) | 71,0 (38,0–94,0) |
| *p = 0.015 |
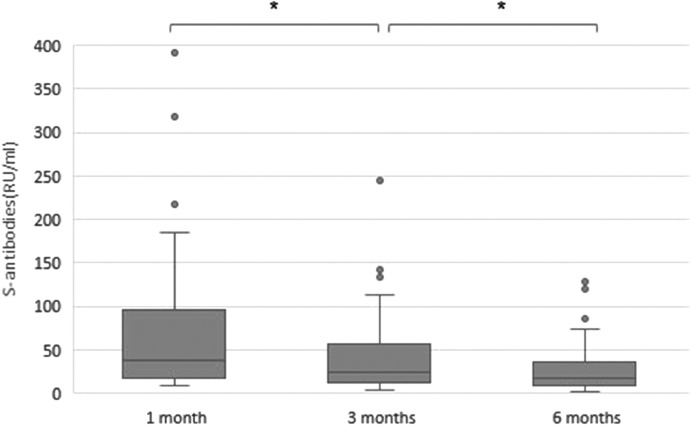
Antibodies against s-protein were detected in 88,1% (n = 52/59) at 1 month after diagnosis and a significant decrease in the anti-spike levels were seen from 1 to 3 to 6 months (median value 37,6, 24,1 and 18,2 RU/ml respectively, p<0.001), see Fig. 2 . No differences between men and women (p>0.05), health care workers or non-health care workers (p>0.05) or between those with duration of symptoms more or less than 2 months (p>0.05) were seen, see table 2.
Fig. 2.
Levels of s-antibodies (RU/ml), time after positive PCR. *p<0.001.
Median value for negative controls in Elecsys Anti-SARS-CoV-2 (Roche e801 Cobas) was 0,078 s/co (range 0,071–2,80) and for Anti-SARS-CoV-2 QuantiVac ELISA (Euroimmun) median value was 1,05 RU/ml (range 0,00–4,64).
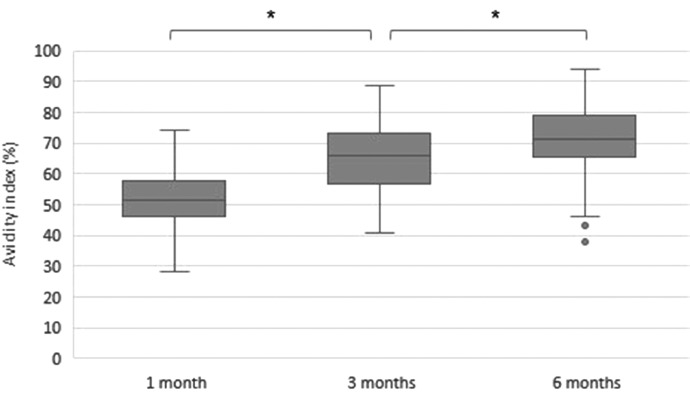
Avidity significantly increased from 1 to 3 to 6 months (median values 51,6, 66,0 and 71,0% p<0.001), see Fig. 3 . No differences between health care workers or non-health care workers (p>0.05) or between those with duration of symptoms more or less than 2 months (p>0.05) were seen. Between men and women there was a significant difference at 3 months (median value 61,4 vs 66,7% p = 0,015) but no significant differences were seen at 1 and 6 months, see table 2. The level of avidity and s-antibodies did not correlate (p>0.05).
Fig. 3.
Avidity index (%) for IgG spike-antibodies, time after positive PCR. *p<0.001.
5. Discussion
To establish long-lasting immunity and to avoid reinfections, memory B cells must generate high avidity IgG antibodies [11,16,29]. In seasonal corona-virus infection, a decline in antibody concentration as well as low avidity is seen and it is believed that this non-sustained immune response causes the risk of reinfection already after 9–12 months [18,30]. In the light of this, determining the dynamics and levels of avidity for antibodies against SARS-CoV-2 are of importance to understand how immunity can be achieved.
Our main finding was that avidity maturation is an ongoing process with significant increase of avidity more than 3 months after infection when the levels of antibodies are declining. A few other studies have also shown increasing avidity after infection but are limited by shorter time of follow-up, small cohorts or having participants without a PCR-confirmed Covid-19 infection [23,[31], [32], [33]]. Benner et al. also showed that increasing levels of avidity correlates with higher titres of neutralizing antibodies [23]. This suggest that avidity, or the absence of avidity maturation, might be of clinical value to indicate long-term immunity and to identify patients that will have higher risk of reinfection.
We showed a considerable variation in the avidity levels between patients. Why the development of high avidity IgG antibodies varies among patients remains to be understood. We could not find any differences in maturation of the avidity in relation to long-term symptoms (>2 months), between health care workers and non-health care workers or in relation to the levels of s-antibodies. Other studies have not reported sex differences in avidity [31,33]. We found a significant difference between men and women, but only at 3 months. Since the actual difference in avidity index is less than 10% and not found after 6 months, it will most likely not affect the overall immunity and risk of reinfection but may reflect a sex difference in the immune response which is present for other infections as well as for autoimmune diseases and malignancies [34].
Since avidity maturation is time dependent, avidity testing has been suggested as a diagnostic tool to estimate the time of acquisition of infection [11,35]. In our study we found a considerable variation over time in the maturation of avidity and the majority did not reach >80% in avidity even after 6 months of follow-up. Based on these findings, it seems difficult to attain a cut-off level to distinguish between a new or previous infection and it seems like avidity may not be a useful diagnostic tool in that perspective.
As others have shown [26,36,37], we could see that the majority of the participants seroconverted after covid-19 with detectable anti-spike and anti-nucleocapsid and that the antibodies were still detectable six months after infection. Also in line with other studies, we could clearly show a decline in antibody-levels over time, for spike-antibodies the decline was significant from 1 to 3 to 6 months and for nucleocapsid-antibodies there was first a significant increase from 1 to 3 months, with peak levels at 3 months, and thereafter a significant decrease [22].
Others have found that male sex and longer duration of symptoms correlate with increased antibody titres [26,27]. We could not find any significant differences in neither anti-spike nor anti-nucleocapsid antibody levels between patients with symptoms lasting more than 2 months or less than 2 months nor between health-care-workers and non-health care workers. There was a significant difference in the level of n-antibodies between men and women after three and six months, but this was not seen in the anti-spike levels. Previous studies have found that men have higher serum levels of antibodies and also a higher risk of severe covid-19 [7,26,38]. These differences in antibody-levels and risk of severe infection implies a sex differences in the immune response but it remains to be investigated if a similar difference exists for the risk of reinfection.
This study has several limitations. Due to lack of resources, the serum samples were not tested in a neutralizing assay and therefore the avidity maturation cannot be correlated to neutralizing capacity. Still, the avidity maturation is significantly increasing and implies an adjustment of the immune system which at least might be one part of achieving long-lasting immunity. Another factor, which we have not studied, is the presence and relevance of different virus variants. It was not possible to sequence the detected virus in the individual specimen used for inclusion in the study but surveillance sequence data in Sweden of circulating variants during the same time period reveals that the B.1.1 and B.1 were dominating, with the Swedish variant B.1.1.302 having minor circulation [39]. According to studies done by the manufacturer of the antibody assay (unpublished data), different variants do not affect the performance of the test.
In conclusion, we found a significant ongoing increase in avidity maturation after Covid-19, whilst the serum levels of spike- and nucleocapsid- antibodies were declining. Avidity, or the absence of avidity maturation, might be of clinical value to indicate long-term immunity and risk of re-infection.
6. Funding
This work was supported by The Region Halland Research Council and The Foundation of Sparbanken Varberg.
Emma Löfström and Anna Eringfält equally contributed as first authors. We would like to thank Anna Malm, Ingela Salomonsson, Ali Kawaz, Reman Matti, Irene Forsell, Annelie Bajkusa, Christina Engström and Eva Haake at the Department of Clinical Microbiology, Halmstad, for their work with the serum samples in this study.
Declaration of Competing Interest
The authors declare no conflicts of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Emma Löfström and Anna Eringfält equally contributed as first authors. We would like to thank Anna Malm, Ingela Salomonsson, Ali Kawaz, Reman Matti, Irene Forsell, Annelie Bajkusa, Christina Engström and Eva Haake at the Department of Clinical Microbiology, Halmstad, for their work with the serum samples in this study.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. https://Covid19.who.int (accessed 15 June 2021).
- 2.Angus D.C., Derde L., Al-Beidh F., Annane D., Arabi Y., Beane A., et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19. The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA. 2020;324(13):1317–1329. doi: 10.1001/jama.2020.17022. Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborative Group RECOVERY. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Centre for Diseases Prevention and control, weekly surveillance report on Covid-19. https://www.ecdc.europa.eu/en/covid-19/surveillance/weekly-surveillance-report (accessed 31 June 2021).
- 6.Galanis P., Vraka I., Fragkou D., Bilali A., Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J. Hosp. Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu B., Guo H., Zhou P., Shi Z. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. Feb 24. [DOI] [PubMed] [Google Scholar]
- 9.Guan W., Ni Z., Hu Y., Hu Y., Liang Z., Liang W., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long Q., Liu B., Deng H., Wu G., Deng K., Chen Y., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. Jun. [DOI] [PubMed] [Google Scholar]
- 11.Bauer G., Bauer G., Bauer G. The variability of the serological response to SARS-corona virus-2: potential resolution of ambiguity through determination of avidity (functional affinity) J. Med. Virol. 2020;93(1):311–322. doi: 10.1002/jmv.26262. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedman K., Lappalainen M., Söderlund M., Hedman L. Avidity of IgG in serodiagnosis of infectious diseases. Rev. Med. Microbiol. 1993;4(3):123–129. July. [Google Scholar]
- 13.Chan P.K.S., Lim P., Liu E.Y.M., Cheung J.L.K., Leung D.T.M., Sung J.J.Y. Antibody Avidity Maturation during Severe Acute Respiratory Syndrome–Associated Coronavirus Infection. J. Infect. Dis. 2005;192(1):166–169. doi: 10.1086/430615. Jul 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer G. The potential significance of high avidity IgG for protective immunity towards SARS-CoV-2. Int. J. Infect. Dis. 2021:61–64. doi: 10.1016/j.ijid.2021.01.061. May; 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prince H.E., Lapé-Nixon M. Role of Cytomegalovirus (CMV) IgG Avidity Testing in Diagnosing Primary CMV Infection during Pregnancy. Clin. Vaccine Immunol. 2014;21(10):1377–1384. doi: 10.1128/CVI.00487-14. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junker A.K., Tilley P. Varicella-zoster virus antibody avidity and IgG subclass patterns in children with recurrent chickenpox. J. Med. Virol. 1994;43(2):119–124. doi: 10.1002/jmv.1890430204. June. [DOI] [PubMed] [Google Scholar]
- 17.Paunio M., Hedman K., Davidkin I., Valle M., Heinonen O.P., Leinikki P., et al. Secondary measles vaccine failures identified by measurement of IgG avidity: high occurrence among teenagers vaccinated at a young age. Epidemiol. Infect. 2000;124(2):263–271. doi: 10.1017/s0950268899003222. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eidge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Loens K., et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020;26(11):1691–1693. doi: 10.1038/s41591-020-1083-1. Nov. [DOI] [PubMed] [Google Scholar]
- 19.Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J., et al. Antibody Detection and Dynamic Characteristics in Patients with COVID-19. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2020 Apr 19,. [DOI] [PMC free article] [PubMed]
- 20.Bauer G., Struck F., Schreiner P.P., Staschik E.E., Soutschek E.E., Motz M. The challenge of avidity determination in SARS-CoV-2 serology. J. Med. Virol. 2021;93(5):3092. doi: 10.1002/jmv.26863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chia W.N., Zhu F., Ong S.W.X., Young B.E., Fong S., Le Bert N., et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2(6):e240–e249. doi: 10.1016/S2666-5247(21)00025-2. June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccoli L., Park Y., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell. 2020;183(4):1024–1042. doi: 10.1016/j.cell.2020.09.037. Nov 12e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benner S.E., Patel E.U., Laeyendecker O., Pekosz A., Littlefield K., Eby Y., et al. SARS-CoV-2 Antibody Avidity Responses in COVID-19 Patients and Convalescent Plasma Donors. J. Infect. Dis. 2020;222(12):1974–1984. doi: 10.1093/infdis/jiaa581. Nov 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y.R., Chakraborty I., Yun C., Wu A.H.B., Lynch K.L. Kinetics of SARS-CoV-2 Antibody Avidity Maturation and Association with Disease Severity. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1389. Sep 14Published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strömer A., Rose R., Grobe O., Neumann F., Fickenscher H., Lorentz T., et al. Kinetics of Nucleo- and Spike Protein-Specific Immunoglobulin G and of Virus-Neutralizing Antibodies after SARS-CoV-2 Infection. Microorganisms (Basel) 2020;8(10):1572. doi: 10.3390/microorganisms8101572. Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.den Hartog G., Vos E.R., van den Hoogen Lotus L., van Boven M., Scheep R.M., Smits G., et al. Persistence of antibodies to SARS-CoV-2 in relation to symptoms in a nationwide prospective study. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab172. Feb;ciab 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein S.L., Pekosz A., Park H., Ursin R.L., Shapiro J.R., Benner Sarah E, Kirsten Littlefield, et al. Sex, age and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J. Clin. Invest. 2020;130(11):6141–6150. doi: 10.1172/JCI142004. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue T., Moran I., Shinnakasu R., Phan T.G., Kurosaki T. Generation of memory B cells and their reactivation. Immunol. Rev. 2018;283(1):138–149. doi: 10.1111/imr.12640. May. [DOI] [PubMed] [Google Scholar]
- 30.Bauer G., Struck F., Schreiner P., Staschik E., Soutschek E., Motz M. The serological response to SARS corona virus-2 is characterized by frequent incomplete maturation of functional affinity (avidity) Res. Square. 2020 https://doi.org.10.21203/rs.3.rs-104847/v1. [Google Scholar]
- 31.Harrington W.E., Trakhimets O., Andrade D.V., Dambrauskas N., Raappana A., Jiang Y., et al. Rapid decline of neutralizing antibodies is associated with decay of IgM in adults recovered from mild COVID-19. Cell Rep. Med. 2021;2(4) doi: 10.1016/j.xcrm.2021.100253. Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo Y.R., Yun C., Chakraborty I., Wu A.H.B., Lynch K.L. A SARS-CoV-2 Label-Free Surrogate Virus Neutralization Test and a Longitudinal Study of Antibody Characteristics in COVID-19 Patients. J. Clin. Microbiol. 2021 doi: 10.1128/JCM.00193-21. Apr 07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pichler D., Baumgartner M., Kimpel J., Rössler A., Riepler L., Bates K., et al. Marked increase in avidity of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) antibodies 7-8 months after infection is not diminished in old age. J. Infect. Dis. 2021:jiab300. doi: 10.1093/infdis/jiab300. Jun 4Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. Oct. [DOI] [PubMed] [Google Scholar]
- 35.Valdivia A., Torres I., Huntley D., Alcaraz M.J., Albert E., Colomina J., et al. Qualitative assessment of SARS-CoV-2-specific antibody avidity by lateral flow immunochromatographic IgG/IgM antibody assay. J. Med. Virol. 2020;93(2):1141. doi: 10.1002/jmv.26344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W., Du R., Li B., Zheng X., Yang X., Hu B., et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.L'Huillier A.G., Meyer B., DO Andrey, Arm-Vernez I., Baggio S., Didierlaurent A., et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clin. Microbiol. Infect. 2021;27(5) doi: 10.1016/j.cmi.2021.01.005. Jan 20784.e1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haitao T., Vermunt J.V., Abeykoon J., Ghamrawi R., Gunaratne M., Jayachandran M., et al. COVID-19 and Sex Differences. Mayo Clin. Proc. 2020;95(10):2189–2203. doi: 10.1016/j.mayocp.2020.07.024. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folkhälsomyndigheten (The Public health agency, Sweden) Helgenomsekvensering av svenska SARS-CoV-2 som orsakar Covid-19, del 3. 2020 Dec 23. Article number: 20232. https://www.folkhalsomyndigheten.se/contentassets/088dbf03a535495ba9df441577eea7ec/helgenomsekvensering-svenska-sars-cov-2-orsakar-covid-19-del-3.pdf.