Abstract
Background
Mechanical ventilators are essential biomedical devices for the respiratory support of patients with SARS-CoV-2 infection. These devices can be transmitters of bacterial pathogens. Therefore, it is necessary to implement effective disinfection procedures. The aim of this work was to show the impact of the modification of a cleaning and disinfection method of mechanical ventilators of patients with SARS-CoV-2 and ventilator-associated pneumonia.
Methods
A total of 338 mechanical ventilators of patients infected with SARS-CoV-2 and ESKAPE bacteria were divided in two groups. Group A and B were subjected to cleaning and disinfection with superoxidation solution-Cl/enzymatic detergent and isopropyl alcohol, respectively. Both groups were cultured for the detection of ESKAPE bacteria. The isolates were subjected to tests for identification, resistance, adherence, and genomic typing.
Results
Contamination rates of 21.6% (n = 36) were identified in group A. The inspiratory limb was the circuit involved in most cases of postdisinfection contamination. Acinetobacter baumanni, Pseudomonas aeruginosa, and multi-resistant Klebsiella pneumoniae were the pathogens involved in the contamination cases. The pathogens were highly adherent and in the case of A. baumanni, clonal dispersion was detected in 14 ventilators. Disinfection with enzymatic detergents allows a 100% reduction in contamination rates.
Conclusions
The implementation of cleaning and disinfection with enzymatic detergents/isopropyl alcohol of mechanical ventilators of patients with SARS-CoV-2 and ESKAPE bacteria had a positive impact on postdisinfection microbial contamination rates.
Key Words: Bacterial contamination, ESKAPE bacteria, Medical devices, SARS-CoV-2
The control of health care-associated infections (HAI) in COVID-19 patients is one of the main challenges of current critical medicine, since its control directly impacts the recovery of the patient.1 HAI are adverse events, and to our knowledge, no health institution in the whole world is free of them, and in the current SARS-CoV-2 pandemic they are of greater attention.2 The most frequent HAI in the adult intensive care unit (AICU) is the ventilator-associated pneumonia (VAP) caused by bacteria from the ESKAPE group, where mechanical ventilators play an important role.3 , 4 According to the World Health Organization (WHO) and the Pan American Health Organization (PAHO), mechanical ventilators are essential biomedical devices for the respiratory support of patients with SARS-CoV-2 infection.5 Since there is no specific treatment for COVID-19, mechanical oxygen therapy is vital, and maintaining oxygenation in critically ill patients is vital to their recovery. Therefore, the mechanical ventilators, in addition to providing adequate performance in terms of oxygenation, it must also be guaranteed that they are free of bacterial pathogens that could cause VAP. It has been shown that the AICU of the Hospital Juarez de Mexico that cares for COVID-19 patients is colonized by ESKAPE bacteria causing VAP in medical devices, patients, and medical personnel, where Acinetobacter baumannii, Citrobacter freundii, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus were the most predominant also with molecular markers of antimicrobial resistance.1 , 6 Even when the installation of filters for bacteria and viruses in mechanical ventilators is recommended, patients develop VAP.7 Therefore, the implementation of comprehensive and standardized high-level cleaning and disinfection procedures for mechanical ventilators is essential. For this, it must be taken into account that the spread of nosocomial pathogens, including SARS-CoV-2, occurs due to its permanence on plastic and stainless-steel surfaces, materials with which the mechanical ventilators are built; therefore, the cleaning and disinfection process is crucial.8 , 9 Among the components of a mechanical ventilator that are crucial in disinfection is the “patient circuit,” since they are pieces of constant contact by the patient/health personnel.10 In general, this section of the mechanical ventilator connects the patient with the main system with two limbs: the inspiratory one that leaves the equipment and reaches the patient, and an expiratory one that goes from the patient to the expiratory valve.11 , 12 Therefore, the choice of effective cleaning and disinfection protocols, as well as postdisinfection microbiological analysis, is relevant for the control of VAP in COVID-19 patients. Under this antecedent, the aim of this work was to show the impact on reducing the incidence of cases of contamination after disinfection of mechanical ventilators after changing the disinfection method. The foregoing, derived from the implementation of a new disinfection protocol for mechanical ventilators used as respiratory therapy in COVID-19 patients with VAP. Alternatively, the phenotype/genotype characteristics of the ESKAPE members isolated from mechanical ventilators prior to the implementation of the new disinfection protocol are shown. The importance of the disinfection processes of mechanical ventilators used in COVID-19 patients, microbiological control, and the implications on the acquisition of VAP associated with contaminated mechanical ventilators are discussed.
Methods
Ethical considerations
The institutional Committee of Research, Ethics, and Biosafety from Hospital Juarez de Mexico (HJM) approved the protocol under the registration number HJM 0432/18-I in accordance with the Regulation of the General Health Law on Research for Health.13
Mechanical ventilators of the study population
A prospective and observational analysis over 12 months (June 2020 to May 2021) of mechanical ventilators used as respiratory support in COVID-19 patients of Intensive Care Unit Adult (ICUA) of the HJM was performed. A total of 338 mechanical ventilators with record of contamination by nosocomial bacteria (ESKAPE group) from patients infected with SARS-CoV-2 and VAP were included in this study.
Preparation of mechanical ventilators before the disinfection process
After extubation of the COVID-19 patient, the disposable circuit that communicates to the inspiratory and expiratory limbs of the mechanical ventilator was disconnected. This single-use circuit was placed in an infectious contagious biological waste container for final disposal. Before transport mechanical ventilator from ICUA to Inhalation Therapy service, a sodium hypochlorite solution (1,000 ppm) was used as an external disinfectant. The excess sodium hypochlorite was removed with a towel moistened with distilled water. Finally, the mechanical ventilator was covered with a plastic bag, labeled as “mechanical ventilator not internally disinfected” and transported to the cleaning and disinfection and reprocessing area of medical devices of the HJM Inhalation Therapy service.
Implementation of a new disinfection protocol of mechanical ventilators
The need for the implementation of the new disinfection protocol for mechanical ventilators was due to the recurrent identification of postdisinfection bacterial contamination (ESKAPE group) of mechanical ventilators used in patients coinfected with SARS-CoV-2 and ESKAPE bacteria. The disinfection method that was replaced was based on the rotation of a single high-level disinfectant (method A), formulated based on an electrolyzed superoxidation solution with neutral pH at 0.004% active Cl (HCLO/CLO) (Estericide QX, Esteripharma, Mexico city, Mexico).
Cleaning and new disinfection procedure of mechanical ventilators
Inspiratory limb
All cleaning and disinfection procedures were performed wearing a coverall protective gown. Prior to the cleaning and disinfection process, the expiratory limb and its oxygen membrane were disconnected from the main system of the mechanical ventilator to undergo the first cleaning process as follows. The components described above were friction washed with distilled water to remove visible traces of organic matter. The follow step was second cleaning, for this purpose, pieces were immersed for 40 minutes in a solution of Endozime AW Plus Premium enzymatic detergent (Ruhof, Mineola, NY), in a proportion of 50 mL per 4 L of distilled water. After this time, the pieces were washed with distilled water and immersed for 40 minutes in a second solution of Alkazyme enzymatic detergent (Alkapharm UK, Penkridge, Staffordshire, UK) in a proportion of 20 g per 4 L of water. Finally, they were washed with distilled water and dried manually with a sterile cotton blanket. Final disinfection was performed using a gauze impregnated with absolute isopropyl alcohol. Additional accessories, such as pressure transduction and oxygen turbine connectors were removed from the main ventilator system and treated in the same way in the cleaning and disinfection process.
Expiratory limb
In the case of the inspiratory limb, a manual cleaning was performed to remove visible organic material by using gauze impregnated with distilled water. The disinfection of this limb was carried out by introducing a sterile gauze impregnated with absolute isopropyl alcohol for 10 minutes. This procedure was performed three times. At the end, a final gauze impregnated with isopropyl alcohol was left for 24 hours. The main system (monitor, pipes, among others) was externally cleaned by manual friction using a gauze impregnated with Estericide QX disinfectant solution (super oxidized solution) (Esteripharma, Mexico). This disinfectant contains active chlorine equivalent to 0.004% (40 parts per million [ppm]), has a neutral pH (6.4-7.5) and oxidation reduction potential (ORP) of 650-900 mV. After finishing the cleaning process of both limbs, the fan was reassembled and subjected to microbiological tests as follows.
Bacterial sampling of mechanical ventilators postdisinfection
All the procedures for taking and processing samples for their microbiological analysis were carried out with strict adherence to good laboratory practices (use of sterile gloves between each sample collection by mechanical ventilator, use of sterile material and others). Two sampling sites for each mechanical ventilator were chosen to determine the bacterial bioburden (inspiratory and expiratory limb) postdisinfection. Bacteriological sampling was performed by using Sanicult sampling swabs (Starplex Scientific, Etobicoke, Ontario, Canada). Samples were transported at 4°C to the research laboratory for their microbiological culture.
Isolation and identification of bacteria
Samples were streaked on selective MacConkey, Mannitol Salt agar (Becton Dickinson & Co., Franklin Lakes, NJ) and bile-esculin azide agar (Hardy Diagnostics, Santa Maria, CA). The plates were incubated aerobically at 37°C for 24-48 hours. Subsequently, typical microbial strains belonging to the ESKAPE bacteria were purified in LB agar. Identification strains were performed by using BD Phoenix (Brea, CA) according to the manufacturer's protocol.
Susceptibility/resistance assays
The antimicrobial resistance/susceptibility was performed by using the disk diffusion method according to the guidelines set by ‘‘The Clinical and Laboratory Standards Institute” (CLSI, 2019).14 Twelve antimicrobial agents were used: amikacin (AN, 30 μg), ampicillin (AM, 10 μg), gentamicin (GM, 10 μg), trimethoprim/sulfamethoxazole (SXT, 23.75/1.25 μg), ceftriaxone (CRO, 30 μg), cefotaxime (CTX, 30 μg), dicloxacillin (CLOX, 1 μg), cephalotin (CF, 30 μg), chloramphenicol (C, 30 μg), penicillin (10, U), nitrofurantoin (NF, 100 μg), and netilmycin (NET, 30 μg) (BD, Brea, CA). Pseudomonas aeruginosa ATCC 27853 and A. baumannii ATCC 19606 were used as controls. Results were inferred as susceptible, intermediate, or resistant by measuring the diameter of the inhibition zone. The frequency of antibiotic resistance was calculated and represented in percentages (%).
Molecular typing of A. baumannii by ERIC-PCR
Isolates belonging to A. baumannii were subjected to molecular typing by ERIC-PCR, by using the primers ERIC1R and ERIC2. Conditions of PCR reactions were performed according to Versalovic et al. (1991).15 Genetic profiles were run in 1 × TBE buffer, pH 8.3, separated in horizontal electrophoresis in 2.0% agarose gels, visualized, photographed under UV illumination, and analyzed by intragel pattern comparison.
Quantification of the biofilm‐forming ability
Biofilm‐forming ability of all strains was performed according to Qi et al. (2016)16; and Rodríguez‐Baño et al. (2008)17 with minor modifications. In brief, all strains were cultured overnight in 3 mL of LB broth with shaking at the appropriate temperature. Overnight cultures were subcultured one more time in 3 mL of LB broth, overnight. Bacterial cultures were centrifuged, and cold isotonic solution saline was added to the bacterial pellet and adjusted to 0.5 McFarland nephelometer. Moreover, 150 μL of fresh LB broth were inoculated into wells of a sterile 96‐well polystyrene microtiter flat bottom plate and 50 μL of each bacterial suspension was added (per triplicate). The plates were sealed and aerobically incubated at 37°C for 48 hours. The broth was removed from the microplate with a quick tap on a container with a disinfectant solution. Wells plates were gently washed with 1 × PBS (pH 7.4) 3 times and stained with 200 μL of 0.1% crystal violet for 30 min at room temperature. The biofilm was quantified by measuring the corresponding OD570 of the supernatant by using an Epoch, BioTek spectrophotometer (Winooski, VT) following biofilm solubilization with 200 μL of 30% glacial acetic acid. Acinetobacter baumannii ATCC 19606 and P. aeruginosa ATCC 27853 strains were used as a positive control. Uninoculated well with LB media was used as a negative control.
Results
Impact of the modification of a cleaning and disinfection method of mechanical ventilators
Due to the recurrent persistence of microbial contamination by bacteria belonging to ESKAPE group after cleaning and disinfection of mechanical ventilators of COVID-19 and VAP patients, a modification of a cleaning and disinfection method was implemented to guarantee the efficacy of disinfection of mechanical ventilators due to ESKAPE nosocomial contamination. A total of 338 mechanical ventilators used for respiratory support in COVID-19 patients of the ICUA of the HJM were subjected to microbiological analysis after the disinfection process.
The first group of mechanical ventilators (n = 166) was subjected to cleaning and disinfection by method “A” and microbiologically analyzed. This group was analyzed in a period of six months (June 2020 to November 2020). During this analysis period, contamination rates of 21.6% were identified, corresponding to 36 contaminated mechanical ventilators. Of the total population of mechanical ventilators analyzed (n = 338), this number of mechanical ventilators corresponds to a frequency of 10.6%. Table 1 shows the contamination rate of mechanical ventilators per month for this first group of medical devices (June 2020 to November 2021). During this first semester of analysis, higher contamination rates were identified for the inspiratory limbs (20/55%) after of method A. Derived from the persistence of nosocomial contamination in mechanical ventilators, it was decided to implement a modification of cleaning and disinfection method (method B), described in Materials and Methods. The second group of mechanical ventilators (n = 172) was microbiologically analyzed during December 2020 to May 2020 postdisinfection with the modified method implemented (method B). The postdisinfection microbial contamination rates of the analyzed mechanical ventilators were 0%. Table 1 summarizes the findings in the incidences of microbial contamination on a temporary basis.
Table 1.
Monthly contamination and percentage rate of the mechanical ventilators from June 2020 to January 2021 used for respiratory support in COVID-19 patients of the Intensive Care Unit Adults of the Hospital Juárez México after the disinfection process
| Year | Month | Mechanical ventilators analyzed | Bacterial culture (Contamination rate) |
Clean and disinfection methods | |||
|---|---|---|---|---|---|---|---|
| Positive |
Negative n(%) | ||||||
| n(%) | Port n(%) |
||||||
| Inspiratory | Expiratory | ||||||
| 2020 | June | 8 | 2(25.0) | 0(0) | 2(100) | 6(75) | Superoxidation/Cl-Isopropyl alcohol |
| July | 25 | 8(32.0) | 6(75) | 2(25) | 17(68) | ||
| August | 35 | 5(14.20) | 0(0) | 5(100) | 30(85.80) | ||
| September | 42 | 8(19.04) | 2(25) | 6(75) | 34(80.96) | ||
| October | 22 | 1(4.54) | 0(0) | 1(100) | 21(95.45) | ||
| November | 34 | 12(35.29) | 12(100) | 0(0) | 22(64.7) | ||
| December | 30 | 0(0) | 0(0) | 0(0) | 30(100) | Enzymatic detergents-Isopropyl alcohol | |
| 2021 | January | 30 | 0(0) | 0(0) | 0(0) | 30(100) | |
| February | 28 | 0(0) | 0(0) | 0(0) | 28(100) | ||
| March | 27 | 0(0) | 0(0) | 0(0) | 22(100) | ||
| April | 32 | 0(0) | 0(0) | 0(0) | 32(100) | ||
| May | 25 | 0(0) | 0(0) | 0(0) | 25(100) | ||
| Total | 338 | 36(10.6) | 20(55.5) | 16(44.44) | 302(89.34) | ||
Distribution of the 36 contaminated mechanical ventilators of a total of 338 biomedical devices in this period.
Isolation and identification of ESKAPE bacteria in mechanical ventilators
The 36 bacterial strains isolated from the postdisinfection mechanical ventilators with method “A” belong to three bacterial groups of the ESKAPE group, made up of Acinetobacter baumannii (n = 14), Pseudomonas aeruginosa (n = 12) and Klebsiella pneumoniae (n = 10). As shown in Figure 1 , the most frequent families of isolated organisms belong to Moraxellaceae, Pseudomonadaceae, and Enterobacteriaceae.
Fig. 1.
Bacterial rate contamination distribution (ESKAPE group) from mechanical ventilators used as respiratory support in COVID-19 patients of the Intensive Care Unit of the Hospital Juarez de Mexico. Bacterial isolation percentage was obtained from 226 mechanical ventilators.
Susceptibility/resistance assays
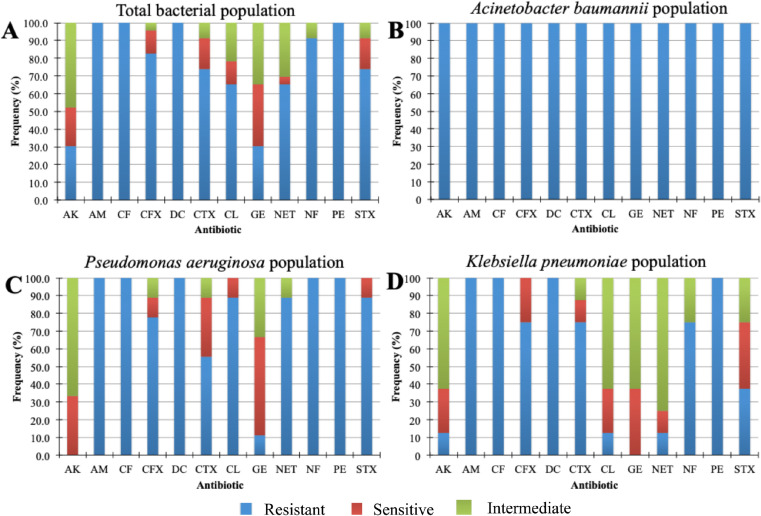
Strains isolated from mechanical ventilators were subjected to antimicrobial resistance assays. The results showed differences in susceptibility and resistance to the twelve antimicrobials tested. In the first group (Fig 2 A), only aminoglycosides (amikacin and gentamicin) were the drugs with the best antimicrobial activity against those strains. The other antibiotics showed lower or null inhibitory activity on the tested strains. An analysis of antimicrobial resistance including only A. baumannii strains was performed (2B). The results revealed that all strains were multidrug-resistant (MDR), therefore, this suggested that all A. baumannii are conformed in a clonal group. In Fig 2C and D, the resistance phenotype of the group of isolates of P. aeruginosa and K. pneumoniae, is shown, respectively. It is observed that only aminoglycosides (amikacin and gentamicin) were the drugs with the best antimicrobial activity against those strains. Only for the group of K. pneumoniae isolates other antibiotics such as: chloramphenicol, netilmicin, nitrofurantoin, and trimethoprim/sulfamethozaxole showed inhibitory activity.
Fig. 2.
Antimicrobial resistance of bacterial strains (ESKAPE group) isolated from mechanical ventilators used as respiratory support in COVID-19 patients of the Intensive Care Unit of the Hospital Juarez de Mexico. (A) Total Gram-negative bacteria (B) Acinetobacter baumannii strains MDR, (C) Pseudomonas aeruginosa strains, and (D) Klebsiella pneumoniae strains.
Molecular typing of A. baumannii by ERIC-PCR
Genomic diversity analysis of A. baumannii MDR strains was carried out by using the ERIC-PCR fingerprinting method with ERIC-type primers. The electrophoretic analysis of PCR reaction products (amplicons) of the evaluated strains revealed that the number of bands ranged from 5 to 7 in different profiles. The sizes of the amplicons ranged from slightly more than 130 bp to about 3,000 bp. Products in the range of 130, 250, and 1,000 bp were more frequently found. ERIC-PCR profiles allowed differentiation of fourteen strains which were clustered in three clonal groups (Fig 3).
Fig. 3.
Clonal groups identified in Acinetobacter baumannii strains by ERIC-PCR isolated from mechanical ventilators used as respiratory support in COVID-19 patients of the Intensive Care Unit of the Hospital Juarez de Mexico. Lane: M: molecular size marker 100 bp (AXYGEN, Bioscience), CI: Internal control (Pseudomonas aeruginosa PAO1), 1-7: Clonal group 1, 8-10: Clonal group 2, 11-14: Clonal group 3.
Quantification of the biofilm-forming ability
To examine the ability of the 36 isolates to form mature biofilm they were classified as follows: A) OD ≤ ODc = negative; B) ODc < OD ≤ 2ODc = weakly positive; C) 2ODc < OD ≤ 4ODc = positive; and D) 4OD < ODc = strongly positive. ODc was the mean OD value of the control wells, whereas OD is the value of the problems. The quantification of the adherent capacity of the isolates can be observed in Table 2 . Regardless of the genus and species, all the isolates were classified as strongly positive biofilm producers.
Table 2.
Biofilm formation ability of bacterial strains (ESKAPE group) isolated from mechanical ventilators used as respiratory support in COVID-19 patients of the Intensive Care Unit Adults of the Hospital Juarez de Mexico
| Bacterial strain | Strains (n) | Percentage (%) | OD570 (mean ±SD) | Biofilm formation ability |
|---|---|---|---|---|
| Acinetobacter baumannii MDR clonal group 1 | 7 | 19.44 | 0.99 ± 0.06 | Strong positive |
| Acinetobacter baumannii MDR clonal group 2 | 4 | 19.44 | 0.73 ± 0.04 | Strong positive |
| Acinetobacter baumannii MDR clonal group 2 | 3 | 27.77 | 0.66 ± 0.06 | Strong positive |
| Pseudomonas aeruginosa | 12 | 5.55 | 1.06 ± 0.07 | Strong positive |
| Klebsiella pneumoniae | 10 | 19.44 | 0.90 ± 0.06 | Strong positive |
Discussion
Mechanical ventilators are part of the essential biomedical equipment for the respiratory support of critically ill patients who are infected by the new coronavirus SARS-CoV-2, the causal agent of COVID-19.18, 19, 20 The foregoing highlights the importance of implementing strategies that guarantee the effective disinfection of mechanical ventilators before being used in the respiratory therapy of COVID-19 patients. The interest of this work is based on the prevention of VAP in COVID-19 patients, since it has been shown that these respiratory complications are of high prevalence in the ICU with high rates of morbidity and mortality and high economic burden. This could be attributed to the inadequate cleaning and disinfection of mechanical ventilators The use of the mechanical ventilator represents a high risk of contamination of pathogens that cause VAP if adequate cleaning and disinfection protocols are not implemented, and they have been recognized as vehicles for the transmission of pathogens.21 , 22 The present work shows the impact of the implementation of an effective cleaning and disinfection method of mechanical ventilators at the Hospital Juarez de Mexico for COVID-19 patients who had VAP due to ESKAPE bacteria. Additionally, experimental evidence is presented of the phenotypes of resistance, adherence, and clonal dispersion of the ESKAPE pathogens isolated from mechanical ventilators. The WHO has recommended that all biomedical devices used in respiratory therapy must be subjected to cleaning and disinfection by removing organic matter from external and internal surfaces, with water, detergents, or enzymatic products. However, it does not specify what type of cleaning agents and disinfectants should be used in situations where contaminants are difficult to eradicate. The Environmental Protection Agency recommended (EPA) the use of ethyl or isopropyl alcohol, sodium hypochlorite, hydrogen peroxide, detergents based on quaternary ammonium salts, phenolic solutions, and iodophors for the elimination of SARS-CoV-2.23 Therefore, we speculate that ESKAPE bacteria could also be susceptible to this type of treatment. Nevertheless, as shown in Table 1, during the first semester (June to November 2020) the use of electrolyzed solutions of active Cl superoxidation were not effective enough in the elimination of A. baumannii, K. pneumoniae, and P. aeruginosa. Our experience in the use of double rotations of enzymatic detergents as cleaning methods (method B), and the use of isopropyl alcohol as a disinfectant agent, showed a frequency of 0% of bacterial contamination of mechanical ventilators during the second semester analyzed (Table 1). The detection of pathogens that cause VAP after disinfection of mechanical ventilators, highlights the importance of continuing with prospective epidemiological surveillance through the inclusion of mandatory microbiological analyses that guarantee the safety of the mechanical ventilator admitted to the ICUA. Among the components of a mechanical ventilator that are crucial in disinfection is the “patient circuit” since they are parts of constant contact by the patient or health personnel. The contamination events for the inspiratory limb were higher compared to the expiratory limb. In a previous work, this observation on the contamination rates for each of the limbs was reported, with the inspiratory limb being the one with the highest contamination in ex-tubed patients.24 Our results suggest that the cases of failed cleaning and disinfection in the inspiratory and expiratory limbs during the first semester were related to the effectiveness of the cleaning agent, as well as the difficult access to these parts of the mechanical ventilator by the operating personnel. The pathogens identified in this work (A. baumannii, P. aeruginosa, and K. pneumoniae) had a similar isolation frequency (Fig 1) and have previously been reported as causative agents of VAP in the HJM, in addition to having been identified as pathogens that colonize medical devices.1 , 6 The coincidence of the isolation of the pathogen of the mechanical ventilator and the pathogen that causes VAP in COVID-19 patients, confirms the participation of the mechanical ventilator as a vehicle for the transmission of pathogens. These three pathogens are among the most frequent pathogens causing VAP in other hospitals around the world.27 Alternatively, the characteristics of the antimicrobial resistance profiles identified clearly demonstrate the potential risk of acquiring VAP, which is difficult to eradicate by ineffective antimicrobial therapies, which would also be reflected in high institutional costs due to the long hospital stay. Particularly, for A. baumannii, multidrug resistance has been previously reported as a nosocomial pathogen in hospitals in Wuhan, China that treat COVID-19 patients.25 , 28 By identifying identical resistance profiles in A. baumannii isolates, we speculate the presence of possible clonal dissemination of this pathogen among COVID-19 patients and the mechanical ventilators with which they received respiratory support. Hence, molecular typing was performed to confirm this hypothesis (Fig 2). The results of the ERIC-PCR typing demonstrated the existence of 3 clonal groups of A. baumannii, distributed in the 14 mechanical ventilators (Fig 3 ). The clonal distribution identified is closely related to the poor clinical practices of the health personnel, for example, the inadequate or null disinfection of hands during manipulation between mechanical ventilators among different COVID-19 patients, lack of replacement of gloves after the manipulation of other medical devices, leading to cross contamination. These practices have been previously reported and have shown that they are the main cause of pathogen dissemination in the ICUA.26 , 6 Biofilms are biological structures that, in addition to providing adhesion capacities for bacteria to inert surfaces, they also provide a resistance mechanism for avoiding the action of antibiotics or preventing the entry of chemical agents used for disinfection. We speculate that the resistance to disinfection of the 36 pathogens identified in mechanical fans was directly related to their adhesion capacity due to the formation of biofilms in the circuits of mechanical ventilators. To test our hypothesis, in vitro biofilm formation tests were performed on inert material, such as polystyrene. Adherence tests showed that all isolates had adherent characteristics, which allowed them to be classified as “strongly biofilm producers,” which is why we believe they were resistant to the disinfection process (method A). This confirms the importance of using alternative methods, such as the use of enzymatic detergents to induce the breakdown of biofilms of pathogens that could be installed in the mechanical ventilator circuit. Unfortunately, there is no scientific literature on the proper methods of cleaning and disinfecting mechanical ventilators, there are only recommendations on these procedures in the CDC and WHO guidelines. However, these recommendations have not been updated in terms of efficacy against pathogens of importance in the ICUA, such as ESKAPE members causing NAV and even SARS-CoV-2. These guidelines only mention that the materials of the ventilator components should be considered to choose the cleaning and disinfection method, considering the following: resistance temperature, contact with the patient, contact with aerosols, vapors (exhalation) of the patient, among others. The substitution of the mechanical ventilator disinfection method together with prospective epidemiological surveillance (postdisinfection microbiological cultures) aim to show an effective strategy for the control of healthcare-associated infections, such as VAP in COVID-19 patients.
Financial support
This study is part of the project “CONACyT 313771″: Análisis del efecto del ozono sobre SARS-CoV-2 como alternativa de producto desinfectante en equipos de protección del personal de salud de alta demanda,” and funding of the 022 budget (years 2020, 2021) of the Hospital Juárez de México.
Declaration of competing interest
All authors report no conflicts of interest relevant to this article.
Acknowledgments
M.A.C.D, G.I.C., G.C.E and J.M.B.L. received support “Sistema Nacional de Investigadores (SNI)” from CONACyT. G.C.E. received support from Estímulos al Desempeño en Investigación, Comisión de Operación y Fomento de Actividades Académicas del IPN. M.A.L.C. received grant-aided support from CONACyT and actually is PhD student of Biomedicine and molecular Biotechnology program of IPN.
References
- 1.Sosa-Hernández O, Matías-Téllez B, Estrada-Hernández A, et al. Incidence and costs of ventilator-associated pneumonia in the adult intensive care unit of a tertiary referral hospital in Mexico. Am j Infect Control. 2019;47:e21–e25. doi: 10.1016/j.ajic.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 2.Ghossoub E, Newman WJ. COVID-19 and the duty to protect from communicable diseases. J Am Acad Psychiatry Law. 2020;48:155–160. doi: 10.29158/JAAPL.200012-20. [DOI] [PubMed] [Google Scholar]
- 3.Emonet S, Lazarevic V, Refondini CL, et al. Identification of respiratory microbiota markers in ventilator-associated pneumonia. Intensive Care Med. 2019;45:1082–1092. doi: 10.1007/s00134-019-05660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhodes NJ, Cruce CE, O'Donnell JN, et al. Resistance trends and treatment options in gram-negative ventilator-associated pneumonia. Curr Infect Dis Rep. 2018;20:1–15. doi: 10.1007/s11908-018-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CEPAL N. Restrictions on the export of medical products hamper efforts to contain coronavirus disease (COVID-19) in Latin America and the Caribbean. 2020. Available at: https://repositorio.cepal.org/handle/11362/45511. Accessed May 28, 2021.
- 6.Durán-Manuel EM, Cruz-Cruz C, Ibáñez-Cervantes G, et al. Clonal dispersion of Acinetobacter baumannii in an intensive care unit designed to patients COVID-19. J Infect Dev Ctries. 2021;15:58–68. doi: 10.3855/jidc.13545. [DOI] [PubMed] [Google Scholar]
- 7.Ari A, Fink JB, Pilbeam SP. Secondhand aerosol exposure during mechanical ventilation with and without expiratory filters: an in-vitro study. Ind J Resp Care. 2016;5:677–682. [Google Scholar]
- 8.Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities. Available at: https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html#tableb1. Accessed May 28, 2021.
- 9.Suman R, Mohd J, Abid H, et al. Sustainability of coronavirus on different surfaces. J Clin Exp Hepatol. 2020;10:386–390. doi: 10.1016/j.jceh.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YC, Lin HL, Liao FC, et al. Potential risk for bacterial contamination in conventional reused ventilator systems and disposable closed ventilator-suction systems. Plos one. 2018;13 doi: 10.1371/journal.pone.0194246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatburn RL. Understanding mechanical ventilators. Expert Rev Respir Med. 2010;4:809–819. doi: 10.1586/ers.10.66. [DOI] [PubMed] [Google Scholar]
- 12.Dellaca RL, Veneroni C. Trends in mechanical ventilation: are we ventilating our patients in the best possible way? Breathe. 2017;13:84–98. doi: 10.1183/20734735.007817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ley General de Salud (Diario Oficial de la Federación, 1987). Reglamento de la Ley General de Salud en Materia de Investigación para la Salud. Secretaria de Salud. Available at: http://www.salud.gob.mx/unidades/cdi/nom/compi/rlgsmis.html. Accessed: May 28, 2021.
- 14.Clinical and Laboratory standard institute (CLSI). Performance standards for antimicrobial susceptibility testing, 28th ed. CLSI Supplement M100. (ISBN: 1-56238-839-8). 2019.
- 15.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi L, Li H, Zhang C, et al. Relationship between antibiotic resistance, biofilm formation, and biofilm-specific resistance in Acinetobacter baumannii. Front Microbiol. 2016;7:483. doi: 10.3389/fmicb.2016.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Baño J, Martí S, Soto S, et al. Biofilm formation in Acinetobacter baumannii: Associated features and clinical implications. Clin Microbiol Infect. 2008;14:276–278. doi: 10.1111/j.1469-0691.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 18.Abba A, Accorsi C, Agnes P, et al. The novel Mechanical Ventilator Milano for the COVID-19 pandemic. Phys Fluids. 2021;33 doi: 10.1063/5.0044445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wunsch H. Mechanical ventilation in COVID-19: interpreting the current epidemiology. Am J Respir Crit Care Med. 2020;202:1–21. doi: 10.1164/rccm.202004-1385ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holanda MA, Pinheiro BV. COVID-19 pandemic and mechanical ventilation: facing the present, designing the future. J Bras Pneumol. 2020;46 doi: 10.36416/1806-3756/e20200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sui YS, Wan GH, Chen YW, et al. Effectiveness of bacterial disinfectants on surfaces of mechanical ventilator systems. Respir Care. 2012;57:250–256. doi: 10.4187/respcare.01180. [DOI] [PubMed] [Google Scholar]
- 22.Perea EJ, Criado A, Moreno M, et al. Mechanical ventilators as vehicles of infection. Acta Anaesthesiol Scand. 1975;19:180–186. doi: 10.1111/j.1399-6576.1975.tb05238.x. [DOI] [PubMed] [Google Scholar]
- 23.List N: Products with emerging viral pathogen AND human coronavirus claims for use against SARS-CoV-2. Available at: https://www.epa.gov/coronavirus/about-list-n-disinfectants-coronavirus-covid-19-0. Accessed May 28, 2021.
- 24.Kalantar-Motamedi MH, Jarineshin H, Mehrvarz S, et al. Bacterial contamination of ventilators in the Intensive Care Unit. Trauma Mon. 2018;23:e43738. [Google Scholar]
- 25.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilonetto M, Rosa EAR, Brofman PRS, et al. Hospital gowns as a vehicle for bacterial dissemination in an intensive care unit. Braz J Infect Dis. 2004;8:206–210. doi: 10.1590/s1413-86702004000300003. [DOI] [PubMed] [Google Scholar]
- 27.Sharifipour E, Shams S, Esmkhani M, et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis. 2020;20:1–7. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa RLd, Lamas CdC, Simvoulidis LFN, et al. Superinfections in a cohort of patients with COVID-19 admitted to intensive care: impact of gram negative resistance [e-pub ahead of print]. Res Sq. 10.21203/rs.3.rs-403577/v1. Accessed May 28, 2021.