Abstract
IMPORTANCE
There is tremendous interest in using immunotherapy to treat breast cancer, as evidenced by the more than 290 clinical trials ongoing at the time of this narrative review. The objective of this review is to describe the current status of immunotherapy in breast cancer, highlighting its potential in both early-stage and metastatic disease.
OBSERVATIONS
After searching ClinicalTrials.gov on April 24, 2018, and PubMed up to June 30, 2018, to identify breast cancer immunotherapy trials, we found that immune checkpoint blockade (ICB) is the most investigated form of immunotherapy in breast cancer. Use of ICB as monotherapy has achieved objective responses in patients with breast cancer, with higher rates seen when administered in earlier lines of therapy. For responding patients, those responses are durable. More recent data suggest clinical efficacy when ICB is given in combination with chemotherapy. Ongoing studies are evaluating combination strategies pairing ICB with additional chemotherapeutic agents, targeted therapy, vaccines, and local ablative therapies to enhance response. To date, robust predictive biomarkers for response to ICB have not been established.
CONCLUSIONS AND RELEVANCE
It is anticipated that combination therapy strategies will be the way forward for immunotherapy in breast cancer, with an improved understanding of tumor, microenvironment, and host factors informing treatment combination decisions. Thoughtful study design incorporating appropriate end points and correlative studies will be critical in identifying optimal strategies for enhancing the immune response against breast tumors.
There is currently great enthusiasm for immunotherapeutic strategies to treat cancer. Several immunotherapeutic agents have received US Food and Drug Administration (FDA) approval, including adoptive cell therapies, vaccines, onco-lytic viruses, and most notably, immune checkpoint blockade (ICB). Agents of ICB such as inhibitors of cytotoxic T-lymphocyte–associated antigen (CTLA-4), programmed cell death receptor 1 (PD-1), and programmed cell death 1 ligand 1 (PD-L1) have been approved by the FDA for use in various solid tumors, refractory cancers with microsatellite instability, and classical Hodgkin lymphoma.1 The first approval of an ICB agent for treatment of breast cancer came in March 2019 when the anti–PD-L1 antibody atezolizumab was approved for use in combination with nab-paclitaxel for patients with triple-negative breast cancer (TNBC) that is metastatic. This initial approval has increased enthusiasm for investigating immunotherapy agents to treat patients with breast cancer.
One perceived challenge for immunotherapy in breast cancer is that breast tumors have previously been considered immunologically quiescent compared with other tumor types such as melanoma and non–small cell lung cancer (NSCLC). Melanoma and NSCLC have high somatic mutation rates, which can lead to neoantigen generation, which may stimulate antitumor immune responses.2 In these tumor types, higher nonsynonymous mutational burden is associated with improved response, durable clinical benefit, and progression-free survival (PFS) with ICB.3,4 The mutational burden in breast cancer is lower than in these other tumor types and varies by subtype, with ERBB2 (formerly HER2)-positive and basal-like tumors having higher burden than hormone receptor (HR)-positive tumors.5,6 Consistent with this, tumor-infiltrating lymphocyte (TIL) rates are higher in ERBB2-positive and TNBCs when compared with HR-positive tumors.7–11 Notably, higher TIL levels are associated with improved prognosis in ERBB2-positive breast cancer and TNBC, with each approximately 10% increase in TIL being associated with a 15% to 25% decrease in risk of relapse and death.8,9,12,13 Inaddition, increased TIL predicts pathologic response to neoadjuvant therapy.11,14 For example, 4% to 20% of breast tumors are lymphocyte predominant (defined by the presence of either ≥50% or ≥60% lymphocytic infiltration, depending on the study). Previous reports showed that lymphocyte-predominant breast cancers have significantly higher pathologic complete response (pCR) rates than breast tumors with fewer TILs (40% vs 5%).7,14 Interestingly, in a recent report of ERBB2-positive patients treated with trastuzumab/pertuzumab-based chemotherapy in the TRYPHAENA trial,15 TILs were associated with improved event-free survival but not pCR. These data underscore the immunogenicity of some breast tumors. Moreover, the findings highlight the opportunity to develop rational combinations of immune modulation with conventional or novel strategies for breast tumors that have minimal or no lymphocytic infiltrate.
Herein, we review the current status of immunotherapy in breast cancer, highlighting its potential in both early-stage and metastatic disease.
Immune Checkpoint Inhibition
Anti–PD-1/PD-L1 Monotherapy in Breast Cancer
Initial phase 1 ICB monotherapy studies enrolled patients with advanced TNBC that was PD-L1 positive (definedas ≥ 1% of tumor cells and/or tumor-infiltrating immune cells expressing PD-L1). Importantly, the PD-L1 assay used, and whether expression was evaluated in the tumor vs in the infiltrating immune cells, was determined by the trial sponsor. In a study evaluating 5 trial-validated PD-L1 antibodies for immunohistochemical analysis of lung cancer specimens, investigators found highly comparable PD-L1 staining in tumor cells, with 3 of the 5 antibodies demonstrating strong reliability among pathologists in PD-L1 scoring.16 There was less reliability for PD-L1 scoring in infiltrating immune cells. This underscores the challenge of using PD-L1 expression as a biomarker.
The initial trials, which evaluated the anti–PD-1 antibody pembrolizumab (n = 27) and the anti–PD-L1 antibody atezolizumab (n = 21), reported objective response rates (ORRs) of 18.5%17 and 19%,18 respectively. Furthermore, some responses were durable, a hallmark of ICB-mediated immune modulation. An update of the phase 1 atezolizumab trial, reported after accrual of 112 patients, including patients with PD-L1–negative tumors, revealed a 10% ORR6.19 Pembrolizumab was further evaluated in a multicohort, phase 2 study enrolling patients with metastatic TNBC. Cohort A (n = 170), which enrolled patients who had received prior chemotherapy, regardless of tumor PD-L1 status, reported a 4.7% ORR that did not differ by tumor PD-L1 status (4.8% for PD-L1–positive, 4.7% for PD-L1–negative tumors).20 In cohort B (n = 84), which enrolled first-line patients with PD-L1–positive tumors, the ORR was 23%,21 suggesting greater responsiveness with earlier treatment. Importantly, responses tended to be durable, with the median duration of response not reached at data cutoff (range, 1.2–21.5 months, unpublished data via written communication from Sylvia Adams, MD, September 1, 2018) in cohort A. Also, ICB has been evaluated in patients with metastatic HR-positive/ERBB2-negative and ERBB2-positive breast cancer. In a trial evaluating the anti–PD-L1 antibody avelumab, the ORR was 28% among HR-positive/ERBB2-negative patients (n = 72), while there were no responders in a cohort of 26 ERBB2-positive patients (n = 26).22 The ORR for patients with TNBC (n = 58) in that study was 5.2%. The results of those studies are included in Table 1,17,19–30 which summarizes available trial data for ICB in metastatic breast cancer.
Table 1.
Reported Trials of Anti–CTLA-4 or Anti–PD1/PD-L1 ICB in Metastatic Breast Cancer as of June 30, 2018
| Source (Name) | Population and Line of Therapy | Evaluable Patients, No. | Safety Profile | ORR, % | Median Survival, mo | |
|---|---|---|---|---|---|---|
| PFS | OS | |||||
| Monotherapy | ||||||
| Phase 1b; pembrolizumab; KEYNOTE-01217 | mTNBC, PD-L1+, 15.6% 1st line | 27 | 15.6% Gr 3–5 AEs; 5 Gr 3 AEs: anemia, aseptic meningitis, lymphopenia, headache, pyrexia; 1 death (DIC) | 18.5 | 1.9 | 11.2 |
| Phase 1a; atezolizumab19 | mTNBC, PD-L1+, later expanded to include PD-L1−, 17% 1st line | 115 | 11% Gr 3/4 treatment-related AEs, 2% Gr 5 treatment-related AEs (1 pulmonary hypertension, 1 not specified) | 10 (12 for PD-L1+, 0 for PD-L1− | 1.4 | 8.9 |
| Phase 1b; avelumab; JAVELIN22 | mBC, PD-L1+/− (58 TNBC, 72 HR+/ERBB2−, 26 ERBB2+), 1st to 4th line | 168 | 14.3% Gr 3–5 AEs, immune-related events, autoimmune hepatitis (1.8%); 2 treatment-related deaths (acute liver failure, respiratory distress) | 4.8 (33 for PD-L1+, 2.4 for PD-L1−); 5.2 for TNBC, 2.8 for HR+/ERBB2−, 0 for ERBB2+ | 5.9 | 8.1 |
| Phase 1b; pembrolizumab; KEYNOTE-02823 | HR+ mBC, PD-L1+, prior chemo/endocrine therapy allowed | 25 | 16% Gr 3–4 AEs, immune-related AEs included hepatitis, pneumonitis, hyperthyroidism/hypothyroidism | 12 | 1.8 | 8.6 |
| Phase 2; pembrolizumab; KEYNOTE-086 Cohort A20 | mTNBC, PD-L1+/−, 2nd line and beyond | 170 | 12% with Gr 3–4 AEs; 19% with irAEs of any grade, of which 1.2% were Gr 3–4 (most common: hypothyroidism/hyperthyroidism, pneumonitis) | 4.7 (4.8 PD-L1+, 4.7 PD-L1−) | 2 | 8.9 |
| Phase 2; pembrolizumab; KEYNOTE-086 Cohort B21 | mTNBC, PD-L1+, 1st line | 84 | 10% Gr 3–4 AEs; no discontinuations or deaths due of treatment-related AEs | 23.1 | 2.1 | NA |
| Combination Therapy | ||||||
| Phase 1b; atezolizumab + nab-paclitaxel24 | mTNBC, PD-L1 +/−, 1st to 3rd line | 33 | 73% Gr 3–4 treatment-related AEs, most common: neutropenia, anemia, thrombocytopenia, diarrhea, pneumonia. | 39 | 5.5 | 14.7 |
| Phase 1b/2; pembrolizumab + eribulin mesylate; ENHANCE25 | mTNBC, PD-L1 +/−, 1st to 3rd line | 107 | No DLTs, 66.7% Gr 3–4 AEs; most common, neutropenia and fatigue; most common immune-related AEs hypothyroidism/hyperthyroidism, rash, hyperglycemia, and pneumonitis | 26.4 | 4.2 | 17.7 |
| Phase 1b; pembrolizumab + abemaciclib; JPCE26 | HR+, ERBB2− mBC, treatment line NA | 28 | 28.6% serious AEs: Gr 5 AEs, 3.6%; no Gr 4 AEs; Gr 3 AEs: diarrhea (10.7%), neutropenia (28.6%), AST increase (14.3%), ALT (10.7%) | 28.6 | NA | NA |
| Phase 2; durvalumab + olaparib; MEDIOLA27 | BRCA1/2-mutated, ERBB2− mBC, 1st and later lines | 25 | Gr 3–4 AEs were anemia (8%), neutropenia (8%), hemolysis (4%), dyspnea (4%), pancreatitis (4%), fatigue (4%), lymphopenia (4%), and leukopenia (4%) | 52 (Includes unconfirmed responses) | NA | NA |
| Phase 2; niraparib + pembrolizumab; TOPACIO28 | mTNBC, 1st to 3rd line | 46 | Gr 3–4 AEs were fatigue (7%), anemia (15%), and thrombopenia (13%) | 28 (Includes unconfirmed responses) | NA | NA |
| Phase 1/2; pembrolizumab + trastuzumab; PANACEA29 | ERBB2+ mBC, 2nd line and later | 58 | No DLTs, 19% immune-related AEs (most common, thyroid and pneumonitis) | 15.2 for PD-L1+; 0 for PD-L1− | 2.7 for PD-L1+,; 2.5 for PD-L1− | 17.1 for PD-L1+; 7 for PD-L1− |
| Phase 1 tremelimumab + exemestane30 | HR+ mBC | 26 | 5 DLTs | 0 | NA | NA |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTLA-4, cytotoxic T-lymphocyte–associated antigen 4; DIC, disseminated intravascular coagulation; DLT, dose-limiting toxic effect; Gr, grade; HR, hormone receptor; ICB, immune checkpoint blockade; irAE, immune-related AE; mBC, metastatic breast cancer; mTNBC, metastatic triple-negative breast cancer; NA, not applicable; ORR, objective response rate; OS, overall survival; PD-1, programmed cell death 1; PD-L1, PD-1 ligand 1; PFS, progression-free survival.
Several randomized phase 3 ICB monotherapy trials are ongoing in the metastatic setting as well as a National Cancer Institute–sponsored trial in the adjuvant setting evaluating the efficacy of single-agent pembrolizumab in patients with residual TNBC after neoadjuvant chemotherapy (NCT02954874) (Table 2).
Table 2.
Key Randomized Phase 3 Registration Studies of ICB in Breast Cancer as of July 7, 2018
| Trial Information | Trial Description | Primary End Point(s) | Open (Estimated Completion) Date |
|---|---|---|---|
| A-Brave (NCT02926196) | Status: recruiting Setting: adjuvant TNBC in patients with high-risk disease (including residual cancer post-NAC) (N = 335) Treatment arms: (1) avelumab, once every 2 wk for 1 y; (2) observation |
DFS DFS in PD-L1+ |
June 2016 (June 2023) |
| IMpassion030 (NCT03498716) | Status: active, not yet recruiting Setting: adjuvant TNBC (N = 2300) Treatment arms: (1) atezolizumab once every 2 wk × 10 with paclitaxel weekly × 12 followed by ddAC (or ddEC) × 4 followed by atezolizumab every 3 weeks maintenance to complete 1 y of treatment; (2) weekly paclitaxel × 12 + ddAC (or ddEC) × 4 |
iDFS | May 31, 2018 (December 29, 2024) |
| IMpassion031 (NCT03197935) | Status: recruiting Setting: neoadjuvant TNBC (N = 204) Treatment arms: (1) atezolizumab once every 2 wk + nab-paclitaxel once every 1 wk × 12→atezolizumab once every 2 wk + ddAC × 4→surgery →atezolizumab once every 3 wk to 1 y; (2) placebo once every 2 wk + nab-paclitaxel once every 1 wk→placebo once every 2 wk + ddAC × 4→surgery |
pCR | July 25, 2017 (Sept26, 2021) |
| IMpassion130 (NCT02425891) | Status: active, not recruiting Setting: metastatic TNBC, front-line (N = 900) Treatment arms: (1) atezolizumab once every 2 wk + nab-paclitaxel on days 1, 8, and 15 of 28 d; (2) placebo once every 2 wk + nab-paclitaxel on days 1, 8, and 15 of 28 d |
PFS PFS in PD-L1+ OS OS in PD-L1+ (See text for first analysis reported in October 2018) |
June 23, 2015 (April 30, 2020) |
| IMpassion131 (NCT03125902) | Status: recruiting Setting: inoperable locally advanced or metastatic TNBC, front-line (N = 540) Treatment arms: (1) atezolizumab once every 2 wk + paclitaxel on days 1, 8, and 15 of 28 d; (2) placebo + Paclitaxel on days 1, 8, and 15 of 28 d |
PFS | August 25, 2017 (June 30, 2021) |
| IMpassion132 (NCT03371017) | Status: recruiting Setting: inoperable, recurrent, or metastatic TNBC, front-line (N = 350) Treatment arms: (1) atezolizumab once every 3 wk + chemotherapy (gemcitabine or capecitabine or carboplatin); (2) placebo once every 3 wk + chemotherapy (gemcitabine or capecitabine or carboplatin) |
OS | January 11, 2018 (January 31, 2021) |
| KEYNOTE-119 (NCT02555657) | Status: active, not recruiting Setting: metastatic TNBC, second- or third-line (N = 600) Treatment arms: (1) pembrolizumab once every 3 wk; (2) chemotherapy (capecitabine, eribulin, gemcitabine, vinorelbine) |
OS | October 13, 2015 (May 30, 2019) |
| KEYNOTE-355 (NCT02819518) | Status: recruiting Setting: inoperable, locally recurrent or metastatic TNBC, front-line (N = 858) Treatment arms: Part 1: (1) pembrolizumab once every 3 wk + nab-paclitaxel on days 1, 8, and 15 of 28 d; (2) pembrolizumab once every 3 wk + paclitaxel on days 1, 8, and 15 of 28 d; (3) pembrolizumab once every 3 wk + gemcitabine/carboplatin on days 1 and 8 of 21 d Part 2: (1) pembrolizumab once every 3 wk + chemotherapy (nab-paclitaxel vs paclitaxel vs gemcitabine/carboplatin); (2) placebo + chemotherapy |
Part 1: AE, drug discontinuation due to AE Part 2: PFS, PFS in PD-L1+, OS, and OS in PD-L1+ |
July 27, 2016 (December 30, 2019) |
| KEYNOTE-522 (NCT03036488) | Status: recruiting Setting: neoadjuvant TNBC (N = 1150) Treatment arms: (1) pembrolizumab once every 3 wk (plus weekly paclitaxel + carboplatin) × 4 cycles→pembrolizumab once every 3 wk + AC once every 3 wk × 4→surgery→pembrolizumab once every 3 wk × 9 cycles; (2) placebo once every 3 wk (plus weekly paclitaxel + carboplatin) × 4 cycles→placebo once every 3 wk + AC (or EC) once every 3 wk × 4→surgery→placebo once every 3 wk × 9 cycles |
pCR EFS |
March 7, 2017 (March 14, 2025) |
| NeoTRIPaPDL1 (NCT02620280) | Status: recruiting Setting: neoadjuvant TNBC (N = 272) Treatment arms: (1) atezolizumab once every 3 wk + carboplatin on days 1 and 8 of 21 d + nab-paclitaxel on days 1 and 8 of 21 d→surgery→AC/EC/FEC once every 3 wk; (2) carboplatin on days 1 and 8 of 21 d + nab-paclitaxel on days 1 and 8 of 21 d→surgery→AC/EC/FEC once every 3 wk |
EFS | April 2016 (October 2022) |
| NRG-BR004 (NCT03199885) | Status: active, not yet recruiting Setting: locally recurrent, unresectable, or metastatic ERBB2+, front-line (N = 480) Treatment arms: (1) pertuzumab once every 3 wk + trastuzumab once every 3 wk + paclitaxel on days 1 and 8 of 21 d; (2) pertuzumab once every 3 wk + trastuzumab once every 3 wk + paclitaxel on days 1 and 8 of 21 d + pembrolizumab once every 3 wk |
PFS | July 11, 2018 (December 31, 2020) |
| NSABPB-59 (NCT03281954) | Status: active Setting: neoadjuvant TNBC (N = 1520) Treatment arms: (1) weekly paclitaxel × 12 + carboplatin once every 3 wk × 4 + placebo once every 3 wk × 4→AC (or EC) once every 2–3 wk × 4 →surgery→placebo once every 3 wk until 1 y; (2) weekly paclitaxel × 12 + carboplatin once every 3 wk × 4 + atezolizumab once every 3 wk × 4→AC (or EC) once every 2–3 wk × 4→surgery→atezolizumab once every 3 wk until 1 y |
pCR EFS |
December 19, 2017 (June 30, 2024) |
| SWOGS1418/BR006 (NCT02954874) | Status: recruiting Setting: adjuvant TNBC with residual disease post-NAC (N = 1000) Treatment arms: (1) observation; (2) pembrolizumab once every 3 wk for 1 y |
iDFS iDFSinPD-L1+ |
Nov 15, 2016 (May 31, 2026) |
Abbreviations: AE, adverse event; arrow (→), followed by; dose dense; AC, doxorubicin/cyclophosphamide; EC, epirubicin/cyclophosphamide; EFS, event-free survival; FEC, fluorouracil/epirubicin/cyclophosphamide; ICB, immune checkpoint blockade; iDFS, invasive disease-free survival; NAC, neoadjuvant chemotherapy; OS, overall survival; pCR, pathologic complete response; PD-L1, programmed cell death 1 ligand 1; PFS, progression-free survival; TNBC, triple-negative breast cancer.
Strategies to Enhance Response to ICB
The median PFS or overall survival (OS) in the ICB monotherapy advanced TNBC trials was not longer than historical chemotherapy controls, suggesting that therapeutic benefit is limited to a minority of patients. To optimize use of immunotherapeutic agents in breast cancer, there is a need to better understand defects in the endogenous immune response to breast tumors. Considering the cancer immunity cycle, there are multiple steps required for an effective immune response.31 Tumors must undergo immunogenic cell death with release of antigens, which must then be presented by antigen-presenting cells to prime and activate an immune response. Activated T cells must traffic to the tumor, infiltrate, recognize, and kill tumor cells. If tumor cells express PD-L1 in an effort to avoid immune destruction at this final step of the cycle, it is reasonable to assume that the proximal steps are intact. A lack of response to therapy targeting PD-1 or PD-L1 suggests defects in the initial steps.32
Although a comprehensive review is outside the scope of the present article, there is a growing body of literature detailing the effects of therapeutic strategies (including chemotherapy, targeted therapy, and radiation) on immunologic aspects of the tumor microenvironment, including effects on antigen release, antigen presentation, the presence of immunomodulatory cells and cytokines, and effects on the stroma that affect T-cell trafficking. This improved understanding of the impact of standard and experimental therapies has provided a rationale for evaluating combination approaches to enhance ICB response.
Combination of ICB With Chemotherapy
Several chemotherapeutic agents, including anthracyclines, cyclophosphamide, and microtubule-stabilizing agents, commonly used in breast cancer, promote immunogenic cell death resulting in release of antigens and danger signals that recruit antigen-presenting cells, promote engulfment of dying cells, and foster dendritic cell (DC) maturation, all of which are required for T-cell priming.33,34 Thus, there is great interest in combining chemotherapy with ICB. Table 1 outlines combination trials in metastatic TNBC. A phase 1 trial combining atezolizumab and nab-paclitaxel enrolled 33 women who had undergone 0 to 2 prior lines of chemotherapy.24 No new or additive safety signals were identified. The confirmed ORR was 39.4% (95% CI, 22.9%−57.9%) with responses reported in both PD-L1-positive and PD-L1-negative patients. Responses were more frequent in first- vs later-line settings, 53.8% and 30%, respectively; and they were durable, with a median duration of response of 9.1 months (range, 2.0–20.9 months). The results of a phase 3 trial (IMpassion130, NCT02425891) (Table 2) evaluating nab-paclitaxel/atezolizumab in patients with previously untreated metastatic TNBC was recently reported.35 This trial had co–primary end points of PFS in the overall and PD-L1–positive population, and OS in the overall population and, if significant, in the PD-L1–positive population. The trial randomized 902 patients to nab-paclitaxel/atezolizumab or nab-paclitaxel/placebo, and after a median follow-up of 12.9 months, the PFS in the overall population was 7.2 months vs 5.5 months, favoring the atezolizumabarm (P = .002). In the PD-L1–positive population, the PFS was 7.5 months for patients receiving atezolizumab vs 5.0 months for those receiving placebo (P < .001). The median OS in the overall population was 21.3 months in the atezolizumab arm vs 17.6 months in the placebo arm (P = .08). This OS difference did not reach statistical significance, precluding formal analysis in the PD-L1–positive population. However, it was notable that in that PD-L1–positive population, the median OS was 25.0 months in the atezolizumab arm vs 15.5 months in the placebo arm (hazardratio, 0.62; 95% CI, 0.45–0.86). Based on the results of this study, the combination of atezolizumab plus nab-paclitaxel was recently approved for use in patients with metastatic TNBC. A phase 1/2 trial evaluating pembrolizumab with eribulin mesylate reported a 26.4% ORR in patients who had undergone 0 to 2 prior therapies in the metastatic setting.25 Again, responses were observed regardless of PD-L1 status and were greater in the earlier line of therapy.
Chemotherapy/ICB combinations are also being investigated for earlier disease stages in the neoadjuvant setting (Table 3).36–39 In the I-SPY2 trial,36 69 ERBB2-negative breast cancer patients (40 HR-positive and 29HR-negative) received pembrolizumab with paclitaxel, while 180 patients (95HR-positive/ERBB2-negative and 85TNBC) were treated with paclitaxel alone, followed in all patients by doxorubicin and cyclophosphamide. In the TNBC cohort, estimated pCR rates were 60% and 20%, respectively, and in the HR-positive/ERBB2- cohort, estimated pCR rates were 34% and 13%, favoring those receiving pembrolizumab. An increased incidence of adrenal insufficiency was seen with pembrolizumab, often with delayed onset, after doxorubicin and cyclophosphamide treatment completion. While other trials combining standard neoadjuvant cytotoxic regimens with ICB have not reported increased toxic effects37–39 (Table 3), it should be cautioned that if studies do not require long-term monitoring, underreporting of adverse events may result.
Table 3.
Trials of Neoadjuvant ICB and Chemotherapy in Early Breast Cancer As of June 30, 2018
| Source (Name) | Population and No. | Safety | pCR Rate |
|---|---|---|---|
| Phase 2; pembrolizumab (with paclitaxel, followed by AC), I-SPY36 | ERBB2−, 69 enrolled to investigational arm: TNBC (n = 29) and HR+/ERBB2− (n = 40); 180 controls | Adrenal insufficiency in 6 patients, at least 3 related to hypophysitis (5 late onset, after completion of AC; 1 during pembrolizumab) | yT0/Tis ypN0 46% vs 16% in ERBB2− (60% vs 20% in TNBC), 34% vs 13% in HR+/ERBB2− |
| Phase 2; durvalumab or placebo (with nab-paclitaxel, followed by EC), GeparNUEVO37 | TNBC,cT1b-cT4a-d,174 enrolled | no significant differences in AEs between arms (except more hypothyroidism/hyperthyroidism with durvalumab) | ypT0N0 53.4% vs 44.2% (P = .29) |
| Phase 1b; pembrolizumab (with nab-paclitaxel +/− carboplatin, followed by AC), KEYNOTE-17338 | TNBC; cohort A (n = 10), cohort B (n = 10) | No increased toxic effects | ypT0/Tis ypN0 60% in cohort A and 90% in cohort B |
| Phase 1/2; durvalumab (concomitant with nab-paclitaxel and AC)39 | Stage I-III TNBC; phase 1, n = 7; phase 2, n = 50 | 1 Gr 2 AE, fatigue; 1 Gr 3 AE, dehydration and dyspnea; no Gr 4 or 5 AEs | 71% in phase 1 |
Abbreviations: AC, doxorubicin/cyclophosphamide; AE, adverse event; EC, epirubicin/cyclophosphamide; Gr, grade; HR, hormone receptor; ICB, immune checkpoint blockade; SAE, serious adverse event; TNBC, triple-negative breast cancer.
Interestingly, in the recently reported GeparNuevo trial37 rates of pCR varied by therapy sequencing, with greater efficacy found when a short run-in of single-agent immunotherapy (durvalumab) was followed by combination chemo-immunotherapy. In the overall study population, the pCR rate for patients randomized to receive durvalumab was 53.4% (vs 44.2% for those receiving placebo), which failed to meet the prespecified rate of 66%. Interestingly, in a sub-population receiving the durvalumab run-in, the pCR rate was 61.0%. It is possible that this finding was owing to chance, as the study was not powered to address this question, but it does highlight the need to better understand immunobiology to optimize combinations and sequencing. Several large randomized studies of chemotherapy with ICB are being explored in the curative setting (Table 2).
Combination of ICB With Local Ablative Therapies
Conventional local therapy strategies such as radiotherapy can induce antigen release and facilitate tumor-specific immune responses, and preclinical studies have shown synergy with systemic immune-modulating therapies.40–43 Early-phase trials evaluating metastasis-directed radiotherapy combined with ICB enrolling only patients with solid tumors demonstrated safety and responses outside the irradiation field in approximately 10% of participants.44–46 Several trials evaluating irradiation plus immune modulation in breast cancer are under way, including trials combining pembrolizumab plus irradiation in HR-positive breast cancer and TNBC47 (NCT02303366, NCT02608385, NCT02730130, and NCT03051672), and a trial combining tremelimumab (anti–CTLA-4), with brain radiotherapy with or without trastuzumab in ERBB2-positive disease48 (NCT02563925).48 Given responses in the metastatic setting, trials of curative intent strategies combining preoperative radiotherapy with checkpoint blockade in early-stage TNBC are under way (NCT03872505). Questions remaining unanswered are optimal irradiation timing, dose, and schedule when partnered with ICB, although preclinical data suggest that multiple fractions are superior to single-fraction strategies.49
Another form of local ablative therapy evaluated in combination with ICB is cryoablation.40 In a pilot study evaluating cryoablation with ipilimumab (anti–CTLA-4) in patients with early-stage breast cancer undergoing mastectomy, the combination was shown to be safe, and it induced immunologic effects systemically and in the tumor.50
Combination of ICB With Targeted Therapies
Several targeted agents routinely used in breast cancer, including trastuzumab and cyclin-dependent kinase (CDK) 4/6 inhibitors, have been shown to enhance antitumor immunity and thus are promising partners for ICB. Recently reported as well as ongoing phase 3 combination trials are summarized in Tables 1 and 2. As an example, trastuzumab functions in part via antibody-dependent cell-mediated cytotoxic effects to promote antigen cross-presentation and stimulation of anti-ERBB2 CD8-positive T cells.51 Patients receiving trastuzumab have also been shown to have an increase in circulating anti-ERBB2 CD4-positive T cells as well as anti-ERBB2 antibody responses, providing support for combining trastuzumab with immunotherapy.52,53 The PANACEA trial29 investigated trastuzumab and pembrolizumab in trastuzumab-resistant ERBB2-positive advanced breast cancer and demonstrated a 15.2% ORR in the PD-L1–positive cohort with no responses in the PD-L1–negative cohort. With respect to CDK4/6 inhibitors, preclinical models have shown that these agents stimulate interferon-γ signaling leading to enhanced antigen presentation, increasing effector T-cell infiltration, increasing expression of antigen-processing and -presentation genes, and suppressing regulatory T-cell proliferation.54,55 Preclinical data show synergy between CDK4/6 inhibition and PD-1 blockade.55 Preliminary results of an ongoing phase 1b study of pembrolizumab plus abemaciclib show an acceptable safety profile and suggested clinical benefit, with a 14.3% ORR and a 60% rate of stable disease at 16 weeks.26
PARP inhibitors are also of interest as combination partners for ICB. These agents are approved by the FDA for the treatment of advanced BRCA-mutant breast cancers that harbor a homologous re-combination repair defect leading to accumulation of DNA damage and mutations, possibly resulting in neoantigens. Preclinical models combining PARP inhibitors with ICB have shown augmented effector T -cell function.56–58 Recently, clinical activity has been reported for the combination of olaparib with durvalumab in germline BRCA-mutated metastatic ERBB2-negative breast cancers27 as well as niraparib with pembrolizumab in unselected metastatic TNBC.28
Vaccines and Adoptive Cellular Therapy (ACT)
Historically, breast cancer vaccines were tested as single agents in patients with metastatic disease. Early-phase trials showed that multiple different vaccine formulations could be administered safely and generate antigen-specific immune responses in peripheral blood; however, there was minimal evidence of clinical activity.59–62 Vaccinating patients with metastatic disease is challenging owing to the extent of disease burden and the immunosuppressive tumor microenvironment. To address these limitations, several strategies are being investigated, including vaccinating in the adjuvant setting, where there is minimal disease burden,63,64 and incorporating vaccines in combination strategies. As an example, a phase 2 trial evaluating a CD8 T-cell–eliciting vaccine demonstrated no recurrences after a median follow-up of 34 months in 48 patients with ERBB2-positive breast cancer vaccinated after completion of trastuzumab therapy.63 While the trial’s overall intention-to-treat analysis did not demonstrate benefit of vaccination as mono-therapy, this exploratory subgroup analysis was viewed as hypothesis generating, and ongoing trials are evaluating the combination of vaccine plus trastuzumab (NCT00971737, NCT01570036, and NCT02297698). There is also great interest in using vaccines to elicit a T-cell response that can be augmented by ICB (NCT03362060 and NCT02826434).
To date, most vaccines have targeted defined tumor antigens. With recent advances in genomic profiling and the ability to identify mutations within a tumor, there is interest in identifying neoantigens and developing vaccines to target them.65 It has been reported that neoantigen recognition by T cells plays a role in response to ICB therapy,66 suggesting that a combination therapy strategy with a neoantigen vaccine and ICB may be effective. A trial evaluating a neoantigen DNA vaccine alone or with durvalumab in stage II-III TNBC is currently recruiting (NCT03199040). Given the relatively low rate of mutations in breast tumors, it is possible that this strategy will be less effective in breast cancer than in other more mutagenic tumor types. However, a recent report showed clinical activity in a patient with metastatic breast cancer who was administered TIL-targeting mutated proteins identified in the tumor.67 This report suggests that if the appropriate antigen is identified, an immunotherapeutic strategy targeting it may be effective. Administration of TIL is a type of ACT (reviewed by Rosenberg and Restifo68), a form of immunotherapy that also includes administration of lymphocytes genetically engineered to express T-cell receptors, or lymphocytes engineered to express chimeric antigen receptors (CARs). A CAR involves an extracellular domain derived from an antibody with specificity for a tumor cell surface antigen linked to an intracellular signaling domain that stimulates T-cell activation. There is great enthusiasm regarding CAR T-cell therapy based on FDA approval of CAR T cells targeting the CD19 antigen in non-Hodgkin lymphoma and pediatric acute lymphoblastic leukemia. CAR T-cell therapy is currently being investigated in solid tumors, including breast cancer, with active trials evaluating CAR T cells targeting ERBB2 (NCT02713984), cMET (NCT01837602), mesothelin (NCT02792114) and mucin-1 (NCT02587689). However, caution is urged because an initial trial evaluating a ERBB2-targeted CAR resulted in the death of a patient due to cytokine storm, presumably occurring after the administered cells localized to the lungs where epithelial cells express low levels of ERBB2.69
Appropriate Clinical Trial End Points for Immunotherapy and FDA Regulatory Considerations
Common primary end points for oncology drug approval by the FDA include OS, PFS, disease-free survival/recurrence-free survival, and ORR. Concerns have been raised that conventional response criteria in the assessment of tumor measurement-based end points (eg, ORR) do not adequately capture clinical activity of immunotherapy agents. This is based on observations in a minority of patients who experienced initial disease progression, as defined by conventional criteria, followed by subsequent tumor burden reduction. To address this, a consensus guideline, iRECIST,70 has been published that outlines a standardized approach to solid tumor measurements and definitions for objective change in tumor size for use in tumor-response assessments in immunotherapy studies. For studies planned to support marketing applications, the FDA has recommended continued use of conventional response criteria (RECIST 1.1) in the assessment of primary end points, with consideration of other measurement techniques as supportive information. With proper justification and patient protections, incorporation of immune-based response criteria (eg, iRECIST) into clinical trials for patient management decisions or exploratory end points may be acceptable from a regulatory standpoint. Use of immune-based criteria to guide patient management in clinical trials allows for carefully selected patients with initial disease progression who may be clinically benefitting to continue with this therapy—after consideration of the risks of continued use of a potentially ineffective drug and delay of alternative, potentially effective therapy. Ultimately, prospective inclusion of immune-based response criteria to assess tumor measurement-based end points is needed to determine whether such criteria more fully capture the clinical benefit of immunotherapeutic agents.
Patient Advocate Perspective
Clinical trials should be thoughtful and coordinated in their approach to investigating the safest and most effective ways to harness the immune system against breast cancer. Advocate groups caution that media hype surrounding success of immunotherapy in other disease sites has put patients with breast cancer at risk of having inflated views of potential benefits. As patients consider participating in clinical trials, a thorough explanation of potential risks and benefits is critical. Adequately informing patients about potential toxic effects is especially important for early-stage disease, since data on the long-term impact of these therapies is lacking. Importantly, given the different toxic effects profiles of immune agents compared with more traditional agents, resources should be devoted to patient-reported outcomes and extending the follow-up time during which such measures are assessed. With respect to correlative studies, the advocate community emphasizes using minimally invasive measures and to scheduling tissue collection to coincide with other clinic visits. For approaches combining agents, care should be taken to systematically evaluate options for determining the most effective and least toxic (physically, financially, and emotionally) regimens. It is also important that exclusion criteria be minimized, maintaining only those that are critical to scientific interpretation and/or patient safety. For example, patients with manageable comorbidities, including stable brain metastases, should be included in trials. Additionally, substantial efforts should be made to ensure that underrepresented patient groups have access to trials.
Current Breast Cancer Immunotherapy Landscape
ClinicalTrials.gov was searched on April 24, 2018, to identify breast cancer immunotherapy trials. Figure 1 shows trial breakdown by category and phase of study. A total of 293 open studies were identified, with 65% (n = 191) of those studies actively recruiting. Most studies are phase1, phase2, or phase 1/2. Almost 80% are being conducted in the metastatic setting (n = 229), with 75% of these studies being specifically for breast cancer treatment (vs 25% for general solid tumors). Most trials are for patients with TNBC (n = 106; 46%) followed by ERBB2-positive breast cancer (n = 29; 12%) (Figure 2A). Some breast cancer–specific trials include more than 1 subtype. A breakdown of ICB trials is shown in Figure 2B. Combination trials were more common than single-agent studies, with the most commonly combined modalities being chemotherapy (23%) or targeted therapy (18%).
Figure 1. Breast Cancer Immunotherapy Trials by Type of Immunotherapeutic Agent or Strategy Being Investigated and by Study Phase.
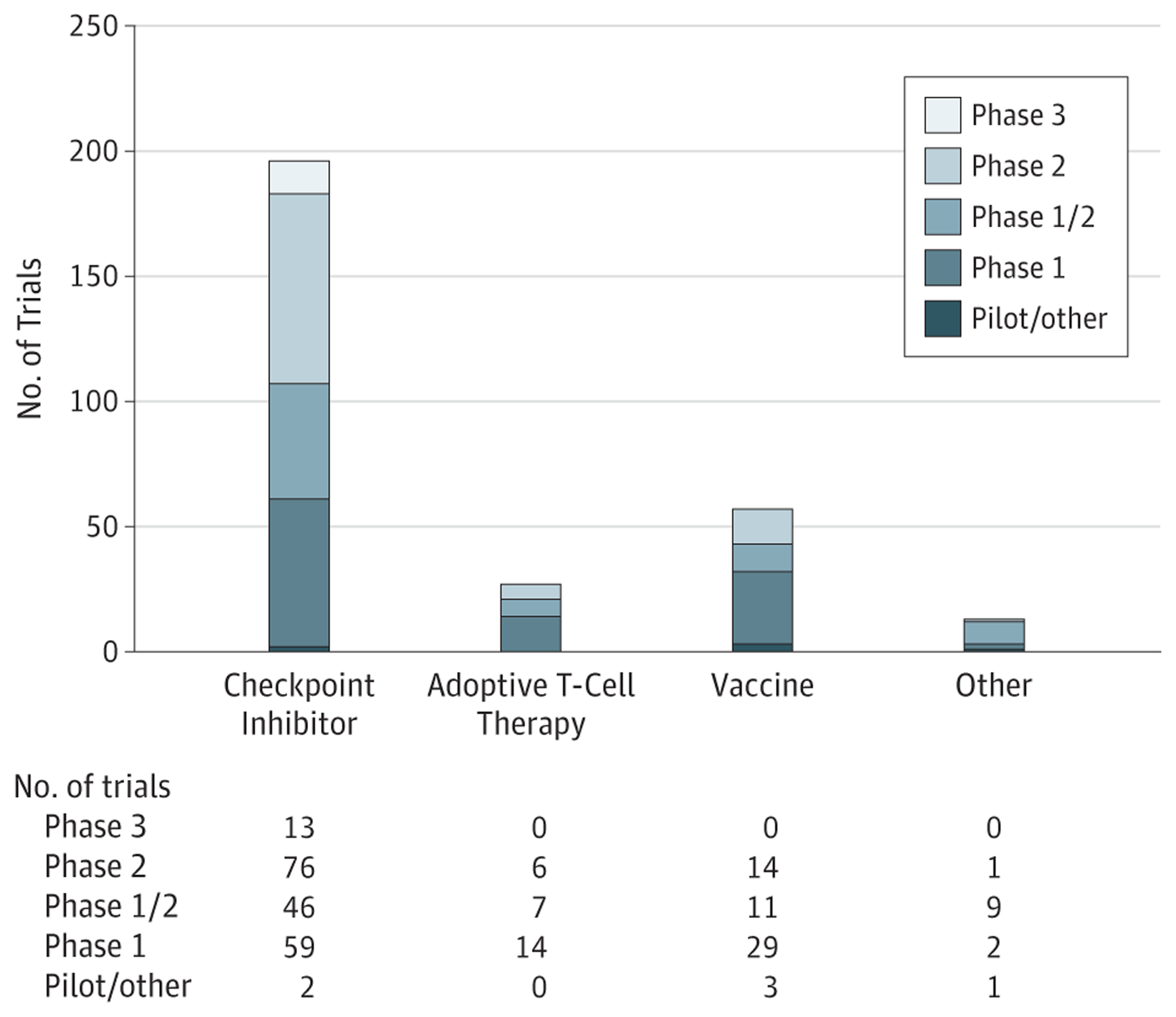
As of April 24, 2018, review of ClinicalTrials.gov identified 293 actively accruing trials evaluating immunotherapeutic agents in breast cancer. “Other” includes natural killer cell therapy, transarterial chemoembolization, and first-in-class agents.
Figure 2. Breast Cancer Immunotherapy Trials.
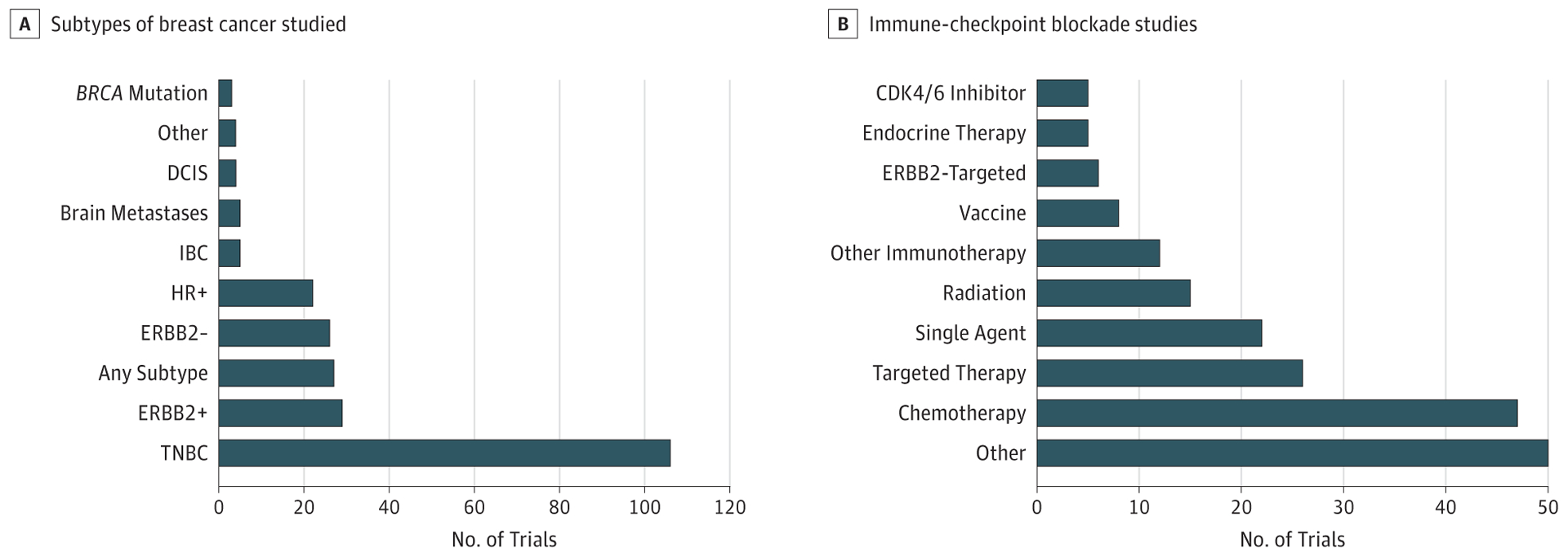
A, Trials by specific subtype of breast cancer being studied. B, Trials investigating immune checkpoint blockade agents alone or in various combinations. “Targeted therapy” includes ERBB2-targeting agents (trastuzumab, pertuzumab, T-DM1), CSFIR inhibitors, HDAC inhibitors, PARP inhibitors, pan-CDK inhibitors, P13K inhibitors, JAK2 inhibitors, adenosine A2 receptor inhibitors, AKT inhibitors, and tyrosine kinase inhibitors. “Other” includes novel monoclonal antibodies (eg, OX40, GITR), first-in-class molecules, as well as combinations with more than 2 active agents (eg, checkpoint inhibitors, vaccines, chemotherapy, irradiation, endocrine therapy, cytokines) in adaptive clinical trials. CDK indicates cyclin-dependent kinase; DCIS, ductal carcinoma in situ; HR, hormone receptor; IBC, inflammatory breast cancer; TNBC, triple-negative breast cancer.
Limitations
Data included in this review, as well as the listing of open clinical trials, was up to date at the time of writing. However, the authors acknowledge that this is a rapidly progressing field, and readers should expect that additional preclinical and clinical trial data will become available, and new trials will be initiated, shortly after publication.
Conclusions
While immunotherapy has yet to realize its full potential in breast cancer, preclinical data and the results of recent clinical trials provide reason for optimism. Success will require an understanding of tumor, microenvironment, and host factors that determine response to immunotherapeutic strategies. Single-arm, mechanistically based trials are important for identifying promising agents and combinations, as well as predictive biomarkers. To date, robust predictive biomarkers for ICB have not been established in breast cancer. Expression of PD-L1 on infiltrating immune cells was required for response to the combination of atezolizumab plus nab-paclitaxel in the IMpassion130 trial.35 Researchers studying other agents have used different strategies: studies evaluating pembrolizumab have looked at PD-L1 expression on the tumor cells.20,21 These trials used different antibodies to assess PD-L1 expression and studies have demonstrated some of the challenges of using multiple different antibodies for PD-L1 detection on tumor and infiltrating immune cells. TILs have shown some promise as predictive biomarkers in exploratory analyses, but larger data sets are needed for confirmation.24,35,71,72 TIL assessment in ongoing and future studies should be performed according to the consensus guidelines established by the International Immuno-Oncology Biomarker Working Group on Breast Cancer.73 And while microsatellite instability74 and tumor mutational burden3 have been identified as biomarkers of response in other tumor types, these alterations are infrequent in breast tumors, suggesting that they are unlikely to be helpful in identifying patients likely to benefit from ICB.
Conflict of Interest Disclosures:
Dr Adams reported institutional research funding from Merck, Genentech, Celgene, and Amgen during the conduct of the study. Dr Kalinsky reported personal fees from Biotheranostics, Eli Lilly, Pfizer, Ipsen, and Novartis, stock ownership in Novartis, and spouse employed by Novartis and Array Biopharma, all outside the submitted work. Dr Bear reported grants and personal fees from Merck during the conduct of the study and grants and personal fees from Genomic Health Inc, outside the submitted work. Dr McArthur reported grants, personal fees, and nonfinancial support from Merck, nonfinancial support from Bristol-Myers Squibb, grants and nonfinancial support from MedImmune/Astra Zeneca, and personal fees from Roche/Genentech, Lilly, Peregrine Pharmaceuticals, TapImmune Inc, Amgen, Puma, Pfizer, Immunomedics, Syndax, Genomic Health, Spectrum Pharmaceuticals, OBI Pharma, Calithera Biosciences, and Celgene during the conduct of the study. Dr Page reported grants, personal fees, and nonfinancial support from Merck and Bristol-Myers Squibb, personal fees and nonfinancial support from Genentech, Nektar, Novartis, Syndax, and Myriad Genetics, grants from MedImmune, and personal fees from Nanostring during the conduct of the study. Dr Vincent reported grants from Merck during the conduct of the study and serving as Scientific Advisory Board Member for Nanostring Inc. Dr Gulley reported that the National Cancer Institutes has several Cooperative Research and Development Agreements (CRADAs) with various biotech and pharma agencies involved in immunotherapy. Dr Litton reported grants from Pfizer, EMD Serono, Genentech, Novartis, GlaxoSmithKline, Astra Zeneca, and Medivation outside the submitted work. Dr Hortobagyi reported personal fees from Novartis outside the submitted work. Dr Chia reported personal fees from Hoffmann LaRoche, Novartis, and Pfizer and grants from Hoffmann LaRoche, Genentech, and BMS during the conduct of the study; and personal fees from Novartis, Pfizer, and Eli Lilly outside the submitted work. Dr Krop reported grants and personal fees from Genentech/Roche and personal fees from Daiichi/Sankyo, Macrogenics, and Taiho outside the submitted work. Dr Sparano reported personal fees, consultancy, and study participation from Roche and personal fees and consultancy Astra Zeneca during the conduct of the study. Dr Disis reported Janssen, Celgene, Pfizer, EMD Serono, EpiThany, and Silverback Therapeutics during the conduct of the study; in addition, Dr Disis had a patent issued, associated with University of Washington. Dr Mittendorf reported funding for a clinical trial for which she was principal investigator from Astra Zeneca, EMD Serono, Galena Biopharma, and Genentech/Roche, and personal fees from Genentech/Roche, Merck, Peregrine Pharmaceuticals, SELLAS Life Sciences, and Tapimmune Inc during the conduct of the study; and personal fees from Physician Education Resource outside the submitted work. No other disclosures were reported.
Footnotes
Publisher's Disclaimer: Disclaimer: Dr Disis is the Editor of JAMA Oncology, but she was not involved in any of the decisions regarding review of the manuscript or its acceptance.
REFERENCES
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359 (6382):1350–1355. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499 (7457):214–218. doi: 10.1038/nature12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budczies J, Bockmayr M, Denkert C, et al. Classical pathology and mutational load of breast cancer—integration of two worlds. J Pathol Clin Res. 2015;1(4):225–238. doi: 10.1002/cjp2.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luen S, Virassamy B, Savas P, Salgado R, Loi S. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016;29: 241–250. doi: 10.1016/j.breast.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 7.Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2(10):1354–1360. doi: 10.1001/jamaoncol.2016.1061 [DOI] [PubMed] [Google Scholar]
- 8.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 9.Dieci MV, Mathieu MC, Guarneri V, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26(8):1698–1704. doi: 10.1093/annonc/mdv239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112 [DOI] [PubMed] [Google Scholar]
- 11.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 12.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27): 2959–2966. doi: 10.1200/JCO.2013.55.0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014;148(3):467–476. doi: 10.1007/s10549-014-3185-2 [DOI] [PubMed] [Google Scholar]
- 14.Salgado R, Denkert C, Campbell C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in her2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1(4):448–454. doi: 10.1001/jamaoncol.2015.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ignatiadis M, Van den Eynden G, Roberto S, et al. Tumor-infiltrating lymphocytes in patients receiving trastuzumab/pertuzumab-based chemotherapy: A TRYPHAENA substudy. J Natl Cancer Inst. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13(9):1302–1311. doi: 10.1016/j.jtho.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34(21): 2460–2467. doi: 10.1200/JCO.2015.64.8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emens LA, Braiteh FS, Cassier P, et al. Abstract 2859: inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC). Cancer Res. 2015;75(15)(suppl):2859–2859. doi: 10.1158/1538-7445.AM2015-2859 [DOI] [Google Scholar]
- 19.Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase 2 KEYNOTE-086 Study. [published online November 26, 2018].Ann Oncol. 2018. doi: 10.1093/annonc/mdy517 [DOI] [PubMed] [Google Scholar]
- 21.Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase 2 KEYNOTE-086 Study. [published online November 26, 2018].Ann Oncol. 2018. doi: 10.1093/annonc/mdy518 [DOI] [PubMed] [Google Scholar]
- 22.Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671–686. doi: 10.1007/s10549-017-4537-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rugo HS, Delord JP, Im SA, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res. 2018;24(12):2804–2811. doi: 10.1158/1078-0432.CCR-17-3452 [DOI] [PubMed] [Google Scholar]
- 24.Adams S, Diamond JR, Hamilton E, et al. Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: a phase 1b clinical trial. JAMA Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolaney S, Kalinsky K, Kaklamani V, et al. Abstract PD6–13: Phase 1b/2 study to evaluate eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer. Cancer Res. 2018;78 (4)(suppl):6–16. [Google Scholar]
- 26.Tolaney SM, Kabos P, Dickler MN, et al. Updated efficacy, safety, & PD-L1 status of patients with HR+, HER2- metastatic breast cancer administered abemaciclib plus pembrolizumab. J Clin Oncol. 2018;36(15)(suppl):1059. doi: 10.1200/JCO.2018.36.15_suppl.1059 [DOI] [Google Scholar]
- 27.Domchek S, Postel-Vinay S, Bang Y-J, et al. Abstract PD6–11: an open-label, multitumor, phase II basket study of olaparib and durvalumab (MEDIOLA): results in germline BRCA-mutated (gBRCAm) HER2-negative metastatic breast cancer (MBC). Cancer Res. 2018;78(4)(suppl):6–16. [Google Scholar]
- 28.Vinayak S, Tolaney SM, Schwartzberg LS, et al. TOPACIO/Keynote-162: Niraparib + pembrolizumab in patients (pts) with metastatic triple-negative breast cancer (TNBC), a phase 2 trial. J Clin Oncol. 2018;36(15)(suppl):1011. doi: 10.1200/JCO.2018.36.15_suppl.1011 [DOI] [Google Scholar]
- 29.Loi S, Giobbie-Hurder A, Gombos A, et al. ; International Breast Cancer Study Group and the Breast International Group. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019; 20(3):371–382. doi: 10.1016/S1470-2045(18)30812-X [DOI] [PubMed] [Google Scholar]
- 30.Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16(13):3485–3494. doi: 10.1158/1078-0432.CCR-10-0505 [DOI] [PubMed] [Google Scholar]
- 31.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 32.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi: 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- 33.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107 [DOI] [PubMed] [Google Scholar]
- 34.Garg AD, More S, Rufo N, et al. Trial watch: immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology. 2017;6(12): e1386829. doi: 10.1080/2162402X.2017.1386829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmid P, Adams S, Rugo HS, et al. ; IMpassion130 Trial Investigators. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 36.Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.506.AccessedMarch 14, 2019.
- 37.Loibl S, Untch M, Burchardi N, et al. Randomized phase II neoadjuvant study (GeparNuevo) to investigate the addition of durvalumab to a taxane-anthracycline containing chemotherapy in triple negative breast cancer (TNBC). J Clin Oncol. 2018;36(15)(suppl):104. doi: 10.1200/JCO.2018.36.15_suppl.104 [DOI] [Google Scholar]
- 38.Schmid P, Park YH, Muñoz-Couselo E, et al. Pembrolizumab (pembro) + chemotherapy (chemo) as neoadjuvant treatment for triple negative breast cancer (TNBC): Preliminary results from KEYNOTE-173. J Clin Oncol. 2017;35(15) (suppl):556. doi: 10.1200/JCO.2017.35.15_suppl.556 [DOI] [Google Scholar]
- 39.Pusztai L, Silber A, Hofstatter EW, et al. Safety of MEDI4736 (anti-PD-L1 antibody) administered concomitant with weekly nab-paclitaxel and dose dense doxorubicin/cyclophosphamide (ddAC) as neoadjuvant chemotherapy for stage I-III triple negative breast cancer (TNBC): a phase I/II trial. J Clin Oncol. 2017;35(15)(suppl):572. doi: 10.1200/JCO.2017.35.15_suppl.572 [DOI] [Google Scholar]
- 40.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1(9):1325–1332. doi: 10.1001/jamaoncol.2015.2756 [DOI] [PubMed] [Google Scholar]
- 41.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4): 256–265. doi: 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124 (2):687–695. doi: 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728–734. [PubMed] [Google Scholar]
- 44.Tang C, Welsh JW, de Groot P, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res. 2017;23(6):1388–1396. doi: 10.1158/1078-0432.CCR-16-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barker CA, Postow MA, Khan SA, et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res. 2013;1(2):92–98. doi: 10.1158/2326-6066.CIR-13-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7): 1813–1821. doi: 10.1093/annonc/mdt107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McArthur HL, Barker CA, Gucalp A, et al. A phase II, single arm study assessing the efficacy of pembrolizumab (Pembro) plus radiotherapy (RT) in metastatic triple negative breast cancer (mTNBC). http://ascopubs.org/doi/abs/10.1200/JCO.2018.36.5_suppl.14.
- 48.McArthur H, Beal K, Halpenny D, et al. Abstract 4705: CTLA4 blockade with HER2-directed therapy (H) yields clinical benefit in women undergoing radiation therapy (RT) for HER2-positive (HER2+) breast cancer brain metastases (BCBM). Vol 772017. http://cancerres.aacrjournals.org/content/77/13_Supplement/4705.AccessedMarch 14, 2019. [Google Scholar]
- 49.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McArthur HL, Diab A, Page DB, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res. 2016;22(23):5729–5737. doi: 10.1158/1078-0432.CCR-16-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gall VA, Philips AV, Qiao N, et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Cancer Res. 2017;77(19):5374–5383. doi: 10.1158/0008-5472.CAN-16-2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knutson KL, Clynes R, Shreeder B, et al. Improved survival of HER2+ breast cancer patients treated with trastuzumab and chemotherapy is associated with host antibody immunity against the HER2 intracellular domain. Cancer Res. 2016;76 (13):3702–3710. doi: 10.1158/0008-5472.CAN-15-3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor C, Hershman D, Shah N, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13(17):5133–5143. doi: 10.1158/1078-0432.CCR-07-0507 [DOI] [PubMed] [Google Scholar]
- 54.Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–475. doi: 10.1038/nature23465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng J, Wang ES, Jenkins RW, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8 (2):216–233. doi: 10.1158/2159-8290.CD-17-0915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Higuchi T, Flies DB, Marjon NA, et al. CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol Res. 2015;3(11):1257–1268. doi: 10.1158/2326-6066.CIR-15-0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang J, Wang L, Cong Z, et al. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1(−/−) murine model of ovarian cancer. Biochem Biophys Res Commun. 2015;463 (4):551–556. doi: 10.1016/j.bbrc.2015.05.083 [DOI] [PubMed] [Google Scholar]
- 58.Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711–3720. doi: 10.1158/1078-0432.CCR-16-3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray JL, Gillogly ME, Przepiorka D, et al. Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTLs by HER-2 peptide E75 (369–377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin Cancer Res 2002;8(11):3407–3418. [PubMed] [Google Scholar]
- 60.Knutson KL, Schiffman K, Cheever MA, Disis ML. Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369–377, results in short-lived peptide-specific immunity. Clin Cancer Res. 2002;8(5):1014–1018. [PubMed] [Google Scholar]
- 61.Kono K, Takahashi A, Sugai H, et al. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8(11):3394–3400. [PubMed] [Google Scholar]
- 62.Disis ML, Gooley TA, Rinn K, et al. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20(11):2624–2632. doi: 10.1200/JCO.2002.06.171 [DOI] [PubMed] [Google Scholar]
- 63.Mittendorf EA, Ardavanis A, Litton JK, et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget. 2016;7(40): 66192–66201. doi: 10.18632/oncotarget.11751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mittendorf EA, Clifton GT, Holmes JP, et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol. 2014;25(9):1735–1742. doi: 10.1093/annonc/mdu211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. doi: 10.1038/nature22991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. doi: 10.1126/science.aaf1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zacharakis N, Chinnasamy H, Black M, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018;24(6):724–730. doi: 10.1038/s41591-018-0040-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–68. doi: 10.1126/science.aaa4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4): 843–851. doi: 10.1038/mt.2010.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seymour L, Bogaerts J, Perrone A, et al. ; RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loi S, Adams S, Schmid P, et al. LBA13 Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): results from KEYNOTE-086. Ann Oncol. 2017;28(suppl 5): mdx440.005. doi: 10.1093/annonc/mdx440.005 [DOI] [Google Scholar]
- 72.Molinero L, Chang C-W, Udyavar A, et al. Abstract P2-09-13: molecular characterization of tumors from metastatic TNBC patients treated with atezolizumab (atezo). Cancer Res. 2018;78(4) (suppl):P2-09-13-P02-09-13. [Google Scholar]
- 73.Salgado R, Denkert C, Demaria S, et al. ; International TILs Working Group 2014. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site—when a biomarker defines the indication. N Engl J Med. 2017;377(15): 1409–1412. doi: 10.1056/NEJMp1709968 [DOI] [PubMed] [Google Scholar]


