Abstract
Objective:
Photobiomodulation (PBM) using diode laser is regarded an effective modality for the repair of tissues and control of pain. Ozone, owing to its biocompatibility, healing, and antimicrobial properties, is used in dentistry as well. This study was carried to clinically compare and evaluate the healing of gingival depigmented wounds using ozonated oil and PBM.
Materials and Methods:
A laser depigmentation procedure was conducted on seven patients exhibiting bilateral upper and lower gingival melanin hyperpigmentation, followed by the application of ozonated oil (Group 1) and laser PBM (Group 2). The clinical parameters are taken namely Visual Analog Scale and Healing Index (HI), were evaluated on the 3rd, 7th, and 15th day.
Results:
Statistical analysis showed better HI in Group I as compared to Group II on the 3rd day, but it was comparable in both groups on the 7th and 15th day.
Conclusion:
The application of ozonated oil was found to be more efficacious in promoting the initial healing of wound in comparison to PBM. Both ozonated oil and PBM also showed the same capabilities in reduction of the postoperative pain.
Keywords: Diode laser, gingival depigmentation, laser depigmentation, ozone therapy, photobiomodulation
INTRODUCTION
Excessive melanin, which is a brown pigment derived from melanocytes, leads to hyperpigmentation of the gingiva causing an unesthetic appearance of brown or dark brown with blackish hue patches of the gingiva.[1]
Gingival pigmentation is considered to be multifactorial, physiological/pathological,[2] but according to Dummett (1946),[3] stimulation by either mechanical, physical, or chemical plays a role in the degree of pigmentation. Due to esthetic reasons, some patients opt for gingival depigmentation[4] and are removed by varied techniques using a scalpel, bur abrasion, electrosurgery, lasers, free gingival graft, and cellular dermal matrix allograft, etc.[5]
Depigmentation using diode lasers are considered to be safe and effective,[6] and they have the advantage of easy usage, convenience in dental clinics, and decreased trauma for the patient[7] and conjointly for its application in photobiomodulation (PBM).
PBM is also known as low-level laser therapy (LLLT), and there have been numerous studies relating to PBM in promoting wound healing[8,9] and are also proven to be a highly effective modality for tissue repair and pain control.[10] Similarly, ozone (O3)-a natural gaseous molecule made up of three oxygen atoms has been trending for use in the promotion of wound healing.[9] The use of O3 has been proposed in dentistry as a result of its antimicrobial, disinfectant, biocompatibility, and healing properties.[11] Ozonated olive oil is pure olive oil that has undergone ozonization using a steady flow of O3-oxygen mixture in the ratio of 5:95% until olive oil transforms from the greenish-colored liquid status to the whitish gel status.[12]
However, there have been limited studies regarding the use of ozonated oil for promoting depigmented wound healing and also its comparison with laser PBM for accelerating depigmented wound healing. Therefore, this study aimed to compare the effects of laser PBM and topical ozonated oil application on wound healing after gingival depigmentation with a 940 nm diode laser.
MATERIALS AND METHODS
Participants were chosen from the Outpatient Clinic of Department of Periodontology and Implantology who were concerned with the unesthetic appearance due to gingival hyperpigmentation. With the help of a literature survey, we have found the expected standard deviation (SD) of Group 1 and Group 2 is 0.08 and 0.09, respectively, and the mean difference is 1.3 of two groups for variables. Using the software Open Epi, Version 3 (OpenEpi: Open Source Epidemiologic Statistics for Public Health, www.OpenEpi.com), we have found the sample size for each group is 7. Hence, a total of seven patients were included in this 15-day split-mouth study. A written and informed consent was obtained from all the participants and patients were also advised for blood investigation which includes complete blood count, hemoglobin%, platelet count, bleeding, and clotting time. The present study was approved by the Institutional Ethical Committee.
The inclusion criteria are as follows: Systemically healthy patient, no antibiotic therapy for the last 6 months, and bilateral gingival pigmentation on the anterior maxillary and mandibular anterior.
The exclusion criteria are as follows: Pregnant/lactating mother, patients who were tobacco user, and noncompliant patient. In Group 1 – 14 sites with gingival hyperpigmentation were treated with laser ablation followed by the application of ozonated oil over the surgical site. In Group 2 – 14 sites with gingival hyperpigmentation were treated with laser ablation followed by PBM application at 1 mm distance from the surgical site for 5 min.
Selected sites were assessed for the following clinical parameters at baseline, 3 days, 7 days, and 15 days – Wound Healing Index (HI),[13] Visual Analog Scale (VAS) score (for pain), and Amount of Epithelialization.[14]
All the patients received scaling and root planning and oral hygiene instructions. Patients were re-evaluated after 1 week for optimal oral hygiene, and only those patients maintaining optimum oral hygiene were included in the study [Figures 1a and 2a].
Figure 1.
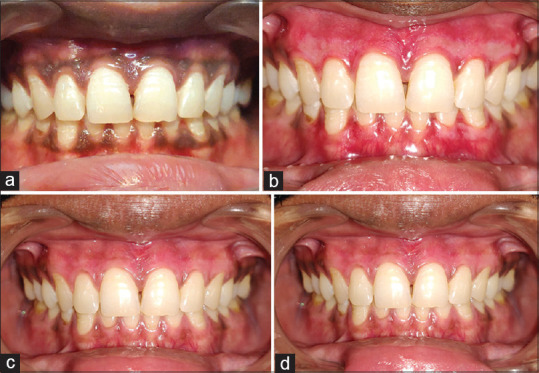
(a) Case 1; preoperative view; (b) Case 1; 3rd day postoperative view; ozonated oil (left), photobiomodulation (right); (c) Case 1; 7th day postoperative view; ozonated oil (left), photobiomodulation (right); (d) Case 1; 15th day postoperative view; ozonated oil (left), photobiomodulation (right)
Figure 2.
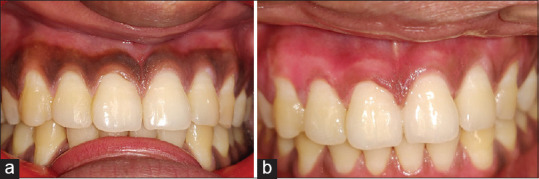
(a) Case 2; preoperative view; (b) Case 2; 15th day postoperative view; ozonated oil (left), photobiomodulation (right)
The diode laser (Biolase EpicX, Biolase Inc., CA, USA), with a fiber optic delivery system and a beam diameter of 400 μm, 940 nm wavelength was used and operated at a 1.5 W irradiation power, in a continuous contact mode. Before starting the surgical procedure, patients were asked to rinse with 0.2% chlorhexidine gluconate. After the area is anesthetized by lidocaine hydrochloride, laser ablation was performed from the mucogingival junction working toward the free gingival margin, including the papillae in a continuous contact mode, and the fiber tip was constantly moved across the site to avoid heat accumulation at any site. Water spray was used to help rinse the tissue as ablation proceeded. The same procedure was repeated until the pigments were finally cleared. Then, finally, the area was wiped with saline-soaked gauze. Following depigmentation, the depigmented sites of each patient were then randomly divided into the left and right halves (split-mouth technique). One half was treated with ozonated oil (Group 1) and the other half by PBM (Group 2). Sterile gauze was used to prevent the ozonated oil from overflowing over the PBM and vice versa during LLLT application. For Group 1, few drops of ozonated oil which come in a syringe system (DENTOZONE, ADC Inc., Mumbai, Maharashtra, India) were delivered on the entire depigmented gingiva and left for 30 s. Ozonated oil was applied immediately after the procedure on the 3rd and 7th day. For Group 2, LLLT using a defocused diode laser at 1 mm distance for 5 min with a power setting at 1 W at continuous mode was introduced to the sites. PBM was initiated immediately after surgery, on the 3rd and 7th day.
Postoperative instructions included avoiding smoking and eating hot and spicy food for the first 24 h. Furthermore, patients were advised not to traumatize the area during the healing period which is 4–7 days after treatment and to take analgesics Ketorolac tromethamine dispersable tablet 10 mg (Ketorol D. T, Dr. Reddy's, India) after the surgery and to continue with the medication for the next 3 days if the pain was experienced.
The patients were then followed up for three subsequent visits, after 3 days, on the 7th day and 15 days. Group 1 and Group 2 sites were compared based on the above-mentioned parameters.
Wound healing was evaluated according to the HI given by Landry et al.[13] The VAS was used to evaluate the subjective pain level experienced by each patient. The VAS consisted of values ranging from 0 to 10, with the left end depicting “no pain” and at the right end “unbearable pain.” The value marked by the patient was used as the VAS score.[9]
The amount of wound epithelialization was determined clinically by applying 3% hydrogen peroxide (H2O2) to the depigmented area. Based on this method, H2O2 diffuses into the connective tissue, where the wound area in the absence of epithelium can be observed clinically by the production of bubbles (oxygen liberation), and the scores were interpreted as 0, 1/3, 2/3, and 3/3.[14]
Statistical analysis
The Mann–Whitney test was used to identify the significant differences between the VAS score, HI, the comparison of bleeding and swelling before and after treatment and Wilcoxon Signed Ranks test was used for intra-group comparison for the same as well. P < 0.05 was set to be statistically significant. The analysis was performed using the SPSS software, Windows version 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The study was conducted on seven patients (28 sites); 3 females (42.8%) and 4 males (57.4%) exhibiting bilateral upper and lower gingival melanin hyperpigmentation with Dummet Oral Melanin Pigmentation (DOPI) score of 1, 2, and 3. The mean ± SD values of age were 22.7 ± 3.1 years with a minimum of 16 years and a maximum of 26 years old. Four cases (57.4%) had moderate pigmentation (DOPI score 2), whereas two cases (28.5%) had mild pigmentation (DOPI score 1) and 1 case (14.2%) had heavy pigmentation (DOPI score 3).
All patients completed the study and complied with the postoperative appointments. The 940 nm diode laser was used to ablate the hyperpigmented gingiva. All the treated sites showed uneventful wound healing.
A white fibrin slough was seen in all patients on the 3rd day [Figure 1b]. At 1 week, the treated gingiva showed fast epithelialization with a healthy appearance but immature healing in all cases [Figure 1c]. After 15 days, complete healing was observed in almost all the cases, and the gingiva exhibited normal appearance [Figures 1d and 2b]. Three sites in Group 2 showed incomplete healing. Postoperative side effects such as gingival recession were not observed in any of the cases during the 15 days' follow-up.
VAS score for pain: There was a drastic reduction in VAS score at day 7 as well as day 15 where the mean VAS score was 0.50 ± 0.760 for Group 1 and 0.71 ± 0.726 for Group 2 at day 7 and 0.00 ± 0.00 for Group 1 and 0.07 ± 0.267 for Group 2 at day 15. However, the results were not statistically significant in the VAS scores of both the groups at baseline, 3rd, 7th, and 15th-day follow-up [Table 1].
Table 1.
Comparison of Visual Analog Scale scores in Group 1 and Group 2
| VAS | Group 1 | Group 2 | P value |
|---|---|---|---|
| Baseline | 0.50±0.519 | 0.64±0.633 | 0.584 (NS) |
| Day 3 | 2.00±1.038 | 1.64±1.151 | 0.328 (NS) |
| Day 7 | 0.71±0.726 | 0.57±0.756 | 0.544 (NS) |
| Day 15 | 0.00±0.000 | 0.07±0.267 | 0.317 (NS) |
Statistical significance at P value < 0.05. NS – Not significant; VAS – Visual Analog Scale
HI – On the evaluation of the HI scores, Group 1 showed statistically significant healing on the 3rd day (P = 0.00); also, it was found to be better in Group 1 on the 7th as well as on the 15th day (P = 0.061 and 0.072, respectively), but the results obtained were not statistically significant. By day 15, excellent healing was observed in all the sites (both Group 1 and Group 2) except on three sites of Group 2, which showed some amount of redness as compared to the adjacent healthy pink tissue [Table 2].
Table 2.
Comparison of healing index score (Landry et al., 1985) in Group 1 and Group 2
| HI | Group 1 | Group 2 | P value |
|---|---|---|---|
| Baseline | 1.00±0.000 | 1.00±0.000 | 1.000 (NS) |
| Day 3 | 3.43±0.514 | 2.50±0.519 | 0.000 (S)* |
| Day 7 | 4.57±0.514 | 4.07±0.730 | 0.061 (NS) |
| Day 15 | 5.00±0.000 | 4.79±0.426 | 0.072 (NS) |
*Statistical significance at P value < 0.05. S – Significant; NS – Not significant; HI – Healing index
Amount of epithelialization – The results were reported as 0, 1/3, 2/3, and 3/3 for each wound site where there was a significant increase in the amount of epithelialization on the 3rd day (P = 0.024) in both the groups but was nonsignificant on the 7th and 15th day (P = 0.366, 1.000 respectively). However, Group 1 showed a greater amount of epithelialization which was statistically significant on the 3rd day which was in agreement with the amount of healing [Table 3].
Table 3.
Comparison of the amount of surface epithelialization in Group 1 and Group 2
| Keratinization | Group 1 | Group 2 | P value |
|---|---|---|---|
| Baseline | 0.00±0.000 | 0.00±0.000 | 1.000 (NS) |
| Day 3 | 1.64±0.497 | 1.21±0.426 | 0.024 (S)* |
| Day 7 | 2.29±0.469 | 2.14±0.363 | 0.366 (NS) |
| Day 15 | 0.366±0.366 | 3.00±0.000 | 1.000 (NS) |
*Statistical significance at P value < 0.05. S – Significant; NS – Not significant
DISCUSSION
Gingival hyperpigmentation often forces patients to demand esthetic removal of the discolored gingiva. One of the most effective, comfortable, and reliable technique for depigmentation of gingiva is laser ablation.[15]
O3 therapy is reported to present beneficial effects on hard- and soft-tissue wound healing. O3 and its products are currently being investigated for their influence on growth factors, cytokines, and cell-cycle regulation in the biological systems.[16] Bocci[17,18,19] conducted a series of studies and showed that the contact of O3 with human blood led to an increased release of transforming growth factor beta 1; interferons-alpha,-beta, and-gamma; interleukins-1,-2,-6, and-8), and tumor necrosis factor-alpha, which are important for human wound healing. The positive effects of healing on O3 application can also be explained by the mechanisms that include enhancing the activity of inflammatory cells during the inflammatory phase, stimulating the production of cytokines and interferons, reactivating the antioxidant system, improving blood circulation and tissue oxygenation processes, and increasing the elasticity of red blood cells.[16,17,19]
There are several investigations done on the influence of O3 therapy on free gingival graft healing and donor site healing.[9,19,20] To the best of our knowledge, the literature lacks information derived from the clinical trials on the efficacy of the application of ozonated oil on depigmented wound sites.
The low output power is also known as LLLT or PBM. Recently, several animal models and human clinical studies have addressed the effects of PBM, which is a promising treatment option that has a beneficial impact during the inflammation, proliferation, and remodeling phases for the acceleration of re-epithelialization and palatal wound healing.[21,22,23,24]
In the present study, the effects of laser PBM and topical ozonated olive oil on gingival depigmented wounds were assessed and compared. Group 1 received ozonated olive oil [Figure 3], and Group 2 received laser PBM [Figure 4]. The results of our study showed a significant difference in HI as well as epithelialization in favor of Group 1 on the 3rd day. Although not statistically significant, the rate was faster in Group 1 at other follow-up visits, i.e., on the 7th day and 15th day. It is hypothesized that the mechanism of action of O3 oil on wound healing may be connected to its antimicrobial effect and promotion of the release of growth factors and activate local antioxidant mechanisms.[25] Bacteria affects wound healing in the oral cavity since the oral wounds are constantly subjected to a diverse commensal flora.[26] Early epithelial healing ultimately results in rapid keratinization and better healing of the gingival tissue. Therefore, the amount of epithelialization was also found to be better on the 3rd day in the ozonated oil group than the PBM group. Thus, the control of bacteria might be due to the direct or indirect effect of ozonated oil in the connective and epithelial tissue cells and resulting in the acceleration of oral wound healing. The effects of LLLT on wound healing after depigmentation were evaluated by Chawla et al., and they reported that LLLT promotes wound healing until the 3rd day, and no statistical difference was observed on the wound healing later.[21] The result for the PBM group in the present study is similar to the study by Ozcelik et al.,[27] who found that LLLT applied sites after gingivectomy procedures have significantly increased healing on the 3rd, 7th, and 15th day.
Figure 3.
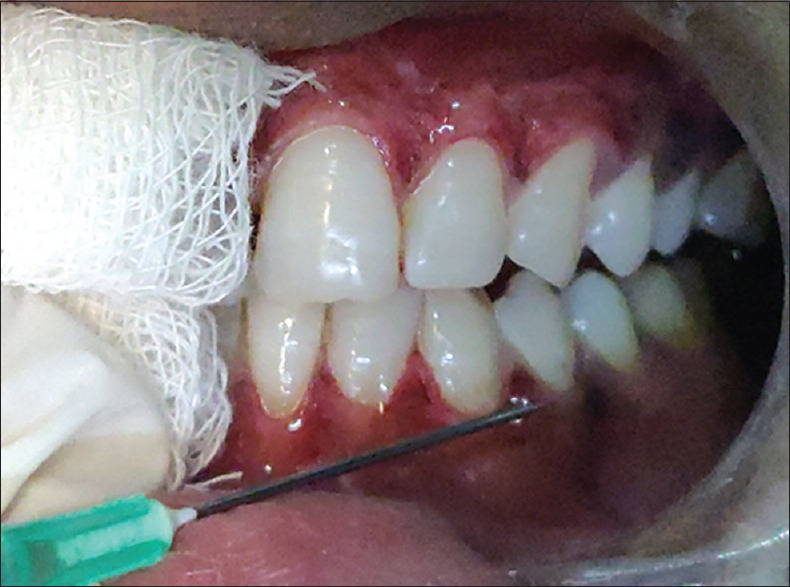
Application of ozonated oil in a syringe system
Figure 4.
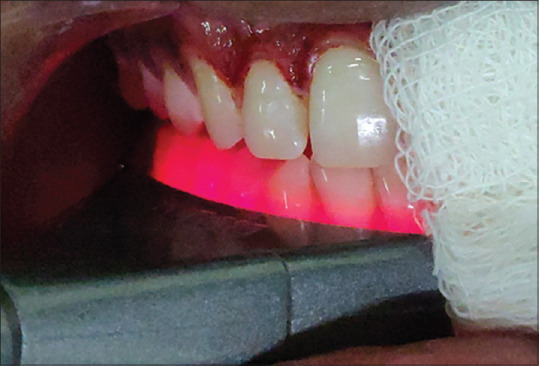
Application of laser photobiomodulation
In the present study, there was no significant difference found between Group 1 and Group 2 in terms of VAS score. The result of our study was similar to the study done by Isler et al.,[9] where they found that both O3 therapy and PBM, reduced postoperative discomfort as compared with spontaneous healing. Taşdemir et al.[20] also stated that O3 application to both donor sites and recipient sites significantly decreases the 1st postoperative week following Free Gingival Grafting FGG. Dias et al.[28] and da Silva Neves et al.[29] had reported no significant differences between laser-treated groups treated by a diode laser and control groups in the reduction of postoperative discomfort. Despite the above-mentioned studies, there are no other clinical trials available to compare the results obtained in the present study. The limitations of our study include no control group (i.e., only laser), a small sample size, and lack of histological examination.
CONCLUSION
Within the limitations of the study, the conclusion drawn from the findings was that overall ozonated oil was found to be more efficacious in promoting early wound healing as well as epithelialization in comparison to PBM. Both ozonated oil and PBM also showed the same capability in terms of reduction of postoperative pain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Abdel Moneim RA, El Deeb M, Rabea AA. Gingival pigmentation (cause, treatment and histological preview) Future Dent J. 2017;3:1–7. [Google Scholar]
- 2.Deepak P, Sunil S, Mishra R, Sheshadri Treatment of gingival pigmentation: A case series. Indian J Dent Res. 2005;16:171–6. doi: 10.4103/0970-9290.29901. [DOI] [PubMed] [Google Scholar]
- 3.Dummett CO. Physiologic pigmentation of the oral and cutaneous tissues in the Negro. J Dent Res. 1946;25:421–32. doi: 10.1177/00220345460250060201. [DOI] [PubMed] [Google Scholar]
- 4.Grover HS, Dadlani H, Bhardwaj A, Yadav A, Lal S. Evaluation of patient response and recurrence of pigmentation following gingival depigmentation using laser and scalpel technique: A clinical study. J Indian Soc Periodontol. 2014;18:586–92. doi: 10.4103/0972-124X.142450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kathariya R, Pradeep AR. Split mouth de-epithelization techniques for gingival depigmentation: A case series and review of literature. J Indian Soc Periodontol. 2011;15:161–8. doi: 10.4103/0972-124X.84387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murthy MB, Kaur J, Das R. Treatment of gingival hyperpigmentation with rotary abrasive, scalpel, and laser techniques: A case series. J Indian Soc Periodontol. 2012;16:614–9. doi: 10.4103/0972-124X.106933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma H, Kaushik M, Tomar N, Wadhawan A, Dureja D. Treatment of gingival melanin hyperpigmentation with diode laser: A report of two cases. Int J Orofac Res. 2018;3:14–6. [Google Scholar]
- 8.Kuffler DP. Photobiomodulation in promoting wound healing: A review. Regen Med. 2016;11:107–22. doi: 10.2217/rme.15.82. [DOI] [PubMed] [Google Scholar]
- 9.Isler SC, Uraz A, Guler B, Ozdemir Y, Cula S, Cetiner D. Effects of laser photobiomodulation and ozone therapy on palatal epithelial wound healing and patient morbidity. Photomed Laser Surg. 2018;36:571–80. doi: 10.1089/pho.2018.4492. [DOI] [PubMed] [Google Scholar]
- 10.Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD. The efficacy of low-power lasers in tissue repair and pain control: A meta-analysis study. Photomed Laser Surg. 2004;22:323–9. doi: 10.1089/pho.2004.22.323. [DOI] [PubMed] [Google Scholar]
- 11.Naik SV, Rajeshwari K, Kohli S, Zohabhasan S, Bhatia S. Ozone – A biological therapy in dentistry- reality or myth? Open Dent J. 2016;10:196–206. doi: 10.2174/1874210601610010196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khare M, Jain KS, Sharma N. Comparative evaluation of effects of subgingival application of ozonated olive oil gel versus chlorhexidine in patients with chronic periodontitis: A clinicomicrobiological randomised trial. J Dent Specialities. 2019;7:95–101. [Google Scholar]
- 13.Landry RG, Turnbull RS, Howley T. Effectiveness of benzydamyne HCl in the treatment of periodontal post-surgical patients. Res Clin Forums. 1988;10:105–18. [Google Scholar]
- 14.Yaghobee S, Rouzmeh N, Aslroosta H, Mahmoodi S, Khorsand A, Kharrazifard MJ. Effect of Topical Erythropoietin (EPO) on palatal wound healing subsequent to Free Gingival Grafting (FGG) Braz Oral Res. 2018;32:e55. doi: 10.1590/1807-3107bor-2018.vol32.0055. [DOI] [PubMed] [Google Scholar]
- 15.Berk G, Atici K, Berk N. Treatment of gingival pigmentation with Er, Cr: YSGG Laser. J Oral Laser Appl. 2005;5:249–53. [Google Scholar]
- 16.Patel PV, Kumar S, Vidya GD, Patel A, Holmes JC, Kumar V. Cytological assessment of healing palatal donor site wounds and grafted gingival wounds after application of ozonated oil: An eighteen-month randomized controlled clinical trial. Acta Cytol. 2012;56:277–84. doi: 10.1159/000336889. [DOI] [PubMed] [Google Scholar]
- 17.Bocci V. Ozone as Janus: This controversial gas can be either toxic or medically useful. Mediators Inflamm. 2004;13:3–11. doi: 10.1080/0962935062000197083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bocci VA. Scientific and medical aspects of ozone therapy.State of the art. Arch Med Res. 2006;37:425–35. doi: 10.1016/j.arcmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Di Paolo N, Bocci V, Gaggiotti E. Ozone therapy. Int J Artif Organs. 2004;27:168–75. doi: 10.1177/039139880402700303. [DOI] [PubMed] [Google Scholar]
- 20.Taşdemir Z, Alkan BA, Albayrak H. Effects of ozone therapy on the early healing period of deepithelialized gingival grafts: A randomized placebo-controlled clinical trial. J Periodontol. 2016;87:663–71. doi: 10.1902/jop.2016.150217. [DOI] [PubMed] [Google Scholar]
- 21.Chawla K, Lamba AK, Tandon S, Faraz F, Gaba V. Effect of low-level laser therapy on wound healing after depigmentation procedure: A clinical study. J Indian Soc Periodontol. 2016;20:184–8. doi: 10.4103/0972-124X.176393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ustaoglu G, Ercan E, Tunali M. Low-level laser therapy in enhancing wound healing and preserving tissue thickness at free gingival graft donor sites: A randomized, controlled clinical study. Photomed Laser Surg. 2017;35:223–30. doi: 10.1089/pho.2016.4163. [DOI] [PubMed] [Google Scholar]
- 23.Fahimipour F, Houshmand B, Alemi P, Asnaashari M, Tafti MA, Akhoundikharanagh F, et al. The effect of He-Ne and Ga-Al-As lasers on the healing of oral mucosa in diabetic mice. J Photochem Photobiol B. 2016;159:149–54. doi: 10.1016/j.jphotobiol.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Wang CY, Tsai SC, Yu MC, Lin YF, Chen CC, Chang PC. Light-emitting diode irradiation promotes donor site wound healing of the free gingival graft. J Periodontol. 2015;86:674–81. doi: 10.1902/jop.2015.140580. [DOI] [PubMed] [Google Scholar]
- 25.Patel PV, Kumar V, Kumar S, Gd V, Patel A. Therapeutic effect of topical ozonated oil on the epithelial healing of palatal wound sites: A planimetrical and cytological study. J Investig Clin Dent. 2011;2:248–58. doi: 10.1111/j.2041-1626.2011.00072.x. [DOI] [PubMed] [Google Scholar]
- 26.Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17:91–6. doi: 10.1097/00001432-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ozcelik O, Cenk Haytac M, Kunin A, Seydaoglu G. Improved wound healing by low-level laser irradiation after gingivectomy operations: A controlled clinical pilot study. J Clin Periodontol. 2008;35:250–4. doi: 10.1111/j.1600-051X.2007.01194.x. [DOI] [PubMed] [Google Scholar]
- 28.Dias SB, Fonseca MV, Dos Santos NC, Mathias IF, Martinho FC, Junior MS, et al. Effect of GaAIAs low-level laser therapy on the healing of human palate mucosa after connective tissue graft harvesting: Randomized clinical trial. Lasers Med Sci. 2015;30:1695–702. doi: 10.1007/s10103-014-1685-2. [DOI] [PubMed] [Google Scholar]
- 29.da Silva Neves FL, Silveira CA, Dias SB, Santamaria Júnior M, de Marco AC, Kerbauy WD, et al. Comparison of two power densities on the healing of palatal wounds after connective tissue graft removal: Randomized clinical trial. Lasers Med Sci. 2016;31:1371–8. doi: 10.1007/s10103-016-1988-6. [DOI] [PubMed] [Google Scholar]


