Abstract
Background:
The role of prenatal diagnosis in reducing neonatal mortality from transposition of the great arteries (TGA) is controversial. Factors affected by prenatal diagnosis such as proximity at birth to a cardiac surgical center (CSC) and CSC volume are associated with mortality in congenital heart disease. The purpose of the study was to determine the associations between prenatal diagnosis, distance from birthplace to a CSC, CSC TGA volume, and neonatal mortality in patients with TGA.
Methods:
The Texas Birth Defects Registry was queried for all live born infants with TGA from 1999 to 2007. Four hundred sixty-eight cases of TGA were included.
Results:
Forty-eight patients (10.3%) were prenatally diagnosed, and 20 patients died before age 28 days (4.3%). Neither prenatal diagnosis nor close proximity to a CSC at birth (p > 0.05) were associated with decreased mortality. Low CSC TGA volume was associated with increased mortality (p < 0.0002). Mortality at the CSCs with <5 patients per year was 9.6%; CSCs with 5 to 10 patients per year had 0% mortality, and those with >10 patients per year had 2.3% mortality. In multivariable logistic regression, only preterm birth (odds ratio, 7.05; 95% confidence interval, 4.13–12.05) and lower CSC volume (p < 0.001) were associated with neonatal mortality, although prenatal diagnosis attenuated the detrimental association of lower volume CSCs with higher mortality (p for interaction = 0.047).
Conclusion:
Lower CSC TGA patient volume was associated with higher neonatal mortality. Prenatal diagnosis may improve survival in lower volume CSCs.
Keywords: transposition of the great arteries, arterial switch operation, prenatal diagnosis, congenital heart disease, surgical outcomes, mortality, hospital volume
Introduction
The question of whether prenatal diagnosis of transposition of the great arteries (TGA) affects patient outcomes is controversial. The rate of prenatal diagnosis of all congenital heart disease has increased over the past 3 decades with improved ultrasound technology and availability (Hill et al., 2015). It seems intuitive that prenatal diagnosis would further improve outcomes of patients with TGA by allowing more rapid resuscitation, yet most studies have not confirmed this hypothesis (Copel et al., 1997; Kumar et al., 1999; Mahle et al., 2001; Verheijen et al., 2001; Bartlett et al., 2004; Brown et al., 2006; Blyth et al., 2008; Atz et al., 2010; Levey et al., 2010; Landis et al., 2013; Morris et al., 2014; Oster et al., 2014; Wright et al., 2014). Three studies found a decrease in hemodynamic compromise in infants with a prenatal diagnosis of TGA with no change in mortality (Kumar et al., 1999; Verheijen et al., 2001; Brown et al., 2006). By contrast, three European studies have found significantly lower mortality in prenatally diagnosed patients with TGA (Bonnet et al., 1999; Khoshnood et al., 2005; van Velzen et al., 2015).
A major outcome of prenatal diagnosis is planned delivery at a cardiac surgical center (CSC). In a previous study, our group reported that increased distance between a birth hospital and a CSC in patients with hypoplastic left heart syndrome (HLHS) was associated with increased neonatal mortality compared with those born close to a CSC (Morris et al., 2014) and also found a significant difference in mortality based on CSC HLHS volume, which is a phenomenon that has previously been described (Scott and Fixler, 2001; Checchia et al., 2005; Hirsch et al., 2008; Welke et al., 2009; Pasquali et al., 2012; Karamlou et al., 2014). Recent studies have suggested that delivery location is critical for mothers with fetuses affected by TGA given the potential need for urgent intervention (Donofrio et al., 2014).
The purpose of this study was to use data from the Texas Birth Defects Registry (TBDR) to determine the associations between prenatal diagnosis, distance from birthplace to a CSC, CSC TGA volume and neonatal mortality in patients with TGA in a large, ethnically and socioeconomically diverse group of such patients over a 9-year period.
Patients and Methods
PATIENT POPULATION AND STUDY DESIGN
Data were collected from the TBDR, which is maintained by the Birth Defects Epidemiology and Surveillance Branch of the Texas Department of State Health Services. Registry staff have conducted active, statewide surveillance of all delivery units and pediatric hospitals throughout Texas since 1999. Detailed birth, diagnostic, and mortality data were available for infants with a registry-monitored birth defect diagnosed within 1 year of delivery to Texas-resident mothers. Clinical information came from abstracted medical records, including echocardiographic, radiographic, and examination records when available. Although operative summaries were available for the majority of patients, detailed surgical information was not universally available.
The TBDR classifies congenital anomalies using six-digit codes based on the British Pediatric Association Classification of Diseases (1979) and the International Classification of Diseases, Ninth Revision, clinical modification (1979). We included live births between January 1, 1999, and December 31, 2007, with the codes of 745.100 (TGA) and 745.110 (TGA with ventricular septal defect); however, these codes included many patients with alternative or more complex cardiac diagnoses like TGA with moderate–severe pulmonary stenosis, double outlet right ventricle, single ventricle anatomy, heterotaxy syndrome, and atrioventricular septal defect. To improve accuracy of diagnosis, two pediatric cardiologists (D.A.L. and S.A.M.) reviewed TBDR clinical data. Patients with TGA with and without ventricular septal defects, with and without coarctation of the aorta, or mild pulmonary stenosis were included; infants with more complex cardiac diagnoses, genetic disorders, major extracardiac birth defects, or multiple congenital anomalies were excluded.
The study was approved by the Institutional Review Boards of Baylor College of Medicine and the Texas Department of State Health Services.
STUDY VARIABLES
The shortest driving time in minutes between a patient’s birth hospital and their cardiac surgical center (CSC) was calculated using geomapping software (CDX technologies and Microsoft MapPoint). We defined a CSC as any hospital in Texas that performed ≥1 arterial switch operation (ASO) during the study period. In the event that the patient died before transfer to a CSC or the CSC was not known, the nearest CSC was used to calculate driving time. Average daily driving times rather than distance were used to help reduce variability due to traffic, speed limits, and urban versus rural roads. Maternal socioeconomic status was estimated by (1) maternal educational level, and (2) percent living in poverty by maternal census tract, according to the 2000 U.S. Census (Pappas et al., 1993; Krieger et al., 2005).
Drive time to a CSC was divided into three groups: <10 min, 10 to 90 min, and >90 min, per our prior study (Morris et al., 2014). Ten minutes was chosen as the lower cutoff to delineate those delivering in or close to a CSC. Ninety minutes was chosen as the upper limit as this is the cutoff when transport by air ambulance would be considered (Roslonski, 2008). Prenatal diagnosis was determined by the presence of a prenatal record mentioning significant cardiac disease in abstracted medical records present in the TBDR.
Volume of TGA admissions was analyzed as both a continuous and categorical variable. Hospital volume was categorized into three groups based on the total number of TGA admissions per CSC during the total 9-year study period: < 5 TGA per year (7 CSCs), 5 to 10 TGA per year (2 CSCs), and >10 TGA per year (2 CSCs). When using CSC volume as a continuous variable in multivariable analysis, volume was log transformed. The number of TGA patients cared for at any included CSC over the course of the study was used in lieu of surgical volume because the surgical date and CSC were missing for some patients. Because admission volume closely mirrors surgical volume, we believed this was appropriate reasonable proxy measure.
Ventricular septal defect (VSD) was coded as present when a VSD was noted in the record, or absent if an intact ventricular septum was mentioned or if a VSD was not mentioned. Preterm birth was defined as <37 weeks, small for gestational age was defined as <10% expected birth weight for gestational age, and low birth weight was defined as < 2.5 kg.
Performance of a balloon atrial septostomy (BAS) was collected when that information was available in the chart. When available, the date of the BAS was also recorded, and the time to septostomy was calculated. A highly restrictive or intact atrial septum (HRAS) was defined as having any of the following reported: (1) intact atrial septum, (2) BAS occurring on the first day life (day of life = 0), (3) severe hypoxia and/or acidosis on the first day of life, or (4) arterial switch operation on the first day of life. This information was not universally reported in the TBDR.
Therefore, presence of an HRAS was not included in any multivariable models that would assume lack of information was equivalent to an unrestrictive atrial septum. Instead, subanalyses were performed limiting evaluation to this population with an HRAS.
OUTCOMES
Neonatal mortality (birth to 27 days) was the primary outcome. This was used to focus on the deceased patients for whom prenatal diagnosis may have made the most impact and to minimize the competing effects of access to healthcare after discharge. Discharge mortality was not calculated as the CSC discharge date was not included in the TBDR. Patients were classified as deceased based on TBDR and vital records. Timing of death was classified as occurring before transfer to a CSC, after transfer but before surgery, or after surgery. Secondary intermediate outcomes included age at diagnosis and age at surgery.
STATISTICAL ANALYSIS
Univariable comparisons were performed using Chi-square, Chi-square trend, Fisher exact, Wilcoxon rank sum, Kruskall-Wallis, and Spearman correlation tests where appropriate. In cases of multiple comparisons, Bonferroni correction was used. The primary analyses included all patients with TGA. Subanalyses were then performed evaluating associations between driving time, prenatal diagnosis, and CSC volume and neonatal mortality only in the subset of patients with an HRAS. For analysis of volume, a receiver operating curve analysis was performed to determine a threshold volume greatest change in mortality.
Multivariable analyses were performed for neonatal mortality. Generalized estimating equations were used to account for correlation between patients at the same CSC. All variables were considered for inclusion if p ≤ 0.20 in association with the outcome. Presence of a VSD or coarctation were included in the initial models given prior literature suggesting association with mortality (Huber et al., 2011). Given the low event rate, variables were removed from the model if p > 0.05, using a backward stepwise approach. History of balloon atrial septostomy was not included in the model given this may be in the causal pathway. Preterm birth and low birth weight were not used in the same multivariable regression equations due to high collinearity. Multivariable analysis was not performed in the subgroup of patients with HRAS given the small number of outcomes.
Given potential effect modification of prenatal diagnosis or driving time by CSC volume, secondary analysis was performed incorporating interactions between prenatal diagnosis, driving time from birth facility to CSC, and CSC volume. p values < 0.05 were considered significant. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
During the years 1999 to 2007, there were 3,401,057 live births to mothers who were residents of the state of Texas, of whom 468 met criteria for inclusion; birth prevalence of TGA was 1.4 cases per 10,000 live births. A VSD was present in 41.9%. Maternal and infant characteristics are listed in Table 1.
TABLE 1.
Maternal, Infant, and Hospital Characteristics and Neonatal Mortality among Infants born with Transposition of the Great Arteries, Texas, 1999 to 2007
| All N = 468 | Survivors N = 448 | Non-Survivors N = 20 | p-Value | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Prenatal diagnosis, no. (%) | 48 (10) | 46 (10) | 2 (10) | 1.00 |
| Median time to CSC, minutes (IQR) | 17.3 (4.9–83) | 17.5 (4.9–83) | 15.3 (9.2–42) | 0.51 |
| Time to CSC, no. (%)a | ||||
| <10 minutes | 163 (35) | 156 (35) | 7 (35) | 0.60 |
| 10–90 minutes | 193 (41) | 183 (41) | 10 (50) | |
| > 90 minutes | 110 (24) | 107 (24) | 3 (15) | |
| Maternal race/ethnicity, no. (%)a | ||||
| White, non-Hispanic | 191 (42) | 182 (42) | 9 (47) | 0.39 |
| Black, non-Hispanic | 33 (7.3) | 31 (7.1) | 2 (11) | |
| Hispanic | 219 (48) | 212 (49) | 7 (37) | |
| Other | 12 (2.6) | 11 (2.5) | 1 (5.3) | |
| Maternal age, no. (%) | ||||
| <20 years | 51 (11) | 48 (11) | 3 (15) | 0.32 |
| 20–29 years | 238 (51) | 231 (52) | 7 (35) | |
| 30–39 years | 167 (36) | 159 (36) | 8 (40) | |
| ≥ 40 years | 12 (2.6) | 10 (2.2) | 2 (10) | 0.72 |
| Maternal education, no. (%)a | ||||
| > 12th grade | 142 (31) | 134 (31) | 8 (40) | |
| 12th grade | 121 (26) | 118 (27) | 3 (15) | |
| < 12th grade | 195 (43) | 186 (43) | 9 (45) | |
| Poverty by census tract >20%, no. (%)a | 139 (30) | 131 (30) | 8 (40) | 0.32 |
| Maternal birth country, no. (%)a | ||||
| United States | 310 (67) | 296 (67) | 14 (70) | 0.47 |
| Mexico | 111 (24) | 108 24) | 3 (15) | |
| Other | 42 (9.1) | 39 (8.8) | 3 (15) | |
| Infant characteristics | ||||
| Birth year, no. (%) | 0.54 | |||
| 1999–2002 | 195 (42) | 188 (42) | 7 (35) | |
| 2003–2007 | 273 (58) | 260 (58) | 13 (65) | |
| Preterm, no. (%) | 54 (12) | 48 (11) | 6 (30) | 0.02 |
| Birth weight < 2500 g, no. (%) | 34 (7.3) | 29 (6.6) | 5 (25.0) | 0.01 |
| SGA, no. (%) | 36 (7.8) | 33 (7.5) | 3 (15) | 0.20 |
| Male sex, no. (%) | 310 (66) | 298 (67) | 12 (60) | 0.55 |
| Twin gestation, no. (%) | 18 (3.9) | 15 (3.4) | 3 (15.0) | 0.04 |
| VSD, no. (%)a | ||||
| Absent | 269 (58) | 259 (58) | 10 (50) | 0.47 |
| Present | 196 (42) | 186 (42) | 10 (50) | |
| Coarctation, no. (%) | 27 (5.8) | 26 (5.8) | 1 (5.0) | 1.00 |
| Mild pulmonary stenosis, no. (%) | 22 (4.7) | 21 (4.7) | 1 (5.0) | 1.00 |
| Hospital characteristics | ||||
| CSC Volume, no. (%)a | ||||
| > 10 TGA/year | 220 (47) | 215 (48) | 5 (25) | <0.0002 |
| 5–10 TGA/year | 98 (21) | 98 (22) | 0 (0.0) | |
| < 5 TGA/year | 125 (26) | 113 (25) | 12 (60) | |
| Intermediate outcomes | ||||
| Age at diagnosisa | 1.00 | |||
| <1 day | 300 (64) | 287 (64) | 13 (65) | |
| 1–2 days | 112 (24) | 107 (24) | 5 (25) | |
| >2 days | 54 (12) | 52 (12) | 2 (10) | |
| Age at surgery in days, median (IQR)a | 6 (4–8) | 6 (4–8) | 6 (5–8) | 0.76 |
| Age at surgery in daysa | 0.21 | |||
| 0–2 days | 49 (13) | 47 (13) | 2 (17) | |
| 3–4 days | 80 (22) | 80 (22) | 0 (0.0) | |
| 5–7 days | 133 (36) | 127 (36) | 6 (50) | |
| >7 days | 107 (29) | 103 (29) | 4 (33) |
Data not available for all patients.
IQR, interquartile range; CSC, cardiac surgical center; SGA, small for gestational age; VSD, ventricular septal defect.
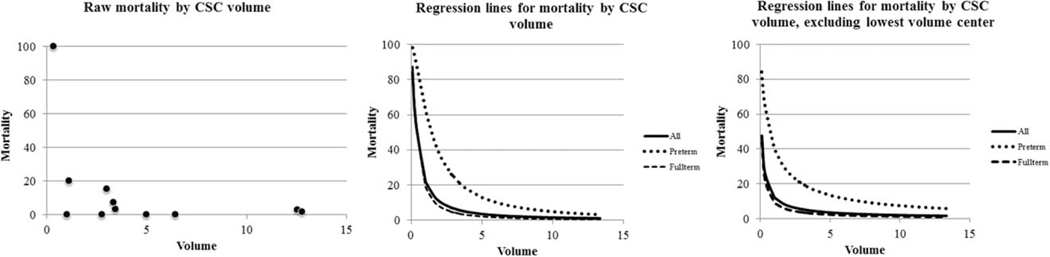
Forty-eight patients (10.3%) were prenatally diagnosed. Median driving time from birth hospital to a CSC was 17.3 min (interquartile range, 4.9–82.6 min). Thirty-five percent of the infants were born <10 min from a CSC, 41.4% were born 10 to 90 min away, and 23.6% were born >90 min away (Table 1). For the group with estimated driving times > 90 min, median driving time was 141 min, with range 91 to 598 min. During the study period, 11 CSCs performed the arterial switch procedure, and TGA volume ranged from 3 to 112 patients over the 9-year study period (Fig. 1). For 25 remaining patients, CSC was not documented in the registry, and these were excluded from the remainder of the analysis of hospital volume.
FIGURE 1.
Percent neonatal mortality per Cardiac Surgical Center based on TGA volume. There was a significant difference in neonatal mortality (p < 0.0001) based on hospital TGA volume.
Two-hundred nine infants were documented as having undergone balloon atrial septostomy. Age at septostomy was available in 199 infants. For these infants, 57 (29%) underwent BAS on day of life 0, 73 (37%) on day of life 1, and the other 69 underwent BAS on day of life 2 or later. Sixty-four infants in the cohort (14%) met study criteria for HRAS.
MORTALITY
Twenty infants (4.3%) died in the neonatal period. Two neonates died before transfer to a CSC with clinical signs of a highly restrictive atrial septum, one died at the CSC before surgery with hypoxia and a diagnosis of respiratory distress syndrome who was premature and low birth-weight, and 12 neonates died in the perioperative period (8 on the same day as surgery). The registry data did not include information on whether the remaining 5 patients had surgery before death; they died between ages 15 and 23 days.
In univariable analysis, closer proximity to a CSC at birth was not associated with lower mortality (p = 0.51, Table 1). Prenatal diagnosis was not associated with lower mortality (p = 1.00). There was no difference in neonatal mortality based on patient birth year (p = 0.537), anatomic factors, or maternal socio-demographic variables (Table 1).
In univariable analysis, the highest volume CSCs (> 10 patients per year) had a mortality of 2.27% (range, 1.8–2.8%), medium volume CSCs (5–10 patients per year) had 0% mortality, and lowest volume group (< 5 patients per year) had 9.6% mortality (range 0 – 100%). When hospital volume was included as a continuous variable, lower volume was significantly associated with increased neonatal mortality (p < 0.001; Fig. 1). Receiver operating curve analysis (area under the curve 0.725; p = 0.002) showed a threshold volume of <38 patients over the study period (4.2 per year), as having the most significant increased risk of mortality (sensitivity and specificity for mortality <38: 74% and 71%, respectively), with only 1.6% mortality for centers with ≥ 38 patients over the study period. The volume/mortality association remained significant even after excluding the lowest volume hospital with 100% mortality from the analysis (p = 0.006; Fig. 1). Of note, four of the five deaths in the highest volume centers were in patients with VSDs, but the difference was not statistically significant (mortality 3.0% in VSD vs. 0.6% without, p = 0.08 in high volume CSCs). This pattern was not present in CSCs with < 5 patients per year (9.5% without VSD vs. 9.8% with VSD, p = 0.95). Neither age at diagnosis nor age at surgery was associated with mortality (Table 2).
TABLE 2.
Association of Prenatal Diagnosis and Time to Surgical Center with Intermediate Secondary Outcomes of Age at Diagnosis and Age at Surgery for neonates with Transposition of the Great Arteries (TGA), Texas, 1999 to 2007
| Intermediate outcomes Age at diagnosis (days) | ||||
|---|---|---|---|---|
|
|
||||
| <1 | 1–2 | >2 | p-Value | |
| Time to CSC | 0.0002 | |||
| <10 min | 120 (74%) | 32 (20%) | 10 (6%) | |
| 10–90 min | 108 (56%) | 48 (25%) | 36 (19%) | |
| >90 min | 72 (65%) | 31 (28%) | 7 (6%) | |
| Age at surgery (days)a | |||||
|---|---|---|---|---|---|
|
|
|||||
| 0–2 | 3–4 | 5–7 | >7 | ||
| Prenatal Dx | 0.15 | ||||
| Yes | 4 (11%) | 5 (13%) | 20 (53%) | 9 (24%) | |
| No | 45 (14%) | 75 (23%) | 113 (34%) | 98 (30%) | |
| Time to CSC | 0.82 | ||||
| <10 min | 22 (16%) | 29 (22%) | 45 (34%) | 38 (28%) | |
| 10–90 min | 20 (13%) | 33 (21%) | 56 (36%) | 46 (30%) | |
| >90 min | 7 (9%) | 16 (21%) | 32 (41%) | 23 (29%) | |
Data not available for all patients.
In a subanalysis of infants meeting criteria for HRAS (n = 64), six infants died in the neonatal period (9.4%). Neonatal mortality was 0% in those with prenatal diagnosis, versus 10.3% in those with a postnatal diagnosis, p = 1.00). Mortality did not differ by distance at birth from a surgical center (<10 min: 8.3%, 10–90 min: 12.5%, >90 min: 8.3%; p = 1.00). When evaluating by hospital volume, the lowest volume CSCs had the highest mortality in infants with HRAS (3/16 = 18.8%), while the mid-volume and high volume CSCs had 0% (0/15) and 3.3% (1/30) mortality (lowest volume versus others p = 0.052). Only two prenatally diagnosed patients were born at the lowest volume CSCs; they both survived.
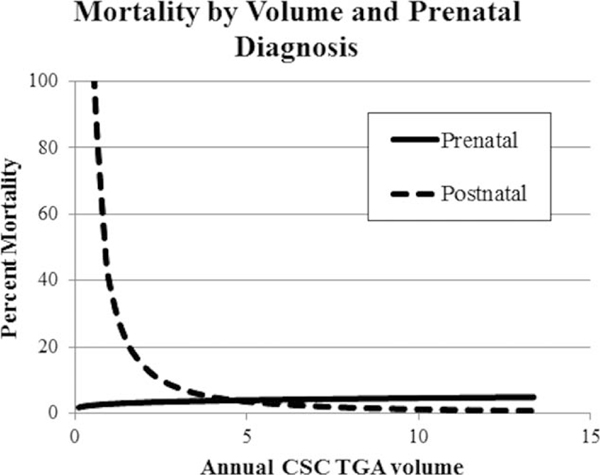
In the main effects multivariable analysis (Table 3, Model 1), only preterm birth (odds ratio, 7.05; 95% confidence interval, 4.13–12.05) and low hospital TGA volume remained significantly associated with higher neonatal mortality. In the lowest volume hospital group, mortality among those with prenatal diagnosis was 4.7% (1 death/21 patients), while those with a postnatal diagnosis had a mortality of 10.6% (11 deaths/104 patients) (p = 0.40). However, when we assessed for an interaction between prenatal diagnosis and volume as a continuous variable, the interaction was significant (p = 0.047) (Table 3, Model 2; Fig. 2).
TABLE 3.
Multivariable Models from Generalized Estimating Equations of Neonatal Mortality Association with Preterm Birth and Hospital Transposition of the Great Arteries (TGA) Volume, Texas, 1999 to 2007
| Model 1 | Model 2 | |||
|---|---|---|---|---|
|
|
|
|||
| Variable | Parameter estimate ± SE | p-Value | Parameter estimate ± SE | p-Value |
| Intercept | 1.95 ± 1.43 | 0.172 | 2.51 ± 1.43 | 0.079 |
| Preterm birth | 1.96 ± 0.27 | <0.001 | 1.94 ± 0.28 | <0.001 |
| Hospital TGA volume (per case, log transformed) | −1.52 ± 0.45 | <0.001 | −1.66 ± 0.43 | <0.001 |
| Prenatal diagnosis | −7.35 ± 4.02 | 0.067 | ||
| Interaction: TGA volume X prenatal diagnosis | 1.97 ± 0.99 | 0.047 | ||
FIGURE 2.
Subgroup analysis of neonatal mortality by cardiac surgery center TGA volume and prenatal diagnosis. There is an interaction (or effect modifier) between low volume centers and prenatal diagnosis (p = 0.047); there were no deaths in the prenatally diagnosed patients at low volume centers.
Discussion
Our findings show that in the overall Texas TGA population, prenatal diagnosis and decreased distance at birth from a CSC were not associated with improved neonatal survival. However, prenatal diagnosis may have the potential to modify outcomes at lower volume CSCs. The mechanism of this interaction could be twofold. First, prenatal diagnosis may allow lower volume centers to intervene rapidly with BAS in cases of an HRAS, where if postnatally diagnosed, deployment of resources may be slower than in institutions that perform these interventions more commonly. Indeed, neither of the prenatally diagnosed patients with an HRAS who delivered at a low-volume institution died in the neonatal period versus 3 of the 14 (21%) postnatally diagnosed patients. In the mid- and high-volume institutions, mortality in those prenatally diagnosed with an HRAS was 0/4 (0%) compared with 1/41 (2.4%) in those postnatally diagnosed. While these numbers are too low to prove the benefit of prenatal diagnosis in patients with HRAS at low-volume institutions, they are supportive of this theory. Second, it is possible that, for infants with TGA, prenatal diagnosis may in fact improve survival in patients cared for at low volume hospitals by prompting fetal cardiologists to refer more complex patients to higher volume centers and less complex patients to lower volume centers.
Prenatal diagnosis was not associated with overall lower mortality in our study as it was in some European studies (Bonnet et al., 1999; Khoshnood et al., 2005; van Velzen et al., 2015) (Table 4) possibly because of our lower rate of prenatal diagnosis and lower mortality limited our power to show a significant difference. However, even descriptive statistics and unadjusted analyses did not indicate a difference. Furthermore, there was enough power to demonstrate a significant interaction.
TABLE 4.
Studies Specifically Analyzing the Association between TGA Mortality and Prenatal Diagnosis
| Author | Location | Dates | No. of patients | Pre Dx rate (%) | Pre-op mortality Pre Dx /Post Dx | p-Value | Post-opmortality Pre Dx / Post Dx | p-Value |
|---|---|---|---|---|---|---|---|---|
| Bonnet | Paris | 1988–1997 | 318 | 21.3 | 0/7.4 | <0.05 | 0/10.6 | <0.01 |
| Kumar | Boston | 1988–1996 | 422 | 4.0 | 0/0.5 | 1.00 | 0/0 | 1.00 |
| Khoshnooda | Paris | 1983–2000 | 84 | 53.0 | 0/15.4 | 0.01 | na | na |
| Blyth | Wessex, UK | 1994–2006 | 72 | 6.9 | 0/7.7 | na | 0/5.0 | 0.11b |
| van Velzen | Netherlands | 2002–2012 | 144 | 36.1 | 0/4.9 | 0.19 | 0/5.3 | 0.17 |
| Lara | Texas | 1999–2007 | 468 | 10.3 | 0/0.7 | 0.57 | 4.2/3.5 | 0.80 |
First week mortality was reported.
Overall survival p value.
Pre Dx, prenatal diagnosis; Post Dx, postnatal diagnosis.
As shown in Table 4, the prenatal diagnosis rate in Texas during this period (10.3%) was much lower than some comparison studies. To determine whether this was due to under ascertainment of prenatal diagnoses by the TBDR, we conducted an independent study on a cohort of patients at Texas Children’s Hospital with TGA during the same time period and found a prenatal diagnosis rate of 15.4%. Given that Texas Children’s Hospital is a large referral center with its own fetal cardiology program, it is unsurprising that its rate would be higher; however, this rate is still relatively close to the statewide prenatal diagnosis rate obtained from the TBDR. Furthermore, while there are many population-based studies of prenatal detection of TGA, with rates ranging from 0% to 72.5% (Montana et al., 1996; Garne et al., 2001; Jaeggi et al., 2001; Stoll et al., 2001, 2002; Khoshnood et al., 2005; Khoo et al., 2008; Friedberg et al., 2009; Marek et al., 2011; Nielsen et al., 2014), a study by Pinto and colleagues using data from the Utah Birth Defects Network, a state with similar demographics to Texas, reported a prenatal detection rate of 14% (Pinto et al., 2012).
Despite the overall low mortality rate, there was a significantly higher mortality in the lower volume CSCs. Our findings are similar to those of Hirsch and colleagues who found an inverse relationship between surgical volume and postoperative mortality (Hirsch et al., 2008). They demonstrated a mortality rate in CSCs performing ≤2 ASOs per year of 9.4%, compared with 27.8% in our study, and a mortality rate of 3.2% in CSCs performing over 10 ASOs per year, compared with 2.3% in our study. Other studies have found an overall in-hospital mortality of 1.4 to 11.4% (Blume et al., 1999; Sarris et al., 2006; Lalezari et al., 2011; Fricke et al., 2012; Khairy et al., 2013; Anderson et al., 2014; Karamlou et al., 2014). Karamlou et al found that CSC volume and surgeon experience matter more in ASO surgeries, compared with Norwood procedures (Karamlou et al., 2010). This finding was strengthened in another study by Karamlou and colleagues, which showed that surgeon volume plays a larger role than center volume in outcomes of the ASO (Karamlou et al., 2014). Unfortunately, surgeon data were not available in the TBDR.
The previously cited studies describing the association between low surgical volume and increased mortality in congenital heart surgery were from large national data-bases in the United States (Scott and Fixler, 2001; Checchia et al., 2005; Hirsch et al., 2008; Welke et al., 2009; Pasquali et al., 2012; Karamlou et al., 2014). Although the data were consistent, application of these findings has been difficult in that often the selection of represented institutions was not consistent with real world options available to families, in that these hospitals are from many different states. This highlights one of the unique aspects of this study: our dataset was a complete 9-year birth cohort of TGA in a single state. Given that over half of neonates in Texas are covered by Medicaid, analysis of the relationship between volume and mortality among all CSCs within the state allows for examination of disparities in care among a similar payer pool (State of Texas, 2012). Our findings suggest that surgical site is a modifiable risk factor of significant importance, but one that may be offset by increased prenatal diagnosis.
It is difficult to determine why there was a higher mortality rate at the larger volume CSCs compared with the medium volume centers. Only one of the five deaths at the highest volume centers was associated with an HRAS, versus none at the medium volume centers. Both high and low volume centers had 11% of infants that were born preterm. Furthermore, it is unclear why patients with VSDs at high volume center had higher mortality. It may be that the patients at the larger CSCs (especially those with VSDs) had greater complexity not otherwise captured in the dataset.
Distance from a CSC was not associated with neonatal mortality among neonates with TGA, in contrast to our previous study in HLHS (Morris et al., 2014). In the HLHS cohort, the majority of deaths that were associated with increased distance from a CSC were presurgical. In comparison, the presurgical mortality in this study was quite low. Part of this difference in presurgical mortality is likely due to the fact that TGA is easier to diagnose postnatally than HLHS, as patients with TGA tend to be more cyanotic initially. This may increase early detection and offset the distance to a CSC phenomenon seen with HLHS. Also, patients with HLHS who decompensate develop shock, whereas patients with TGA most often present with severe hypoxemia, which is often less fatal and more responsive to prostaglandin therapy.
It will be interesting to see if the new recommendations for universal postnatal screening for critical congenital heart disease change outcomes with respect to prenatal diagnosis in TGA (Martin, 2013). Pulse oximetry screening detects hypoxia in those for whom it was not previously recognized. However, the primary contributors to mortality in TGA appear to be either an HRAS, which presents very early with severe hypoxia and is only improved with rapid intervention, or surgical complications. Neither of these would expect to be altered by routine screening. Also, age at diagnosis was not associated with mortality. In contrast, pulse oximetry screening may be critical in predominantly ductal-dependent lesions, which may not clinically present until closure of the ductus arteriosus. Early studies of pulse oximetry screening in TGA suggest that screening may prevent hospital discharge before diagnosis for some patients with TGA, but prenatal screening would still be critical in preventing adverse neurological outcomes and circulatory instability present in the sickest neonates with TGA (Bartos et al., 2015; Eckersley et al., 2015).
Limitations
While the total number of patients in this study is large, the primary outcome of neonatal mortality is rare. This limited our ability to develop a multivariable regression model for neonatal mortality that included all potential confounders and covariates. For example, four of the five patients who died in higher volume CSCs had a VSD (p = 0.08); a larger study sample or higher mortality would have increased our statistical power and may have resulted in this being statistically significant.
Also, we were unable to reliably determine the degree of atrial septal restriction for all patients. While evidence of an HRAS was apparent in the medical records for 64 of the infants as defined, we could not reliably determine who did not have an HRAS, so did not believe this was appropriate to include in the multivariable analysis. For example, in some patients, there is only a record of an echocardiogram with TGA and a patent foramen ovale, BAS on day of life 1 and an arterial switch operation some days later. Without clinical records suggesting hypoxia, acidosis, or changes on x-ray in patients like this, it is challenging to know whether the BAS was performed somewhat electively or for clinical deterioration. As almost half of the population underwent BAS before ASO, this is too nonspecific of an indicator of an HRAS. Given this, we thought it was most appropriate to perform stratified analyses in the population which had evidence of an HRAS, and believe this subanalysis adds to the study.
This study used abstracted registry data and not full medical charts and some of our variables are missing values; for example, age at surgery was available for only 78.9% and age at diagnosis for 96.7% of the patients. To use a uniform population, patients with extracardiac birth defect or chromosomal abnormalities were excluded from analysis; the outcomes of these patients would be an interesting future analysis.
Coronary artery anatomy, which has been shown to be a risk factor for mortality in TGA was not available in this dataset (Pasquali et al., 2002).
CONCLUSIONS
There was no association between prenatal diagnosis or proximity to a CSC and neonatal mortality in infants with isolated TGA. However, lower CSC TGA volume was highly associated with higher neonatal mortality. We found an interaction between prenatal diagnosis and hospital volume that may confer a protective effect for prenatally diagnosed patients at low volume centers that has not, to our knowledge, been previously described. Focused efforts to improve prenatal diagnosis may improve survival for newborns with TGA.
Acknowledgments
We thank the staff from the Texas Birth Defects Registry who identify cases and collect/abstract information from medical records. We also acknowledge partial support from the Title V Block Grant at the Texas Department of State Health Services.
Funding source: No funding was secured for this study.
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose.
Clinical Trial Registration: None.
References
- Anderson BR, Ciarleglio AJ, Hayes DA, et al. 2014. Earlier arterial switch operation improves outcomes and reduces costs for neonates with transposition of the great arteries. J Am Coll Cardiol 63:481–487. [DOI] [PubMed] [Google Scholar]
- Atz AM, Travison TG, Williams IA, et al. 2010. Prenatal diagnosis and risk factors for preoperative death in neonates with single right ventricle and systemic outflow obstruction: screening data from the Pediatric Heart Network Single Ventricle Reconstruction Trial(*). J Thorac Cardiovasc Surg 140:1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JM, Wypij D, Bellinger DC, et al. 2004. Effect of prenatal diagnosis on outcomes in D-transposition of the great arteries. Pediatrics 113:e335–e340. [DOI] [PubMed] [Google Scholar]
- Bartos M, Lannering K, Mellander M. 2015. Pulse oximetry screening and prenatal diagnosis play complementary roles in reducing risks in simple transposition of the great arteries. Acta Paediatr 104:557–565. [DOI] [PubMed] [Google Scholar]
- Blume ED, Altmann K, Mayer JE, et al. 1999. Evolution of risk factors influencing early mortality of the arterial switch operation. J Am Coll Cardiol 33:1702–1709. [DOI] [PubMed] [Google Scholar]
- Blyth M, Howe D, Gnanapragasam J, Wellesley D. 2008. The hidden mortality of transposition of the great arteries and survival advantage provided by prenatal diagnosis. BJOG 115:1096–1100. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Coltri A, Butera G, et al. 1999. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation 99:916–918. [DOI] [PubMed] [Google Scholar]
- Brown KL, Ridout DA, Hoskote A, et al. 2006. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart 92:1298–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checchia PA, McCollegan J, Daher N, et al. 2005. The effect of surgical case volume on outcome after the Norwood procedure. J Thorac Cardiovasc Surg 129:754–759. [DOI] [PubMed] [Google Scholar]
- Copel JA, Tan AS, Kleinman CS. 1997. Does a prenatal diagnosis of congenital heart disease alter short-term outcome? Ultrasound Obstet Gynecol 10:237–241. [DOI] [PubMed] [Google Scholar]
- Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. 2014. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 129:2183–2242. [DOI] [PubMed] [Google Scholar]
- Eckersley L, Sadler L, Parry E, et al. 2015. Timing of diagnosis affects mortality in critical congenital heart disease. Arch Dis Child [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Fricke TA, d’Udekem Y, Richardson M, et al. 2012. Outcomes of the arterial switch operation for transposition of the great arteries: 25 years of experience. Ann Thorac Surg 94:139–145. [DOI] [PubMed] [Google Scholar]
- Friedberg MK, Silverman NH, Moon-Grady AJ, et al. 2009. Prenatal detection of congenital heart disease. J Pediatr 155:26–31. [DOI] [PubMed] [Google Scholar]
- Garne E, Stoll C, Clementi M, Euroscan Group. 2001. Evaluation of prenatal diagnosis of congenital heart diseases by ultrasound: experience from 20 European registries. Ultrasound Obstet Gynecol 17:386–391. [DOI] [PubMed] [Google Scholar]
- Hill GD, Block JR, Tanem JB, Frommelt MA. 2015. Disparities in the prenatal detection of critical congenital heart disease. Prenat Diagn 35:859–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JC, Gurney JG, Donohue JE, et al. 2008. Hospital mortality for Norwood and arterial switch operations as a function of institutional volume. Pediatr Cardiol 29:713–717. [DOI] [PubMed] [Google Scholar]
- Huber C, Mimic B, Oswal N, et al. 2011. Outcomes and re-interventions after one-stage repair of transposition of great arteries and aortic arch obstruction. Eur J Cardiothorac Surg 39: 213–220. [DOI] [PubMed] [Google Scholar]
- Jaeggi ET, Sholler GF, Jones OD, Cooper SG. 2001. Comparative analysis of pattern, management and outcome of pre- versus postnatally diagnosed major congenital heart disease: a population-based study. Ultrasound Obstet Gynecol 17:380–385. [DOI] [PubMed] [Google Scholar]
- Karamlou T, Jacobs ML, Pasquali S, et al. 2014. Surgeon and center volume influence on outcomes after arterial switch operation: analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg 98:904–911. [DOI] [PubMed] [Google Scholar]
- Karamlou T, McCrindle BW, Blackstone EH, et al. 2010. Lesion-specific outcomes in neonates undergoing congenital heart surgery are related predominantly to patient and management factors rather than institution or surgeon experience: a Congenital Heart Surgeons Society Study. J Thorac Cardiovasc Surg 139: 569–577. [DOI] [PubMed] [Google Scholar]
- Khairy P, Clair M, Fernandes SM, et al. 2013. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation 127:331–339. [DOI] [PubMed] [Google Scholar]
- Khoo NS, Van Essen P, Richardson M, Robertson T. 2008. Effectiveness of prenatal diagnosis of congenital heart defects in South Australia: a population analysis 1999–2003. Aust N Z J Obstet Gynaecol 48:559–563. [DOI] [PubMed] [Google Scholar]
- Khoshnood B, De Vigan C, Vodovar V, et al. 2005. Trends in prenatal diagnosis, pregnancy termination, and perinatal mortality of newborns with congenital heart disease in France, 1983–2000: a population-based evaluation. Pediatrics 115:95–101. [DOI] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, et al. 2005. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health 95:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RK, Newburger JW, Gauvreau K, et al. 1999. Comparison of outcome when hypoplastic left heart syndrome and transposition of the great arteries are diagnosed prenatally versus when diagnosis of these two conditions is made only postnatally. Am J Cardiol 83:1649–1653. [DOI] [PubMed] [Google Scholar]
- Lalezari S, Bruggemans EF, Blom NA, Hazekamp MG. 2011. Thirty-year experience with the arterial switch operation. Ann Thorac Surg 92:973–979. [DOI] [PubMed] [Google Scholar]
- Landis BJ, Levey A, Levasseur SM, et al. 2013. Prenatal diagnosis of congenital heart disease and birth outcomes. Pediatr Cardiol 34:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A, Glickstein JS, Kleinman CS, et al. 2010. The impact of prenatal diagnosis of complex congenital heart disease on neonatal outcomes. Pediatr Cardiol 31:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahle WT, Clancy RR, McGaurn SP, et al. 2001. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with the hypoplastic left heart syndrome. Pediatrics 107: 1277–1282. [DOI] [PubMed] [Google Scholar]
- Marek J, Tomek V, Skovranek J, et al. 2011. Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21-year experience. Heart 97:124–130. [DOI] [PubMed] [Google Scholar]
- Martin GR, Beekman RH 3rd, Mikula EB, Fasules J, Garg LF, Kemper AR, et al. 2013. Implementing recommended screening for critical congenital heart disease. Pediatrics 132:e185–e92. [DOI] [PubMed] [Google Scholar]
- Montana E, Khoury MJ, Cragan JD, et al. 1996. Trends and outcomes after prenatal diagnosis of congenital cardiac malformations by fetal echocardiography in a well defined birth population, Atlanta, Georgia, 1990–1994. J Am Coll Cardiol 28:1805–1809. [DOI] [PubMed] [Google Scholar]
- Morris SA, Ethen MK, Penny DJ, et al. 2014. Prenatal diagnosis, birth location, surgical center, and neonatal mortality in infants with hypoplastic left heart syndrome. Circulation 129:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DE, Vejlstrup N, Jorgensen C, et al. 2014. Prenatal detection of congenital heart disease in a low risk population undergoing first and second trimester screening. Prenat Diagn 35:325–330. [DOI] [PubMed] [Google Scholar]
- Oster ME, Kim CH, Kusano AS, et al. 2014. A population-based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol 113:1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G, Queen S, Hadden W, Fisher G. 1993. The increasing disparity in mortality between socioeconomic groups in the United States, 1960 and 1986. N Engl J Med 329: 103–109. [DOI] [PubMed] [Google Scholar]
- Pasquali SK, Hasselblad V, Li JS, et al. 2002. Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries: a meta-analysis. Circulation 106:2575–2580. [DOI] [PubMed] [Google Scholar]
- Pasquali SK, Li JS, Burstein DS, et al. 2012. Association of center volume with mortality and complications in pediatric heart surgery. Pediatrics 129:e370–e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto NM, Keenan HT, Minich LL, et al. 2012. Barriers to prenatal detection of congenital heart disease: a population-based study. Ultrasound Obstet Gynecol 40:418–425. [DOI] [PubMed] [Google Scholar]
- Roslonski D. 2008. Guidelines for air medical dispatch. Lenexa, KS: American College of Emergency Physicians and National Association of EMS Physicians. [Google Scholar]
- Sarris GE, Chatzis AC, Giannopoulos NM, et al. 2006. The arterial switch operation in Europe for transposition of the great arteries: a multi-institutional study from the European Congenital Heart Surgeons Association. J Thorac Cardiovasc Surg 132:633–639. [DOI] [PubMed] [Google Scholar]
- Scott WA, Fixler DE. 2001. Effect of center volume on outcome of ventricular septal defect closure and arterial switch operation. Am J Cardiol 88:1259–1263. [DOI] [PubMed] [Google Scholar]
- State of Texas Health and Human Services Commission. 2012. 1115(a) research and demonstration waiver, Texas Women’s Health Program. Austin, TX: State of Texas Health and Human Services Commission. [Google Scholar]
- Stoll C, Dott B, Alembik Y, De Geeter B. 2002. Evaluation and evolution during time of prenatal diagnosis of congenital heart diseases by routine fetal ultrasonographic examination. Ann Genet 45:21–27. [DOI] [PubMed] [Google Scholar]
- Stoll C, Garne E, Clementi M, Euroscan Study Group 2001. Evaluation of prenatal diagnosis of associated congenital heart diseases by fetal ultrasonographic examination in Europe. Prenat Diagn 21:243–252. [DOI] [PubMed] [Google Scholar]
- van Velzen CL, Haak MC, Reijnders G, et al. 2015. Prenatal detection of transposition of the great arteries reduces mortality and morbidity. Ultrasound Obstet Gynecol 45:320–325. [DOI] [PubMed] [Google Scholar]
- Verheijen PM, Lisowski LA, Stoutenbeek P, et al. 2001. Prenatal diagnosis of congenital heart disease affects preoperative acidosis in the newborn patient. J Thorac Cardiovasc Surg 121: 798–803. [DOI] [PubMed] [Google Scholar]
- Welke KF, O’Brien SM, Peterson ED, et al. 2009. The complex relationship between pediatric cardiac surgical case volumes and mortality rates in a national clinical database. J Thorac Cardiovasc Surg 137:1133–1140. [DOI] [PubMed] [Google Scholar]
- Wright LK, Ehrlich A, Stauffer N, et al. 2014. Relation of prenatal diagnosis with one-year survival rate for infants with congenital heart disease. Am J Cardiol 113:1041–1044. [DOI] [PubMed] [Google Scholar]