Purpose of review
To discuss why severe COVID-19 should be considered sepsis and how co-infection and secondary infection can aggravate this condition and perpetuate organ dysfunction leading to high mortality rates.
Recent findings
In severe COVID-19, there is both direct viral toxicity and dysregulated host response to infection. Although both coinfection and/or secondary infection are present, the latest is of greater concern mainly in resource-poor settings. Patients with severe COVID-19 present a phenotype of multiorgan dysfunction that leads to death in an unacceptable high percentage of the patients, with wide variability around the world. Similarly to endemic sepsis, the mortality of COVID-19 critically ill patients is higher in low-income and middle-income countries as compared with high-income countries. Disparities, including hospital strain, resources limitations, higher incidence of healthcare-associated infections (HAI), and staffing issues could in part explain this variability.
Summary
The high mortality rates of critically ill patients with severe COVID-19 disease are not only related to the severity of patient disease but also to modifiable factors, such as the ICU strain, HAI incidence, and organizational aspects. Therefore, HAI prevention and the delivery of best evidence-based care for these patients to avoid additional damage is important. Quality improvement interventions might help in improving outcomes mainly in resource-limited settings.
Keywords: acute respiratory distress syndrome, bacterial pneumonia, coronavirus disease 2019, ICU, sepsis, viral pneumonia
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to enormous pressure on health systems around the world with a surge in hospitalizations for pneumonia. Critical disease (i.e. respiratory failure, septic shock, and/or multiple organ dysfunction) has been reported in approximately 5% of the symptomatic patients [1]. These patients meet criteria for sepsis [2,3]. Additionally, bacterial co-infection or secondary infection can aggravate the condition and perpetuate organ dysfunction [4,5]. In this narrative review, we discuss the concept that COVID-19 with organ dysfunction is sepsis, the importance of co-infection and secondary infections, and the severity of sepsis in COVID-19 patients.
Box 1.
no caption available
SEVERE CORONAVIRUS DISEASE 2019 IS SEPSIS
According to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), sepsis is a ‘life-threatening organ dysfunction caused by a dysregulated host response to infection’ [6]. Sepsis can be caused by several pathogens, such as bacteria, viruses, fungi, or parasites. In COVID-19 sepsis, the causative agent is the SARS-CoV-2. Although there is undoubtedly direct viral toxicity, a dysregulated host response to infection is also present [2]. Although there is controversy about the role of the ‘cytokine storm’ in the pathogenesis of COVID-19, there is clearly an increase in the proinflammatory cytokines and chemokines including tumor necrosis factor (TNF), interleukin 1a (IL-1a), interleukin 6 (IL-6), granulocyte-colony-stimulating factor, interferon gamma-induced protein-10, monocyte chemoattractant protein-1, and macrophage inflammatory proteins-1 [7▪]. The deregulated inflammatory response together with the direct virus damage lead to endothelial cell activation. Given the pivotal role of these cells in maintaining homeostasis and in the control of vascular permeability and coagulation, the dysregulation of these processes contributes to the generation of thrombotic disorders and further compromise in oxygen delivery leading to organ dysfunction [8▪]. Although the most concerning clinical feature of COVID-19 is acute respiratory distress syndrome requiring mechanical ventilation [9], it can also result in several extrapulmonary manifestations [10▪]. Severe cases can progress to organ dysfunction syndrome (MODS) including distributive shock, coagulation abnormalities, renal injury, neurological and cardiac dysfunction may ensue both because of the direct viral toxicity as to the nonhomeostatic host response to viral infection. In fact, MODS accounts for most of the deaths from COVID-19 [11,12].
Sepsis is an umbrella term, which describes a clinical syndrome caused by different infectious agents that share common mechanisms of disease but significant differences exist and contribute to specific clinical manifestations and complications [13▪]. Thus, patients with COVID-19 share pathophysiological and clinical features already described in patients with sepsis, including long-term disabilities [14,15], However, some peculiarities are present, such as lymphopenia or relative lymphopenia, hypoxemia in the presence of almost normal lung compliance and a profound COVID-19-induced coagulopathy affecting not only the microcirculation but also resulting in large vessel thrombosis and major thromboembolic complications [16,17]. Viral sepsis and death in COVID-19 can occur in otherwise healthy individuals of any age but predominantly occurs in adults with advanced age or with underlying medical comorbidities [1]. As with bacterial sepsis, biological and medical heterogeneity of the patients affect the multifaceted host response to infection, leading to different spectrum of disease severity and clinical presentations. Subphenotypes have been described in critically ill patients with COVID-19 [18].
Different from bacterial and fungal sepsis, specific therapy directed to the causative agent is still substantially less effective for COVID-19, being one of the potential explanations for the high mortality in patients with severe forms of the disease [19]. However, whichever the infectious agent is, once established, MODS has high mortality. The best management of COVID-19 sepsis should not be focused only on respiratory failure but supportive care and monitoring [20▪]. It is important to assure the maintenance of tissue perfusion and infection control to interrupt the pathogenesis of MODS, in addition to prevention of iatrogenic complications including secondary infection until recovery occurs.
CO-INFECTION AND SECONDARY INFECTIONS
Bacterial infections, especially Streptococcus pneumoniae and Staphylococcus aureus, and even viral or fungal co-infections, have been commonly reported in previous epidemic and pandemic outbreaks of viral respiratory infections, such as influenza. During the H1N1 pandemic influenza in 2009, bacterial co-infection was identified in 30% of cases admitted to ICUs [21]. Bacterial complications are associated with a higher severity of illness and use of healthcare resources, and increased risk of death [22,23].
Mechanisms by which viral infections, including SARS-Co-V-2, may predispose to concomitant and secondary bacterial infections involve the damage of respiratory airways and simultaneously defects in both innate and acquired immune response providing a favorable environment for bacterial growth, adherence, and invasion into healthy sites of the respiratory tract [17,19,24].
Data regarding coinfections and secondary infections in COVID-19 pneumonia are emerging. There are several published articles, ranging from letters to the editor to systematic reviews and meta-analyses [23,25]. Co-infection and secondary infection are distinct clinical entities, although used interchangeably in medical literature and clinical practice. Although both are present in COVID-19, the issue of secondary infection and multidrug resistance are of greater concern. Langford et al. demonstrated in a living meta-analysis and systematic review the presence of co-infection in 3.5% (95% CI 0.4–6.7%) and secondary infection in 14.3% (95% CI 9.6–18.9%) of patients with COVID-19 [23]. When pooling all included studies, the proportion of COVID-19 patients with bacterial infection was 6.9% (95% CI 4.3–9.5%), ranging from 5.9% in hospitalized patients to 8.1% in critically ill patients. Despite an overall low rate of bacterial infections, over 70% of patients received antibiotics, suggesting overuse. Specific species of bacterial co-pathogens were reported in 11 of 24 studies (45.8%), representing a low isolating proportion of less than 14% of the patients with reported infections. The most common organisms reported were Mycoplasma spp. (n = 11 patients), Haemophilus influenzae (n = 5 patients), and Pseudomonas aeruginosa (n = 5 patients) [23].
In a recent preprint systematic review and meta-analysis, including 48 articles, the pooled prevalence of co-infection was 12% (95% CI 6--18%) and of superinfection was 14% (95% CI 9--21%), with the highest prevalence of 17% (95% CI 1--43%) among ICU patients. Among those with co-infections, the three most frequently identified bacteria were Strep. pneumoniae (17.9%), Klebsiella pneumoniae (16.7%) and Haemophilus influenza (12.4%). The three most frequently identified viruses among co-infected patients were Influenza type A (8.1%), Rhinovirus (6.3%) and non-SARS-CoV-2 coronaviruses (3.7%). For fungi, only Candida spp. (0.7%) and Mucor spp. (0.7%) were identified. Among those with secondary infections, the three most frequently identified bacteria were Acinetobacter spp. (22%), Escherichia coli (18%) and Pseudomonas (16%). The only reported virus was Human Metapneumovirus (4%) and for fungi only Candida spp. (14%) was identified [25].
However, there is conflicting data. Several studies evidenced high incidence of bacterial secondary infection and sepsis because of HAI in COVID-19 patients [5,26▪▪,27–29]. A review by Lai et al. reported that the prevalence of COVID-19-associated secondary infections could be as high as 50% among nonsurvivors [28]. In a multicenter retrospective analysis of prospectively collected data including 774 adult patients with severe COVID-19 in 8 Italian hub hospitals, 359 (46%) patients developed 759 hospital-acquired infections (44.7 infections/1000 ICU patient-days) [26▪▪]. The authors reported a high prevalence of multidrug-resistant (MDR) bacteria (35% of all isolated agents). As expected, ventilator-associated pneumonia (VAP) (389, 50%), bloodstream infections (183, 34%), and catheter-associated bloodstream infections (CABSI) (74, 10%) were the most frequent HAIs. Gram-negative bacteria (especially Enterobacteriaceae) and Staph. aureus caused 64 and 28% of VAPs. The vast majority of the patients, 534 (69%) received at least one antibiotic at admission, of them 240 (44.9%) were broad-spectrum antibiotics. HAIs prolonged mechanical ventilation and hospitalization, and HAIs complicated by septic-shock almost doubled mortality [26▪▪].
There is no robust data from low-income and middle-income countries (LMICs) regarding HAIs in COVID-19 patients. A systematic review reported 44% of nosocomial infection in these patients in China suggesting that the impact might be greater than in developed countries [29]. Previous data already suggested that the rates for HAI are higher in resource-poor settings. In non-COVID patients, data from the International Nosocomial Infection Control Consortium (INICC) clearly show higher rates for CABSI, VAP and catheter-associated urinary tract infections [30]. These findings suggest that there is a lower infection risk in countries with higher socioeconomical level. Considering the level of ICU strain throughout the pandemic, the situation of LMIC is worrisome. The burden of COVID-19 exposed important healthcare deficits around the world. Over-crowding in ICUs, temporary ICU beds, lack of trained and experienced healthcare workers, low nurse-to-patient staffing ratios, burnout syndrome, insufficient medical equipment and supplies, antibiotics stewardship personnel workload, infection prevention resource diversion may contribute to increased rates of healthcare-associated infections, antibiotic overuse, and increased multidrug resistance.
SEVERITY OF SEPSIS IN CORONAVIRUS DISEASE 2019 PATIENTS
Regardless of the causative agent of sepsis, the virus or a bacterial coinfection or secondary infection, the patients with severe COVID-19 present a phenotype of multiorgan dysfunction that leads to death in an unacceptable high percentage of the patients. A recent systematic review including 32 studies showed the prevalence of several organ dysfunctions and support in COVID-19 patients. As expected, respiratory support was the main life-sustaining therapy in ICU patients admitted with COVID-19, with high-flow nasal therapy being used by 20% of the patients, noninvasive ventilation by 25.5% and almost 60% of the patients being under invasive mechanical ventilation. Extracorporeal membrane oxygenation was used in only 2.5% of the cases. Nearly 30% of the cases had hemodynamic dysfunction and 16.6% of the patients needed renal replacement therapy [31▪▪].
Until now, no specific severity score for ICU patients with COVID-19 was developed and validated; however, almost all studies in critically ill patients reported the SOFA score on admission. Gupta et al.[32▪▪] showed that the PaO2/FiO2 ratio, liver and renal dysfunction as described in SOFA score were associated with mortality. Interestingly, in this study, the authors found that shock and coagulation dysfunction on day 1 were not related to mortality. There was a clear association between respiratory dysfunction and death; however, the concomitant presence of other dysfunctions, such as cardiovascular and acute kidney injury significantly increased the risk of death [31▪▪,33].
There are conflicting data on the mortality of COVID-19 critical patients [31▪▪,34]. Early data from China showed mortality rates in critical ill patients ranging from 14 to 61% [32▪▪]. In Italy, Grasseli et al.[4] reported an ICU mortality around 25%. Data from United States of America showed that almost one in each four patients died after ICU admission [34]. Serafim et al.[31▪▪] analyzed 65 383 ICU admissions from several countries and reported an ICU mortality of 32%; however, when considering only mechanically ventilated patients, the mortality was 59%. Karagiannidis et al. reported an in-hospital mortality in ventilated patients requiring dialysis of 73% in Germany [33]. There is also lack of robust data on the causes of death. Although ARDS has a high mortality rate, death because of refractory hypoxemia is reportedly rare. Even in cohorts of ARDS patients treated in the 1990s, less than 20% of death were because of refractory hypoxemia, being most of the deaths secondary to multiorgan failure [35]. Reported causes of deaths in COVID-19 patients are scarce but differently from non-COVID-19 ARDS respiratory failure seems to play a major role. Small studies reported respiratory failure as cause of death in 53% [36] and 45% [37] of the cases with smaller percentage of the patients dying from shock and multiorgan failure. Ketchem et al. evaluated the cause of death from 82 COVID-19 patients. The authors reported that the most common organ dysfunction prior to death were pulmonary (81.7%), neurologic (57.3%), and renal (39%). Interestingly, septic shock was present in 40.2% cases. However, septic shock was considered the primary cause of death only in 26.8% [38▪]. Gupta et al.[32▪▪] analyzed the cause of death from 787 patients and found that 92.7% of them died from respiratory failure; however, almost 40% also had septic shock.
One possible explanation for the inconsistence in the mortality rates and in the causes of death might be the influence of health disparities in the outcomes of COVID-19 patients as previously reported for sepsis. A recent systematic review estimated that the sepsis mortality rate for hospitalized patients is 26.7%, being 41.9% for ICU-treated sepsis [39]. However, as there is a paucity of data coming from LMICs, these numbers might be underestimating the sepsis burden as data from these settings showed higher mortality rates [40–42]. In a recent analysis from the Global Burden of Disease Study based on death certificates, Rudd et al. demonstrated that sepsis incidence and mortality varied substantially across regions with higher rates in countries with lower sociodemographic index. The highest burden was found in sub-Saharan Africa, Oceania, south Asia, east Asia, and southeast Asia [43]. In the current pandemic, disparities related to socioeconomical status have also been demonstrated. Ranzani et al. in a retrospective study over 250 000 hospital admissions in Brazil evidenced a high incidence of mortality in patients admitted to ICU (55%) and even higher for those submitted to invasive mechanical ventilation (80%). This study also demonstrated that the mortality was influenced by regional disparity in healthy system [44▪]. Kurtz et al.[45] reported a trend in mortality reduction possibly related with more liberal use of noninvasive respiratory support (e.g. noninvasive ventilation and high nasal flow catheter), resources with variable availability in Brazilian ICUs [46]. Disparities were also reported in developed settings. Recently, Chuperk et al. found significant interhospital variation in mortality for critically ill patients with COVID-19 in United States. This variation was mostly explained by hospital-level socioeconomic status, strain, and physiologic differences [47▪▪].
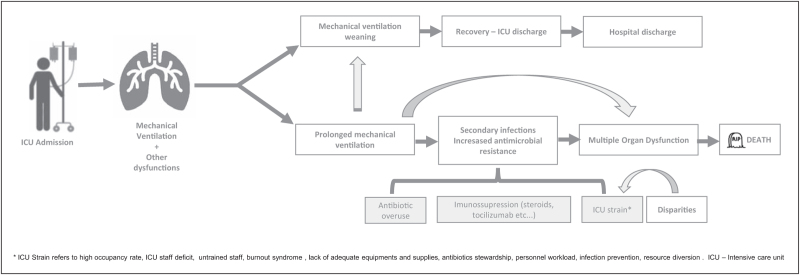
Disparities might influence some of the major determinants of mortality in COVID-19 ICU patients. Secondary infections and the consequent bacterial sepsis multiorgan dysfunction syndrome seem to be one of the major causes of death in patients with prolonged ICU stay under mechanical ventilation, at least in resource poor settings. A higher incidence of HAI is expected with a higher ICU strain, characterized by overcrowded ICU, inadequate staffing, lack of supplies, and inadequate antibiotics stewardship leading to a higher incidence of HAI, and these factors are influenced by disparities in healthcare. In addition, COVID-19 patients can have infection complications from treatments, such as immunosuppression from corticosteroids or tocilizumab. Multidrug resistance can arise as a consequence of antibiotics overuse. The current ICU strain also exposes patients to other serious adverse events that might increase the risk of death [48]. Figure 1 presents the possible trails to death in COVID-19 patients. Therefore, is of upmost importance to deliver the best evidenced-based care for these patients to avoid additional damage [49]. HAI prevention with the use of validated bundles and antimicrobial stewardship, the early diagnosis and proper management of secondary infections are necessary to reduce the impact of sepsis derived from secondary infections, Similarly, quality improvement initiatives aiming to reduce the gap between evidence-based medicine and bedside management should be implemented. Recent studies demonstrate that the use of quality improvement interventions is associated with improved outcomes in LMICs [50].
FIGURE 1.
Possible trails related to death in mechanically ventilated coronavirus disease 2019 critically ill patients.
CONCLUSION
The severe forms of COVID-19 should be considered as viral sepsis. More data about co-infection and secondary bacterial infections is necessary mainly from resource-limited settings. Mortality rates for COVID sepsis either caused by the virus or complicated by secondary infection is high. Notwithstanding the severity of the viral disease, the ICU strain, the incidence of HAI, and organizational aspects are important factors that determine mortality. These factors are influenced by disparities, which can contribute to the variability in outcomes worldwide. HAI prevention, the use of evidenced-based treatment, and the proper management of sepsis through quality improvement are important initiatives to improve outcomes.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet Lond Engl 2020; 395:1517–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltrán-García J, Osca-Verdegal R, Pallardó FV, et al. Sepsis and coronavirus disease 2019: common features and anti-inflammatory therapeutic approaches. Crit Care Med 2020; 48:1841–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Greco M, Zanella A, et al. COVID-19 Lombardy ICU Network. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180:1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardi T, Pintado V, Gomez-Rojo M, et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis 2021; 490:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪.Osuchowski MF, Winkler MS, Skirecki S, et al. COVID-19: pathophysiology of acute disease 1. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med 2021; 9:622–642. [DOI] [PMC free article] [PubMed] [Google Scholar]; Great review about pathophysiology and clinical phenotypes COVID-19 patients.
- 8▪.Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care 2020; 24:353. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review described the central role of vascular endothelium in COVID-19.
- 9.Wilcox SR. Management of respiratory failure due to covid-19. BMJ 2020; 369:m1786. [DOI] [PubMed] [Google Scholar]
- 10▪.Gupta A, Madhavan M, Sehgal K, et al. Extrapulmonary manifestation of COVID-19. Nat Med 2020; 26:1017–1032. [DOI] [PubMed] [Google Scholar]; This nice review described the several extrapulmonary manifestations of COVID-19.
- 11.Vincent J-L. COVID-19: it's all about sepsis. Future Microbiol 2021; 16:131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent J-L, Taccone FS. Understanding pathways to death in patients with COVID-19. Lancet Respir Med 2020; 8:430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪.Dolin HH, Papadimos TJ, Chen X, Pan ZK. Characterization of pathogenic sepsis etiologies and patient profiles: a novel approach to triage and treatment. Microbiol Insights 2019; 12: 1178636118825081. [DOI] [PMC free article] [PubMed] [Google Scholar]; Interesting review of the typical situations leading to the three major types of pathogenic sepsis: bacterial, viral, and fungal.
- 14.Herridge MS, Moss M, Hough CL, et al. Recovery and outcomes after the acute respiratory distress syndrome (2) in patients and their family caregivers. Intensive Care Med 2016; 42:725–738. [DOI] [PubMed] [Google Scholar]
- 15.Zettersten E, Engerström L, Bell M, et al. Long-term outcome after intensive care for COVID-19: differences between men and women—a nationwide cohort study. Crit Care 2021; 25:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olwal CO, Nganyewo NN, Tapela K, et al. Parallels in sepsis and COVID-19 conditions: implications for managing severe COVID-19. Front Immunol 2021; 12:602848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iba T, Levy JH, Levi M, et al. Coagulopathy of coronavirus disease. Crit Care Med 2020; 48:1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasquez CR, Gupta S, Miano TA, et al. Identification of distinct clinical subphenotypes in critically ill patients with COVID-19. Chest 2021; S0012-3692(21)00874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yataco AOC, Simpson SQ. Coronavirus disease 2019 sepsis: a nudge toward antibiotic stewardship. Chest 2020; 158:1833–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med 2020; 46:854–887. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence-based guidelines on the management of COVID-19 in critically ill patients.
- 21.MacIntyre CR, Chughtai AA, Barnes M, Ridda I, et al. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect Dis 2018; 18:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman C, Anderson R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia 2021; 13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26:1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bengoechea JA, Bamford CG. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med 2020; 12:e12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musuuza J, Watson L, Parmasad V, et al. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. medRxiv 2020.10.27.20220566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪▪.Grasselli G, Scaravilli V, Mangioni D, et al. Hospital-Acquired Infections in Critically Ill Patients With COVID-19. Chest 2021; S0012-3692(21)00679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This multicenter retrospective study described the characteristics and impact on outcome of hospital-acquired infections in a large cohort of patients with COVID-19 admitted to the ICU in Italian centers.
- 27.Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest 2020; 50:e13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai C-C, Wang C-Y, Hsueh P-R. Co-infections among patients with COVID-19: The need for combination therapy with nonanti-SARS-CoV-2 agents? J Microbiol Immunol Infect 2020; 53:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q, Gao Y, Wang X, et al. COVID-19 Evidence and Recommendations Working Group. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med 2020; 8:629–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenthal VD, Duszynska W, Ider BE, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2013-2018, Adult and Pediatric Units, Device-associated Module. Am J Infect Control 2021; S0196-6553(21)00272-8. [DOI] [PubMed] [Google Scholar]
- 31▪▪.Serafim RB, Póvoa P, Souza-Dantas V, et al. Clinical course and outcomes of critically ill patients with COVID-19 infection: a systematic review. Clin Microbiol Infect 2021; 27:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nice systematic review about organ dysfunction, clinical support, and outcomes of COVID-19 ICU patients.
- 32▪▪.Gupta S, Hayek SS, Wang W, et al. STOP-COVID Investigators. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020; 180:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]; Interesting study that described clinical and hospital-level factors associated with death in United States.
- 33.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med 2020; 8:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quah P, Li A, Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care 2020; 24:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ketcham SW, Sedhai YR, Miller HC, et al. Causes and characteristics of death in patients with acute hypoxemic respiratory failure and acute respiratory distress syndrome: a retrospective cohort study. Crit Care 2020; 24:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Contou D, Cally R, Sarfati F, et al. Causes and timing of death in critically ill COVID-19 patients. Crit Care 2021; 25:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪.Ketcham SW, Bolig TC, Molling DJ, et al. Causes and Circumstances of Death among Patients Hospitalized with COVID-19: A Retrospective Cohort Study. Ann Am Thorac Soc 2021; 18:1076–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study described the cause and circumstances of death in patients with COVID-19 related respiratory failure.
- 39.Fleischmann-Struzek C, Mellhammar L, Rose N, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med 2020; 46:1552–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Machado FR, Cavalcanti AB, Bozza FA, et al. SPREAD Investigators, Latin American Sepsis Institute Network. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis 2017; 17:1180–1189. [DOI] [PubMed] [Google Scholar]
- 41.Baykara N, Akalin H, Arslantaş MK, et al. Sepsis Study Group. Epidemiology of sepsis in intensive care units in Turkey: a multicenter, point-prevalence study. Crit Care 2018; 22:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis JM, Feasey NA, Rylance J. Aetiology and outcomes of sepsis in adults in sub-Saharan Africa: a systematic review and meta-analysis. Crit Care 2019; 23:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 2020; 395:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44▪.Ranzani OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med 2021; 9:407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]; Retrospective study that observed a high COVID-19 burden in Brazil.
- 45.Kurtz P, Bastos LSL, Dantas LF, et al. Evolving changes in mortality of 13,301 critically ill adult patients with COVID-19 over 8 months. Intensive Care Med 2021; 47:538–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taniguchi LU, Azevedo LCPde, Bozza FA, et al. Availability of resources to treat sepsis in Brazil: a random sample of Brazilian institutions. Rev Bras Ter Intensiva 2019; 31:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47▪▪.Churpek MM, Gupta S, Spicer AB, et al. Hospital-Level Variation in Death for Critically Ill Patients with COVID-19. Am J Respir Crit Care Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]; Multicenter cohort study that described the interhospital variation in mortality for critically ill patients.
- 48.Salluh JIF, Lisboa T, Bozza FA. Challenges for the care delivery for critically ill COVID-19 patients in developing countries: the Brazilian perspective. Crit Care 2020; 24:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salluh JIF, Ramos F, Chiche JD. Delivering evidence-based critical care for mechanically ventilated patients with COVID-19. Lancet Respir Med 2020; 8:756–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machado FR, Ferreira EM, Sousa JL, et al. Quality improvement initiatives in sepsis in an emerging country: does the institution's main source of income influence the results? An analysis of 21,103 patients∗. Crit Care Med 2017; 45:1650–1659. [DOI] [PubMed] [Google Scholar]