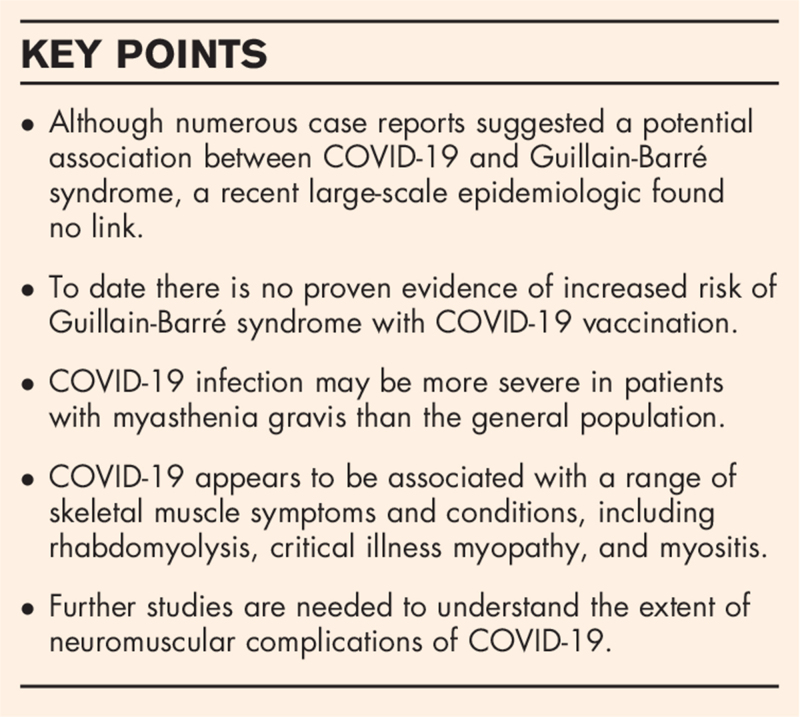
Abstract
Purpose of review
Since its outbreak in Wuhan, China in late 2019, coronavirus disease-19 (COVID-19) has become a global pandemic. The number of affected cases and deaths continues to rise. Primarily a respiratory illness, COVID-19 is now known to affect various organ systems including peripheral nerve and skeletal muscle. The purpose of this review is to discuss the scope of neuromuscular manifestations and complications of COVID-19.
Recent findings
Several neuromuscular conditions, including Guillain-Barré syndrome, rhabdomyolysis, and myositis, have been reported in patients infected with COVID-19, but even with a temporal association, a causal relationship remains unproven. Direct invasion of neurons or myocytes by the virus, and immune-mediated injury have been speculated but not consistently demonstrated. In addition to potentially causing the above conditions, COVID-19 can trigger exacerbations of preexisting neuromuscular conditions such as myasthenia gravis, and severe infections can lead to critical illness myopathy/polyneuropathy.
Summary
COVID-19 appears to be potentially associated with a wide range of neuromuscular manifestations and complications. Further studies are needed to examine these possible associations, understand the pathogenesis, and develop preventive and treatment strategies.
Keywords: coronavirus disease-19, myasthenia gravis, myopathy, neuropathy, severe acute respiratory syndrome coronavirus 2
INTRODUCTION
Coronavirus disease-19 (COVID-19) has become a global pandemic since its origination in Wuhan, China in late 2019, with now more than 125 million cumulative cases and more than 2.7 million deaths worldwide [1]. Although COVID-19 primarily causes a respiratory disease, it has become apparent over time that the infection can have far reaching consequences on various organ systems. A wide breadth of neurologic complications has been reported and investigations into the epidemiology, pathogenesis, and therapies are ongoing. In this review, we focus on the neuromuscular complications of COVID-19 infection and their proposed mechanisms.
Box 1.
no caption available
Peripheral nerve complications of coronavirus disease-19
To date, there are more than 60 published case reports on Guillain-Barré syndrome (GBS) arising in COVID-19-infected patients. However, a link between COVID-19 and GBS is debatable. Case reports assumed a relationship based on the fact that GBS occurred with symptoms and signs of COVID-19 or soon thereafter, suggesting a para-infectious or postinfectious process. Retrospective studies found a higher frequency of GBS in COVID-19-infected patients with an odds ratio (OR) of 4.6–6.3 [2,3]. Another study found that the incidence of GBS during the pandemic (0.202/100,000/month) was 2.6 times higher compared to the same period during the prior year [4]. In contrast, a different study did not find a substantial increase in the number of patients hospitalized with GBS during the pandemic [5]. A study from Italy found that GBS in patients with COVID-19 was more commonly the acute inflammatory demyelinating polyneuropathy variant, caused more weakness, and more frequently led to intensive care unit (ICU) admission and hypotension [4]. However, the response to therapy (intravenous immunoglobulin [IVIG] or plasma exchange [PLEX]) was similar in patients with and without COVID-19.
The most extensive study to date, an epidemiologic study from the United Kingdom (UK), found that the incidence of GBS was actually lower during the pandemic compared to prior years and found no correlation between the number of COVID-19 and GBS cases when comparing different regions of the UK [6▪▪]. Moreover, the study found no significant differences in clinical features of GBS, disease severity or outcomes between patients with or without COVID-19 except for a higher rate of mechanical ventilation in patients with definite COVID-19, which was ascribed to pulmonary effects of COVID-19 rather than neuromuscular weakness from GBS [6▪▪].
Molecular mimicry, which plays a critical role in the pathogenesis of GBS following C. jejuni infection, has been speculated for GBS arising after COVID-19 infection [7]. Studies have searched for similarities between peripheral nerve and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins. For host cell infection, the SARS-CoV-2 spike protein binds the angiotensin converting enzyme 2 (ACE-2) receptor as well as sialic acids linked to host cell gangliosides [8–10]. Accordingly, cross-reactivity between epitopes of the complex formed by the viral spike protein binding to host cell gangliosides and epitopes of peripheral nerve gangliosides has also been speculated [11,12]. However, ganglioside antibodies (GM1, GM2, GD1a, GD1b, GD3, asialo GM1, GT1b) were only rarely present in COVID-19-positive patients who developed GBS [13–17].
One study found homologous peptide sequences between SARS-CoV-2 and the neuronal cell adhesion molecule, and another found similarities with heat shock proteins 60 and 90, which may be associated with GBS [18,19]. However, a different study that compared the SARS-CoV-2 and human genomes found no significant similarities. Additionally, individual proteins encoded by SARS-CoV-2 RNA had no similarity to neuronal proteins [6▪▪]. In short, studies have not consistently identified similar epitopes between SARS-CoV-2 and peripheral nerve that may cause cross-reactivity.
Direct invasion of peripheral nerves by the virus has also been hypothesized as a cause of GBS and other neuropathies. One autopsy study found SARS-CoV-2 immunostaining in cranial nerves IX and X in 2 of 40 patients without GBS who died from COVID-19, raising the possibility of direct nerve infection [20]. However, SARS-CoV-2 RNA in CSF has only rarely been reported in GBS [3]. SARS-CoV-2 IgG was found in one case, but the authors commented it may have originated from the serum [21]. There are no reports of SARS-CoV-2 being detected in peripheral nerves aside from the autopsy paper mentioned above. Cranial mononeuropathies (III, VI, VII) arising in patients with acute COVID-19 infection have been reported in several case reports, but it is not clear whether they were caused by COVID-19 or arose coincidentally with COVID-19 infection [22–27]. Ischemia due to endotheliopathy associated with COVID-19, immune-mediated injury and direct viral infection are potential mechanisms.
Several COVID-19 vaccinations (virus-vector and mRNA vaccines) are currently in use. Whether they can cause peripheral nerve complications is not definitively known. Bell's palsy was reported in vaccine trials, however, the incidence did not exceed the expected rate in the general population [28,29▪]. One case of GBS arose after the Pfizer vaccination and the authors suggested an association [30]. We reported a case of GBS that occurred following vaccination in the Johnson & Johnson trial, however, a placebo-injected patient also developed GBS shortly thereafter [31,32]. We and others have emphasized the point that temporal association does not prove causality [32–34]. The FDA recently issued an alert that there may be an increased risk of GBS with the Johnson & Johnson vaccine based on ongoing surveillance data.
GBS was not reported as an adverse event in the clinical trials for the two mRNA COVID-19 vaccines approved by the United States Food and Drug Administration [28,35]. Accumulation of safety data as more people receive vaccinations will help elucidate whether a causal link exists. A history of GBS or Bell's palsy is currently not a contraindication for COVID-19 vaccination [29▪], and the benefits of vaccination in reducing morbidity and mortality associated with COVID-19 infection certainly outweigh the risks in our opinion.
Coronavirus disease-19 and neuromuscular junction disorders
A few papers reported new onset of myasthenia gravis (MG) in patients infected with COVID-19 [36,37]. However, perhaps a more likely explanation is that these cases represent patients with subclinical MG or subtle symptoms whose symptoms became unmasked in the setting of an infection. Infection is a known trigger for acute MG exacerbation [38]. Additionally, patients with COVID-19 infection may receive medications that can cause MG exacerbation. Early in the pandemic, azithromycin or hydroxychloroquine were often used when thought to be potentially effective for COVID-19. An interim analysis from an international, physician-reported registry found that 36 of 91 (40%) MG patients with COVID-19 experienced worsening of MG requiring rescue therapy [39]. Most patients were treated with IVIG and/or corticosteroids. Twenty-four percentage of patients died, whereas 43% had complete recovery and/or were discharged home.
Ongoing investigations are examining the impact of COVID-19 infection on neuromuscular junction (NMJ) disorders, mainly MG. Patients with MG are at risk of disease exacerbation with infection, and are often on immunosuppressive therapy (IST), which raises concerns about the risk of COVID-19 infection and the ability to mount an effective immune response. Studies are examining (1) the incidence of COVID-19 infection in patients with MG, (2) outcomes in patients with MG infected with COVID-19 compared to the general population, and (3) the effect of IST on outcomes following COVID-19 infection.
The true incidence of COVID-19 infection in patients with MG is unknown, but a large American database study found that 380 of 40,392 patients with MG (0.94%) were diagnosed with COVID-19 as of December 2020 [40]. Similarly, a French database study found that the cumulative incidence of symptomatic COVID-19 among MG patients was 0.96% (34 of 3,558 patients) [41]. The rate of hospitalization ranged from 27 to 69% [39–41]. In total, 10–26% of patients required ICU admission, and the mortality rate ranged from 7 to 24% [40,41].
The reported incidence rates are likely under-estimates, whereas the rates of hospitalization, ICU admission, and mortality are likely over-estimates, as asymptomatic infections (estimated at 5–32%) or mildly symptomatic infections may go undiagnosed or unreported in registries [42]. Nevertheless, one study found that compared to the general population, patients with MG were at higher risk of hospitalization with an OR of 3, ICU admission (OR 5.2), intubation (OR 4.6), and death (OR 4.3) [40]. The elevated risk remained even when compared to age- and gender-matched controls.
A few retrospective studies examined the impact of immunosuppressive therapies for MG on the risk of COVID-19 infection and outcomes. One study showed that IST did not increase the risk of COVID-19 infection in patients with chronic autoimmune neuromuscular disorders, including those with MG [43▪]. Patients with chronic autoimmune neuromuscular disorders on IST were more likely than those not on IST to be hospitalized (OR 2.86) but were not at increased risk of being admitted to an ICU. Another study showed that severe baseline MG status, but not IST use, was associated with severe COVID-19 infection (defined as requiring ICU care or resulting in death) [41].
Guidelines for the management of NMJ disorders during the pandemic, namely, MG and Lambert-Eaton myasthenic syndrome (LEMS), recommend continuing treatment without alteration with strict social-distancing measures [44▪▪,45]. For patients considering B-cell depleting therapy (e.g. rituximab), delaying initiation until after the peak of the outbreak in the patient's region is advised. In our opinion, the local incidence and prevalence of COVID-19, ability to strictly socially isolate, and vaccination status should be considered at a minimum. In unvaccinated patients, postponing the initiation of B-cell depleting therapy to at least 2 weeks after vaccination may be advisable to allow for a sufficient antibody response.
IVIG is thought unlikely to affect the antibody response to vaccination, thus there is no recommended minimum interval between IVIG treatment and vaccination at the time of writing this review [46]. Although not definitively known, PLEX is likely to remove antibodies that form in response to vaccination. Reduction in antibodies against COVID-19 was observed in a patient with severe COVID-19 infection who underwent PLEX [47]. Therefore, in patients with MG on maintenance PLEX or requiring rescue therapy, the risk of COVID-19 must be carefully weighed against the benefit of PLEX over alternative treatment options for MG.
For patients with MG or LEMS who develop COVID-19 infection, guidelines suggest that current therapy should be continued, but a temporary pause may need to be considered in patients with severe infection especially if there are concurrent infections or sepsis [44▪▪].
In sum, retrospective studies suggest that while the incidence of symptomatic COVID-19 in patients with MG is low, the risk of hospitalization, ICU admission and death are relatively high. The use of IST appears to increase the risk of hospitalization, but not the risk of ICU admission or death. Further studies are needed to understand the impact of COVID-19 on NMJ disorders.
Coronavirus disease-19-associated myopathy
Early on in the pandemic, it became apparent that COVID-19 can affect skeletal muscle. Myalgia and fatigue, although not specific for skeletal muscle injury, occur in 11–70% of patients and creatine kinase (CK) is elevated in 9–33% [48–52]. Fatigue or muscle weakness are common lingering symptoms and are present in 63% as is myalgia in 2% of patients 6 months after acute infection [53]. Literature continues to expand on this topic with studies reporting various myopathic processes potentially associated with COVID-19, including rhabdomyolysis, myositis, and critical illness myopathy (CIM).
To date, there are more than 30 published case reports on rhabdomyolysis occurring in patients with COVID-19. One study found that rhabdomyolysis occurred in 1.1% of patients hospitalized with COVID-19 [48]. Patients with COVID-19 infection may be prone to known causes of rhabdomyolysis, such as severe electrolyte imbalance, ischemia, prolonged immobility or myotoxicity from medications, but immune-mediated damage or direct invasion of myocytes by the virus are additional possible mechanisms and are discussed further below.
Myositis has been described in a few case reports. Beydon et al. reported a patient who presented with diffuse myalgia and lower extremity weakness. The patient tested positive for COVID-19 and CK was 25,384 U/L [54]. MRI of the thighs showed bilateral thigh edema suggestive of myositis but a biopsy was not performed. Zhang et al. described a patient who presented with respiratory symptoms, myalgia, and bulbar and generalized weakness [55▪]. CK was elevated to 700 U/L. Anti-SAE-1, a dermatomyositis-specific antibody, was detected in the serum (along with anti-SSA and anti-Ku). Muscle biopsy revealed perivascular inflammation with endomysial extension, and abnormal sarcolemmal and sarcoplasmic expression of major histocompatibility antigen 1 (MHC-1). These biopsy and laboratory findings led to a speculation that COVID-19-associated myositis may be dermatomyositis [56]. We also found muscle biopsy findings compatible with this hypothesis in a patient who presented with fever, myalgia, proximal greater than distal weakness, and an elevated CK of 29,800 U/L [57▪]. Muscle biopsy revealed mild perivascular inflammation. Immunohistochemistry showed abnormal expression of myxovirus resistance protein A (MxA) and MHC-1 on the sarcolemma and sarcoplasm of perifascicular muscle fibers, and MxA on capillaries, suggestive of type-1 interferonopathy as seen in dermatomyositis. Both patients with myositis on muscle biopsy received steroids with clinical improvement and decrease in CK over a couple weeks [55▪,57▪]. However, by no means does this imply that a type-1 interferonopathy is the cause of most myopathies associated with COVID-19.
Whether SARS-CoV-2 directly infects muscle is unclear. The ACE2 receptor, which is used by SARS-CoV-2 to infect host cells, is also expressed in skeletal muscle, raising the possibility of direct infection [8,9]. An autopsy study that examined the diaphragm muscles of 26 patients who died of COVID-19 found ACE2 expression predominantly in the sarcolemma [58]. In 4 cases, COVID-19 RNA was found in myocytes, indicating that viral infection is possible. In contrast, Zhang et al. did not find viral particles by electron microscopy in their case [55▪]. Notably, two large autopsy studies of patients dying from COVID-19 revealed inflammatory cell infiltrates, necrotic muscle fibers, MHC-1 expression on muscle fibers and MxA expression on capillaries but no evidence of direct viral invasion by immunohistochemistry [59,60].
Finally, ICU acquired weakness (ICUAW), which includes CIM and critical illness polyneuropathy (CIP), is not specific to COVID-19 but deserves mention. Risk factors include prolonged immobility, sepsis, systemic inflammatory response syndrome and multiorgan failure, which can complicate severe COVID-19 infection [61]. In published cases, ICUAW was suspected when critically ill patients with COVID-19 had diffuse weakness and/or difficulty weaning from mechanical ventilation [62–65]. CK was normal or mildly elevated, and nerve conduction studies/electromyography showed myopathic features in CIM or axonal sensorimotor polyneuropathy in CIP. One study reported muscle biopsies in 3 patients; scattered necrotic and regenerating fibers were seen in one patient, and atrophic and regenerating fibers in 2, which are not specific for but can be seen in CIM [65]. MHC-1 and membrane attack complex were not observed. In patients who survived, strength improved over weeks to months [62,63]. Features of CIM/CIP following severe COVID-19 infection appear to be comparable to CIM/CIP from other severe illnesses. Type 2 muscle fiber atrophy was present in most cases one large autopsy series of patients dying from COVID-19 [59].
CONCLUSION
With rising numbers of cumulative COVID-19 cases by the day and increasing literature on its manifestations, we are discovering more about the spectrum of neuromuscular effects of COVID-19. Some of these complications can have long-lasting consequences or a protracted recovery period after the acute illness. As the pandemic continues on, these issues are likely to be encountered in clinical practice in the acute and recovery phase. Further research is needed to fully understand the spectrum of neuromuscular consequences of COVID-19, pathogenesis, preventive strategies and treatment.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
J.S.: none.
A.A.A. has served on medical advisory boards for Johnson and Johnson, Alexion, Sarepta, CSL Behring, Strongbridge Pharma.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/. [Accessed 27 March 2021]
- 2.Miró Ò, Llorens P, Jiménez S, et al. Frequency of five unusual presentations in patients with COVID-19: results of the UMC-19-S(1). Epidemiol Infect 2020; 148:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fragiel M, Miro O, Llorens P, et al. Incidence, clinical, risk factors and outcomes of Guillain-Barre in Covid-19. Ann Neurol 2021; 89:598–603. [DOI] [PubMed] [Google Scholar]
- 4.Filosto M, Cotti Piccinelli S, Gazzina S, et al. Guillain-Barre syndrome and COVID-19: an observational multicentre study from two Italian hotspot regions. J Neurol Neurosurg Psychiatry 2021; 92:751–756. [DOI] [PubMed] [Google Scholar]
- 5.Garnero M, Del Sette M, Assini A, et al. COVID-19-related and not related Guillain-Barré syndromes share the same management pitfalls during lock down: the experience of Liguria region in Italy. J Neurol Sci 2020; 418:117114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪▪.Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barre syndrome. Brain 2021; 144:682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first large-scale epidemiologic study that examined the relationship between GBS and COVID-19. An association was not identified.
- 7.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet 2016; 388:717–727. [DOI] [PubMed] [Google Scholar]
- 8.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu H, Yan P, Xiong W, et al. Genomic characterization of a novel virulent phage infecting Shigella fiexneri and isolated from sewage. Virus Res 2020; 283:197983. [DOI] [PubMed] [Google Scholar]
- 10.Fantini J, Di Scala C, Chahinian H, et al. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents 2020; 55:105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalakas MC. Guillain-Barré syndrome: the first documented COVID-19-triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm 2020; 7:e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costello F, Dalakas MC. Cranial neuropathies and COVID-19: neurotropism and autoimmunity. Neurology 2020; 95:195–196. [DOI] [PubMed] [Google Scholar]
- 13.Diez-Porras L, Vergés E, Gil F, et al. Guillain-Barré-Strohl syndrome and COVID-19: case report and literature review. Neuromuscul Disord 2020; 30:859–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrelli C, Scendoni R, Paglioriti M, et al. Acute motor axonal neuropathy related to COVID-19 infection: a new diagnostic overview. J Clin Neuromuscul Dis 2020; 22:120–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Civardi C, Collini A, Geda DJ, et al. Antiganglioside antibodies in Guillain-Barré syndrome associated with SARS-CoV-2 infection. J Neurol Neurosurg Psychiatry 2020; 91:1361–1362. [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 2020; 95:e601–e605. [DOI] [PubMed] [Google Scholar]
- 17.Lantos JE, Strauss SB, Lin E. COVID-19-associated miller fisher syndrome: MRI findings. AJNR Am J Neuroradiol 2020; 41:1184–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morsy S. NCAM protein and SARS-COV-2 surface proteins: in-silico hypothetical evidence for the immunopathogenesis of Guillain-Barré syndrome. Med Hypotheses 2020; 145:110342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucchese G, Flöel A. SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperones 2020; 25:731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matschke J, Lutgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a postmortem case series. Lancet Neurol 2020; 19:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helbok R, Beer R, Löscher W, et al. Guillain-Barré syndrome in a patient with antibodies against SARS-COV-2. Eur J Neurol 2020; 27:1754–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belghmaidi S, Nassih H, Boutgayout S, et al. Third cranial nerve palsy presenting with unilateral diplopia and strabismus in a 24-year-old woman with COVID-19. Am J Case Rep 2020; 21:e925897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascual-Goni E, Fortea J, Martinez-Domeno A, et al. COVID-19-associated ophthalmoparesis and hypothalamic involvement. Neurol Neuroimmunol Neuroinflamm 2020; 7:e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei H, Yin H, Huang M, et al. The 2019 novel cornoavirus pneumonia with onset of oculomotor nerve palsy: a case study. J Neurol 2020; 267:1550–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lima MA, Silva MTT, Soares CN, et al. Peripheral facial nerve palsy associated with COVID-19. J Neurovirol 2020; 26:941–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goh Y, Beh DLL, Makmur A, et al. Pearls & Oy-sters: facial nerve palsy in COVID-19 infection. Neurology 2020; 95:364–367. [DOI] [PubMed] [Google Scholar]
- 27.Homma Y, Watanabe M, Inoue K, et al. Coronavirus disease-19 pneumonia with facial nerve palsy and olfactory disturbance. Intern Med 2020; 59:1773–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪. Centers for Disease Control. Vaccine Considerations for People with Underlying Medical Conditions. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/underlying-conditions.html. [Accessed 3 April 2021]; This website provides updated information on COVID-19 vaccination for patients with underlying medical conditions.
- 30.Waheed S, Bayas A, Hindi F, et al. Neurological complications of COVID-19: Guillain-Barre Syndrome following Pfizer COVID-19 vaccine. Cureus 2021; 13:e13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. JanssenMD. Use of Janssen COVID-19 Vaccine – Adverse Event Demyelinating Disorders (Guillain-Barré Syndrome). Available from: https://www.janssenmd.com/janssen-covid19-vaccine/safety/adverseevents/neurological/use-of-janssen-covid19-vaccine-adverse-event-demyelinating-disorders-guillainbarr-syndrome. [Accessed 3 April 2021]
- 32.Márquez Loza AM, Holroyd KB, Johnson SA, et al. Guillain-Barré Syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality. Neurology 2021; 96:1052–1054. [DOI] [PubMed] [Google Scholar]
- 33.Lunn MP, Cornblath DR, Jacobs BC, et al. COVID-19 vaccine and Guillain-Barre syndrome: let's not leap to associations. Brain 2021; 144:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bourdette D, Killestein J. Quelling Public Fears about Guillain-Barre Syndrome and COVID-19 Vaccination Neurology. [DOI] [PubMed]
- 35.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Restivo DA, Centonze D, Alesina A, et al. Myasthenia gravis associated with SARS-CoV-2 infection. Ann Intern Med 2020; 173:1027–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sriwastava S, Tandon M, Kataria S, et al. New onset of ocular myasthenia gravis in a patient with COVID-19: a novel case report and literature review. J Neurol 2020; doi: 10.1007/s00415-020-10263-1 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gummi RR, Kukulka NA, Deroche CB, et al. Factors associated with acute exacerbations of myasthenia gravis. Muscle Nerve 2019; 60:693–699. [DOI] [PubMed] [Google Scholar]
- 39.Muppidi S, Guptill JT, Jacob S, et al. COVID-19-associated risks and effects in myasthenia gravis (CARE-MG). Lancet Neurol 2020; 19:970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy B, Kovvuru S, Nalleballe K, et al. Electronic health record derived-impact of COVID-19 on myasthenia gravis. J Neurol Sci 2021; 423:117362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sole G, Mathis S, Friedman D, et al. Impact of coronavirus disease 2019 in a French Cohort of Myasthenia Gravis. Neurology 2021; 96:e2109–e2120. [DOI] [PubMed] [Google Scholar]
- 42. Centers for Disease Control and Prevention. Estimated COVID-19 Burden. 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html. [Accessed 7 March 2021]
- 43▪.Kovvuru S, Nalleballe K, Onteddu SR, et al. Immunosuppression in chronic autoimmune neurological disorders during the COVID-19 pandemic. J Neurol Sci 2021; 420:117230. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examined the effect of immunosuppressive therapy use in patients with autoimmune neurologic disorders on the outcomes of COVID-19 infection.
- 44▪▪.Jacob S, Muppidi S, Guidon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci 2020; 412:116803. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides guidance on the management of neuromuscular junction disorders based on expert opinion.
- 45.Sole G, Salort-Campana E, Pereon Y, et al. Guidance for the care of neuromuscular patients during the COVID-19 pandemic outbreak from the French Rare Healthcare for Neuromuscular Diseases Network. Revue Neurol 2020; 176:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Centers for Disease Control and Prevention. COVID-19 Vaccines. Available from: https://www.cdc.gov/vaccines/covid-19/infobyproduct/clinical-considerations.html. [Accessed 3 April 2021]
- 47.Stahl K, Bode C, David S. First do no harm—beware the risk of therapeutic plasma exchange in severe COVID-19. Crit Care 2020; 24:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romero-Sanchez CM, Diaz-Maroto I, Fernandez-Diaz E, et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology 2020; 95:e1060–e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beydon M, Chevalier K, Al Tabaa O, et al. Myositis as a manifestation of SARS-CoV-2. Ann Rheum Dis 2020; 80:e42. [DOI] [PubMed] [Google Scholar]
- 55▪.Zhang H, Charmchi Z, Seidman RJ, et al. COVID-19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve 2020; 62:E57–E60. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes a patient with COVID-19 who presented with myopathy and was found to have SAE-1 antibody in serum, and features on muscle biopsy compatible with dermatomyositis.
- 56.Tanboon J, Nishino I. COVID-19-associated myositis may be dermatomyositis. Muscle Nerve 2021; 63:E9–e10. [DOI] [PubMed] [Google Scholar]
- 57▪.Manzano GS, Woods JK, Amato AA. Covid-19-associated myopathy caused by type I Interferonopathy. N Engl J Med 2020; 383:2389–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes a patient presenting with COVID-19 and clinical myopathy, whose muscle biopsy findings suggsted type-1 interferonopathy and dermatomyositis.
- 58.Shi Z, de Vries HJ, Vlaar APJ, et al. Diaphragm pathology in critically ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern Med 2021; 181:122–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suh J, Mukerji SS, Collens SI, et al. Skeletal muscle and peripheral nerve histopathology in COVID-19. Neurology 2021; DOI 10.1212/WNL.0000000000012344. [DOI] [PubMed] [Google Scholar]
- 60.Aschman T, Schneider J, Meinhardt J, et al. Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol 2021; doi: 10.1001/jamaneurol.2021.2004 [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 61.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011; 10:931–941. [DOI] [PubMed] [Google Scholar]
- 62.Madia F, Merico B, Primiano G, et al. Acute myopathic quadriplegia in patients with COVID-19 in the intensive care unit. Neurology 2020; 95:492–494. [DOI] [PubMed] [Google Scholar]
- 63.Bagnato S, Boccagni C, Marino G, et al. Critical illness myopathy after COVID-19. Int J Infect Dis 2020; 99:276–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tankisi H, Tankisi A, Harbo T, et al. Critical illness myopathy as a consequence of Covid-19 infection. Clin Neurophysiol 2020; 131:1931–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cabanes-Martinez L, Villadoniga M, Gonzalez-Rodriguez L, et al. Neuromuscular involvement in COVID-19 critically ill patients. Clin Neurophysiol 2020; 131:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]