Abstract
Scorpionism is a relevant medical condition in Brazil. It is responsible for most accidents involving venomous animals in the country, which leads to severe symptoms that can evolve to death. In recent years, an increase of almost 50% in the incidence of scorpionism has been observed in the Northern Region, where the highest severity of envenoming has been notified since the beginning of the 21st century. This review aims to provide an in-depth assessment of public data and reports on symptoms and epidemiology of envenoming, ecological aspects of scorpions, and characterization of venoms and toxins to access the gaps that need to be filled in the knowledge of the scorpion species of medical importance from the Brazilian Amazon. A systematic search using the string words “Amazon” and “scorpion” was performed on 11 databases. No restriction on date, language or status of the publication was applied. Reports not related to the Brazilian Amazon were excluded. Therefore, 88 studies remained. It is shown that populations of scorpions of medical importance, even of the same species, may present significant toxic variations peculiar to some regions in the Brazilian Amazon, and commercial scorpion antivenoms were not able to shorten the intensity and duration of neurological manifestations in patients stung by T. silvestris, T. apiacas or T. obscurus. It is also highlighted that the toxins responsible for triggering these alterations have not been elucidated yet and this is a fruitful field for the development of more efficient antivenoms. Furthermore, the geographic distribution of scorpions of the genus Tityus in the Brazilian Amazon was revised and updated. The cumulative and detailed information provided in this review may help physicians and scientists interested in scorpionism in the Brazilian Amazon.
Keywords: Brazilian Amazon, Endemic scorpions, Arboreal scorpions, Venom, Tityus metuendeus, Tityus silvestris, Brotheas amazonicus, Tityus strandi, Tityus apiacas, Tityus obscurus
Background
To date, there are 2,584 scorpion species worldwide distributed into 23 families (according to [1] and updated by [2-6]). So far, four scorpion families (Bothriuridae, Buthidae, Chactidae and Hormuridae), 23 genera and about 160 species have been reported in Brazil [7-9], which represents 6.3% of the worldwide diversity of these arachnids.
Scorpionism remains a serious public health problem. More than 1.2 million scorpion stings and 3,000 deaths caused by scorpion envenoming are registered annually worldwide, and about 2.3 billion people live in areas of scorpionism risk [10, 11].
Buthidae, the largest of the scorpion families, is distributed in several regions around the world, except Antarctica and New Zealand [3]. This family comprises 1,225 species (updated on January 22nd, 2021) [12], including about 50 species considered dangerous to humans [13].
Around 95% of the scorpion envenomings are caused by species of the family Buthidae C. L. Koch, which includes the genera Tityus, Centruroides, Mesobuthus, Parabuthus, Leiurus, Buthus, Hottentota and Androctonus [14]. Scorpions belonging to Chactidae family are capable of causing mild and local toxicity in humans [15, 16]. However, a greater number of scorpion species can be potentially harmful to humans. Thus far, 104 scorpion species have been considered medically significant in the literature. However, there is a lack of reports of symptoms induced by the venom of most of these species [17].
Although scorpions are present on all continents, except Antarctica, the severity and incidence of envenoming are higher in the northern Saharan Africa, African Sahel, South Africa, Middle East, southern India, Mexico, Brazil and the Amazon basin area [18].
The Amazon region spans territories of France (French Guyana) and eight countries (Bolivia, Brazil, Colombia, Ecuador, Guyana, Peru, Suriname and Venezuela) [19]. The Amazon biome covers 49.5% of the Brazilian territory [20], which holds around 60% of the Amazon rainforest [21]. The Brazilian Amazon occupies the states of Acre (AC), Amapá (AP), Amazonas (AM), Roraima (RR), Pará (PA), Rondônia (RO), and parts of the state of Tocantins (TO), encompassing 93.2% of the Northern Region. The rainforest also comprises parts of the states of Maranhão (MA) and Mato Grosso (MT) in the Northeast and Central-West regions, respectively [20].
The North and Northeast regions comprise 52% and 26%, respectively, of the Brazilian scorpion fauna [22]. The twenty-six states of Brazil and the Federal District are geopolitically divided into five macroregions: North, Northeast, Central-West, Southeast, and South [23]. More than 70 scorpion species were identified in the North region [7, 22, 24], of which 48 species were recorded in the state of Amazonas [25-27]. The geographic distribution of the 28 species of the genus Tityus will be shown in the section “3.1. Ecological aspects of scorpions from the Brazilian Amazon region”.
The increasing number of envenomings and deaths caused by scorpions, mainly in urban centers, has been a public health problem in Brazil for years [28, 29]. The accelerated process of urbanization and the lack of basic infrastructure (such as water, electricity, sewage treatment, and regular collection of garbage) have provided conditions for the proliferation of opportunistic and invasive scorpions of high ecological plasticity, such as T. stigmurus and T. serrulatus [28, 29]. The later species is responsible for the most serious envenomings and deaths in Brazil [30].
Scorpion stings are worrisome because they represent the majority of incidents and can be of high severity, which makes it difficult for sanitary agencies to manage cases [31].
Since 2004, the number of scorpion envenoming cases in Brazil has exceeded those caused by snakebites [30], and in 2018, these figures were 156,833 against 28,946 [32]. However, some Brazilian states provide updated notification records with a time lag, sometimes one or two years after the report [33]. Scorpion stings were the most frequent accidents caused by venomous terrestrial animals in Brazil (46%), and responsible for almost 31% of deaths from 2000 to 2018, when compared to accidents caused by snakes, spiders, bees and caterpillars [34, 35]. It is noteworthy that the number of cases of scorpion accidents increased about 70% from 2016 to 2018 [35].
In Brazil, since the beginning of the 21st century, the highest severity of scorpion envenoming has been notified in the Northern region [33, 36-38]. However, it is important to highlight that not all parts of the Brazilian Amazon have records about the species of scorpions involved in accidents.
Although the North region comprises 52% of the Brazilian scorpion fauna [22], only the venom of T. obscurus has been extensively studied [39-48]. T. apiacas, T. metuendus, T. silvestris and T. strandi are also species of medical interest in this region [36, 37, 49-52], but there are no studies on the biochemical and molecular characterization of the toxins present in these venoms.
According to the clinical manifestations, the scorpion envenoming is classified into mild (local pain and paresthesia), moderate (intense local pain associated with one or more systemic manifestations) or severe (cardiac and hemodynamic changes, cardiogenic shock and pulmonary edema that can evolve to death) [11, 36]. Local manifestations are classified as mild symptoms and represent about 87% of the recorded scorpionism cases [29, 30]. Because of the pain, patients may experience nausea, agitation, and mild tachycardia, which will disappear after local treatment. In these cases, the patient is kept under observation for at least 6 hours, and any worsening of the symptoms requires hospitalization for clinical management [30]. Among the severe manifestations are countless vomiting episodes, profuse sweating, tachypnea, increased blood pressure, tachycardia or bradycardia, and symptoms compatible with acute congestive heart failure due to increased vascular resistance and acute lung edema [11, 29, 53]. Patients presenting systemic manifestations of scorpion envenoming are managed with symptomatic treatment, antivenom serum, and cardiorespiratory support [30].
From 2013 to 2017, about 83% of deaths resulting from scorpionism occurred within 48 hours after the sting [29]. The severity of scorpion envenoming is related to cardiac and hemodynamic changes, with cardiogenic shock and pulmonary edema contributing to the main causes of death [30]. Most victims of lethal scorpion stings die from cardiac or respiratory failure [11].
The notified scorpionism cases analyzed during the period from 2001 to 2012 revealed the highest severity of scorpion envenoming in the northern Brazil [33]. Only 60% of the envenomings were asymptomatic or mild in the North region (against 80-90% in the rest of the country). Moderate and severe symptoms accounted, respectively, for 35% and 4% in the North region, against, respectively, less than 15% and 2.5 % in the other four macroregions [33]. This discrepancy is due to the scarcity of studies on the epidemiology and venom characterization of the main scorpion species causing accidents and clinical manifestations. Such lack of data is due to, among other factors, incorrect species identification, inaccurate diagnosis and limited accessibility to antivenom treatment. These aspects make scorpionism a relevant public health problem, especially in the Brazilian Amazon region [54, 55].
The specific treatment for moderate and severe scorpion stings, in Brazil, consists of antivenoms produced against T. serrulatus venom, species that does not belong to the Brazilian Amazon region [56]. A case report showing that severe symptoms caused by T. silvestris sting were refractory to anti-Tityus antivenom [50] illustrates the need to develop new antivenoms or improve the effectiveness of those available. Despite the territorial extension of the North region, the specific clinical care and professional support to taxonomically distinguish venomous animals that are life-threatening is usually carried out in the capitals of the Brazilian states of Amazonas and Pará, respectively, Manaus (03°05′S 60°02′W) and Belém (1°26′S 48°29′W) [9, 50]. The severity of the systemic effects caused by Tityus species depends on the venom composition and the patient's clinical condition [29]. Therefore, it is essential to identify and characterize the components within the scorpion venom to produce more effective antivenoms.
It is important to highlight that many animal venoms, including those from non-health threatening scorpions, may provide diagnostic tools, experimental molecules to validate postulated therapeutic targets, drug libraries and prototypes for the design of drugs and therapeutic agents [57]. Furthermore, studies on the biology of scorpions can contribute to anticipate the risk of envenoming and reduce the severity of accidents [58].
In view of this scenario, this manuscript systematically reviews the reported symptoms and epidemiology of envenoming, ecological aspects of scorpions, and characterization of venoms and toxins to access the gaps that need to be filled in the knowledge of scorpion species of medical importance that occur in the Brazilian Amazon.
Methods
A systematic review was carried out following the rules and guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [59].
Search strategy and data collection
An electronic search was performed in the following general databases: Web of Science all databases (Zoological Record, Web of Science Core Collection, Current Contents Connect, and Scielo Citation Index), Scopus, Virtual Health Library (VHL, which hosts Medline and the Latin American and Caribbean Center on Health Sciences Informational - LILACS), Embase, Pubmed and Cochrane Library. An electronic search was also performed on the Brazilian Digital Library of Theses and Dissertations (BDTD) (https://bdtd.ibict.br/vufind/). The search string used in these bases was “Amazon* AND *scorpi* AND NOT (pseudoscorpi* OR pseudoescorpi* OR scorpioides)”. Concerning Embase, “$scorpi*” was used instead of “*scorpi*”.
An electronic search was performed on the gray literature base Opengrey (https://opengrey.eu), and on the preprint servers BioRxiv (https://www.biorxiv.org/) and MedRxiv (https://www.medrxiv.org/), using the keywords “Amazon scorpion”.
Manual search and some alternative sources, such as reference lists from other studies and reviews, were consulted to ensure the inclusion of relevant articles.
No restrictions on date (from 1864 to 2020), language or status (abstract or full text) of the publication were used. The searches were carried out on December 11th, 2020.
Criteria
The study selection process was carried out by two independent reviewers, and any disagreement was solved by consensus. The selection process was verified by a third reviewer, ensuring the specificity and quality of the process. The selection of studies was carried out in two stages. In the first stage, the titles and abstracts of the references identified through the search strategy. Articles that did not meet the inclusion criteria or met the exclusion criteria were removed and the potentially eligible studies were pre-selected. In the second stage, the full text evaluation of the pre-selected studies was carried out to confirm eligibility. The selection process was carried out through the StArt (State of the Art through Systematic Review) tool (http://lapes.dc.ufscar.br/tools/start_tool) [60].
When the searches from all the bibliographic databases were combined, 522 records were obtained (Fig. 1). A total of 272 repeated records were found in the combined dataset, leaving 250 papers to be examined for inclusion/exclusion criteria. All articles related to Amazonian scorpions were included. The articles were excluded from the final analysis if they met any of the following exclusion criteria: (a) articles not about scorpions, (b) concerning non-Amazonian scorpions, (c) full text not available, (d) not from Brazilian Amazon, (e) ecological aspects of species with no accidents (except Tityus), and (f) repeated (thesis published as an article).
Figure 1. PRISMA flowchart showing the total number of records identified and filtered at each stage of the selection process obtained from the literature search of a systematic review on Amazonian scorpions.
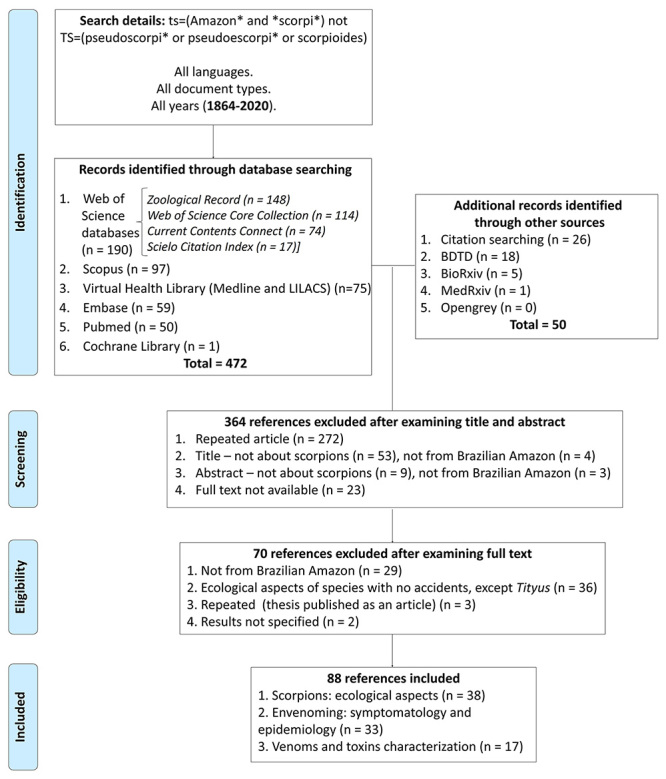
After title and abstract screening, 181 relevant papers were obtained for full paper screening. Three theses were removed because they were published as papers and 23 papers could not be obtained. During full text screening, a total of 88 studies didn’t meet the exclusion criteria. These remaining articles were divided into three groups, considering the following inclusion criteria: (a) ecological aspects of scorpions, (b) symptomatology and epidemiology of envenoming, or (c) venoms and toxins characterization, as shown in the PRISMA flowchart in Figure 1.
Results
Ecological aspects of scorpions from the Brazilian Amazon region
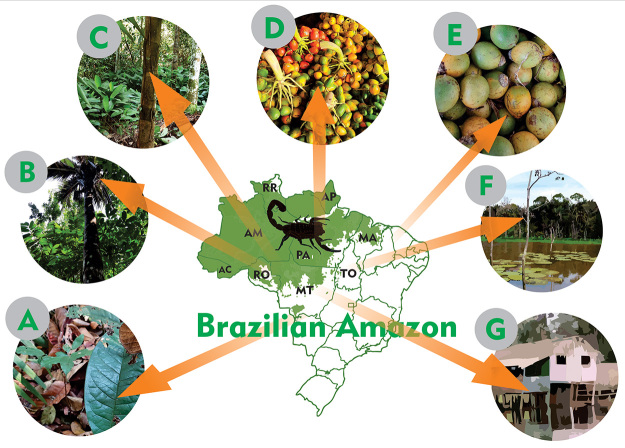
Scorpions native from Amazon basin are equilibrium species that depend on natural conditions [61, 62]. Such animals inhabit stable environments and demonstrate habitat and microhabitat specificity [61]. Populations of Tityus scorpions, in the Brazilian Amazon, are most abundant in terra firme forest (non-flooded area) (Table 1), which comprises the largest Amazonian vegetation cover [61]. Places where scorpion envenoming are more likely to occur in the Brazilian Amazon are shown in Figure 2 (A-G).
Table 1. Ecological, morphological and reproductive aspects of scorpions from the Brazilian Amazon.
| Species | Morphology | Body size (mm) | Habitat | Micro-habitat | Brood size | Ref. |
|---|---|---|---|---|---|---|
| Brotheas amazonicus (Chactidae), Lourenço, 1988 | General coloration dark brown and reddish telson. Carapace moderately granular; tergites I to IV punctuate; VII moderately granular. Pectines with 8 to 11 teeth. | 60-70 | Terra firme forest (non-flooded zone) | These scorpions hide mainly under decaying trunks. During the night, they are detected near burrows in the leaf litter (~20 cm depth). Specimens are found in the urban region or near rural settlements. | 8-21 | [7, 15, 64, 65] |
| Rhopalurus laticauda (Buthidae), Thorell, 1876 (synonym R. amazonicus Lourenço, 1986 ; R. crassicauda Lourenço, 2002) | Yellowish-brown coloration with metasomal segment V and dark telson. Exhibits phenotypic plasticity in size and the intensity of infuscation on the carapace, tergites, metasoma, and pedipalps. | 50 | Amazonian savannah | Under rocks, wood barks and fallen trunks. | 19 | [66, 67] |
| T. (Archaeotityus) bastosiLourenço, 1984 | Dark yellow background densely covered with dark reddish-brown variegated spots. Dorsolateral keels of metasomal segments I to IV with a very strong spinoid posterior granule. | 33-41 | Terra firme forest | Specimens hide under leaves and wood barks in the leaf litter. | 36 | [68] |
| T. (Archaeotityus) clathratus Kosch, 1844 | Dentate margins of pedipalp-chela fingers composed of 12 to 14 oblique rows of granules. | 25-30 | Terra firme forest | Leaf litter. | 8-18 | [25] |
| T. (Archaeotityus) grahamiLourenço, 2012 | Yellowish to reddish-yellow with brown to dark brown spots over the body and appendages. Metasomal segment V granulated; vesicle with ventral and lateral carinae. | 25-37 | Terra firme forest | These scorpions can be found in the leaf litter or in tree trunks. | - | [26] |
| T. (Archaeotityus) maranhensis Lourenço et al. 2006 | Coloration yellowish to reddish-yellow. Chelicerae with residual spots, and sternites pigmented, dorsal carinae of metasomal segments I to IV with spinoid. granules, internal carinae of patella with marked granules. | 32-34 | Terra firme forest | Leaf litter. | - | [26] |
| T. (Archaeotityus) mattogrossensis Boreli, 1901 | Yellowish with dark pigmentation on the body. Dentate margins of pedipalp-tibia fingers composed of 15/16 oblique rows of granules. | 30-36 | Open vegetation | In termite mounds or on Arecaceae. | 12 | [68, 69] |
| T. (Archaeotityus) silvestris Pocock, 1897 | Yellowish with dark spots over the body. Dorsolateral keels of metasomal segments I to IV without a spinoid posterior. | 25-45 | Terra firme forest and floodable forest (igapó and várzea) | They are detected in leaf litter, in the trunk of Arecaceae or among fruits in the Amazonian canopy. Specimens are found inside houses in the urban and rural areas. | 5-14 | [62, 65, 70-72] |
| T. (Atreus) anori Lourenço, Rossi and Wilmé, 2019 | Reddish-brown to dark brown. I) Chela with an inconspicuous scalloping of the proximal dentate margin of fixed finger in male; III) better marked carinae on mesosoma; IV) very weak chetotaxy on pedipalps; V) sternites III and V with a white triangular zone on posterior edge. | 88 | Floodable forest | They hide under tree trunks or in the Amazonian canopy. | - | [25] |
| T. (Atreus) apiacas Lourenço, 2002 | General coloration reddish-brown, with some yellowish zones on the sternites. Metasomal segments I to V blackish-brown, with 10-8-8-6(8)-5 darker carinae. Dentate margins of pedipalp-chela fingers with 16 oblique rows of granules. | 75-100 | Terra firme forest. | These scorpions can be detected in the leaf litter or in tree trunks. They are found in rural communities, in fruit and vegetable plantations. | - | [51, 73] |
| T. (Atreus) dinizi Lourenço, 1997 | Blackish, but with some pale regions on the sternites. Basal middle lamellae of pectines of males slightly dilated. Dentate margins of pedipalp-chela fingers with 16 rows of granules; pectines with 20 teeth. | 85-100 | Floodable forest | They hide under tree trunks or in the Amazonian canopy. | - | [68, 73, 74] |
| T. (Atreus) elizabethae Lourenço and Ramos, 2004 | Reddish to dark reddish overall. Metasomal segments with 10-10-10-8(7)-5 carinae. | 72 | Amazonian savannah | They are found in the leaf litter or in tree trunks. | - | [75] |
| T. (Atreus) generaltheophiloi Lourenço, 2017 | Blackish-brown to dark blackish, particularly on the legs and pedipalps. Metasomal segments I to V, with 10-8-8-8-5 carinae, crenulated. Basal tooth on fixed finger of chelicera has a particular trifid morphology. | 70.5 | Terra firme forest (Mountain forest at altitudes of 600 m) | These scorpions can be detected in the leaf litter or in tree trunks. | - | [24] |
| T. (Atreus) matthieseni Pinto da Rocha and Lourenço, 2000 | General coloration brownish with some dark spots over the body and pedipalps; legs brownish with yellowish spots. Metasomal segments I to V and telson uniformly blackish-brown; with 10-10-8-8-5 carinae. | 75-85 | Terra firme forest | They are found under wood bark in the leaf litter or in palm leaf sheaths. | - | [76] |
| T. (Atreus) metuendus Pocock, 1897 | Blackish-brown to blackish. Metasomal segments I to III blackish-brown, IV and V blackish; ventral keels of metasomal segments I to IV parallel. | 80-90 | Terra firme forest and floodable forest | They are detected in leaf litter, in the trunk of Arecaceae or among fruits in the Amazonian canopy. Specimens are found in the urban region or near rural settlements. | 25-35 | [7, 65, 67, 68, 73, 77, 78] |
| T. (Atreus) neblina Lourenço, 2008 | General coloration reddish-yellow to reddish-brown overall. Metasomal segments with 10-8-8-8(7)-5 carinae. Dorsal carinae of metasomal segments III and IV have 1 to 3 spinoid granules. | 45-52 | Terra firme forest (Mountain forest at altitudes of 850-2200 m) | Scorpions can be detected in the leaf litter or in tree trunks. | - | [76] |
| T. (Atreus) obscurus Gervais, 1843 (synonym: T. cambridgei Pocock, 1897; T. paraensis Kraepelin, 1896) | Blackish. Metasomal segments I to V and telson uniformly blackish; with 10-10-8-8-5 carinae. Trichobothria* et and est on fixed finger of chela only slightly proximal; Est in the chela not basal to Esb. | 75-100 | Terra firme forest and floodable forest (igapó and várzea) | Specimens are found in leaf litter, in the trunk of Arecaceae, on Amazonian canopy or near rural settlements. | 15-25 | [15, 69, 71, 79] |
| T. (Atreus) tucurui Lourenço, 1988 | Coloration blackish-brown dentate margins of pedipalp-chela fingers with 16 oblique rows of granules; pectines with 20 to 21 teeth. | 85-100 | Terra firme forest | These arthropods hide in the vegetation or in the palm leaf sheaths. | - | [68, 73] |
| T. (Atreus) unusPinto da Rocha and Lourenço, 2000 | Blackish-brown. Trichobothria* et and est on fixed finger of chela proximal; Est in the chela basal to Esb. | 70-80 | Terra firme forest | They are detected in the leaf litter or in tree trunks. | - | [76] |
| T. (Brazilotityus) adisi Lourenço & Pézier, 2002 | Yellow-variegated pigmentation. The dentate margins of the pedipalp-tibia fingers are composed of 12/13 oblique rows of granules. | 20 | Floodable forest (igapó) | Hide in tree trunks or in the Amazonian canopy. | - | [76] |
| T. (Brazilotityus) lokiae Lourenço, 2005 | Coloration yellowish with variegated brown spots over the body and appendages. Subaculear tubercle very and spinoid. | 27 | Floodable forest (igapó) | Hide in tree trunks or in the Amazonian canopy. | - | [76] |
| T. (Brazilotityus) rionegrensis Lourenço, 2006 | General color variegated from pale yellow to reddish. Fixed and movable finger cutting edge of pedipalp hands with 10-11 longitudinal series of granules. | 30 | Floodable forest | Amazonian canopy. | - | [76] |
| T. (Tityus) canopensis Lourenço & Pézier, 2002 | Generally pale yellow without spots or pigmented regions on the body and its appendages. | 10.3 | Floodable forest (igapó) | Hide in tree trunks or in the Amazonian canopy. | - | [76] |
| T. (Tityus) carvalhoi | Coloration reddish-brown; pedipalps without spots; dentate margins of pedipalp-chela fingers composed of 16 oblique rows of denticles; pectines with 23 to 24 teeth. | 45-50 | Terra firme | - | - | [76] |
| T. (Tityus) gasci Lourenço, 1981 | General coloration yellowish or with diffuse spots; basal middle lamellae of female pectines at the same level as the anterior tooth. | 63 | Terra firme. | Leaf litter. | - | [76] |
| T. (Tityus) marajoensis Lourenço & Silva, 2007 | Coloration yellow-wish, with carapace and tergites reddish-brown to brown, much darker than appendages. The tergites are divided by a yellow longitudinal strip. | 49 | Marajó island (0°58′S 49°34′W) | - | - | [80] |
| T. (Tityus) nelsoni Lourenço, 2005 | Yellowish to reddish yellow, without any marked spots over the carapace. Basal middle lamellae of female pectines not dilated. | 55-60 | Terra firme. | - | - | [76] |
| T. (Tityus) raquelae Lourenço, 1988 | Coloration yellowish without spots; pedipalp-chela fingers composed of 15 oblique rows of denticles; pectines with 17 to 18 teeth. | 55-60 | Terra firme forest. | These animals hide in tree trunks or in palm leaf sheaths. | - | [65, 78, 81] |
| T. (Tityus) strandi Werner, 1939 | General coloration yellowish-brown; tergites with confluent pale brown spots; basal middle lamellae of female pectines with only half of its surface at the same level as the anterior tooth. | 50-70 | Terra firme forest. | They hide in the vegetation, mainly Arecaceae. They are found in the urban region or near rural settlements. | 12 | [7, 65, 68] |
| T. (Tityus) sylviae Lourenço, 2005 | Coloration yellowish to reddish yellow, with confluent dark spots over the carapace the tergites. Fixed finger with 15 and chela movable finger with 16 oblique rows of granulates. | 45-50 | Terra firme forest | Specimens can be found in fallen trunks. | - | [76] |
Subgenus is shown in parentheses: Genus (subgenus) species; -: no information; Trichobothria*: trichobothrial notations (et, est, Est, Esb) used to distinguish the two species; Ref.: reference.
Figure 2. Places where scorpion envenoming are more likely to occur in the Brazilian Amazon. (A) Forest leaf litter. (B) Amazon canopy. (C) Arecaceae trunks. During the harvesting and handling of fruits, such as (D) Bactris spp. and (E) Astrocaryum spp. (F) Floodable forests (known as igapó and várzea in Brazil). (G) Houses in rural and urban areas. Figure by Nícolas da Silva Garcia.
In the states of Pará and Amazonas, Brazil, which together have a large area of terra firme forest, scorpions such as, respectively, T. obscurus and T. metuendus, can be found in leaf litter (dead plant material, including leaves, bark, needles, and twigs, that has fallen to the ground) (Fig. 2A), in palm leaf sheaths, on the canopy (which may be over 100 feet (30 m) above the ground) (Fig. 2B) or in Arecaceae trunks (Fig. 2C). Among the species found in the Brazilian Amazon region, twenty-eight species of the genus Tityus are reported in Table 1.
In different areas of the rainforest, it is possible to find four scorpion subgenera: Archaeotityus Lourenço, 2006, Atreus Gervais, 1843, Tityus C. L. Koch, 1836 (subgenus) and Brazilotityus Lourenço, 2006 [63]. All these subgenera are notified in the Brazilian Amazon and their ecological, reproductive and morphological aspects are displayed in Table 1.
Scorpions Tityus can live about two meters from the ground, in the insertion of palm leaves or inside bromeliads suspended in trees over 30 meters above the ground [82]. Specimens of the subgenera Atreus and Brazilotityus can be found in micro-habitats at a height of up to 40 m from the ground in some regions with dense forest, suggesting that they can reproduce on the Amazon canopy [65, 69]. The availability of hiding places for arboreal scorpions is huge in the Amazon. It is estimated that 16,000 species of trees occur in the Amazon rainforest [83]. However, scorpions dangerous to humans, such as T. metuendus, T. silvestris and T. obscurus, are found mainly in Arecaceae (previously designated as Palmae) [69, 84], the most common botanical family in the Amazon region [85] that comprises 38 genera and 270 species [86]. Palm trees produce a great variety of fruits that serve as daily food for the population of the Amazon basin [87].
Tityus specimens are usually found in contact with the trunk of palm trees (Fig. 2C), including Bactris and Astrocaryum [70, 84]. T. silvestris can be detected in the trunk of Bactris gasipaes Kunth or may prefer to hide among their fruits produced at a height of about 20 m [29, 70, 87]. Palms of genera Mauritia, Bactris, Euterpe and Astrocaryum are micro-habitats for harmful scorpions (Table 1). Some scorpions, such as T. obscurus, T. strandi, T. silvestris, T. apiacas and T. metuendus, can hide among fruits, such as “açaí” (Euterpe spp.), “pupunha” (Bactris spp.) (Fig. 2D) and “tucumã” (Astrocaryum spp.) (Fig. 2E) [38, 69, 70]. The palm fruits usually come from dense forests or from rural plantations in the Amazon, which are geographical areas of greatest contact with venomous animals. Arecaceae species usually develop their fruits in clusters [87], which serve as shelter for scorpions and other arthropods. The harvest of these fruits and especially the handling in open markets or at home, can increase the risk of stings [38, 70].
In igapó and várzea forests (flooding zones) (Fig. 2F), invertebrates, including scorpions (Tityus), migrate vertically to the tree trunks or canopy before the annual flood period [65, 67, 88]. This seasonal event prevents the terrestrial activity of several venomous animals and the risk of human envenoming can decrease in these areas, usually for 5 to 7 months [65, 88]. Furthermore, the ecological conditions of these forests during the flood period lead to a decrease in animal hunting, cultivation of plants, fruit collections and, consequently, contact with venomous animals that hide in leaf litter and in tree trunks. However, several groups of vertebrates and invertebrates, such as scorpions, can move to human communities that are established a few meters from rivers. In the Brazilian Amazon, Atreus scorpions are the species with the greatest presence in flooded forest (igapó) (Table 1). These animals can be found even inside the houses or in the surroundings of the houses (Fig. 2G), favoring the risk of accidents [54] during all the year, since the seasonal incidence of scorpion stings is steady in North region [33].
Chactidae family concentrates a large number of species in the Northern region of Brazil living on terra firme forest, mainly under fallen trunks and burrows in the ground [64]. Populations of B. amazonicus Lourenço, 1988 (Fig. 3A), a member of the Chactidae family, can be found mainly in the region of Manaus [64]. The specimen of B. amazonicus shown in Figure 3A was found between roots of Mauritia flexuosa L. f. palm, popularly known as buriti, in the city of Manaus, Amazonas state capital, Brazil. Fewer and less severe accidents caused by the scorpions Ananteris sp. (Buthidae) and B. amazonicus have been registered in the Brazilian Amazon region [89]. Although the venom of B. amazonicus has no medical relevance, it caused rapid paralyzing effects in adult crickets Gryllus assimilis and in larvae of tenebrioid beetles Zophobas morio. In larvae, this effect persisted for 24 hours when higher doses of venom (20 µg) were used [90]. The first report of a caecilian amphibian (Siphonopidae: Microcaecilia sp.) being preyed upon by a scorpion, whose genus was identified as Brotheas, was recently published [91].
Figure 3. Scorpion species of the Brazilian Amazon region: (A) Brotheas amazonicus; (B) Tityus apiacas; (C) Tityus metuendus; (D) Tityus obscurus (photo by Pedro P. O. Pardal, reprinted with permission); (E) Tityus silvestris (photo by Bruno R. R. Almeida, reprinted with permission); (F) Tityus strandi. Scale bar (A-G) = 1 cm.
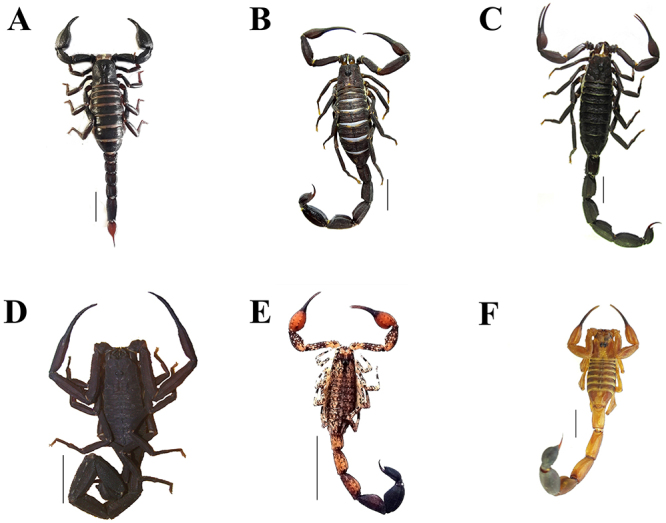
Local manifestations have also been registered after stings of Rhopalurus laticauda Thorell, 1876 (synonym R. amazonicus Lourenço, 1986 and R. crassicauda Lourenço, 2002) (Buthidae) [92], which is endemic to Amazon savannah [66]. The venom and major β-neurotoxin Rc1 from R. laticauda show pro-inflammatory activities in vitro and a nociceptive response in vivo [93].
The geographic distribution of the genus Tityus in the Brazilian Amazon
The geographic distribution of a total of 28 species belonging to four subgenera of the genus Tityus in the Brazilian Amazon is shown in Table 2, including some species found on Amazon canopy that have no reported envenoming cases yet [25, 26, 54] (Table 1).
Table 2. Geographic distribution of scorpions of the genus Tityus in the Brazilian Amazon.
| Species of medical importance | Locations (State: Municipality) | References |
|---|---|---|
| T. apiacas |
Amazonas: Apuí. Mato Grosso: Apiacás |
[74, 94, 95] |
| T. metuendus |
Amazonas: Silves, Rio Preto da Eva, Autazes,
Manacapuru, Itacoatiara, Novo Airão, Manaquiri, Itapiranga,
Careiro da Várzea, Beruri, Iranduba, Tabatinga, Parintins,
Presidente Figueiredo, Manaus, Novo Aripuanã. Acre: Rio Branco, Mâncio Lima, Senador Guiomard, Cruzeiro do Sul, Brasiléia, Xapuri Pará: Juriti, Óbidos, Santarém. Mato Grosso: Alta Floresta. Rondônia: Guajará-Mirim, Porto Velho. Roraima: Amajari. |
[72, 74, 77, 94, 96-99] |
| T. obscurus |
Pará: Belém, Santarém, Barcarena, Ananindeua,
Juruti, Almeirim, Jacundá, Bujaru, Benevides, Ourém, Ipixuna
do Pará, Colares do Pará, Alenquer, Santa Izabel do Pará,
São Francisco do Pará, Primavera, São Félix do Xingu, Acará,
Conceição do Araguaia, Breves, Melgaço, Parauapebas,
Tomé-açu, Salvaterra, Tucuruí, Belterra, Uruará, Afuá, Santa
Bárbara do Pará, Santo Antônio do Tauá, Rurópolis,
Abaetetuba, Novo Progresso, Traírão, Aveiro, Traquateua,
Castanhal, Óbidos. Amapá: Serra do Navio. |
[9, 70, 95-97, 100-102] |
| T. silvestris |
Amazonas: São Paulo de Olivença, Novo Airão,
Benjamin Constant, Tabatinga, Tefé, Maraã, Manaus,
Presidente Figueiredo, Rio Preto da Eva, Iranduba,
Manacapuru, Itacoatiara. Pará: Juriti, Belém, Santarém, Tucuruí, Bragança, Acará, São João de Pirabas, Parauapebas, Melgaço, Almeirim, Altamira, Itaituba, Benevides, Ananindeua, Mocajuba, Óbidos, Baião, Rurópolis, Santo Antônio do Tauá. Amapá: Serra do Navio. Mato Grosso: Aripuanã, Cláudia. Rondônia: Ji-paraná, Guajará-Mirim. Acre: Senador Guiomard, Rio branco. |
[72, 74, 94, 95, 97, 100, 103, 104] |
| T. strandi |
Amazonas: Manacapuru, Beruri, Barcelos, Uarini,
Coari, Manaus. Pará: Melgaço, Altamira, Vitória do Xingu, Santarém, Rurópolis, Tucuruí, Juruti, Monte alegre. Mato Grosso: Cotriguaçu, Aripuanã. |
[74, 95, 97, 100, 103, 105] |
| Species with no recorded accidents | Locations (State: Municipality) | References |
| T. adisi | Amazonas: Tarumã Mirim, Manaus. | [65] |
| T. anori | Amazonas: Anori. | [25] |
| T. bastosi | Amazonas: Tefé, São Paulo de Olivença, Tabatinga. | [74, 95, 97] |
| T. canopensis | Amazonas: Tarumã Mirim, Manaus. | [65] |
| T. carvalhoi |
Pará: Piracuruca. Mato Grosso (no municipality specified) |
[97, 99] |
| T. clathratus | Roraima: Amajari, Boa Vista. | [95, 104] |
| T. dinizi | Amazonas: Novo Airão. | [74, 94] |
| T. elizabethae | Roraima: Pacaraima. | [73, 75] |
| T. gasci |
Amazonas: Urucará. Acre: Mâncio Lima, Rio Branco, Sena Madureira, Senador Guiomard. Pará (no municipality specified) |
[74, 106] |
| T. generaltheophiloi | Roraima: Parque Nacional da Serra da Mocidade | [24] |
| T. grahami | Amazonas: Santa Isabel do Rio Negro, Barcelos. | [26, 95] |
| T. lokiae | Amazonas: Tarumã Mirim, Manaus. | [65, 67] |
| T. marajoensis | Pará: Marajó island (0°58′S 49°34′W). | [80] |
| T. maranhensis | Maranhão: Caxias (Inhamum Ecological Reserve). | [107] |
| T. matthieseni | Amazonas: Manicoré. | [74, 76] |
| T. mattogrossensis | Mato Grosso: Cuiabá, Alto Araguaia. | [104] |
| T. neblina | Amazonas: Parque Nacional do Pico da Neblina (0°48'01"N 66°0'25"O). | [73] |
| T. nelsoni | Amazonas: São Gabriel da Cachoeira. | [108] |
| T. raquelae | Amazonas: Manaus, Rio Preto da Eva, Presidente Figueiredo. | [74, 81] |
| T. rionegrensis | Amazonas: São Gabriel da Cachoeira. | [54] |
| T. sylviae | Amazonas: Barcelos. | [108] |
| T. tucurui | Pará: Baião, Tucuruí. | [95, 96, 100, 103] |
| T. unus | Amazonas: Santa Isabel do Rio Negro. | [74, 76, 95] |
Table 2 indicates a higher number of Tityus species in the state of Amazonas (n = 19), followed by the state of Pará (n = 8), and lower in the states of Roraima (n = 4), Mato Grosso (n = 4), Acre (n = 3), Rondônia (n = 2) and Amapá (n = 2). In Maranhão, it was considered only the record of T. maranhensis, which was found in an area in the state with Amazonian vegetation. In Tocantins, the occurrence of T. (Atreus) sp. was registered [109]; however, the specimens of the collected arachnids were not identified. The region of Manaus stands out for the highest number of species of Tityus notified (n = 7). In the Eastern Amazon, comprised by the states of Pará, Maranhão, Amapá, Tocantins and Mato Grosso, T. obcurus was recorded in 39 municipalities (Table 2). However, this species is found mainly in the state of Pará, where it is widely distributed and more related to moderate and severe cases of scorpionism [99, 102, 110] (Tables 2 and 3). T. silvestris and T. metuendus have a wide geographic reach in the Northern region (Table 2), as proposed previously by other studies [7, 31, 69]. T. strandi, which has been recorded in the state of Amazonas, also occurs in the states of Mato Grosso and Pará [81]. On the other hand, there are no new geographic occurrences for T. apiacas. Tityus scorpions with no confirmed records for the Brazilian Amazon are T. thelyacanthus, T. magnimanus and T. rufofuscus [75, 97, 111]. Although cases of accidents with Atreus can evolve into severe manifestations, the toxic potential of these arachnids is still unknown. Despite the geographic reach of the genus Tityus being underestimated in the Brazilian Amazon, the spatial distribution of scorpions of medical importance showed in Table 2 can assist health agencies in the prevention and prediction of moderate and severe accidents. On the other hand, surveillance should be extended to species still with no notification of stings (Table 2).
Table 3. Symptomatology of victims stung by scorpions of medical interest from the Brazilian Amazon region and in vivo effects of venoms on experimental animals.
| Species | Symptomatology of envenoming | Ref. |
|---|---|---|
| T. apiacas | Immediate local pain, electric shock sensation (Apuí, AM), local erythema, local edema, sweating, vomiting, diarrhea, pallor, tremors, myosis and agitation. | [51] |
| T. metuendus | Cause human envenoming. Humans:
local pain, hyperemia, paresthesia, edema, tachypnea,
lethargy, psychomotor agitation, mental confusion,
myoclonia, sweating, sialorrhea, dyspnea, nausea, vomiting
and tachycardia. Mice: restlessness, piloerection, sialorrhea, hyperactivity, respiratory difficulties, partial paralysis of limbs, exophthalmos, loss of equilibrium, convulsions and death. |
[36, 52] [113] [98] |
| T. obscurus | Humans: Belém (PA) - Local manifestations: local and radiating pain, paresthesia, edema, erythema, sweating, piloerection and burning. Santarém region (PA) - Neurological manifestations: electric shock sensation, dysdiadochokinesia, dysmetria, dysarthria, dyslalia, nausea and vomiting, compatible with acute cerebellar dysfunction. Rats: hemorrhagic patches in the lung parenchyma and no pulmonary edema; decrease in general activity; no changes in the occurrence and intensity of induced convulsions; no hippocampal neuronal loss. Mice: edematogenic and moderate nociceptive activity; decreased locomotion, breathing difficulty, piloerection, palpebral ptosis and excessive oral and nasal secretions. The effects began 30 min after venom injection and persisted for about 3 h, while the respiratory changes persisted for 6 h. | [9, 49, 102, 114] [115] [115] |
| T. silvestris | Local manifestations: local pain, paresthesia and edema. Systemic manifestations: nausea, vomiting, somnolence, malaise, dyspnea, tachycardia, headache, myoclonia, hyper/hypotension, hypothermia, abdominal pain and generalized muscle spasms. | [50, 70, 113] |
| T. strandi | Local pain, paresthesia, erythema, edema, dysesthesia with a tingling sensation and electric shock sensation (Santarém, PA) of variable extension. | [37, 105] |
Ref.: reference. The atypical sensation of electric shock registered in cases of scorpionism in the Brazilian Amazon is underlined.
Scorpion envenoming in the Brazilian Amazon - symptoms and epidemiology
The genus Tityus is of medical importance [30], and four species (T. serrulatus, T. bahiensis, T. stigmurus and T. obscurus) are capable of causing serious accidents in Brazil [89, 112]. The incidence of envenoming with scorpion stings is increasing in the Brazilian Amazon, mainly due to the species T. metuendus, T. apiacas, T. silvestris and T. obscurus [54]. Recently, the first three cases of envenoming by T. strandi were recorded in Santarém (2°25′48″S 54°43′12″W), state of Pará [37]. These five scorpion species (T. apiacas, T. metuendus, T. silvestris, T. obscurus and T. strandi) of medical relevance in the Brazilian Amazon region [36, 37, 49-52] are reported in Table 3.
Many patients, who seek public health care in the Brazilian Amazon after being envenomed, report they were stung by a black scorpion [49, 114]. T. (Atreus) obscurus Gervais, 1843, is known as the black scorpion of the Amazon [112]. However, the animal color may not contribute to the elucidation of the cases. Depending on the geographical area, different dark-colored species, such as T. anori, T. dinizi, T. generaltheophiloi, T. unus, T. matthieseni, T. elizabethae, T. tucurui, T. apiacas, T. metuendus and T. obscurus, may be related to human envenoming. These large (65 to 110 mm total length) Amazonian scorpions belong to the subgenus Atreus and are very similar when adults (Fig. 3B-D, Table 1). The similarity between Atreus scorpions in the Brazilian Amazon region is complex (Table 1) and can induce misidentifications [99]. Throughout the ontogenetic development of T. metuendus, the species exhibits a remarkable difference in the coloration of juveniles and adults (Fig. 4A-F) and the main responsible for human envenoming are adult specimens (Fig. 4D-F) [113]. During this stage of life, T. metuendus can be widely detected in terra firme forest and in rural and urban areas of the Amazon [64, 66]. Juveniles scorpions of T. metuendus (Fig. 4B-C) may be mistakenly confirmed as T. silvestris, T. grahami (morphological data in Table 1), or as juveniles of T. obcurus [99]. Besides Atreus scorpions, the Amazon encompasses complexes models of polymorphism observed for species of Tityus, such as T. silvestris, T. gasci and T. bastosi [13, 104, 116], which may hinder species identification. The correct distinction of scorpions involved in accidents helps in the diagnosis and in the prediction of serious complications, depending on the causative species [36, 113]. Surprisingly, in several cases of scorpion envenoming, the animals responsible for the stings are not identified in the Brazilian Amazon region (as will be shown later in Table 4) [36, 117-119]. In the Northern region, the identification of venomous animals is usually carried out in Manaus and Belém, respectively, the capitals of the Brazilian states of Amazonas and Pará [9, 50]. Other cities lack a professional qualification structure to taxonomically distinguish venomous animals that are dangerous to humans. It is also worth highlighting that out of 369 presumed scorpion stings treated in Manaus between June 2014 and December 2019, about 61% (225 cases) had no identified causative agent [113]. Scorpion species capable of causing moderate and severe human envenoming accidents in the Brazilian Amazon region are shown in Figure 3 (B-F). Some listed species possess populations widely distributed in the Amazon basin [7, 62]. T. metuendus is a monomorphic species and can use parthenogenesis for producing offspring [120], without needing a sexual partner. Such mechanism of reproduction contributes to the high dispersion of animals and incidence of scorpion stings [61].
Figure 4. Ontogenetic development of T. metuendus. (A) A 3-day old newborn (red circle). (B, C) Juveniles of 20 and 75 days old. (D, E) Adults aged between 390 and 515 days. (F) Male and female adults of undetermined age. T. metuendus adults are responsible for many cases of envenoming in the Brazilian Amazon. Photos of panels A, B and F by Francisco José Ramos Prestes, reprinted with permission.
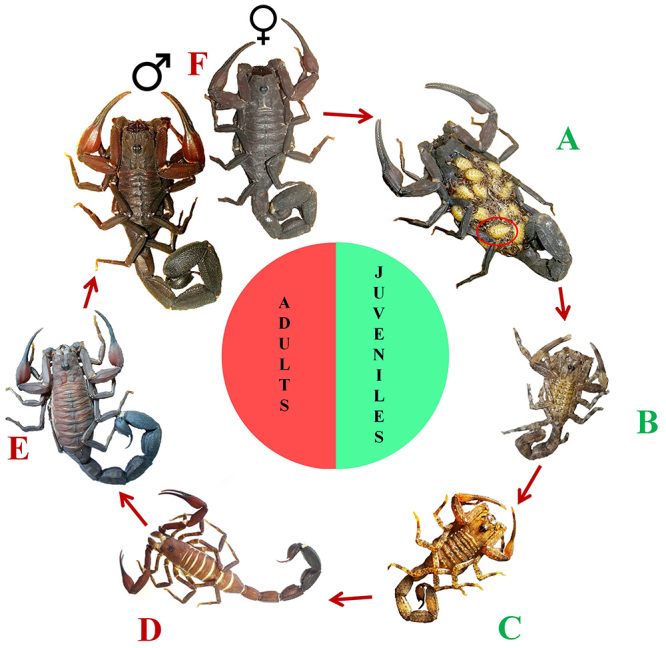
Table 4. Epidemiological aspects of scorpionism in the Brazilian Amazon region.
| Scorpion species/Animal description | Region | Number of cases/Period/Time of day and year | Gender/Age of the victims (years) | Sting location | Time between the accident and the medical assistance | Main signs and symptoms | Clinical manifestations | Treatment administered | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| T. obscurus and T. silvestris | Belém (Pará, Brazil) (1°26′S 48°29′W) | 61 Jan. to Dec.1996 | 54.1% female Age range 0-7 (21.4%) 7-55 (65.3%) ˃ 55 (13.3%) | Upper limbs - hand (40.9%); neck (3.3%); elbow (1.6%) Lower limbs - foot (31.2%); thigh (14.7%) | - |
Local symptoms - pain (83.9%), edema (47.5%),
hiperemia (36%), paresthesia (6.6%) Systemic symptoms - vomiting (13%), tachycardia (3.2%), nausea (3.2%), somnolence (3.2%), paleness (3.2%), shock (1.6%), sweating (1.6%) |
Mild (86.9%) Moderate (11.4%), Severe (1.7%) | Specific antivenom serum (26.2%) | [110] |
| T. obscurus (8.3%) Designation - “lacrau” (a synonym in Portuguese for the word scorpion) (87.5%); scorpion (4.2%), did not know (8.3%). Description - black (5.6%), black and large (59.7%), yellow (1.4%), and did not know inform (33.3%). Took the animal (8.3%). | Municipal Hospital of Santarém - Santarém (2°25′48″S 54°43′12″W) (94.4%), Belterra (02°38′09″S 54°56′13″W) (4.2%), Prainha (1°48′0″S 53°28′48″W) (1.4%) (Pará, Brazil) | 72 Feb. 2000 to Feb. 2001 Morning (40.3%) Afternoon (41.6%) 19-6 h (18.1%) The highest incidences occurred in Mar. (18.1%), Aug. (18.1%) and Apr. (11.1%) | 83.3% male Age range (median 33.6 ± 18.3 years) 2-14 (13%) 84.1%) > 65 (2.8%) | Upper limbs (51.5%) - hands (41.1%) Lower limbs (43.1%) - foot (38.9%) Other body parts (5.4%). | Time ranged from 30 min to 14 h, with an average of 4.6 ± 3.2 h. |
Local symptoms (91.7%) - paresthesia (79.2%),
pain (52.8%), edema (26.4%). Neurological signs (97.2%) - myoclonia (93%), electric shock sensation (88.9%); dysmetria (86.1%), dysarthria (80.6%), ataxia (70.8%) |
Moderate (76.4%), with no serious cases | Specific antivenom serum was not administered in 32.7% of the moderate cases (unavailable). Antiscorpionic serum (63.6%) and antiaracnidic serum (3.7%), with an average of 3.5 ± 0.8 ampoules. Patients discharged cured - within 24 hours (98.6%), in 3 days (1.4%). | [49] |
| T. obscurus Specimens were taken by the patients and identified. | Eastern and western areas of the state of Pará (Brazil) | 48 Jan. 2008 to July 2011 | Eastern: 50% male Western: 64.3% male Age range Eastern: < 5 (2.9%) 11.8%) > 15 (85.3%) Western: < 5 (7.1%) 6-14 (7.1%) > 15 (85.8%) | Eastern: upper limbs (70.6%); lower limbs (23.5%); other parts (5.9%) Western: upper limbs (57.2%); lower limbs (35.7%); other parts (7.1%) | Eastern: < 1 h (64.7%) 2-3 h (14.7%) > 3 h (20.6%) Western: < 1 h (57.1%) 2-3 h (14.3%) > 3 h (28.6%) |
Eastern: Local symptoms - pain (88.2%),
radiating pain (5.9%), paresthesia (47.1%); systemic
manifestations - sweating (5.9%), somnolence (2.9%) Western: local symptoms - pain (100%), radiating pain (64.3%), paresthesia (85.7%); systemic manifestations - sweating (35.7%), somnolence (28.6%), tremors (35.7%), agitation (28.6%), electric shock sensation (50%), myoclonus (64.3%), dysarthria (42.8%) |
Eastern: Level 1 (76.5%) Level 2 (17.6%) Dry sting (5.9%) Western: Level 1 (35.7%) Level 2 (64.3%) | Eastern: Painkillers (76.5%) Antivenom (17.6%) Western: Painkillers (35.7%) Antivenom (64.3%) | [9] |
| - | State of Amazonas - highest incidence in Apuí (7°11′49″S 59°53′27″W) and Rio Preto da Eva (2°41′56″S 59°42′0″W)) | 2,120 (56.6% from rural areas; 38.7% work-related accidents; 72.4% farmer/fisher) Jan. 2007 to Dec. 2014 | 63.9% male 0-10 (14.8%) 11-20 (18.6%) 21-30 (19.7%) 31-40 (18.1%) 41-50 (14.8%) 51-60 (8.2%) > 61 (5.7%) | Upper limbs (47.9%); lower limbs (46.5%), body (3.3%), head (2.3%) | < 3 h (69.6%) 4-6 h (17.1%) 7-12 h (6.9%) 13-24 h (3.4%) > 24 h (2.8%) | - | Mild (68.6%) Moderate (26.8%), Severe (4.6%) Death (0.3%) | - | [36] |
| - | State of Pará (Brazil) | 13,453 2007 to 2014 | 65.8% male Age ranged from 1 to > 60 years old 1-14 (20.2%) 15-19 (9.5%) 20-39 (38.6%) 40-59 (23.9%) >60 (7.8%) | - | 0-1 h (37.6%) 1-3 h (30.4%) 3-6 h (17.2%) 6-12 h (8.2%) > 12 h (6.6%) | - | Mild (55.8%) Moderate (39.1%) Severe (51%) | - | [124] |
| - | Cruzeiro do Sul (Acre, Brazil) (7°37′51″S 72°40′12″W) | 164 2012 to 2017 | 68.2% male Age range 0-10 (13.5%) 11-20 (10.1%) 21-30 (15.2) 31-40 (25.4%) 41-50 (10.1%) 51-60 (6.4%) ˃ 60 (18.6%) | Upper limbs - forearm (1.3%), upper arm (1.3%), hand (55.7%); Lower limbs - foot (35.3%), lower leg (4.0%), thigh (0.6%) | 0-1 h (45.1%) 1-3 h (27.0%) 3-6 h (15.2%) 6-12 h (6.9%) 12-24 h (2.0%) ˃24 h (3.4%) |
Local symptoms (100%) - pain (81.7%), edema (66.8%); Systemic manifestations (5.4%) - neuroparalytics (4.7%); Vomiting/diarrhea (2.0%) |
Mild (67.3%) Moderate (25%) Severe (7.6%) | Serum therapy (68.9%) | [117] |
| T. obscurus Black | Santarém (Pará, Brazil) (2°25′48″S 54°43′12″W) | 28 2013 to 2014 | 60.7% male Age range 1 - 10 (7.1%) 11 - 20 (7.1%) 21 - 30 (32.1%) 31 - 40 (14.3%) 41 - 50 (17.9%) 51 - 60 (7.1%) 61 - 70 (14.3%) | Upper limbs - hand; lower limbs foot | - | Neurological manifestations - electric shock sensation, ataxia, dysarthria, dysmetria | - | No antivenom available (1%), antivenom administered 10 hours after the accident (1%) | [38] |
-: no information. Ref.: reference.
Epidemiological data indicate that Atreus scorpions are responsible for most Tityus envenoming and lethality reports in the Brazilian Amazon [9, 49, 51, 52, 113]. However, the number of species involved in the scorpionism in the Brazilian Amazon may be greater than reported in the literature. This macroregion encompasses 52% of the Brazilian scorpion fauna [22], where new species are continually being discovered [24-26]. However, displacement challenges faced by the envenomed victim, such as rivers on the way, dirt roads, areas with poor road access and long distances to reach the local health units, can result in under-notification [54]. Despite geographical barriers, it is important to raise awareness of scorpionism, including prevention, pathophysiological effects and treatment, especially in the Brazilian Amazon region, where a large number of scorpion-endemic venoms has not been studied yet and whose potential for lethality is unknown. For instance, the upper Rio Negro (0°58'26"S 62°55'32"W) region (state of Amazonas, Brazil) concentrate scorpion species with high degree of endemism and biology poorly known [121]. This is an area with complex floristic composition, which can hinder human access for sample collections and studies of scorpion fauna [108]. Although T. matthieseni may be related to some envenoming cases in the state of Amazonas [27], no clinical description has been reported for this species [54].
Human populations living in close contact with the forest, such as indigenous people, rubber tappers and rural workers, are the most susceptible to accidents with venomous animals [56, 118]. In 2017, the incidence coefficient (per 100,000 workers) due to work accidents involving scorpions in the rural areas, forest and waters was higher in municipalities in the North region, mainly in the states of Amazonas, Pará, Amapá and Tocantins, than in the other four Brazilian macroregions [122]. About 10% of tappers and 14% of Amerindians from the state of Acre were stung by scorpions at least once in their lifetime [118]. Scorpion stings occurred in the forest are the most underreported accidents and the species that caused the envenoming is generally not identified [118]. Nevertheless, a technical effort to elucidate the dynamics of accidents in the forest and urban-forestry areas indicated that the stings occur most often during the day, especially in the workdays. However, night accidents (18.1% of the cases) also occurred, when scorpions tend to be more active and imperceptible to people [49]. Many scorpion stings have occurred in the comfort of home in many parts of the Brazilian Amazon. From 1998 to 2005, 52% of accidents in the region of Belém, state of Pará, occurred at home and the most affected members were hands and feet [119]. Infrastructure problems, such as the garbage collection and disposal, sanitation (sewage and storm drain systems), are responsible for scorpions’ dispersion in large Brazilian cities [29, 123]. Although the introduction of these venomous animals into human space in the Amazon region should be investigated, the harvesting of fruits and vegetables in rural areas and the exploitation of wood in native forest probably contribute to the dispersion of scorpions to urban areas [70]. People who live especially in rural settlements in the Amazon rainforest often climb trees several meters high to harvest fruits. This practice increases the risk of stings, mostly on the victims’ hands and feet, by arboreal scorpions that hide in trunks and among bunches of fruits [11, 13]. The first case of a male adult stung by T. serrulatus, a non-native species to Pará, while unloading bananas at the supply center in Belém (Amazon region) was recently reported. The banana bunches from Bahia (Northeast region) were transported by truck to Belém. If the species were introduced to the Northern region, it could cause ecological disturbances and become a public health problem [101].
Mild and moderate symptoms are frequently recorded after scorpion envenoming in the Brazilian Amazon [33, 36, 113, 117]. Intense pain, paresthesia, edema and erythema are the most reported local symptoms [9, 113]. Depending on the scorpion species, neurological manifestations stand out among the systemic effects [49, 114]. Epidemiological aspects of scorpionism in the Brazilian Amazon region are shown in Table 4.
Although cardiac and hemodynamic changes may culminate in fatal outcomes from cardiogenic shock and pulmonary edema, complementary exams that aid in diagnosis, such as biochemical tests, electrocardiogram, chest radiography and echocardiography [30], are rarely performed.
The prognosis depends on the time between a sting and the patient’s arrival at the hospital/appropriate treatment [29]. The time before arrival at hospital was later in North region when compared to the other four Brazilian regions. Only about 35% of the patients in North region were admitted at the hospital during the first hour after the scorpion envenoming (against 50% in the other four macroregions) [33]. Pharmacokinetic assays showed that biodistribution of Androctonus australis hector scorpion venom from the injection site to the tissues is within 15 min [125], which reinforces the need for early health care.
Severe symptoms of scorpionism in the Brazilian Amazon region (Table 3) mainly affects people living in precarious conditions [126]. Most of these people are farmers, rubber tappers, traders, domestic workers and hunters who work directly with the resources extracted from the forest [118]. From an epidemiological point of view, these occupations are more vulnerable to scorpion envenoming [118].
Most victims of lethal scorpion stings die from cardiac or respiratory failure [11] and a direct relationship among the inflammatory process, neuronal activation, neurotransmitter storm, cardiac dysfunction, and mortality induced by scorpion venom was recently established [127]. Mild or severe scorpion envenomings, such as those caused by, respectively, R. laticauda and T. serrulatus, can activate the canonical nuclear factor-kappa B (NF-(B) pathway that mediates inflammatory responses [93, 128] and is supposed to be one important mechanism of enhancing the immune responses after scorpion envenoming [129].
In the Brazilian Amazon region, mortality is also associated with delayed access to care [130], lower literacy levels and income than in the Southeast region [130], due to geographical barriers and long distances to reach local health units [54], the delay in receiving immunotherapy [33, 36, 54] and sometimes the specific antivenom is not available (Table 4) [49]. Pharmacokinetic studies showed that venom concentrations were maximal at 15 min in the kidney and liver, and at 30 min in serum, lung, heart and spleen, after subcutaneous injection of T. serrulatus venom [131]. The antivenom therapy is frequently administered in patients in the western region of the state of Pará, where more than 97% of the patients had systemic and neurological signs (Table 4) [49].
People living in the Amazon region have their daily lives altered by accidents with several venomous animals, which can even compromise the livelihood of their families [50]. Tityus stings can cause a person to stay from a few hours to several days in the hospital. For instance, a man spent 9 days in care at the Manaus Tropical Medicine Foundation after being stung by T. silvestris [50].
Populations of T. metuendus are abundant in different parts of the Amazon basin, and adult specimens (Fig. 4D-F) are the main responsible for human envenoming, especially in the region of Manaus. The venom of this arachnid causes paresthesia, nausea, blurred vision, respiratory failure, myoclonia, edema, pain, sialorrhea and tachycardia [113]. Several patients with similar or more severe symptoms face the difficulty of locating hospitals in the Amazon with specific antivenom and clinical support with experience in this type of emergency.
Strikingly, harmful scorpions which belong to different subgenera, such as T. obscurus and T. strandi (Table 1), depending on the Amazon region where they live [126], can cause cerebellar-muscular changes in the envenomed victims. The reported neurological manifestations are not observed in patients stung by scorpions in other regions of Brazil [38, 49], where typical autonomic disturbances prevail, such as those caused by T. serrulatus [132].
The reported neurological symptoms caused by T. obscurus (synonymous species T. cambridgei and T. paraensis) [133], in the region of Santarém, state of Pará, such as myoclonus, fasciculations and a sensation of shock, evidenced a regional symptomatology [49, 89]. A clinical, mitochondrial (16S rRNA), morphometric and proteomic study with populations of T. obscurus indicated that distinct lineages occur in the eastern and western regions of the state of Pará [126]. In the western region (Santarém), one of the symptoms most reported by patients is the sensation of electric shock [49, 114], usually reported when T. obscurus is involved in the accidents [49, 114]. This symptom is also reported after envenomings caused by T. apiacas and T. strandi (Table 3) [37, 51]. Significant toxic variations in these venoms may be peculiar to some Amazon regions [38, 49, 51]. Since several populations of Tityus live in microregions in the dense forest [25, 27], assessing the toxicity of these species’ venoms can be complex. Clinical manifestations compatible with neuromuscular/somatosensory dysfunction and few manifestations compatible with adrenergic/cholinergic stimulation were also reported after T. strandi stings [37]. Three sudden fatal cases caused by T. obscurus stings in Guyana's remote jungle areas over a 12-month period indicate the potential for gravity caused by local populations of this species [134].
In the Amazon region, many victims of scorpion accidents needed immunotherapy (Table 4) and the number of cases requiring antivenom therapy is growing in the last years [51, 113, 114]. It is important to highlight that Brazilian scorpion antivenom is produced by immunizing horses with T. serrulatus antigen [56]. The literature reports a clinical case of T. silvestris envenoming refractory to the antiscorpionic serum produced against T. serrulatus. A 39-year-old patient, envenomed by T. silvestris in the urban area of Manaus, showed cerebellar-muscular changes (usually observed after accidents with T. obscurus) and required treatment with benzodiazepines, in addition to supportive therapy with hydantoins and antihistamines [50].
A study comparing the transcriptomic-proteomic profiles of the venoms from T. serrulatus and T. obscurus revealed that differences at primary sequence may reflect in different epitopes for the same protein classes in these two allopatric species, resulting in the poor recognition of T. obscurus venom by the Brazilian scorpion antivenom [47]. Furthermore, variation in toxicity due to the diversity of T. obscurus venom in different areas of the Amazon has been suggested [102] and most of the 320 NDBPs detected by a peptide profile from T. obscurus venom do not correspond to any known toxin [135]. Similarly, eight sequenced peptides, among 201 molecular species from 800 to 17,000 Da detected in the venom from the Amazonian scorpion B. amazonicus, showed no similarity degree with any known molecule [136]. This remarkable number of unknown toxins from Amazonian scorpions highlights the need to characterize these venoms for the development of more effective therapies.
Furthermore, the available Brazilian scorpion and arachnid antivenoms were not able to recognize R. laticauda venom and its fractions (with exception of hyaluronidase) [93]. These antivenoms were also not able to shorten the intensity and duration of the neurological manifestations in patients stung by T. apiacas or T. obscurus [113]. On the other hand, phage display technique allowed the isolation of scFv from a human library of antibodies against Ts1. This antibody fragment specific for Ts1 toxin from T. serrulatus also recognized toxins from the scorpions T. packyurus and T. obscurus from the Amazonian region [137]. The design of efficient serotherapies is challenged by the structural and antigenic polymorphisms reported in the α-toxin family [138]. For instance, T. obscurus and T. serrulatus venoms have toxins with distinct epitopes for the same protein classes [47].
To compare reactivity from medically important Tityus populations inhabiting Brazil, Colombia, Costa Rica, Ecuador, Panama, Trinidad and Tobago, and Venezuela against commercial antivenoms from Brazil, Venezuela, and Mexico, in vivo cross-reactivity studies and molecular assays, including MALDI-TOF mass spectrometry, cDNA sequencing, competitive ELISA, immunoblotting, and phylogenetic analyses were performed [139]. Based on venom composition and immunochemical criteria, Tityus spp. fauna inhabiting the Caribbean, Lower Central America (LCA) and South America was grouped into four venom antigenic regions. Species inhabiting Region I (LCA/Colombia/Amazonia) produce venoms that were not significantly reactive against available antivenoms [139]. In view of this scenario, further studies are needed to identify and characterize compounds from Amazonian scorpion venoms to improve the design of efficient antivenoms.
Characterization of venoms and toxins of scorpions from the Brazilian Amazon region
Old World scorpion genera of Buthidae, including Androctonus and Leiurus, have very potent neurotoxins specific for mammalian or insect Na+ channels, whereas New World scorpion genera, such as Centruroides and Tityus, have potent toxins acting on both mammalian and insect channels [63]. Despite the dangerous, painful and fatal effects caused by scorpionism, therapeutic properties of scorpion venoms have been explored for thousands of years and several scorpion venom compounds may represent promising leads for the development of new pharmaceuticals [11, 57].
Scorpion toxins have been explored as antiangiogenic [140], insecticide [141], tumor binding [142], antithrombotic peptide [143], potential intranuclear delivery tool to target cancerous cells [144], tools to understand the mechanisms triggered in chronic pain [145], models to study the mechanisms involved in sterile inflammation [129], and to treat autoimmune diseases [57, 146, 147].
The T. obscurus venom was firstly reported in 1998 and its first potassium channel toxin (Tc1) was characterized in 2000 [39]. Up to now, there are 48 and 33 transcripts from T. obscurus that have similarities with known, respectively, sodium and potassium channel toxins [47]. Among them, 9 NaTx and 1 KTx had been described before, of which 3 NaTx and 1KTx showed proteomic evidence [47]. There are three [39, 40] and four [41-43, 46, 48], respectively, potassium and sodium channel toxins from T. obscurus tested on electrophysiological assays (Table 5). A peptide profiling from T. obscurus venom detected 320 non-disulfide bridged peptides (NDBPs), which represents 5% of the crude venom, including thirteen novel peptides with inflammatory activities, identified as fragments of hypotensins, potassium channel toxins and the allergen 5 protein [135]. Interestingly, transcripts of phospholipase C were identified in species of T. obscurus and T. serrulatus, although no proteomic evidence has been detected. There is proteomic evidence of phospholipase A2 transcripts for T. obscurus venom only [47].
Table 5. Peptides and proteins identified in scorpion venoms from the Brazilian Amazon.
| Scorpion | Toxin (synonym) | UniProt ID | MW (Da) | Sequence | Class | Tested channels/Characterization assays | Activity | Ref. |
|---|---|---|---|---|---|---|---|---|
| Tityus. obscurus | Tc1 | P83243 | 2446 | ACGSCRKKCKGSGKCINGRCKCY | α-KTx 13.1 | Shaker B K+-channels | Blocks Shaker B K+-channels | [39] |
| Tityus obscurus | Tc30 | P60210 | 3878 | VFINVKCRGSKECLPACKAAVGKAAGKCMNGKCKCYP | α-KTx 4.4 | Kv1.3 and Shaker B K+ (analog of the mammalian Kv1.1) | Blocks Shaker B( Kv1.1) and Kv1.3 | [40] |
| Tc32 | P60211 | 3521 | TGPQTTCQAAMCEAGCKGLGKSMESCQGDTCKCKA | α-KTx 18.1 | Kv1.3 and Shaker B K+ (analog of the mammalian Kv1.1) | Blocks Shaker B( Kv1.1) and Kv1.3 | ||
| Tityus obscurus | To2 (Tc48a) | - | 7310 | DKDGYLMEGDGCMNGCLTRKASYCVDQCKEVGGKNGY... | NaTx | - | - | [41] |
| To3 (Tc49a) | - | 7141 | KDGYLVGNDGCKYNCLTRPGHYCANECSRVKGAD... | NaTx | - | - | ||
| To1 (Tc49b) | - | 7405 | KKEGYLVGNDGCKYGCITRPHQYCVHECELKKGTDGYCAYWLACYCYNMPDWVKTWSSATNKCK | NaTx | Shaker B K+-channels; Na+ currents of granular cells | Changes Na+ currents of granular cells | ||
| To4 (Tc54) | - | 7259 | KDGYLMEYGGCKMSCLMKKGTFCAEECT... | NaTx | - | - | ||
| Brotheas amazonicus | Venom peptide 1 | P86341 | 978 | IWSGIQGAF | - | - | - | [136] |
| Venom peptide 2 | P86340 | 1008 | IWSGIQSAF | - | - | - | ||
| Venom peptide 3 | P86344 | 1045 | IGDIWSGIQG | - | - | - | ||
| Venom peptide 4 | P86339 | 1087 | IIDFIPQIE | - | - | - | ||
| Venom peptide 5 | P86343 | 1192 | FIGDIWSGIQG | - | - | - | ||
| Venom peptide 6 | P86342 | 1249 | GFIGDIWSGIQG | - | - | - | ||
| Venom peptide 7 | P86338 | 1429 | VAIRIIWSDIQD | - | - | - | ||
| Venom peptide 8 | P86337 | 1449 | ISDDIQSIIQGIF | - | - | - | ||
| Tityus obscurus | Tc1 | P83243 | 2446 | ACGSCRKKCK... | KTx | - | - | [42] |
| Tc27 | P84676 | 4103 | DEGPKSDCKP... | - | - | - | ||
| Tc29 | P84677 | 4150 | FNGAVXIW... | - | - | - | ||
| Tc30 | P60210 | 3871 | VFINVKCRGS... | KTx | - | - | ||
| Tc31 | P84678 | 4304 | CSTCLDKP... | - | - | - | ||
| Tc32 | P60211 | 3521 | TGPQTTCQAA... | - | - | - | ||
| Tc33 | P84679 | 3807 | ILNRCCNDDN... | - | - | - | ||
| Tc35 | P84680 | 3926 | TGPQTXXQAA... | - | - | - | ||
| Tc37 | P84681 | 7265 | TAIRKCNPRT... | - | - | - | ||
| Tc39 | P84682 | 2744 | DDDDLEGFSE... | - | - | - | ||
| Tc40 (~To13)1 | P84683 | 7796 | IKNGYPRDS... | - | - | - | ||
| Tc41 (~To14)1 | P84684 | 7109 | KDDYPVDTAK... | - | - | - | ||
| To6 (Tc43) | P84685 | 7266 | LDGYPLSKNN... | - | - | - | ||
| Tc46 | P84686 | 6032 | KEGYLFGSRG... | - | - | - | ||
| To2 (Tc48a) | P60212 | 7318 | NKDGYLMEGDGCKMGCLTRKASYCVDQCKEVGGKDGYCYAWLSCYCYNMPDSVEIWDSKNNKCGK | NaTx | Na+-channel | Changes Na+-channel | ||
| To3 (Tc48b) | P69213 | 7385 | KDGYLVGNDG... | NaTx | - | - | ||
| To3 (Tc49a) | P69213 | 7152 | KDGYLVGNDG... | NaTx | - | - | ||
| To1 (Tc49b) | P60214 | 7405 | KKEGYLVGND... | NaTx | - | - | ||
| Tc50 | P84688 | 7073 | LDGYPLSKIN... | - | - | - | ||
| To4 (Tc54) | P60215 | 7253 | KDGYLMEYGG... | NaTx | - | - | ||
| Tc56 | P84689 | 7299 | EKGKEILGKI... | - | - | - | ||
| Tc58 | P84690 | 5504 | KKFGGFLXXI... | - | - | - | ||
| Tc61 (~To8)1 | P84691 | 7105 | KEGYLLGSRG... | - | - | - | ||
| Tc64 | P84692 | 7628 | GLRQKVQSLV... | - | - | - | ||
| To5 (Tc66) | P84693 | 6935 | SYSGYPVTQK... | - | - | - | ||
| Tc83 | P84694 | 25402 | NDQCLVIEIL... | - | - | - | ||
| Tityus obscurus | To3 (Tc48b) | P69213 | 7385 | KDGYLVGNDGCKYNCLTRPGHYCANECSRVKGKDGYCYAWMACYCYSMPDWVKTWSRSTNRCGR | α-toxin | Na+-currents of cultured rat pituitary GH3 cells | Changes Na+-currents of cultured rat pituitary GH3 cells | [43] |
| Tityus obscurus | To1 (Tc49b) | P60214 | 7405 | MTRFVLFISCFFLIDMIVECKKEGYLVGNDGCKYGCITRPHQYCVHECELKKGTDGYCAYWLACYCYNMPDWVKTWSSATNKCKGK | All proteins shared sequence identity with NaTx. | - (proteomic) | - | [44] |
| To2 (Tc48a) | P60212 | 7318 | MIRFVLFISCFFLIGTVVECNKDGYLMEGDGCKMGCLTRKASYCVDQCKEVGGKDGYCYAWLSCYCYNMPDSVEIWDSKNNKCGKGK | |||||
| To3 (Tc48b/Tc49a) | P69213 | 7385 | MTRFVLFISCFFVIGMVVECKDGYLVGNDGCKYNCLTRPGHYCANECSRVKGKDGYCYAWMACYCYSMPDWVKTWSRSTNRCGRGK | |||||
| To4 (Tc54) | P60215 | 7253 | MTRFVLFISCFFLIGMIVECKDGYLMEYGGCKMSCLMKKGTFCAEECTRMKGKDGYCYAWLACYCYNMPDWVKIWNRATNKCGKRK | |||||
| To5 | P84693 | 6937 | MKAIIFFIGCLMLIDLVAGSRSGYPVTQKGCVYSCFWGSNWWCNAECTALGGSSGYCAWPSCWCYSLPDNRNIWGSYPNNCGKK | |||||
| To6 (Tc43) | P84685 | 7266 | MSIFPIILALLLIGLDEGEALDGYPLSKNNYCKIYCPDEKVCKWSCKHRAGATNGKGDCINKGCYCYDVAPGTEMYPGRLPCNPY | |||||
| To7 (Tc50) | P84688 | 7073 | MSIFPIVLALLLIGLEETEALDGYPLSKINNCKIYCPDDDVCKWTCKHRAGATNGKGDCIWYGCYCYDVAPGTKMYPGSSPCYA | |||||
| To8 | H1ZZH7 | 7050* | MTRFVLFISCFFLIGMVVECKEGYLLGSRGCKMNCLTRPEKFCELECSLVGGENGYCAYWLACYCYNVPESVKLWESDTNECGKRK | |||||
| To9 | H1ZZH8 | 7155* | MNYSTLIAVASLLTAGTESKKDGYPVKEGDCAFPCGYDNEYCDKLCKERKADSGYCYWGNILCYCYGLPDKAAIKGYGRCRPGKK | |||||
| To10 | H1ZZH9 | 6940* | MNYSTLIAVASLLTAGTESKKDGYPVEGSCAFPCGYDNAYCDKLCKERKADSGYCYWVNILCYCYGLPDNAAIKGYGRCKPGKK | |||||
| To11 | H1ZZI0 | 7154* | MTRFVLFISCFFLIGMIVECKDGYLVGNDGCKYNCLTRPGHYCANECSRVKGKDGYCYAWMACYCYNMPNWVKTWSRATNKCGKRK | |||||
| To12 | H1ZZI1 | 7171* | MKGLILFICGFMMIGVILAKEGYPMDHEGCKFSCFIRPSGFCERYCKTHLSASTGYCAWPACYCYGVPANQKVWDYYNNKCGK | |||||
| To13 | H1ZZI2 | 8054* | MKTLFLIITSFILLEVEGIKNGYPRDSKGCTFECGQDAKHGDDYCDKMCKTTLKGEGGDCDFEYAECWCDNIPDTVVTWKNKEPKCKQI | |||||
| To14 | H1ZZI3 | 7953* | MNCLMLIFVVFLLAFGVECKKDDYPVDTAKRNCMLDCNVWDDEGYCDKFCKGRKADSGYCYKLKAACYCYGLPDDSPTKTSGRCNPNVR | |||||
| To15 | H1ZZI4 | 7195* | MKGIILLISCLMLIEVVVGGKEGYPLDSSGCKAGCFFGTNSWCNTECKRKSAAKGYCAWPSCYCYEFTDDSKIWNAKTNKCYK | |||||
| Tityus obscurus | Recombinant toxin Tc32 | P60211 | - | GSTGPQTTCQAAMCEAGCKGLGKSMESCQGDTCKCKA | α-KTx | Electrophysiological assays on periglomerular cells of olfactory bulb; three-dimensional (3D) solution structure determined by 1H NMR spectroscopy. | Blocks Kv1.1 and Kv1.3 | [45] |
| Tityus obscurus | ToPI1 | - | 3807 | DDCKDVCKARKGKCEFGICKC... | Serine peptidase inhibitor | Trypsin and chymotrypsin inhibitory assays; viability of the tumor cells HeLa (from human cervical cancer) and B16F10 (from murine melanoma) and non-tumor murine fibroblasts (NIH-3T3); EAG1, EAG2, hKv1.4, hKv1.1, hERG1, hERG2, hERG3 (5 uM) | Potent trypsin inhibitory activity; did not reduce the viability of tumor cells; no visible behavioral and/or physiological changes in mice; stable at the range of pH 3.0 to 9.0 even at 95 °C; | [148] |
| Brotheas amazonicus | - | - | - | - | Serine proteases | Proteolytic activity using SDS-PAGE | Proteolytic activity inhibited by PMSF | [149] |
| Tityus obscurus | ToAp1 | A0A1D3IXR7 | - | FIGMIPGLIGGLISAFK-NH2 | AMP, NDBP subfamily 4 | Antifungal activity against planktonic cells of Candida spp. and Cryptococcus neoformans and Candida albicans biofilms | Active against biofilm formation. Lower than 50% of hemolysis in all the tested concentrations. Active against C. neoformans and all Candida spp. (except for C. glabrata). | [150] |
| ToAp2 | A0A1D3IXJ5 | - | MQFKKQLLVIFFAYFLVVNESEAFFGTLFKLGSKLIPGVMKLFSKKKERSLMKRELKNLYDPYQRSVEMERLLKELPLY | AMP, NDBP subfamily 3 | Active against biofilm formation and all strains tested (MIC 3.12 to 200 μM). Hemolysis maintained at about 50%. | |||
| ToAP2S1 | - | - | FFGTLFKLLSKLIPGLMKLFSKLLER-NH2 | AMP, NDBP subfamily 3 | Hemolysis percent higher than 50% in concentrations up to 25 μM. No antifungal activity. | |||
| ToAp3 | - | - | FIGMIPGLIGGLISAIK-NH2 | AMP, NDBP subfamily 4 | Active against C. neoformans and all Candida spp. (except for C. glabrata). | |||
| ToAp4 | - | - | FFSLIPSLIGGLVSAIK-NH2 | AMP, NDBP subfamily 4 | No antifungal activity. | |||
| NDBP-4.23 | S6D3A7 | - | FLGMIPGLIGGLISAFK-NH2 | AMP, NDBP subfamily 4 | Active against biofilm formation. Lower than 50% of hemolysis in all the tested concentrations. | |||
| ToAcP | A0A1D3IY23 | - | EEDDLLGFSEEDLKAIKEHRAKNA-NH2 | AMP, NDBP subfamily not designated | No antifungal activity. | |||
| Tityus obscurus | To4 (Tc54) | P60215 | - | KDGYLMEYGGCKMSCLMKKGTFCAEECTRMKGKDGYCYAWLACYCYNMPDWVKIWNRATNKC | β-toxin | hNav (1.1- 1.7) | Changes hNav 1.1, hNav 1.2 and hNav 1.4 | [46] |
| Tityus obscurus | Transcripts with proteomic evidence | - | - | - | Angiotensin converting enzyme like Cysteine-rich protein, allergen V5/Tpx-1-related Endothelin-converting enzyme-like Hyaluronidase Metalloproteinase Serine and cysteine proteinases Phospholipase A2 KTx Proteinase inhibitors NaTx | - | - | [47] |
| Tityus metuendus | 1 (24.41 min) | - | 3927 | TPFRYCNPRNCAKECQGRCKETTYCDEVCKCSGW | KTx | - | - | [98] |
| 2 (24.66 min) | - | 1735 | KVLAPAEEAPAEAPAAAE | bradykinin-potentiating peptide | - | - | ||
| 3 (28.33 min) | - | 4004 | TAIGNCNPFTCDKECKTKGNKRGYCENYNCECSKW | - | Shaker-type ion-channels | - | ||
| 4 (30.07 min) | - | 7796/7767 | KKNDYPVDTAKRNCMLDCNVWDDEGYCDNFCKGRKAESGYCYKLDAACYCYGLPDDSPTKTSGRCNPNV | NaTx | - | - | ||
| 5 | - | 7318/7385 | NKDGYLMEGDGCKMGCLTRKASYCVDQCKEVGGKDGYCYAWLSCYCYNMPDSVEIWDSKNNKCGK | NaTx | - | - | ||
| 37.26 min | - | 6961 | KEGYLLGSRGCKMNCLTxPGNYCELECSLVGGxNG... | - | - | - | ||
| 42.10 min | - | 7153 | SRRGYPVTQKGxVYSSFWGSN... | - | - | - | ||
| - | - | - | - | Angiotensin converting enzyme like Endothelin-converting enzyme-like Hyaluronidase Metalloproteinase | - | - | ||
| Tityus obscurus | - | - | - | 320 non-disulfide bond-containing peptides (NDBPs) | Fragments of hypotensins, KTx and the allergen 5 protein | - | - | [135] |
| Tityus obscurus | To1 (Tc49b) | P60214 | - | KKEGYLVGNDGCKYGCITRPHQYCVHECELKKGTDGYCAYWLACYCYNMPDWVKTWSSATNKCK | β-toxin | hNaV (1.1-1.7), BgNaV1, VdNaV1 | Changes NaV 1.3, NaV 1.6, BgNaV1, VdNaV1 | [48] |
| Tityus obscurus | Synthetic peptide ToAP2 (P6) | - | - | FFGTLFKLGSKLIPGVMKLFSKKKER | AMP, NDBP subfamily 3 | Antiretroviral and cytotoxic activities | Active against simian immunodeficiency virus (SIV) replication in the HUT-78 cell line and in primary human leukocytes | [151] |
(~To)1: N-terminal with which it shares identity (this work); *theoretical molecular mass reported (other molecular masses were determined experimentally); …: sequence not complete (N-terminal fragment); AMP: antimicrobial peptide; BgNav: Nav from the German cockroach Blattella germanica; EAG: ether-à-go-go channel; 1H NMR: proton nuclear magnetic resonance; hERG: the human Ether-à-go-go-related gene; hNav: Nav from humans; KTx: potassium channel toxin; Kv: voltage-gated potassium channel; MIC: minimum inhibitory concentration; NaTx: sodium channel toxin; Nav: voltage-gated sodium channel; NDBP: non-disulfide-bridged peptide; Ref.: reference; VdNav: Nav from the mite Varroa destructor.
A comparative assay indicated that the dose of T. obscurus venom required to kill 50% (LD50) of mice (18-22 g) is 12.136 mg/kg, being classified as moderately toxic [15], whereas B. amazonicus (LD50 = 90.909 mg/kg) was practically nontoxic [15]. Other study reported the LD50 of 3.13 mg/kg and 0.99 mg/kg (intraperitoneal injection in mice (18-20g)) for T. obscurus and T. serrulatus, respectively [115]. It is noteworthy that the lethal dose (LD50) of the venoms of most scorpion species found in igapó, várzea and terra firme is still unknown.
Table 5 shows two studies on B. amazonicus venom, one study on the venom from T. metuendus and the remaining 14 reports (82%) are on T. obscurus venom. B. amazonicus venom degraded Aα and Bβ subunits of fibrinogen in sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and its proteolytic activity was inhibited in the presence of phenylmethylsulfonyl fluoride (PMSF), indicating the presence of serine proteases in the venom [149].
Electrophysiological assays using T. metuendus venom on seven sub-types of human voltage gated sodium channels (hNav1.1 to 1.7) revealed that it presents α- and β-scorpion toxins [98]. In vivo assays showed that T. metuendus venom was lethal to mice strain CD1 (25 g body weight) intraperitoneally injected with 200 μg and 300 μg of venom, respectively, within 80 min and 38 min after injection [98].
A recent proteomic analysis of the total soluble T. metuendus venom identified sodium and potassium channel toxins, metalloproteinases, endothelin and angiotensin-converting enzymes, bradykinin-potentiating peptide and hyaluronidases [98]. Arthropod venom hyaluronidases show potential medical and biotechnological applications [152]. The intranasal inoculation of hyaluronidase from T. serrulatus venom is a promising tool for pulmonary fibrosis treatment once it induces mononuclear increase in the bronchoalveolar space [153]. The use of hyaluronidase inhibitors can be used as a novel first-aid strategy in envenoming, since the enzyme has a key role in the scorpion venom spreading and biodistribution [154]. The toxic effects of scorpion envenoming were reduced by anti-hyaluronidase serum, which inhibited and delayed mouse death after subcutaneous injection of a lethal dose (13.2 (g) of T. serrulatus venom [155].
Scorpion venoms have a great diversity of molecules with potent and specific biological actions and, therefore, with possible biotechnological applications, justifying their study.
Discussion
Biases in this study
Systematic reviews must avoid publication biases as much as possible, including time lag, location, language, multiple publication, citation, and selective outcome reporting biases [156]. To avoid these publication biases, the following strategies were used in this manuscript: articles from 1864 to 2020 were retrieved; the searches were performed on 11 indexation databases (including grey literature, preprint databases and manual search); published and unpublished studies were searched; all languages available for the theme (English, French, Portuguese and Spanish), in the databases consulted, were included; theses were excluded if they were published as articles; all public (positive and negative) results available were presented in the text or in tables. The 88 reports included in this study were grouped as follows: 38 manuscripts about ecological aspects of scorpions; 33 reports about envenoming, including symptomatology, epidemiology and therapies; and 17 studies about venoms and toxins characterization.
Although none of the 88 reports included in this study discussed publication biases or any limitations of the study, we can point some of them. Concerning ecological information about the scorpion species studied, there is a lack of accurate geographical coordinates for different species, and the absence of a detailed description of the habitat and micro-habitat in which the species were found. These factors are impediments to conclusions about the biology of scorpions in the Brazilian Amazon. Regarding the cited epidemiological reports, they are observational studies which showed scorpion accidents refractory to antiscorpionic serum. Because correlation does not imply causation [157], an experimental randomized trial would be required in order to support a conclusion of cause and effect. On the basis of venoms and toxins characterization, proteomic reports identify several components, but lack functional characterization. And it is not possible to predict the function of these components, since most of them are novel molecules that do not share sequence identity with any reported primary sequence in databanks. Furthermore, there are some studies on the activity of enzymes that have not been isolated, impairing their structural characterization.
Difficulties and limitations in the study
This review article was based on data and articles available on public databases in which information on complications in patients was very limited. The process of searching for information on accidents caused by venomous animals in the Brazilian Notifiable Diseases Information System (SINAN) database is a difficult task. Data are updated continuously in the website and some Brazilian states provide records with a lag, sometimes one or two years after the envenoming, which can result in variations in the presented data depending on the date the search was performed [33]. Much of the data relevant to epidemiology of scorpionism is not published and/or quite difficult to access or retrieve from public databases. It is noteworthy that data for the period 2016 to 2018 were retrieved from SINAN database by the Ministry of Health from May to September 2019 and are still subject to review (please see [158]). In addition, there are no data on accidents with scorpions in 2019 in the Ministry of Health website, showing that scorpionism is still a neglected public health problem in Brazil.
Challenges related to research on scorpions and their toxins
The hardship in obtaining large amounts of venom and purified toxins is one obstacle faced when studying toxins from rare or small venomous species, such as scorpions [57]. For example, first venom extraction yields an average of 0.4 mg of venom from each scorpion and this amount is gradually reduced in the subsequent extractions [159]. In the Brazilian Amazon, the challenges to study the components from scorpion venoms begin initially with the collection of animals in a region of dense forest. Depending on the species, the capture of these arthropods may involve logistics that meet the conditions of the Amazon biome. Scorpions such as T. metuendus, T. silvestris and T. obscurus are abundant species in certain areas of the Brazilian Amazon, which offers opportunities for capture and reproduction in the laboratory for further assays. However, in an artificial environment, a large number of juvenile scorpions die, reducing the opportunity for more detailed experimental studies. In this case, the active collection of scorpions may present greater opportunities. The heterologous expression and chemical synthesis strategies have been used to overcome the limitation of amount of purified toxin.
After overcoming the challenge of obtaining enough quantity of the toxin, different cutting-edge technologies are used to integrate all data obtained through the “venomics” strategy, paving the way to explore novel compounds. The use of proteomics supported by mass spectrometry to explore animal venoms can elucidate the molecular mass of the toxin, fragments of its primary sequence, post translational modifications and their localization within the primary sequence [160]. However, the price of sophisticated instrumentation, maintenance of equipment and the need for qualified personal operating the instruments are usually costly. A difficulty faced by Brazilian researchers, mainly from the Northern and Northeast regions, is that the freight price and the waiting time for receiving an accessory or equipment may make the purchase of the product or maintenance of the equipment unfeasible, impairing the achievement of results. In the Northern region, few research groups study molecules originating from venomous animals, mainly due to the lack of specialists, “omic” tools, and financial support needed to discover the therapeutic value of venoms and achieve biotechnological development. A pioneering study of characterization of venom compounds from T. metuendus collected in the city of Manaus was made possible through a partnership abroad [98].
The next step involves experimental studies which mimics changes in the human body, since they have a key role for scientific evidence [161]. Animal experiments are needed to predict therapeutic doses, drug toxicity, pharmacokinetics, pharmacodynamics, and mechanistic information, but the use of mammalian models is being significantly reduced due to several reasons, including ethical and social dilemmas. In view of this scenario, the use of alternative models (such as brine shrimp, fruit fly, greater wax moth, roundworm, and zebra fish), development of non-animal methods and in silico modelling are required [162].
Regarding scorpionism, under-notification and low funding for investigating scorpion accidents result in the scarcity of data in the Brazilian Amazon. In addition to the lack of funding, epidemiological studies may be discontinued due to successive health programs that are ineffective in combating scorpionism.
Research needs to fill knowledge gaps about scorpion species of medical importance in the Brazilian Amazon
In the Northern region lack a professional qualification structure to taxonomically distinguish venomous animals that are dangerous to humans. The identification of scorpions is usually carried out in Tropical Medicine Foundation Heitor Vieira Dourado (Fundação de Medicina Tropical Heitor Vieira Dourado, FMT-HVD), Manaus, Amazonas (03°05′S 60°02′W) and Laboratory of Medical Entomology and Venomous Animals, which is part of the Center of Tropical Medicine at the Federal University of Pará, Belém, Pará (1°26′S 48°29′W) [9, 50], but in some cases the scorpions were sent for identification or double check at the Special Laboratory of Ecology and Evolution at the Butantan Institute, São Paulo, Brazil (23°34′S 46°43′W) [37, 49, 51, 114].
Reported studies showed that some populations of T. obscurus in certain areas of the Brazilian Amazon may be more lethal, suggesting an intraspecific variability in the venom [9, 49], being notified a high recurrence of severe cases in the Santarém region [9, 29]. The atypical sensation of electric shock (Table 3) is reported after scorpion accidents in the states of Pará and Amazonas [30, 49]. In these last two states, new cases of scorpionism with neurological effects have been reported [37, 51, 113].
The epidemiology of scorpionism in the Brazilian Amazon is related to five species with varying colors and sizes (Fig. 3 B-F). These scorpions are responsible for envenomings that often evolve to a moderate and, in some cases, severe condition (Table 3). To the best of our knowledge, there are no studies showing the establishment of populations of scorpions Tityus in urban areas of the Brazilian Amazon, which is reported for populations of T. serrulatus and T. stigmurus in other regions of Brazil [61, 82].
In northern Brazil, forest regions host polymorphic populations of scorpions, mainly in the states of Amazonas and Pará [7, 103]. For example, the specimen of T. silvestris (Fig. 3E) is a representative of a population that occurs in the municipality of Santo Antônio do Tauá (01º09'S 48º08'00"W), state of Pará. However, it is not known if different polymorphic groups of T. silvestris in the Amazon have developed venoms capable of triggering clinical effects with regional symptoms. The sensation of electric shock reported by victims of T. strandi in Santarém (2°25′48″S 54°43′12″W), state of Pará [37], has not been reported so far in Cotriguaçu, Aripuanã, Manacapuru, Manaus, Beruri, Barcelos, Monte Alegre or in other municipalities where the species occurs (Table 2). The absence of these data can be related to the lack of knowledge about local scorpions, a small percentage (8.3%) of the envenomed victims take the scorpions to be identified [49] and a few health units are capable of registering and preserving the specimens brought by the victims.
Among the potential reasons for the under-notification of scorpion sting reports are the lack of scorpion antivenom (that leads people to doubt the usefulness of the surveillance system), lack of health professionals for operational issues, or patients being unable to access a health center for antivenom therapy. The improvement of systems of collecting data on scorpion accidents may improve the knowledge regarding scorpion envenoming, especially in areas with poor road access and geographical barriers.
Two studies showed that the available scorpion antivenoms have not been able to shorten the severity of neurological changes in patients stung by T. apiacas, T. obscurus or T. silvestris. Several studies worldwide have been performed to improve the manufacture and quality of available therapies. Next-generation antivenoms based on recombinant monoclonal antibodies and antibody fragments are expected to be more effective, safer, and cost-competitive therapies [11]. Studies on inhibitors, such as peptides, small molecules or bioactive components from plant sources, are also an interesting field to be explored as alternative approaches to improve the current limitations of serum therapy [154, 163-165].
Furthermore, there are few studies evaluating the immunogenic effect of Amazonian scorpion venoms on experimental animals (Table 3). These biological assays could contribute to the production of more effective therapeutic antibodies for the treatment of scorpion envenoming.
Experimental models and the knowledge about the scorpion toxins and biomarkers can be useful tools to help in early diagnosis in patients stung by scorpions, explain the clinical picture in humans, improve treatment and reduce fatal complications. Epidemiological and experimental studies are necessary to assess the relationship of an injury after an animal envenoming.
The venoms of scorpions from the Brazilian Amazon are still underexploited, lacking information on the chemical structure, physiological role and therapeutic application of their biologically active compounds. For example, there are eight peptides sequenced from B. amazonicus whose biological functions have not been evaluated. Although serine proteinase activity has been detected in this venom, the enzyme has not been isolated and no fragments of its sequence have been identified. Many compounds from T. metuendus and T. obscurus were identified through “omic” approaches, but no biological assays were performed (Table 5). Strikingly, 15 proteins from T. obscurus shared sequence identity with NaTx, but only four toxins (To1 to To4) were tested on electrophysiological assays. The study of venom components could increase the chances of discovering new molecules with great pharmaceutical potential and understanding the pathophysiological effects of the scorpion envenoming in the Brazilian Amazon.
Conclusions
The critical assessment in this review showed that a meticulous study design is necessary to minimize bias during clinical and case reports about scorpion envenoming. We suggest that researchers become familiar with reporting guidelines such as the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE), which are delineated to investigate the associations between an exposure and a health outcome [166].
More investment from the government and research foundations is required to raise awareness of the society on the importance of scorpion accident notification. Despite the highest severity of scorpion envenoming notified in the Northern region, there is a data scarcity on scorpionism in the Brazilian Amazon region, especially in remote areas.
T. obscurus is widely studied when compared to other Amazonian scorpions. However, data on its venom and its effects on human body are still little explored when compared to T. serrulatus. Furthermore, we still have dozens of scorpion species from the Brazilian Amazon with unexplored venoms. Although five species have been reported to be of medical importance in the northern region, the diversity of the scorpion fauna in this macroregion suggests that more species are involved in the scorpionism in the Brazilian Amazon.
Time, intellectual, technological and financial resources are also necessary to untangle the interaction of venom peptides with their target during the development of potential new engineered therapeutic molecules and more effective serum therapies. Antivenoms capable of neutralizing neurological changes caused by Amazonian scorpions are required, as well as mechanistic information about which and how the toxins present in the venoms of T. apiacas, T. obscurus and T. silvestris trigger these manifestations. Therefore, studies on venoms and toxins of scorpions from the Brazilian Amazon are fruitful tools for future research.
Abbreviations
1H NMR: proton nuclear magnetic resonance; 3D: three-dimensional; AC: Acre; AM: Amazonas; AMP: antimicrobial peptide; AP: Amapá; BDTD: Brazilian Digital Library of Theses and Dissertations; BgNav: Nav from the German cockroach Blattella germanica; CPP: cell penetrating peptide; EAG: ether-à-go-go channel; hERG: the human Ether-à-go-go-related gene; IBGE: Brazilian Institute of Geography and Statistics; KTx: potassium channel toxin; Kv: voltage-gated potassium channels; LCA: Lower Central America; LD50: lethal dose required to kill 50%; LILACS: Latin American and Caribbean Center on Health Sciences Informational; MA: Maranhão; MIC: minimum inhibitory concentration; MT: Mato Grosso; NaTx: sodium channel toxin; Nav: voltage-gated sodium channels; NDBP: non-disulfide bridged peptide; NF-(B: nuclear factor-kappa B; PA: Pará; PMSF: phenylmethylsulfonyl fluoride; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; Ref.: reference; RO: Rondônia; RR: Roraima; SDS-PAGE: sodium dodecyl sulfate-polyacrylamide gel electrophoresis; SINAN: Brazilian Notifiable Diseases Information System; SIV: simian immunodeficiency virus; TO: Tocantins; VdNav: Nav from the mite Varroa destructor; VHL: Virtual Health Library.
Acknowledgements
The authors would like to thank Dr. Pedro Pereira de Oliveira Pardal and MSc. Bruno Rafael Ribeiro de Almeida for the photos of the scorpions, respectively, T. obscurus (Fig. 3D) and T. silvestris (Fig. 3E), and Dr. Maria das Graças Vale Barbosa Guerra, who provided specimens of T. apiacas for photography. Thanks are also due to Nícolas da Silva Garcia for his help in making the Figure 2 and biologist Francisco José Ramos Prestes for the photos of T. metuendus scorpions (Fig. 4A, B and F).
Footnotes
Availability of data and materials: All data generated or analyzed during this study are included in this article.
Funding: This study was supported by the São Paulo Research Foundation (FAPESP, grant n. 2019/10173-6, and scholarships to GCS 2019/27544-7) and the National Council for Scientific and Technological Development (CNPq, grant n. 306479/2017-6).
Ethics approval: Not applicable.
Consent for publication: Not applicable.
References
- Prendini L, Wheeler WC. Scorpion higher phylogeny and classification, taxonomic anarchy, and standards for peer review in online publishing. Cladistics. 2005;21(5):446–494. doi: 10.1111/j.1096-0031.2005.00073.x. [DOI] [PubMed] [Google Scholar]
- Rein JO. The scorpion files. 2021. [2021 Jan 22]. Available from: http://www.ntnu.no/ub/scorpion-files. [Google Scholar]
- Mullen GR, Sissom WD. In: Medical and veterinary entomology. 3rd. Mullen GR, Durden LA, editors. London: Elsevier; 2019. Scorpions (Scorpiones) pp. 489–504. [Google Scholar]
- Tropea G, Onnis C. A remarkable discovery of a new scorpion genus and species from Sardinia (Scorpiones: Chactoidea: Belisariidae) Arachnida. 2020;26:3–25. [Google Scholar]
- Santibanez-Lopez CE, Ojanguren-Affilastro AA, Sharma PP. Another one bites the dust: taxonomic sampling of a key genus in phylogenomic datasets reveals more non-monophyletic groups in traditional scorpion classification. Invert Syst. 2020;34(2):133–143. [Google Scholar]
- Prendini L, Loria SF. Systematic revision of the Asian forest scorpions (Heterometrinae Simon, 1879), revised suprageneric classification of Scorpionidae Latreille, 1802, and revalidation of Rugodentidae Bastawade et al., 2005. Bull Am Mus Nat Hist. 2020;442(1):1–480. [Google Scholar]
- Lourenço WR. Scorpions of Brazil. 1st. Les editions de l'If; Paris: 2002. pp. 307–307. [Google Scholar]
- Lourenço WR, Eickstead VRD. In: Animais Peçonhentos no Brasil: Biologia, Clínica e Terapêutica dos Acidentes. 1st. Cardoso JLC, França FOS, Wen FH, Málaque CMS, Jr. V Haddad, editors. São Paulo: Sarvier; 2003. Escorpiões de importância médica; pp. 182–197. [Google Scholar]
- Pardal PPO, Ishikawa EAY, Vieira JLF, Coelho JS, Dorea RCC, Abati PAM, Quiroga MMM, Chalkidis HM. Clinical aspects of envenomation caused by Tityus obscurus (Gervais, 1843) in two distinct regions of Para state, Brazilian Amazon basin: a prospective case series. J Venom Anim Toxins incl Trop Dis. 2014;20(3) doi: 10.1186/1678-9199-20-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: A global appraisal. Acta Trop. 2008;107(2):71–79. doi: 10.1016/j.actatropica.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Ahmadi S, Knerr JM, Argemi L, Bordon KCF, Pucca MB, Cerni FA, Arantes EC, Caliskan F, Laustsen AH. Scorpion venom: detriments and benefits. Biomedicines. 2020;8(5):118. doi: 10.3390/biomedicines8050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein JO. The scorpion files - Buthidae. 2020. [2021 Jan 22]. Available from: https://www.ntnu.no/ub/scorpion-files/buthidae.php. [Google Scholar]
- Lourenco WR. Scorpion incidents, misidentification cases and possible implications for the final interpretation of results. J Venom Anim Toxins incl Trop Dis. 2016;22:21. doi: 10.1186/s40409-016-0075-6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco WR. The evolution and distribution of noxious species of scorpions (Arachnida: Scorpiones) J Venom Anim Toxins incl Trop Dis. 2018;24:1. doi: 10.1186/s40409-017-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa AK, Caricati CP, Lima M, Dossantos MC, Kipnis TL, Eickstedt VRD, Knysak I, da Silva MH, Higashi HG, da Silva WD. Antigenic cross-reactivity among the venoms from several species of Brazilian scorpions. Toxicon. 1994;32(8):989–998. doi: 10.1016/0041-0101(94)90377-8. [DOI] [PubMed] [Google Scholar]
- Goyffon M, Kovoor J. In: Arthropod venoms. Bettini S, editor. Berlin/Heidelberg: Springer-Verlag; 1978. Chactoid venoms; pp. 395–418. [Google Scholar]
- Ward MJ, Ellsworth SA, Nystrom GS. A global accounting of medically significant scorpions: Epidemiology, major toxins, and comparative resources in harmless counterparts. Toxicon. 2018;151:137–155. doi: 10.1016/j.toxicon.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Santos MSV, Silva CGL, S B, Neto, Grangeiro CRP, Lopes VHG, Teixeira AG, Bezerra DA, Luna JVCP, Cordeiro JB, Gonçalves J, Jr, Lima MAP. Clinical and epidemiological aspects of scorpionism in the world: A systematic review. Wild Environ Med. 2016;27(4):504–518. doi: 10.1016/j.wem.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Tigre MA. Building a regional adaptation strategy for Amazon countries. Int Environ Agreem-P. 2019;19(4-5):411–427. [Google Scholar]
- Institute of Geography and Statistics - IBGE IBGE launches unprecedented map of Biomes and Coastal-Marine System 2019. 2019. https://agenciadenoticias.ibge.gov.br/en/agencia-press-room/2185-news-agency/releases-en/25812-ibge-lanca-mapa-inedito-de-biomas-e-sistema-costeiro-marinho-2
- Divino JA, McAleer M. Modelling sustainable international tourism demand to the Brazilian Amazon. Environ Model Softw. 2009;24(12):1411–1419. [Google Scholar]
- Brazil TK, Porto TJ. Os escorpiões. Salvador: EDUFBA; 2010. [Google Scholar]
- Institute of Geography and Statistics - IBGE Regional divisions of Brazil. [2021 Jan 22]. Available from: https://www.ibge.gov.br/en/geosciences/territorial-organization/regional-division/21536-regional-divisions-of-brazil.html?=&t=o-que-e.
- Lourenço W. Scorpions from Brazilian Amazonia, with a description of two new species from'Serra da Mocidade'National Park in the State of Roraima (Scorpiones: Buthidae, Chactidae) Arachnida. 2017;12:2–17. [Google Scholar]
- Lourenço W, Rossi A, Wilmé L. Further clarifications on species of Tityus C. L. Koch, 1836, subgenus Atreus Gervais, 1843 (Scorpiones: Buthidae), from Amazonia, with the description of a new species. Arachnida. 2019;21:11–25. [Google Scholar]
- Lourenço WR. Boletín de la SEA. 50. 2012. Further considerations on Tityus (Archaeotityus) clathratus CL Koch, 1844 and description of two associated new species (Scorpiones: Buthidae) pp. 277–283. [Google Scholar]
- Costa CLSO, Fe NF, Sampaio I, Tadei WP. A profile of scorpionism, including the species of scorpions involved, in the State of Amazonas, Brazil. Rev Soc Bras Med Trop. 2016;49(3):376–379. doi: 10.1590/0037-8682-0377-2015. [DOI] [PubMed] [Google Scholar]
- Torrez PPQ, Dourado FS, Bertani R, Cupo P, Franca FOD. Scorpionism in Brazil: exponential growth of accidents and deaths from scorpion stings. Rev Soc Bras Med Trop. 2019;52:e20180350. doi: 10.1186/1678-9199-20-46.. [DOI] [PubMed] [Google Scholar]
- Reckziegel GC, Pinto VL. Scorpionism in Brazil in the years 2000 to 2012. J Venom Anim Toxins incl Trop Dis. 2014;20:46. doi: 10.1590/0037-8682-0350-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupo P. Clinical update on scorpion envenoming. Rev Soc Bras Med Trop. 2015;48(6):642–649. doi: 10.1590/0037-8682-0237-2015. [DOI] [PubMed] [Google Scholar]
- Porto TJ, Brazil TK. Brazil TK, Porto TJ. Os escorpiões. Salvador: EDUFBA; 2010. Os escorpiões de importância médica e seus venenos: Escorpionismo no Brasil; pp. 65–69. [Google Scholar]
- SVS/MS (Secretaria de Vigilância em Saúde/Ministério da Saúde) Casos de acidentes por serpentes e por escorpiões. Brasil 2000-2018. 2019. [2021 Mar 19]. Available from: https://antigo.saude.gov.br/images/pdf/2019/outubro/16/1--Dados-Epidemiologicos-SiteSVS--Setembro-2019-OFIDISMO-CASOS.pdf. [Google Scholar]
- Chippaux JP. Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: from obvious facts to contingencies. J Venom Anim Toxins incl Trop Dis. 2015;21(13) doi: 10.1186/s40409-015-0011-1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil - Secretaria de Vigilância em Saúde/Ministério da Saúde . Óbitos por animais peçonhentos. Brasil 2000-2018. 2019. [2021 Mar 19]. Available from: https://antigo.saude.gov.br/images/pdf/2019/outubro/16/3--Dados-Epidemiologicos-SiteSVS--Setembro-2019-ANIMAIS-PE--ONHENTOS---BITOS.pdf. [Google Scholar]
- SVS/MS (Secretaria de Vigilância em Saúde/Ministério da Saúde) Incidência de acidentes por animais peçonhentos. Brasil 2000-2018. 2019. [2021 Mar 19]. Available from: https://antigo.saude.gov.br/images/pdf/2019/outubro/16/2--Dados-Epidemiologicos-SiteSVS--Setembro-2019-ANIMAIS-PE--ONHENTOS-INCID--NCIA.pdf. [Google Scholar]
- Queiroz AM, Sampaio VS, Mendona I, Fe NF, Sachett J, Ferreira LCL, Feitosa E, Wen FH, Lacerda M, Monteiro W. Severity of scorpion stings in the Western Brazilian Amazon: A case-control study. Plos One. 2015;10(6):e0128819. doi: 10.1371/journal.pone.0128819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira SMS, Bertani R, Torrez PPQ, de Sousa PRL, Quiroga MMM, Bertolozzi MR, Franca FOS. Electric shock sensation in the first reports of envenomations by Tityus strandi in the Brazilian Amazon. Toxicon. 2020;178:8–12. doi: 10.1016/j.toxicon.2020.01.005. [DOI] [PubMed] [Google Scholar]
- Torrez PPQ, Bertolozzi MR, de Siqueira Franca FO. Vulnerabilities and clinical manifestations in scorpion envenomations in Santarem, Para, Brazil: a qualitative study. Rev Esc Enferm USP. 2020;54:e03579. doi: 10.1590/s1980-220x2018050403579. [DOI] [PubMed] [Google Scholar]
- Batista CVF, Gomez-Lagunas F, Lucas S, Possani LD. Tc1, from Tityus cambridgei, is the first member of a new subfamily of scorpion toxin that blocks K+-channels. Febs Lett. 2000;486(2):117–120. doi: 10.1016/s0014-5793(00)02253-5. [DOI] [PubMed] [Google Scholar]
- Batista CVF, Gomez-Lagunas F, de la Vega RCR, Hajdu P, Panyi G, Gaspar R, Possani LD. Two novel toxins from the Amazonian scorpion Tityus cambridgei that block Kv1.3 and Shaker BK+-channels with distinctly different affinities. Biochim Biophys Acta Proteins Proteom. 2002;1601(2):123–131. doi: 10.1016/s1570-9639(02)00458-2. [DOI] [PubMed] [Google Scholar]
- Batista CVF, Zamudio FZ, Lucas S, Fox JW, Frau A, Prestipino G, Possani LD. Scorpion toxins from Tityus cambridgei that affect Na+-channels. Toxicon. 2002;40(5):557–562. doi: 10.1016/s0041-0101(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Batista CVF, del Pozo L, Zamudio FZ, Contreras S, Becerril B, Wanke E, Possani LD. Proteomics of the venom from the Amazonian scorpion Tityus cambridgei and the role of prolines on mass spectrometry analysis of toxins. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;803(1):55–66. doi: 10.1016/j.jchromb.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Murgia AR, Batista CVF, Prestipino G, Possani LD. Amino acid sequence and function of a new alpha-toxin from the Amazonian scorpion Tityus cambridgei. Toxicon. 2004;43(6):737–740. doi: 10.1016/j.toxicon.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Guerrero-Vargas JA, Mourao CBF, Quintero-Hernandez V, Possani LD, Schwartz EF. Identification and phylogenetic analysis of Tityus pachyurus and Tityus obscurus novel putative Na+-channel scorpion toxins. PloS One. 2012;7(2):e30478. doi: 10.1371/journal.pone.0030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehling EG, Sforca ML, Zanchin NIT, Oyama S, Jr., Pignatelli A, Belluzzi O, Polverini E, Corsini R, Spisni A, Pertinhez TA. Looking over toxin-K+ channel interactions. Clues from the structural and functional characterization of alpha-KTx toxin Tc32, a Kv1.3 channel blocker. Biochemistry. 2012;51(9):1885–1894. doi: 10.1021/bi201713z. [DOI] [PubMed] [Google Scholar]
- Duque HM, Farias Mourao CB, Tibery DV, Barbosa EA, Campos LA, Schwartz EF. To4, the first Tityus obscurus beta-toxin fully electrophysiologically characterized on human sodium channel isoforms. Peptides. 2017;95:106–115. doi: 10.1016/j.peptides.2017.07.010. [DOI] [PubMed] [Google Scholar]
- de Oliveira UC, Nishiyama MY, Jr., Viana dos Santos MB, AdP Santos-da-Silva, HdM Chalkidis, Souza-Imberg A, Candido DM, Yamanouye N, Dorce VAC, Junqueira-de-Azevedo ILM. Proteomic endorsed transcriptomic profiles of venom glands from Tityus obscurus and T. serrulatus scorpions. PloS One. 2018;13(3):e0193739. doi: 10.1371/journal.pone.0193739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibery DV, Campos LA, Farias Mourao CB, Peigneur S, Cruz e Carvalho A, Tytgat J, Schwartz EF. Electrophysiological characterization of Tityus obscurus beta toxin 1 (To1) on Na+-channel isoforms. Biochim Biophys Acta Biomembr. 2019;1861(1):142–150. doi: 10.1016/j.bbamem.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Pardal PPO, Castro LC, Jennings E, Pardal JS, Monteiro MR. Epidemiological and clinical aspects of scorpion envenomation in the region of Santarém, Pará, Brazil. Rev Soc Bras Med Trop. 2003;36(3):349–353. doi: 10.1590/s0037-86822003000300006. [DOI] [PubMed] [Google Scholar]
- Monteiro WM, de Oliveira SS, Pivoto G, Alves EC, Sachett JDG, Alexandre CN, Fé NF, Guerra MGVB, Silva IM, Tavares AM, Ferreira LCL, Lacerda MVG. Scorpion envenoming caused by Tityus cf. silvestris evolving with severe muscle spasms in the Brazilian Amazon. Toxicon. 2016;119:266–269. doi: 10.1016/j.toxicon.2016.06.015. [DOI] [PubMed] [Google Scholar]
- da Silva BAJ, Fe NF, Gomes AAD, Souza AD, Sachett JDG, Fan HW, Melo GC, Monteiro WM. Implication of Tityus apiacas (Lourenco, 2002) in scorpion envenomations in the Southern Amazon border, Brazil. Rev Soc Bras Med Trop. 2017;50(3):427–430. doi: 10.1590/0037-8682-0490-2016. [DOI] [PubMed] [Google Scholar]
- Souza ARB, Arakian SKL, Buhrnhein PF. Estudo clínico-epidemiológico dos acidentes escorpiônicos atendidos no Instituto de Medicina Tropical de Manaus no periódo de 1986 a 1994. In: XXXI Congresso da Sociedade Brasileira de Medicina Tropical: Resumos, 1995. Rev Soc Bras Med Trop. 1995;28(1):167 [Google Scholar]
- Amaral CFS, Derezende NA, Freiremaia L. Acute pulmonary-edema after Tityus serrulatus scorpion sting in children. Am J Cardiol. 1993;71(2):242–245. doi: 10.1016/0002-9149(93)90746-y. [DOI] [PubMed] [Google Scholar]
- Monteiro WM, Gomes J, Fe N, da Silva IM, Lacerda M, Alencar A, Farias AS, Val F, Sampaio VS, Melo GC, Pardal P, Silva AM, Bernarde OS, Ferreira LCL, Gutierrez JM, Sachett JAG, Fan HW. Perspectives and recommendations towards evidence-based health care for scorpion sting envenoming in the Brazilian Amazon: A comprehensive review. Toxicon. 2019;169:68–80. doi: 10.1016/j.toxicon.2019.09.003. [DOI] [PubMed] [Google Scholar]
- Silva EMRd. Scorpionic accident in the municipality of Santarém PA: epidemiological characteristics and access of the patients to the health service. São Paulo, SP: São Paulo, SP: Universidade de São Paulo; 2017. dissertation. [Google Scholar]
- Wen FH, Monteiro WM, da Silva AMM, Tambourgi DV, da Silva IM, Sampaio VS, dos Santos MC, Sachett J, Ferreira LCL, Kalil J, Lacerda M. Snakebites and scorpion stings in the Brazilian Amazon: identifying research priorities for a largely neglected problem. PloS Negl Trop Dis. 2015;9(5):e0003701. doi: 10.1371/journal.pntd.0003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordon KCF, Cologna CT, Fornari-Baldo EC, Pinheiro EL, Cerni FA, Amorim FG, Anjolette FAP, Cordeiro FA, Wiezel GA, Cardoso IA, Ferreira IG, Oliveira IS, Boldrini-França J, Pucca MB, Baldo MA, Arantes EC. From animal poisons and venoms to medicines: achievements, challenges and perspectives in drug discovery. Front Pharmacol. 2020;11:1132. doi: 10.3389/fphar.2020.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux JP. Impact of the environment on envenomation incidence and severity. Med Sci (Paris) 2009;25(10):858–862. doi: 10.1051/medsci/20092510858. Article in French. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandes E, Zamboni A, Fabbri S, Thommazo AD. Using GQM and TAM to evaluate StArt-a tool that supports Systematic Review. CLEI Electr J. 2012;15(1) Paper 2. [Google Scholar]
- Lourenço W, Cloudsley-Thompson J, Cuellar O, Vv Eickstedt, Barraviera B, Knox M. The evolution of scorpionism in Brazil in recent years. J Venom Anim Toxins incl Trop Dis. 1996;2(2):121–134. https://www.scielo.br/j/jvat/a/jTLDYBWjLTKBGdPvM7MvGhS/?lang=en [Google Scholar]
- Lourenço WR. The biogeography of scorpions. Rev Suisse Zool. 1996;103:437–448. [Google Scholar]
- Lourenco WR. What do we know about some of the most conspicuous scorpion species of the genus Tityus? A historical approach. J Venom Anim Toxins incl Trop Dis. 2015;21:20. doi: 10.1186/s40409-015-0016-9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer H, Wollscheid E, Gasnier T. The relative abundance of Brotheas amazonicus (Chactidae, Scorpiones) in different habitat types of a central Amazon rainforest. J Arachnol. 1996;24(1):34–38. [Google Scholar]
- Lourenco WR, Adis J, Araujo JDS. A new synopsis of the scorpion fauna of the Manaus region in Brazilian Amazonia, with special reference to an inundation forest at the Taruma Mirim river. Amazoniana. 2005;18(3-4):241–249. [Google Scholar]
- Fuentes-Silva D, Santos AP, Oliveira JS. Envenomation caused by Rhopalurus amazonicus Lourenco, 1986 (Scorpiones, Buthidae) in Para State, Brazil. J Venom Anim Toxins incl Trop Dis. 2014;20:52. doi: 10.1186/1678-9199-20-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatierra L. Myster RW. Igapó (Black-water flooded forests) of the Amazon Basin. Berlin: Heidelberg; New York: Springer; 2018. Diversity and phenology of arachnids in Igapó forests; pp. 81–97. [Google Scholar]
- Lourenco WR. Reproduction in scorpions, with special reference to parthenogenesis. European Arachnology. 2000;2002:71–85. [Google Scholar]
- Lourenco WR. Addition to the scorpion fauna of the neotropics (Arachnida) Rev Suisse Zool. 1997;104(3):587–604. [Google Scholar]
- Coelho JS, Ishikawa EAY, dos Santos PRSG, Pardal PPO. Scorpionism by Tityus silvestris in eastern Brazilian Amazon. J Venom Anim Toxins incl Trop Dis. 2016;22:24. doi: 10.1186/s40409-016-0079-2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeda-Jorge S, Mello T, Pinto-Da-Rocha R. Litter size, effects of maternal body size, and date of birth in South American scorpions (Arachnida: Scorpiones) Zoologia. 2009;26(1):43–53. [Google Scholar]
- Pinto-da-Rocha R, de Araujo CO, Barreiros JAP, Bonaldo AB. Arthropoda, Arachnida, Scorpiones: Estacao Cientifica Ferreira Penna and Juruti Plateau, Para, Brazil. Check List. 2007;3(2):143–146. [Google Scholar]
- Lourenço WR. The distribution of noxious species of scorpions in Brazilian Amazonia: the genus Tityus CL Koch, 1836, subgenus Atreus Gervais, 1843 (Scorpiones, Buthidae) Entomol Mitt Zool Mus Hamburg. 2011;15(185):287–301. [Google Scholar]
- Costa CLSdO. Análises epidemiológica, de distribuição geográfica e genética dos escorpiões que ocorrem no Estado do Amazonas, Brasil. Manaus, AM: Manaus, AM: Instituto Nacional de Pesquisas da Amazônia - INPA; 2016. thesis. [Google Scholar]
- Lourenço WR, Ramos EB. New considerations on the status of Tityus magnimanus Pocock, 1897 (Scorpiones: Buthidae), and description of a new species of Tityus from the State of Roraima, Brazil. Rev Iber Aracnol. 2004;(10):285–291. [Google Scholar]
- Pinto-da-Rocha R, Lourenco WR. Two new species of Tityus from Brazilian Amazonia (Scorpiones, Buthidae) Rev Arachnol. 2000;13(13):187–195. [Google Scholar]
- Lourenco WR. Contribution to the knowledge of the Amazonian scorpion Tityus metuendus Pocock, 1897 (Buthidae) Stud Neotrop Fauna E. 1983;18(4):185–193. [Google Scholar]
- Lourenço WR. In: Amazonian Arachnida and Myriapoda: identification keys to all classes, orders, families, some genera, and lists of known terrestrial species. Adis J, editor. Moscow: Pensoft Publishers; 2002. Scorpiones; pp. 430–435. [Google Scholar]
- Lourenço WR, Huber D, Cloudsley-Thompson JL, editors. Notes on the ecology, distribution and postembryonic development of Tityus cambridgei Pocock, 1897 (Scorpiones, Buthidae) from French Guyana and Oriental Amazonia. Entomol Mitt Zool Mus Hamburg. 2000;13(162):197–203. [Google Scholar]
- Lourenco WR, da Silva EA. New evidence for a disrupted distribution pattern of the 'Tityus confluens' complex, with the description of a new species from the State of Para, Brazil (Scorpiones, Buthidae) Amazoniana. 2007;19(3-4):77–86. [Google Scholar]
- Lourenco WR. Description of the male of Tityus (Tityus) raquelae Lourenco, 1988 (Scorpiones, Buthidae), a species from the Brazilian Amazon. Rev Iber Aracnol. 2012;21:65–68. [Google Scholar]
- Eickstedt VRDv. O que você sabe sobre escorpiões? Rev Ensino Ciências. 1989;22:35–40. [Google Scholar]
- ter Steege H, Vaessen RW, Cardenas-Lopez D, Sabatier D, Antonelli A, de Oliveira SM, Pitman NCA, Jorgensen PM, Salomão RP. The discovery of the Amazonian tree flora with an updated checklist of all known tree taxa. Sci Rep. 2016;6:29549. doi: 10.1038/srep29549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco WR, Pezier A. Addition to the scorpion fauna of the Manaus region (Brazil), with a description of two new species of Tityus from the canopy. Amazoniana. 2002;17(1):177–186. [Google Scholar]
- Ribeiro JELS, Hopkins MJG, Vicentini A, Sothers CA, Costa MAS, Brito JM. Guia de identificação de plantas vasculares de uma floresta de terra-firme na Amazônia Central. Manaus: INPA-DFID; 1999. Flora da reserva Ducke. [Google Scholar]
- Lorenzi H. In: Flora brasileira: Arecaceae (palmeiras) Lorenzi H, Kahn F, Noblick LR, Ferreira E, editors. Nova Odessa: Instituto Plantarum de Estudos da Flora; 2010. Geonoma; pp. 214–255. [Google Scholar]
- Smith N. In: Palms and people in the Amazon. Geobotany studies - basics, methods and case studies. Smith N, editor. Heidelberg: Springer; 2015. Palms and cultural landscapes; pp. 1–8. [Google Scholar]
- Adis J. Survival strategies of terrestrial invertebrate in Central Amazonian inundation forests; a response to long-term flooding. Acta Amaz. 1997;27(1):43–54. [Google Scholar]
- Brasil - Secretaria de Vigilância em Saúde- Departamento de Vigilância Epidemiológica . Manual de controle de escorpiões. Brasília: Ministério da Saúde; 2009. [Google Scholar]
- Higa AM, JdS Araújo, Araújo TF, Noronha MDN, Martins Marx JP, Medeiros BM, Muniz EG, Aguiar NO, Lopez-Lozano JL, editors. Toxic effects on insects of venoms from Tityus metuendus and Brotheas amazonicus Amazonian scorpions. IX Symposium of the Brazilian Society on Toxinology - posters arthropods. J. Venom Anin Toxins incl Trop Dis. 2007 [Google Scholar]
- Auguste RJ, Deo R, Finnell B, Ali H. First report of a caecilian amphibian (Siphonopidae: Microcaecilia sp.) being preyed upon by a scorpion (Chactidae: Brotheas sp.) Herpetol Notes. 2019;12:661–662. [Google Scholar]
- Esposito LA, Yamaguti HY, Souza CA, Pinto-Da-Rocha R, Prendini L. Systematic revision of the neotropical club-tailed scorpions, Physoctonus, Rhopalurus and Troglorhopalurus, revalidation of Heteroctenus, and descriptions of two new genera and three new species (Buthidae, Rhopalurusinae) Bull Am Mus Nat Hist. 2017;415:1–134. [Google Scholar]
- Abreu CB, Bordon KCF, Cerni FA, Oliveira IS, Balenzuela C, Alexandre-Silva GM, Zoccal KF, Reis MB, Wiezel GA, Peigneur S, Pinheiro-Junior EL, Tytgat J, Cunha TM, Quinton L, Faccioli LH, Arantes EC, Zottich U, Pucca MB. Pioneering study on Rhopalurus crassicauda scorpion venom: isolation and characterization of the major toxin and hyaluronidase. Front Immunol. 2020;11:2011. doi: 10.3389/fimmu.2020.02011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco WR. New additions to the Neotropical scorpion fauna (Arachnida) Rev Suisse Zool. 2002;109(1):127–141. [Google Scholar]
- SpeciesLink. [2021 March 19]. Available from: http://splink.cria.org.br/project?criaLANG=pt.
- Collections of the Museu Paraense Emílio Goeldi. [2021 March 19].
- Fet V, Sissom WD, Lowe G, Braunwalder ME. Catalog of the scorpions of the world (1758-1998) New York: New York Entomological Society; 2000. [Google Scholar]
- Batista CVF, Martins JG, Restano-Cassulini R, Coronas FIV, Zamudio FZ, Procopio R, Possani LD. Venom characterization of the Amazonian scorpion Tityus metuendus. Toxicon. 2018;143:51–58. doi: 10.1016/j.toxicon.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Lourenco WR. Revisão crítica das especies Tityus do Estado do Pará (Scorpiones, Butidae) Bol. Mus. Para. Emilio Goeldi. Sér. Zool. 1984;1(1):5–18. [Google Scholar]
- Lourenco WR, Qi J-X. Additions to the scorpion fauna of the Amapá State in Brazil (Chelicerata, Scorpiones) Rev Suisse Zool. 2007;114(1):3–12. [Google Scholar]
- Costa GG, LdFM Serejo, JdS Coelho, Candido DM, Gadelha MAdC, Pardal PPdO. First report of scorpionism caused by Tityus serrulatus, described by Lutz and Mello, 1922 (Scorpiones, Buthidae), a species non-native to the state of Para, Brazilian Amazon. Rev Soc Bras Med Trop. 2020;53:e20190285. doi: 10.1590/0037-8682-0285-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal PPO, Gadelha MAC, Menezes MMGO, Malheiros RS, Ishikawa EAY, Gabriel MDG. Severe envenomation by Tityus obscurus Gervais, 1843. Rev Pan-Amaz Saúde. 2014;5:65–70. [Google Scholar]
- Lourenco WR. Sinopse da fauna escorpionica do Estado do Para, especialmente as regioes de Carajas, Tucurui, Belem e Trombetas. Bol. Mus. Para. Emilio Goeldi. Sér. Zool. 1988;4(2):155–173. [Google Scholar]
- Mattos VF, Carvalho LS, Carvalho MA, Schneider MC. Insights into the origin of the high variability of multivalent-meiotic associations in holocentric chromosomes of Tityus (Archaeotityus) scorpions. PloS One. 2018;13(2):e0192070. doi: 10.1371/journal.pone.0192070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battirola LD. A case of predation on Ancylometes rufus (Walckenaer, 1837) (Araneae, Ctenidae) by Tityus strandi (Werner, 1939) (Scorpiones, Buthidae) in Southern Amazonia. Acta Biol Parana. 2015;44(3-4):145–151. [Google Scholar]
- Lira AFD, Guilherme E, de Souza MB, Carvalho LS. Scorpions (Arachnida, Scorpiones) from the state of Acre, southwestern Brazilian Amazon. Acta Amaz. 2021;51(1) [Google Scholar]
- Lourenco WR, de MMBG, Jesus Junior, Limeira-de-Oliveira F. A new species of Tityus C.L. Koch, 1836 (Scorpiones, Buthidae) from the State of Maranhao in Brazil. Boletin de la SEA. 2006;38:117–120. [Google Scholar]
- Lourenco WR. Scorpion diversity and endemism in the Rio Negro region of Brazilian Amazonia, with the description of two new species of Tityus C. L. KOCH (Scorpiones, Buthidae) Amazoniana. 2005;18(3-4):203–213. [Google Scholar]
- Kury AB, Chagas-Jr A, Giupponi APL, Gonzalez AP. Amblypygi, Opiliones, Schizomida, Scorpiones and Chilopoda, Tocantins, Brazil. Check List. 2010;6(4):564–571. [Google Scholar]
- Martins MA, Barradas L, Silva RHVd, Pardal PPdO. Clinical and epidemiological study of accidents by scorpions attended in João de Barros Barreto Universitarian Hospital from January to December. Rev Para Med. 2002;16(1):34–38. [Google Scholar]
- Lourenco WR, Leguin EA, Cloudsley-Thompson JL. The status of Tityus (Atreus) rufofuscus Pocock, 1897 (Scorpiones, Buthidae), an enigmatic scorpion described from Brazil. Boletin de la SEA. 2009;45:423–427. [Google Scholar]
- Brasil - SVS/MS. Secretaria de Vigilância em Saúde/Ministério da Saúde Acidentes por animais peçonhentos - Escorpião. [2021 Mar 19]. Available from: ht tps://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z-1/a/acidentes-por-animais-peconhentos-o-que-fazer-e-como-evitar.
- Gomes JV, Fé NF, Santos HLR, Jung B, Bisneto PF, Sachett A, Moura VM, Mendonça I, Melo GC, Pardal PPO, Lacerda M, Sampaio V, Fan HW, Sachett JAG, Monteiro WM. Clinical profile of confirmed scorpion stings in a referral center in Manaus, Western Brazilian Amazon. Toxicon. 2020;187:245–254. doi: 10.1016/j.toxicon.2020.09.012. [DOI] [PubMed] [Google Scholar]
- Torrez PPQ, Quiroga MMM, Abati PAM, Mascheretti M, Costa WS, Campos LP, et al. Acute cerebellar dysfunction with neuromuscular manifestations after scorpionism presumably caused by Tityus obscurus in Santarem, Para/Brazil. Toxicon. 2015;96(1):68–73. doi: 10.1016/j.toxicon.2014.12.012. [DOI] [PubMed] [Google Scholar]
- AdP Santos-da-Silva, Candido DM, Nencioni ALA, Kimura LF, Prezotto-Neto JP, Barbaro KC, Chalkidis HM, Dorce VAC. Some pharmacological effects of Tityus obscurus venom in rats and mice. Toxicon. 2017;126:51–58. doi: 10.1016/j.toxicon.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Lourenço WR. Diversity of the scorpion fauna from Amazonia - centers of endemism - new support for the theory of Pleistocene forest refuges. Amazoniana. 1986;9(4):559–580. [Google Scholar]
- Silva EPd, Monteiro WM, Bernarde PS. Scorpion stings and spider bites in the Upper Juruá, Acre-Brazil. J Hum Growth Dev. 2018;28(3):290–297. [Google Scholar]
- Pierini SV, Warrell DA, dePaulo A, Theakston RDG. High incidence of bites and stings by snakes and other animals among rubber tappers and Amazonian Indians of the Jurua Valley, Acre State, Brazil. Toxicon. 1996;34(2):225–236. doi: 10.1016/0041-0101(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Maestri A, Neto, Guedes AB, SdF Carmo, HdM Chalkidis, Souza Coelho J, Pardal PPO. Aspects of scorpionism in the state of Pará-Brazil. Rev Para Med. 2008;22(1):49–55. [Google Scholar]
- Lourenco WR, Cuellar O. A new all-female scorpion and the first probable case of arrhenotoky in scorpions. J Arachnol. 1999;27(1):149–153. [Google Scholar]
- Lourenco WR. Scorpion biogeographic patterns as evidence for a Neblina-Sao Gabriel endemic center in Brazilian Amazonia. Rev Acad Colomb Cienc Exact Fis Nat. 1994;19(72):181–185. [Google Scholar]
- Brasil - SVS/MS Secretaria de Vigilância em Saúde/Ministério da Saúde Acidentes de trabalho por animais peçonhentos entre trabalhadores do campo, floresta e águas, Brasil 2007 a 2017. [2021 Mar 19];Bol Epidemiol. 2019 50(11):1–14. Available from: https://portalarquivos2.saude.gov.br/images/pdf/2019/marco/29/2018-059.pdf. [Google Scholar]
- Spirandeli-Cruz E, Winther-Yassuda C, Jim J, Barraviera B. The program for controlling the scorpion Tityus serrulatus, Lutz and Mello 1922, in Aparecida, São Paulo state, Brazil (Scorpiones, Buthidae) J Venom Anim Toxins incl Trop Dis. 1999;5(1):119. https://www.scielo.br/j/jvat/a/6HhJhXCmbZJNLjrLDfWKKmR/?lang=en [Google Scholar]
- Pardal PPO, dos Santos PRSG, da Silva Cardoso B, da Silva Lima RJ, da Costa Gadelha MA. Spatial distribution of envenomation by scorpions in Pará state, Brazil. J Trop Pathol. 2017;46(1):94–104. [Google Scholar]
- Hammoudi-Triki D, Lefort J, Rougeot C, Robbe-Vincent A, Bon C, Laraba-Djebari F, Choumet V. Toxicokinetic and toxicodynamic analyses of Androctonus australis hector venom in rats: Optimization of antivenom therapy. Toxicol Appl Pharmacol. 2007;218(3):205–214. doi: 10.1016/j.taap.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Pardal PPO, Ishikawa EAY, Vieira JLF. Contribution to the knowledge of Tityus obscurus Gervais, 1843 (Scorpiones, Buthidae) and scorpionism in two areas of Para State, in the Brazilian Amazon. Rev Pan-Amaz Saude. 2014;5(3):73–74. [Google Scholar]
- Reis MB, Rodrigues FL, Lautherbach N, Kanashiro A, Sorgi CA, Meirelles AFG, Silva CAA, Zoccal KF, Souza COS, Ramos SG, Matsuno AK, Rocha LB, Salgado HC, Navegantes LCC, Kettelhut IC, Cupo P, Gardinassi LG, Faccioli LH. Interleukin-1 receptor-induced PGE2 production controls acetylcholine-mediated cardiac dysfunction and mortality during scorpion envenomation. Nat Commun. 2020;11(1):5433. doi: 10.1038/s41467-020-19232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal KF, Sorgi CA, Hori JI, Paula-Silva FWG, Arantes EC, Serezani CH, Zamboni DS, Faccioli LH. Opposing roles of LTB4 and PGE2 in regulating the inflammasome-dependent scorpion venom-induced mortality. Nat Commun. 2016;7:10760. doi: 10.1038/ncomms10760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis MB, Zoccal KF, Gardinassi LG, Faccioli LH. Scorpion envenomation and inflammation: Beyond neurotoxic effects. Toxicon. 2019;167:174–179. doi: 10.1016/j.toxicon.2019.06.219. [DOI] [PubMed] [Google Scholar]
- Wen FH, Monteiro WM, Scheidt JF, Andrade L, Ye J, Staton CA, Gerardo CJ, Vissoci JRN. Geographical distribution and health care disparities of scorpion stings in Brazil. Toxicon. 2020;182:S24–S25. [Google Scholar]
- Revelo MP, Bambirra EA, Ferreira AP, Diniz CR, ChavezOlortegui C. Body distribution of Tityus serrulatus scorpion venom in mice and effects of scorpion antivenom. Toxicon. 1996;34(10):1119–1125. doi: 10.1016/0041-0101(96)00074-8. [DOI] [PubMed] [Google Scholar]
- Wen FH. Why is so difficult to reduce the burden of scorpionism in Brazil? Toxicon. 2019;168:S8 [Google Scholar]
- Lourenço WR, Leguin E-A. The true identity of Scorpio (Atreus) obscurus Gervais, 1843 (Scorpiones, Buthidae) Euscorpius. 2008;2008(75):1–9. [Google Scholar]
- Iserson KV, Ramcharran SDJ. Black scorpion (Tityus obscurus) fatalities in Guyana and a literature review. J Emerg Med. 2019;57(4):554–559. doi: 10.1016/j.jemermed.2019.07.018. [DOI] [PubMed] [Google Scholar]
- Dias NB, de Souza BM, Cocchi FK, Chalkidis HM, Dorcec VAC, Palma MS. Profiling the short, linear, non-disulfide bond-containing peptidome from the venom of the scorpion Tityus obscurus. J Proteom. 2018;170:70–79. doi: 10.1016/j.jprot.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Ireno IC.Partial peptidomics of the venom from the Amazon scorpion Brotheas amazonicus dissertation Belo Horizonte, MG: Belo Horizonte, MG: Universidade Federal de Minas Gerais; 2009 [Google Scholar]
- Amaro I, Riano-Umbarila L, Becerril B, Possani LD. Isolation and characterization of a human antibody fragment specific for Ts1 toxin from Tityus serrulatus scorpion. Immunol Lett. 2011;139(1-2):73–79. doi: 10.1016/j.imlet.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Martin-Eauclaire MF, Adi-Bessalem S, Hammoudi-Triki D, Laraba-Djebari F, Bougis PE. Serotherapy against voltage-gated sodium channel-targeting α -toxins from Androctonus scorpion venom. Toxins. 2019;11(2):63. doi: 10.3390/toxins11020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges A, Lomonte B, Angulo Y, Acosta de Patino H, Pascale JM, Otero R, Miranda RJ, de Sousa L, Grahan MR, Gómez A, Pardal PPO, Ishikawa E, Bonilla F, Castillo A, de Ávila RAM, Gómez JP, Caro-López JA. Venom diversity in the Neotropical scorpion genus Tityus: Implications for antivenom design emerging from molecular and immunochemical analyses across endemic areas of scorpionism. Acta Trop. 2020;204:105346. doi: 10.1016/j.actatropica.2020.105346. [DOI] [PubMed] [Google Scholar]
- Jacoby DB, Dyskin E, Yalcin M, Kesavan K, Dahlberg W, Ratliff J, Johnson EW, Mousa SA. Potent pleiotropic anti-angiogenic effects of TM601, a synthetic chlorotoxin peptide. Anticancer Res. 2010;30(1):39–46. [PubMed] [Google Scholar]
- DeBin JA, Maggio JE, Strichartz GR. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am J Physiol. 1993;264(2):C361–C3C9. doi: 10.1152/ajpcell.1993.264.2.C361. [DOI] [PubMed] [Google Scholar]
- Cohen-Inbar O, Zaaroor M. Glioblastoma multiforme targeted therapy: The Chlorotoxin story. J Clin Neurosci. 2016;33:52–58. doi: 10.1016/j.jocn.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Song YM, Tang XX, Chen XG, Gao BB, Gao E, Bai L, Lv XR. Effects of scorpion venom bioactive polypolypeptides on platelet aggregation and thrombosis and plasma 6-keto-PG F1alpha and TXB2 in rabbits and rats. Toxicon. 2005;46(2):230–235. doi: 10.1016/j.toxicon.2005.04.012. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Mendes BBR, Horta CCR, do Carmo AO, Biscoto GL, Sales-Medina DF, Leal HG, Brandão-Dias PFP, Miranda SEM, Aguiar CJ, Cardoso VN, Barros ALB, Chávez-Olortégui C, Leite MF, Kalapothakis E. CPP-Ts: a new intracellular calcium channel modulator and a promising tool for drug delivery in cancer cells. Sci Rep. 2018;8(14739) doi: 10.1038/s41598-018-33133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JVL, Emrick JJ, Kelly MJS, Herzig V, King GF, Medzihradszky KF, Julius D. A cell-penetrating scorpion toxin enables mode-specific modulation of TRPA1 and pain. Cell. 2019;178(6):1362–1374.e16. doi: 10.1016/j.cell.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira IS, Ferreira IG, Alexandre-Silva GM, Cerni FA, Cremonez CM, Arantes EC, Zottich U, Pucca MB. Scorpion toxins targeting Kv1.3 channels: insights into immunosuppression. J Venom Anim Toxins incl Trop Dis. 2019;25:e148118. doi: 10.1590/1678-9199-JVATITD-1481-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Huang J, Yuan X, Peng B, Liu W, Han S, He X. Toxins targeting the Kv1.3 channel: Potential immunomodulators for autoimmune diseases. Toxins (Basel) 2015;7(5):1749–1764. doi: 10.3390/toxins7051749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourão CBF. New class of serinopeptidase inhibitor present in Tityus obscurus scorpion venom. Brasília, DF: Brasília, DF: Universidade de Brasília; 2016. thesis. [Google Scholar]
- Higa A, das Dores Noronha M, López-Lozano JL, editors. Degradation of Aα and Bβ chains from bovine fibrinogen by serine proteases of the Amazonian scorpion Brotheas amazonicus. BMC proceedings. 2014;8(4):12 [Google Scholar]
- Guilhelmelli F, Vilela N, Smidt KS, de Oliveira MA, AdC Morales Alvares, Rigonatto MCL, Costa PHS, Tavares AH, Freitas SM, Nicola AM, Franco OL, Derengowski LS, Schwartz EF, Mortari MR, Bocca AL, Albuquerque P, Silva-Pereira I. Activity of scorpion venom-derived antifungal peptides against planktonic cells of Candida spp. and Cryptococcus neoformans and Candida albicans biofilms. Front Microbiol. 2016;7:1844. doi: 10.3389/fmicb.2016.01844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Mata ECG, Ombredane A, Joanitti GA, Kanzaki LIB, Schwartz EF. Antiretroviral and cytotoxic activities of Tityus obscurus synthetic peptide. Arch Pharm. 2020;353(11):e2000151. doi: 10.1002/ardp.202000151. [DOI] [PubMed] [Google Scholar]
- Bordon KCF, Wiezel GA, Amorim FG, Arantes EC. Arthropod venom hyaluronidases: biochemical properties and potential applications in medicine and biotechnology. J Venom Anim Toxins incl Trop Dis. 2015;21:43. doi: 10.1186/s40409-015-0042-7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt CS, Pereira PA, Ramos SG, Sampaio SV, Arantes EC, Aronoff DM, Faccioli LH. Hyaluronidase recruits mesenchymal-like cells to the lung and ameliorates fibrosis. Fibrogenesis Tissue Repair. 2011;4(1):3. doi: 10.1186/1755-1536-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira-Mendes BBR, Miranda SEM, Sales-Medina DF, Magalhães BF, Kalapothakis Y, Souza RP, Cardoso VN, Barros ALB, Guerra-Duarte C, Kalapothakis E, Horta CCR. Inhibition of Tityus serrulatus venom hyaluronidase affects venom biodistribution. PLoS Negl Trop Dis. 2019;13(4):e0007048. doi: 10.1371/journal.pntd.0007048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta CCR, BdF Magalhaes, Ribeiro Oliveira-Mendes BB, do Carmo AO, Duarte CG, Felicori LF, Machado-de-Avila RA, Chavez-Olórtegui C, Kalapothakis E. Molecular, immunological, and biological characterization of Tityus serrulatus venom hyaluronidase: New insights into its Role in envenomation. PLoS Negl Trop Dis. 2014;8(2):e2693. doi: 10.1371/journal.pntd.0002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Hooper L, Loke YK. Publication bias: what is it? How do we measure it? How do we avoid it? Open Access J Clin Trials. 2013;5(1):71–81. [Google Scholar]
- Rohrer JM. Thinking clearly about correlations and causation: Graphical causal models for observational data. Adv Methods Pract Psychol Sci. 2018;1(1):27–42. [Google Scholar]
- Brasil. SVS/MS - Secretaria de Vigilância em Saúde/Ministério da Saúde Casos de acidentes por escorpiões. Brasil, Grandes Regiões e Unidades Federadas. 2000 a 2018. [2021 Mar 19]. Available from: https://antigo.saude.gov.br/images/pdf/2019/outubro/23/Dados-Epidemiologicos-SiteSVS--Setembro-2019-ESCORPI--O-CASOS.pdf.
- Candido DM, Lucas S. Maintenance of scorpions of the genus Tityus Koch (Scorpiones, Buthidae) for venom obtention at Instituto Butantan, Sao Paulo, Brazil. J Venom Anim Toxins incl Trop Dis. 2004;10(1):86–97. doi: 10.1590/S1678-91992004000100007. [DOI] [Google Scholar]
- Favreau P, Menin L, Michalet S, Perret F, Cheyneval O, Stocklin M, Bulet P, Stocklin R. Mass spectrometry strategies for venom mapping and peptide sequencing from crude venoms: Case applications with single arthropod specimen. Toxicon. 2006;47(6):676–687. doi: 10.1016/j.toxicon.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Albuquerque P, Paiva J, Martins AMC, Meneses GC, da Silva GB, Buckley N, Daher EF. Clinical assessment and pathophysiology of Bothrops venom-related acute kidney injury: a scoping review. J Venom Anim Toxins incl Trop Dis. 2020;26:e20190076. doi: 10.1590/1678-9199-jvatitd-2019-0076.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freires IA, de Cassia Orlandi Sardi J, de Castro RD, Rosalen PL. Alternative animal and non-animal models for drug discovery and development: bonus or burden? Pharm Res. 2017;34(4):681–686. doi: 10.1007/s11095-016-2069-z. [DOI] [PubMed] [Google Scholar]
- Pessini AC, Takao TT, Cavalheiro EC, Vichnewski W, Sampaio SV, Giglio JR, Arantes EC. A hyaluronidase from Tityus serrulatus scorpion venom: isolation, characterization and inhibition by flavonoids. Toxicon. 2001;39(10):1495–1504. doi: 10.1016/s0041-0101(01)00122-2. [DOI] [PubMed] [Google Scholar]
- Lima M, Bitencourt MAO, Furtado AA, Torres-Rego M, Siqueira EMD, Oliveira RM, Rocha HAO, Rocha KBF, Silva-Júnior AA, Zucolotto SM, Fernandes-Pedrosa MF. Aspidosperma pyrifolium has anti-inflammatory properties: an experimental study in mice with peritonitis induced by Tityus serrulatus venom or carrageenan. Int J Mol Sci. 2017;18(11):2228. doi: 10.3390/ijms18112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt MAO, Lima M, Torres-Rego M, Fernandes JM, da Silva AA, Tambourgi DV, Zucolotto SM, Fernandes-Pedrosa MF. Neutralizing effects of Mimosa tenuiflora extracts against inflammation caused by Tityus serrulatus scorpion venom. Biomed Res Int. 2014;2014:378235. doi: 10.1155/2014/378235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(1):S31–S34. doi: 10.4103/sja.SJA_543_18. [DOI] [PMC free article] [PubMed] [Google Scholar]