Abstract
Purpose of review
Antimicrobial resistance (AMR) in bacteria poses a major risk to global public health, with many factors contributing to the observed increase in AMR. International travel is one recognized contributor. The purpose of this review is to summarize current knowledge regarding the acquisition, carriage and spread of AMR bacteria by international travelers.
Recent findings
Recent studies have highlighted that travel is an important risk factor for the acquisition of AMR bacteria, with approximately 30% of studied travelers returning with an acquired AMR bacterium. Epidemiological studies have shown there are three major risk factors for acquisition: travel destination, antimicrobial usage and travelers’ diarrhea (TD). Analyses have begun to illustrate the AMR genes that are acquired and spread by travelers, risk factors for acquisition and carriage of AMR bacteria, and local transmission of imported AMR organisms.
Summary
International travel is a contributor to the acquisition and dissemination of AMR organisms globally. Efforts to reduce the burden of AMR organisms should include a focus on international travelers. Routine genomic surveillance would further elucidate the role of international travel in the global spread of AMR bacteria.
Keywords: antimicrobial resistant bacteria, risk factors, travel
INTRODUCTION
Antimicrobial resistance (AMR) is a global public health threat, and its frequency is increasing [1–3]. AMR is directly correlated with antimicrobial usage, and its spread is driven by the transfer of resistant organisms and resistance genes between humans, other animals and the environment [4]. Recent models have suggested that without intervention, deaths attributable to AMR may reach 10 million by 2050, with disproportionate effects on individuals in low and middle-income countries (LMIC) [1,2]. This problem is compounded by the lack of novel antimicrobials and vaccines for many bacterial infections, and by high and inappropriate antimicrobial usage in many parts of the world, driving variable geographic patterns of AMR [1,3] The heavy use of antimicrobials for livestock, aquaculture and in routine clinical practice, and the environmental accumulation of antimicrobials through runoff, are widely recognized as important contributing factors in the rise of AMR [5–16]. As a result, there has been a steady increase in resistance, especially among gram-negative bacteria, to important classes of antimicrobials, including beta-lactams, fluoroquinolones and polymyxins [8,17,18]. The carriage of extended spectrum beta-lactamase (ESBL)-producing bacteria among healthy individuals has increased by 5% each year between 1978 and 2015 [19].
International travel has been identified as playing a role in the spread of resistance genes and organisms [12,20,21▪,22▪]. The ongoing COVID-19 global pandemic has highlighted the ease with which infectious diseases can be disseminated by international travel. Here, we review recent published data regarding the acquisition of AMR bacteria by international travelers and their role in the global spread of AMR.
Box 1.
no caption available
TRAVELER PATTERNS
Except for the slowdown associated with the COVID-19 pandemic, international travel has been steadily increasing for decades. In 2019, there were 1.46 billion international tourist arrivals, representing an increase of 4% from the previous year [23]. This overall increase in travel has been paired with a concomitant increase in travel to and between countries with vulnerable health systems and inadequate public health infrastructure, as assessed by the Fragile States Index (FSI) [24▪]. The FSI categorizes countries as Sustainable, Stable, Warning or Alert, and only countries listed as Sustainable are considered resilient to infectious disease threats [24▪,25]. Currently, 84% of the world's population lives in Warning or Alert countries, and the greatest increase in travel since 2010 has been between Warning countries, with outbound travel from Warning countries increasing by 326 765 passengers per year [24▪]. The widespread global presence of vulnerable health systems potentiates the risks of travel-associated AMR.
ANTIMICROBIAL RESISTANCE IN TRAVELERS
Most studies of AMR in travelers have been small convenience cohorts. To date, a small set of published studies have enrolled a sizable number (i.e. more than 100) of international travelers and have systematically characterized the acquisition of AMR bacteria in these cohorts (Table 1). All have focused on the acquisition of ESBL-producing gram-negative bacteria. More recent studies have assessed the presence of additional resistance genes, including those associated with carbapenem resistance and mcr-mediated colistin resistance. Here, we summarize the findings of seven large studies that have conducted recent analyses (Table 1).
Table 1.
Recent large prospective studies of antimicrobial resistance in international travelers
| Study | Study years | Number of participants (Country of departure) | Top three travel regions | Resistance profiles phenotyped by selective growth | AMR genes assayed by genotyping | Resistance profiles found (genes found by genotyping) | Publications |
| Kantele et al. | 2008–2010 | 430 (Finland) | Sub-Saharan Africa, South East Asia, South Asia | ESBL-P, CP-CR | ESBL-P | [21▪,26,27▪,49,55▪▪,82] | |
| COMBAT | 2012–2013 | 2001 (Netherlands) | East Africa, South Asia, South East Asia | ESBL-P | blaTEM; blaSHV; blaCTX-M; mcr-1; CP-CRE by microarray | ESBL-P, CP-CR (blaTEM; blaSHV; blaCTX-M; mcr-1) | [28,29▪,30,31,52] |
| VOYAG-R | 2012–2013 | 574 (France) | Asia, sub-Saharan Africa, Latin America | ESBL-P, CP-CR, pAmpC production (cefoxitin resistance) | blaCTX-M; blaTEM; blaSHV; blaVEB; pAmpc; blaKPC; blaVIM; blaIMP; blaNDM; blaOXA-48; blaOXA-181 | ESBL-P, CP-CR, pAmpC (blaCTX-M; blaNDM;blaOXA-48) | [32,33,83] |
| Meurs et al. | 2016–2017 | 230 (Germany) | South East Asia, East Africa, South America | ESBL-P | blaCTX-M; blaVIM; blaIMP; blaNDM; blaKPC; blaOXA-48 | ESBL-P | [35▪▪] |
| Tufic-Garutti et al. | 2015–2019 | 210 (Brazil) | Sub-Saharan Africa, South America | ESBL-P, CP-CR | blaTEM; blaGES; blaSHV; blaCTX-M; blaGIM; blaIMP; blaVIM; blaSIM; blaSPM; blaNDM; blaOXA; blaGES; blaKPC; pAmpC; blaMOX; blaCMY; blaLAT; blaDHA; blaACC; blaMIR; blaFOX; PMQR genes (qnrD, qnrVC, qnrC, qnrB, qnrS, qnrA, aac(6’)-Ib, qepA); mcr genes | ESBL-P, CP-CR; mcr genes; PMQR genes | [41▪▪] |
| Dao et al. | 2017–2019 | 382 (France) | South East Asia, Africa, South Asia | ESBL-P, CP-CR | blaTEM; blaSHV; blaCTX-M; blaOXA48; blaNDM; blaVIM; blaIMP; blaKPC; mcr genes (1–6, 8) | ESBL-P, CP-CR, MCR (blaCTX-M; blaTEM; blaSHV; mcr genes (1, 3, 8)) | [37▪▪,38▪▪,40] |
| GTEN | 2017–2019 | 608 (United States) | South Asia, South Africa, South America | ESBL-P, CP-CR, MCR | blaNDM; blaCTX-M; mcr genes; aac(3)-IIa; qnrS | ESBL-P, CP-CR, MCR (blaNDM; blaCTX-M; mcr genes; aac(3)-IIa; qnrS) | [22▪,42▪,44▪] |
ESBL-P, CP-CR, pAmpC production, and MCR were the only resistance profiles phenotypically measured; ESBL-P, extended-spectrum beta-lactamase-producing; CP-CR, carbapenemase-producing carbapenem-resistant; MCR, mcr-mediated colistin-resistant; PMQR, plasmid-mediated quinolone resistance.
Kantele et al.[26] performed a prospective study of pre and posttravel stool samples from 430 Finnish travelers between 2008 and 2010 at a travel clinic in Helsinki. Samples were screened for ESBL and carbapenemase-producing Enterobacterales (ESBL-PE and CPE, respectively). Sub-Saharan Africa was the top destination, followed by South East and South Asia. Twenty-one percent of travelers acquired ESBL-PE, the majority of which were E. coli[27▪]. No carbapenemase-producing Enterobacterales were found [21▪,26]. Of the travelers who acquired ESBL-PE, none had longitudinal carriage after one year.
The Carriage of Multiresistant Bacteria After Travel (COMBAT) study was a longitudinal multicentre cohort study that prospectively sampled stool from 2001 Dutch travelers and their nontravelling household members from November 2012 to November 2013 to investigate the acquisition of AMR Enterobacterales[28]. The most visited destinations by travelers were South East Asia, Eastern Africa and South Asia [28]. Thirty-four percent of travelers acquired ESBL-PE, mostly E. coli. A recent cross-sectional analysis of pre and posttravel ESBL-PE acquisition within the COMBAT cohort found that there were significant levels of coresistance to other antimicrobials found in ESBL-PE from returning travelers compared with ESBL-PE isolated from pretravel samples [29▪]. However, carbapenem resistance was not identified in these returning travelers. The COMBAT study also found 8% onward transmission from ESBL-PE positive returning travelers to ESBL-PE negative household members [30,31].
The VOYAG-R study prospectively followed 574 French international travelers between 2012 and 2013 longitudinally up to 12 months, sampling stool pretravel and posttravel at 1, 2, 3, 6 and 12 months for ESBL-PE, CPE and plasmid-mediated AmpC (pAmpC)-type cephalosporinases [32,33]. The top travel regions were Asia, sub-Saharan Africa and Latin America, and 51% of travelers acquired an AMR organism, 92% of which were ESBL-PE, and which were predominantly E. coli. There was independent and concomitant pAmpC production, and three instances of CPE. There was a 16% carriage rate of travel-acquired bacteria after 1 month, which halved over each subsequent follow-up interval. A more recent analysis of 43 participants in the VOYAG-R cohort investigated microbiota composition pre and posttravel, finding that diarrhea during travel was associated with a higher abundance of Prevotella copri but lower overall microbiome species richness [34▪].
Meurs et al.[35▪▪] conducted a prospective cohort study of 230 German international travelers visiting LMIC between 2016 and 2017, assessing pre and posttravel stool for ESBL-PE. The primary regional destinations were South East Asia, South America, and East Africa [35▪▪]. Twenty-three percent of returning travelers were colonized with ESBL-PE, of which 92% were E. coli. Of the ESBL-PE positive returning travelers, 42 were tested for carriage at 6 months posttravel; 17% remained colonized with ESBL-PE [35▪▪]. This study followed an earlier prospective cohort study of 205 German international travelers through the same travel clinic in 2013–2014, which found a 33% acquisition rate of ESBL-PE and no CPE; 97% of ESBL-PE were E. coli[36].
Dao et al.[37▪▪,38▪▪,39,40] conducted a prospective study of 382 French medical students interning internationally between 2017 and 2019 to assess acquisition of respiratory, enteric and vaginal pathogens. The top three destinations were South East Asia, Africa and South Asia. Twenty-nine percent of travelers acquired ESBL-PE, most of which were E. coli. A small proportion of acquired organisms were CPE (3%), and mcr genes were detected by PCR in 7% of returning participants [37▪▪,38▪▪].
Tufic-Garutti et al.[41▪▪] screened pre and posttravel stool samples of 210 Brazilian international travelers between 2015 and 2019, for ESBL-PE and CPE. Brazilian travelers most frequently traveled to sub-Saharan Africa and South America. Interestingly, over the study period, there was a 5% annual increase in pretravel carriage of ESBL-PE. Twenty-two percent of travelers acquired ESBL-PE; the majority of organisms were E. coli[41▪▪]. Within the ESBL-PE isolates, there was considerable concomitant resistance to other antimicrobials, including several observed instances of trimethoprim-sulfamethoxazole and quinolone resistance determinants [41▪▪].
More recently, the Global TravEpiNet (GTEN) study prospectively screened stool of 608 U.S. international travelers for ESBL-PE, CPE and mcr-mediated colistin-resistant Enterobacterales (MCRE) before and after travel from November 2017 to April 2019 [22▪,42▪–44▪]. Travelers most commonly visited India, South Africa and Peru. Thirty-eight percent of returning travelers acquired ESBL-PE, of which most were E. coli; CPE and MCRE were also detected independently and concomitantly with ESBL-PE [22▪,42▪]. There was a 30% carriage rate of acquired ESBL-PE at 3 months posttravel, and 10% at 1-year posttravel [22▪].
Overall, although there are some differences between these traveler cohort studies, there are clear trends indicating that ESBL-PE acquisition occurs in approximately 30% of travelers. The most recent studies have also observed acquisition of CPE and MCRE. These findings suggest continued evolution of AMR and an increase in circulating resistance genes, which may be readily acquired and transmitted by travelers.
RISK FACTORS
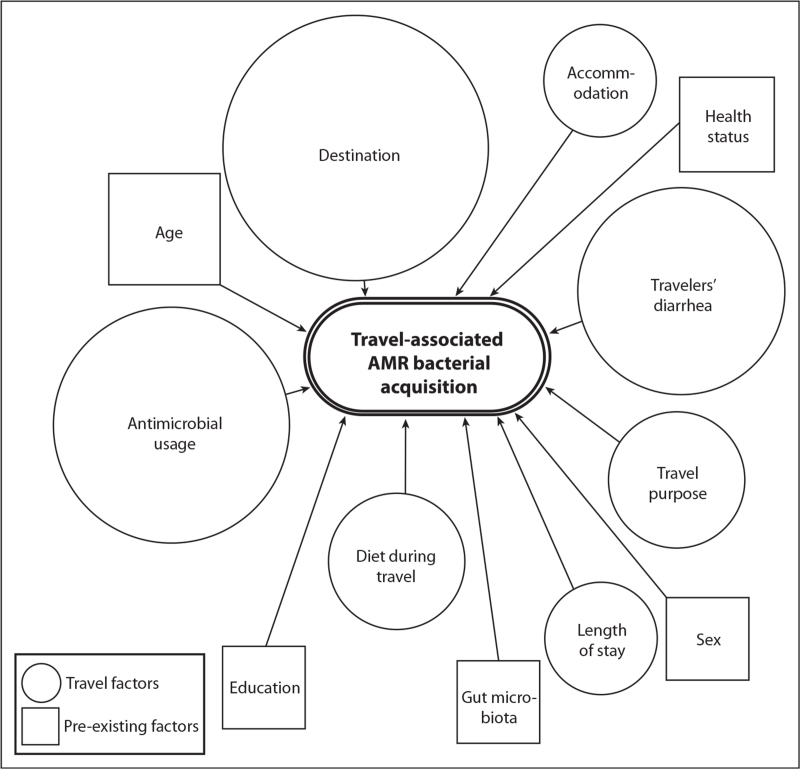
Antimicrobial usage, travelers’ diarrhea and destination have been repeatedly identified as risk factors for AMR bacterial acquisition during travel (Fig. 1) [22▪,26,29▪,37▪▪,41▪▪,45▪].
FIGURE 1.
Risk factors for the acquisition of travel-associated antimicrobial resistant bacteria. The three risk factors most commonly identified are destination, antimicrobial usage during travel and the occurrence of travelers’ diarrhea. Risk factors have been distinguished by type (shape) and relative importance (size). The size of the shapes is a rough estimation of the relative importance of risk factors, based on the number of analyzed studies in which the risk factor was identified. The most common risk factor (destination) was identified in 13 out of 15 analyzed studies [22▪,26,28,29▪,30,32,35▪▪,36,37▪▪,38▪▪,41▪▪,50▪▪,55▪▪,56▪,81▪].
Antimicrobial usage
International travelers who visit travel clinics are frequently prescribed and consume antimicrobials. In 121 295 pretravel consultations at GTEN sites between 2009 and 2018, antimicrobials were prescribed 78% of the time; azithromycin (41%) and fluoroquinolones (35%) were prescribed most frequently [46▪]. An analysis of pretravel colonization by ESBL-PE within the COMBAT cohort found that antimicrobial usage in the three months pretravel was most predictive for ESBL-PE acquisition, and beta-lactam usage correlated most closely with the acquisition of ESBL-PE [29▪]. A meta-analysis of multidrug-resistant Enterobacterales acquisition risk factors found that during travel, the leading risk factor was antimicrobial consumption with an odds ratio of 2.38 [95% confidence interval (95% CI) 1.88–3.0] [47▪]. There was similarly an association between antimicrobial usage and multidrug-resistant Enterobacterales acquisition, and resistance was correlated with the antimicrobial used [41▪▪]. For instance, ciprofloxacin-resistance was found in 29% of Brazilian travelers who consumed ciprofloxacin compared with 11% who did not, and similar rates have been found in other cohorts [22▪,26,41▪▪].
Travel destination
Although E. coli are the most commonly acquired organisms in travelers, the resistance differs geographically. South East Asia, South Asia and North Africa have been identified as the riskiest destinations for AMR acquisition, and many of the travelers surveyed across studies visited one of these regions (Table 1) [22▪,30,35▪▪,37▪▪,48,49,50▪▪,51▪]. In addition, there is country-to-country variation in risk within a travel region; for example, travel to India and Vietnam within South and South East Asia, respectively, appears to be higher-risk than other regional destinations [37▪▪]. Multiple studies have found that acquisition of mcr-mediated colistin-resistant organisms is highest following travel to Peru, although acquisition of mcr genes have also been reported from travelers to Vietnam, China and other destinations [20,22▪,37▪▪,41▪▪,52–54]. A study of 20 European travelers to Laos in 2015 found that 70% of participants were colonized at the end of the study. All participants had at least transiently acquired ESBL-PE, the majority of which were E. coli, and 28% of isolates had plasmid-mediated colistin resistance [45▪]. Further evidence suggests that travel to Africa (other than Southern Africa) and South Asia increased risk of CTX-M group 1 acquisition; there appears to be a regional association with specific CTX genes encoding ESBL's [29▪].
Travelers’ diarrhea
Travelers’ diarrhea has repeatedly been identified as a risk factor for AMR bacterial acquisition. Kantele et al.[55▪▪] found that 68% of travelers in their Finnish cohort experienced travelers’ diarrhea, and there was greater acquisition of an AMR bacterium among those with travelers’ diarrhea than without, regardless of antimicrobial usage. This association between travelers’ diarrhea and AMR bacterial acquisition was also found in U.S. international travelers from the GTEN study, with travelers’ diarrhea and antimicrobial usage independently increasing the likelihood of AMR acquisition [22▪].
ENTERIC FEVER IN INTERNATIONAL TRAVELERS
Multidrug-resistant typhoidal and paratyphoid Salmonella enterica are a special concern with regard to international travel. Two recent studies have investigated travel-associated enteric fever. There were 68 reported cases of imported ESBL Salmonella Typhi and Salmonella Paratyphi in the UK between 2017 and 2020 [56▪]. The majority of cases were associated with travel to Pakistan, where there is an ongoing outbreak of extremely drug-resistant S. Typhi in the Sindh province [57,58]. A separate multisite study of travelers with enteric fever found that 65% of S. Typhi isolates were resistant to multiple classes of antimicrobials [50▪▪]. Of the infected travelers, 59% (522/888) required hospitalization; in children, the rate of hospitalization was 61%. Of the surveyed travelers with S. Typhi (70 travelers), only 10% had received a typhoid fever vaccine. Interestingly, typhoid fever was more common in travelers visiting friends and relatives, whereas paratyphoid fever was more common in leisure travelers [50▪▪].
AREAS IN NEED OF FURTHER INVESTIGATION
Many gaps remain in our understanding of travel-associated AMR. The breakdown in regular healthcare due to COVID-19 may have exacerbated AMR challenges, and this lends urgency to increased efforts to monitor and combat AMR [59]. Here, we highlight four areas in need of further investigation and development.
Genomic surveillance
Recent work has indicated there may be transmission of ESBL Enterobacterales from travelers to previously uncolonized household members, and further genomic investigations could confirm such links [30]. There has also been evidence of secondary transmission of Shigella spp. and E. coli in the Netherlands with genomic similarity to travel-associated clusters [60▪]. Many studies have tracked the spread of New Delhi metallo-beta-lactamase 1 (NDM-1) and more recently the dissemination of mcr-1, showing that these resistance elements have spread geographically and become endemic in some parts of the world [61–72]. One recent example of this is nearly identical plasmids with blaNDM-5 and qepA in E. coli found in Pakistan and Canada [73]. Existing efforts to follow AMR and specific pathogens include GeoSentinel, NARMS and PathogenWatch, and such efforts could be implemented more broadly [50▪▪,74–77]. New genomic surveillance efforts have been implemented during the COVID-19 pandemic, and prioritizing the development and support for such infrastructure is crucial for global AMR control efforts as well [78–80].
Role of the microbiome
Another gap in understanding is how the gut microbiome influences and is influenced by travel-associated AMR. ESBL Enterobacterales acquisition and carriage have been shown to be temporal and highly dynamic, but it is unclear what the ramifications of that are for gut microbial communities and risk of infection [45▪]. There is some evidence that Actinobacteria species richness pretravel lowers the risk of ESBL-E acquisition during travel, and the VOYAG-R study found that higher abundance of Prevotella species is correlated with during-travel diarrhea; further studies would be valuable in determining the relationship between the gut microbiome and travel-associated AMR acquisition, carriage and longitudinal gut health [34▪,81▪].
Broader sampling for antimicrobial resistant organisms
AMR analyses in travelers have largely been conducted from stool samples; however, there may be value in evaluating nasal, skin and vaginal samples, as there may be important organisms and resistance mechanisms missed by focusing solely on stool [37▪▪]. In addition, past traveler studies have been biased in sampling primarily European and American travelers. Analyzing more diverse populations and samples will provide greater insight into AMR patterns.
Clinical strategies and interventions
Travel-associated AMR has implications for clinical practice. Medical practitioners should be aware of travel history as a risk factor for AMR. Clinicians providing pretravel health consultation should also consider antimicrobial stewardship principles and be cautious about the prescription of broad-spectrum antimicrobials for empiric use by travelers. Recent analyses have shown that although the occurrence of travelers’ diarrhea cannot be controlled for, reducing the use of empiric antimicrobial treatment for travelers’ diarrhea may be useful for lowering the acquisition of travel-associated AMR bacteria [21▪,55▪▪,82].
CONCLUSION
Research over the past decade has shown that approximately 30% of international travelers acquire AMR bacteria during travel, indicating that international travel is one component of the global problem of AMR. Surveillance, antimicrobial stewardship in travelers and novel strategies to decrease AMR acquisition by international travelers are essential for mitigating the global spread of AMR.
Acknowledgements
None.
Financial support and sponsorship
S.S., S.E.T. and R.C.L. are supported by the US Centers for Disease Control and Prevention (U01 CK000490). J.B.H. is supported by the National Institutes of Health (R01AI137164 and R01AI099243).
This work was supported by the US Centers for Disease Control and Prevention (U01 CK000490) and the National Institutes of Health (R01AI137164 and R01AI099243).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2017; 3099:318–327. [DOI] [PubMed] [Google Scholar]
- 2. O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. 2016. Online. Available at: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf. [Google Scholar]
- 3. Antimicrobial resistance: global report on surveillance. World Health Organization 2014. Online. Available at: https://www.who.int/publications/i/item/9789241564748. [Google Scholar]
- 4.Da Costa PM, Loureiro L, Matos AJFF. Transfer of multidrug-resistant bacteria between intermingled ecological niches: the interface between humans, animals and the environment. Int J Environ Res Public Health 2013; 10:278–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haulisah NA, Hassan L, Bejo SK, et al. High levels of antibiotic resistance in isolates from diseased livestock. Front Vet Sci 2021; 8:652351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira AR, Paranhos AG, de Aquino SF, Silva SQ. Distribution of genetic elements associated with antibiotic resistance in treated and untreated animal husbandry waste and wastewater. Environ Sci Pollut Res Int 2021; 28:26380–26403. [DOI] [PubMed] [Google Scholar]
- 7.Schar D, Klein EY, Laxminarayan R, et al. Global trends in antimicrobial use in aquaculture. Sci Rep 2020; 10:21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anyanwu MU, Jaja IF, Nwobi OC. Occurrence and characteristics of mobile colistin resistance (Mcr) gene-containing isolates from the environment: a review. Int J Environ Res Public Health 2020; 17:1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharland M, Gandra S, Huttner B, et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use: the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect Dis 2019; 12:1278–1280. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization 2018: WHO Report on Surveillance of Antibiotic Consumption. 2018. Online. Available at: https://apps.who.int/iris/bitstream/handle/10665/277359/9789241514880-eng.pdf?sequence=1&isAllowed=y. [Google Scholar]
- 11.Hsia Y, Sharland M, Jackson C, et al. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: an analysis of sales data from 70 middle-income and high-income countries. Lancet Infect Dis 2019; 19:67–75. [DOI] [PubMed] [Google Scholar]
- 12.Hansen GT. Continuous evolution: perspective on the epidemiology of carbapenemase resistance among enterobacterales and other Gram-negative bacteria. Infect Dis Ther 2021; 10:75–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mughini-Gras L, Kooh P, Fravalo P, et al. Critical orientation in the jungle of currently available methods and types of data for source attribution of foodborne diseases. Front Microbiol 2019; 10:2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow LKM, Ghaly TM, Gillings MR. A survey of sub-inhibitory concentrations of antibiotics in the environment. J Environ Sci 2021; 99:21–27. [DOI] [PubMed] [Google Scholar]
- 15.Felis E, Kalka J, Sochacki A, et al. Antimicrobial pharmaceuticals in the aquatic environment: occurrence and environmental implications. Eur J Pharmacol 2020; 866:172813. [DOI] [PubMed] [Google Scholar]
- 16.Neher TP, Ma L, Moorman TB, et al. Seasonal variations in export of antibiotic resistance genes and bacteria in runoff from an agricultural watershed in Iowa. Sci Total Environ 2020; 738:140224. [DOI] [PubMed] [Google Scholar]
- 17.Khuntayaporn P, Kanathum P, Houngsaitong J, et al. Predominance of international clone 2 multidrug-resistant Acinetobacter baumannii clinical isolates in Thailand: a nationwide study. Ann Clin Microbiol Antimicrob 2021; 20:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bush NG, Diez-Santos I, Abbott LR, Maxwell A. Quinolones: mechanism, lethality and their contributions to antibiotic resistance. Molecules 2020; 25:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karanika S, Karantanos T, Arvanitis M, et al. Fecal colonization with extended-spectrum beta-lactamase-producing enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin Infect Dis 2016; 63:310–318. [DOI] [PubMed] [Google Scholar]
- 20.Hassan J, Kassem II. Audacious Hitchhikers: the role of travel and the International Food Trade in the global dissemination of mobile colistin-resistance (mcr) genes. Antibiotics (Basel) 2020; 9:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Kantele A, Laaveri T. Extended-spectrum beta-lactamase-producing strains among diarrheagenic Escherichia coli-prospective traveler study with literature review. J Travel Med 2021; 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study analyzes diarrheagenic E. coli found within Finnish international travelers, finding that 2.7% of DEC were ESBL-producing, and there was concomitant resistance to other antimicrobials.
- 22▪.Worby CJ, Earl AM, Turbett SE, et al. Acquisition and long-term carriage of multidrug-resistant organisms in US international travelers. Open Forum Infect Dis 2020; 7:ofaa543. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study analyses ESBL-PE, CRE and MCRE acquisition and longitudinal carriage amongst a recent large cohort of U.S. international travelers; 38% of travelers acquired ESBL-PE, and carriage was higher for ESBL-PE than for MCRE.
- 23. World Tourism Organization. International tourism highlights, 2019 ed. World Tourism Organization (UNWTO); 2019, Madrid. Online. Available at: [DOI] [Google Scholar]
- 24▪.Tuite AR, Bhatia D, Moineddin R, et al. Global trends in air travel: implications for connectivity and resilience to infectious disease threats. J Travel Med 2020; 27:1–8. [DOI] [PubMed] [Google Scholar]; This study uses data from the FSI to measure the ability of countries to respond to infectious disease threats.
- 25.Messner JJ, Haken N, Taft P, et al. Fragile States Index Annual Report 2019. Fund for Peace. Online. Available at: https://fragilestatesindex.org/2019/04/07/fragile-states-index-2019-annual-report/. [Google Scholar]
- 26.Kantele A, Lääveri T, Mero S, et al. Antimicrobials increase travelers’ risk of colonization by extended-spectrum betalactamase-producing enterobacteriaceae. Clin Infect Dis 2015; 60:837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪.Lääveri T, Antikainen J, Mero S, et al. Bacterial, viral and parasitic pathogens analysed by qPCR: findings from a prospective study of travellers’ diarrhoea. Travel Med Infect Dis 2021; 40:101957. [DOI] [PubMed] [Google Scholar]; This study uses multiplexed qPCR assays to interrogate pre and posttravel stool samples from 146 Finnish travelers for bacterial, viral and parasitic pathogens, finding that 70% of samples had bacterial pathogens. E. coli were the most commonly identified bacteria.
- 28.Arcilla MS, van Hattem JM, Bootsma MCJ, et al. The Carriage Of Multiresistant Bacteria After Travel (COMBAT) prospective cohort study: methodology and design. BMC Public Health 2014; 14:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪.Arcilla MS, van Hattem JM, Bootsma MCJ, et al. Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in a population of Dutch travellers: a cross-sectional study. Travel Med Infect Dis 2020; 33:101547. [DOI] [PubMed] [Google Scholar]; This study uses the 2008–2010 cohort of Finnish international travelers to identify that pretravel antimicrobial usage and prior travel are risk factors for ESBL-PE, and travel-associated ESBL-PE have greater concomitant AMR.
- 30.Arcilla MS, van Hattem JM, Haverkate MR, et al. Import and spread of extended-spectrum (-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis 2017; 17:78–85. [DOI] [PubMed] [Google Scholar]
- 31.van Hattem JM, Arcilla MS, Bootsma MC, et al. Prolonged carriage and potential onward transmission of carbapenemase-producing Enterobacteriaceae in Dutch travelers. Future Microbiol 2016; 11:857–864. [DOI] [PubMed] [Google Scholar]
- 32.Ruppé E, Armand-Lefèvre L, Estellat C, et al. High rate of acquisition but short duration of carriage of Multidrug-resistant enterobacteriaceae after travel to the tropics. Clin Infect Dis 2015; 61:593–600. [DOI] [PubMed] [Google Scholar]
- 33.Rondinaud E, Ruppé E, Matheron S, et al. Screening methods for intestinal carriage of multidrug-resistant Enterobacterales: interest of enrichment broth. Diagn Microbiol Infect Dis 2020; 97:115079. [DOI] [PubMed] [Google Scholar]
- 34▪.Leo S, Lazarevic V, Gaïa N, et al. The intestinal microbiota predisposes to traveler's diarrhea and to the carriage of multidrug-resistant Enterobacteriaceae after traveling to tropical regions. Gut Microbes 2019; 10:631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses 16S sequencing to evaluate gut microbiota composition pre and posttravel within the VOYAG-R study cohort, finding that higher Prevotella abundance before and after travel is associated with diarrhea during travel.
- 35▪▪.Meurs L, Lempp FS, Lippmann N, et al. Intestinal colonization with extended-spectrum beta-lactamase producing Enterobacterales (ESBL-PE) during long distance travel: a cohort study in a German travel clinic (2016–2017). Travel Med Infect Dis 2020; 33: [DOI] [PubMed] [Google Scholar]; This recent study investigates ESBL-PE and CPE acquisition in German international travelers visiting LMIC. Twenty-three percent of travelers acquired ESBL-PE, and there was longitudinal carriage following travel. They find that risk factors for acquisition of ESBL-PE are travel to Asia, age and accommodation type. This study follows from an earlier German traveller study with similar outcomes.
- 36.Lübbert C, Straube L, Stein C, et al. Colonization with extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacteriaceae in international travelers returning to Germany. Int J Med Microbiol 2015; 305:148–156. [DOI] [PubMed] [Google Scholar]
- 37▪▪.Dao TL, Hoang VT, Magmoun A, et al. Acquisition of multidrug-resistant bacteria and colistin resistance genes in French medical students on internships abroad. Travel Med Infect Dis 2021; 39:101940. [DOI] [PubMed] [Google Scholar]; This study identifies ESBL-PE and CPE acquired by French medical students traveling abroad, identifying destination, amongst others, as a significant risk factor for acquisition. Unique from other traveller studies, Dao et al.[37▪▪] screen for nasal and vaginal AMR bacteria in addition to gut-associated AMR bacteria.
- 38▪▪.Dao TL, Canard N, Hoang VT, et al. Risk factors for symptoms of infection and microbial carriage among French medical students abroad. Int J Infect Dis 2020; 100:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study looks at the acquisition of specific organisms in a cohort of French medical students traveling abroad. In particular, they discuss EAEC and EPEC acquisition. They also find that there are within-region differences in AMR genes and acquisition, and that association with orphanages is a significant risk factor.
- 39.Dao TL, Gautret P. Patterns of diseases in health students abroad: a systematic review. Travel Med Infect Dis 2021; 39:101944. [DOI] [PubMed] [Google Scholar]
- 40.Dao TL, Hoang VT, Ly TDA, et al. Infectious disease symptoms and microbial carriage among French medical students travelling abroad: a prospective study. Travel Med Infect Dis 2020; 34:101548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪▪.Tufic-Garutti SDS, Almeida Ramalho JV, Guilherme de Araújo Longo L, et al. Acquisition of antimicrobial resistance determinants in Enterobacterales by international travelers from a large urban setting in Brazil. Travel Med Infect Dis 2021; 196:3–16. [DOI] [PubMed] [Google Scholar]; This study is the first large prospective cohort study in an LMIC (Brazilian international travelers), finding 22% acquisition of ESBL-PE, with destination and antimicrobial usage to be important risk factors. They additionally find that the rate of community carriage of ESBL-PE increases by 11% over the 4-year study period.
- 42▪.Mellon G, Turbett SE, Worby C, et al. Acquisition of antibiotic-resistant bacteria by U.S. international travelers. N Engl J Med 2020; 382:1372–1374. [DOI] [PubMed] [Google Scholar]; This is the first large prospective cohort study of recent U.S. international travelers, which screened for ESBL-PE, CRE and MCRE acquisition in returning travelers.
- 43▪.Turbett SE, Becker M, Desrosiers L, et al. The effect of transport temperature and time on the recovery of antimicrobial-resistant Enterobacterales in stool. Diagn Microbiol Infect Dis 2021; 99:115210. [DOI] [PubMed] [Google Scholar]; This study demonstrates effective isolation and culture of ESBL-PE across a range of temperatures and transport conditions.
- 44▪.Turbett SE, Desrosiers L, Andrews-Dunleavey C, et al. Evaluation of a screening method for the detection of colistin-resistant enterobacteriaceae in Stool. Open Forum Infect Dis 2019; 6:ofz211. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates an effective method to detect mcr-mediated colistin resistance using spiked stool samples.
- 45▪.Kantele A, Kuenzli E, Dunn SJ, et al. Dynamics of intestinal multidrug-resistant bacteria colonisation contracted by visitors to a high-endemic setting: a prospective, daily, real-time sampling study. Lancet Microbe 2021; 5247:e151–e158. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study samples a small cohort of European travelers to Laos daily, finding that 70% of returning travelers carry an AMR bacterium, and all travelers transiently carried an AMR bacterium during travel.
- 46▪.Gandhi AR, Rao SR, Chen LH, et al. Prescribing patterns of antibiotics for the self-treatment of travelers’ diarrhea in global TravEpiNet, 2009–2018. Open Forum Infect Dis 2020; 7:ofaa376–ofaa1376. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study analyses antimicrobial prescriptions for travelers’ diarrhea in international travelers who visited travel clinics, finding prescription occurred in 75% of visits but an overall decrease over time.
- 47▪.Furuya-Kanamori L, Stone J, Yakob L, et al. Risk factors for acquisition of multidrug-resistant Enterobacterales among international travellers: a synthesis of cumulative evidence. J Travel Med 2019; 27:1–10. [DOI] [PubMed] [Google Scholar]; This meta-analysis analyses and quantifies risk factors for travel-associated multidrug-resistant Enterobacterales based on 20 studies of over 5000 travelers.
- 48.Armand-Lefèvre L, Andremont A, Ruppé E. Travel and acquisition of multidrug-resistant Enterobacteriaceae. Med Mal Infect 2018; 48:431–441. [DOI] [PubMed] [Google Scholar]
- 49.Woerther PL, Andremont A, Kantele A. Travel-acquired ESBL-producing Enterobacteriaceae: impact of colonization at individual and community level. J Travel Med 2017; 24:S29–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50▪▪.Hagmann SHFF, Angelo KM, Huits R, et al. Epidemiological and clinical characteristics of international travelers with enteric fever and antibiotic resistance profiles of their isolates: a GeoSentinel analysis. Antimicrob Agents Chemother 2020; 64:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]; This cross-sectional multisite study evaluates enteric fever incidence and AMR in 889 returned international travelers, finding high levels of fluoroquinolone resistance. They use data from the GeoSentinel surveillance study, and they find that most infections occur when travelers visit friends and relatives in South Asia.
- 51▪.Buchek G, Mende K, Telu K, et al. Travel-associated multidrug-resistant organism acquisition and risk factors among US military personnel. J Travel Med 2021; 28:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This recent study evaluates risk factors for AMR bacterial acquisition in a military cohort traveling abroad; they find that ESBL-PE acquisition is highest in South Asia, but overall risk is lower than in civilian populations.
- 52.Arcilla MS, van Hattem JM, Matamoros S, et al. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 2016; 16:147–149. [DOI] [PubMed] [Google Scholar]
- 53.Matamoros S, van Hattem JM, Arcilla MS, et al. Global phylogenetic analysis of Escherichia coli and plasmids carrying the mcr-1 gene indicates bacterial diversity but plasmid restriction. Sci Rep 2017; 7:15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernasconi OJ, Kuenzli E, Pires J, et al. Travelers can import colistin-resistant Enterobacteriaceae, including those possessing the plasmid-mediated mcr-1 gene. Antimicrob Agents Chemother 2016; 60:5080–5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪▪.Turunen KA, Kantele A. Revisiting travellers’ diarrhoea justifying antibiotic treatment: prospective study. J Travel Med 2021; 28:1–10. [DOI] [PubMed] [Google Scholar]; This recent study analyses data from 370 Finnish travelers from the 2008 to 2010 prospective study. They determine that most cases of travelers’ diarrhea resolve without antimicrobial treatment, highlighting that antimicrobials are overprescribed for this purpose.
- 56▪.Nair S, Chattaway M, Langridge GC, et al. ESBL-producing strains isolated from imported cases of enteric fever in England and Wales reveal multiple chromosomal integrations of blaCTX-M-15 in XDR Salmonella Typhi. J Antimicrob Chemother 2021; 76:1459–1466. [DOI] [PubMed] [Google Scholar]; This recent study investigates cases of imported enteric fever in the UK resulting from travel; all isolates of S. Typhi and Paratyphi were ESBL-producing and primarily associated with travel to South Asia.
- 57.Nizamuddin S, Ching C, Kamal R, et al. Continued outbreak of ceftriaxone-resistant Salmonella enterica serotype Typhi across Pakistan and assessment of knowledge and practices among healthcare workers. Am J Trop Med Hyg 2021; 104:1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klemm EJ, Shakoor S, Page AJ, et al. Emergence of an extensively drug-resistant Salmonella enterica serovar typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio 2018; 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight GM, Glover RE, McQuaid CF, et al. Antimicrobial resistance and COVID-19: Intersections and implications. eLife 2021; 10:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60▪.van den Beld MJCC, Reubsaet FAGG, Pijnacker R, et al. A multifactorial approach for surveillance of Shigella spp. and entero-invasive Escherichia coli is important for detecting (Inter)national clusters. Front Microbiol 2020; 11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study applies multiple strategies to Shigella and EIEC surveillance for the detection of related clusters, and they find that there are overlaps in travel-related and domestic clusters in the Netherlands, suggesting secondary transmission.
- 61.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 2010; 10:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker S, Thomson N, Weill F-X, Holt KE. Genomic insights into the emergenceand spread of antimicrobial-resistantbacterial pathogens. Science 2018; 360:733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain A, Hopkins KL, Turton J, et al. NDM carbapenemases in the United Kingdom: an analysis of the first 250 cases. J Antimicrob Chemother 2014; 69:1777–1784. [DOI] [PubMed] [Google Scholar]
- 64.Peirano G, Pillai DR, Pitondo-Silva A, et al. The characteristics of NDM-producing Klebsiella pneumoniae from Canada. Diagn Microbiol Infect Dis 2011; 71:106–109. [DOI] [PubMed] [Google Scholar]
- 65.Struelens MJ, Monnet DL, Magiorakos AP, et al. New Delhi metallo-beta-lactamase 1-producing Enterobacteriaceae: emergence and response in Europe. Euro Surveill 2010; 15:1–10. [DOI] [PubMed] [Google Scholar]
- 66.Johnson AP, Woodford N. Global spread of antibiotic resistance: the example of New Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. J Med Microbiol 2013; 62:499–513. [DOI] [PubMed] [Google Scholar]
- 67.Leverstein-Van Hall MA, Stuart JC, Voets GM, et al. Global spread of New Delhi metallo-β-lactamase 1. Lancet Infect Dis 2010; 10:830–831. [DOI] [PubMed] [Google Scholar]
- 68.von Wintersdorff CJH, Wolffs PFG, van Niekerk JM, et al. Detection of the plasmid-mediated colistin-resistance gene mcr-1 in faecal metagenomes of Dutch travellers. J Antimicrob Chemother 2016; 71:3416–3419. [DOI] [PubMed] [Google Scholar]
- 69.Romero-Alvarez D, Reyes J, Quezada V, et al. First case of New Delhi metallo-β-lactamase in Klebsiella pneumoniae from Ecuador: an update for South America. Int J Infect Dis 2017; 65:119–121. [DOI] [PubMed] [Google Scholar]
- 70.Nakayama T, Kumeda Y, Kawahara R, et al. Carriage of colistin-resistant, extended-spectrum β-lactamase-producing escherichia coli harboring the mcr-1 resistance gene after short-term international travel to Vietnam. Infect Drug Resist 2018; 11:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Payne M, Croxen MA, Lee TD, et al. mcr-1–positive colistin- resistant Escherichia coli in traveler returning to Canada from China. Emerg Infect Dis 2016; 22:1673–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Tian G-B, Zhang R, et al. Prevalence, risk factors, outcomes, and molecular epidemiology of mcr-1-positive Enterobacteriaceae in patients and healthy adults from China: an epidemiological and clinical study. Lancet Infect Dis 2017; 17:390–399. [DOI] [PubMed] [Google Scholar]
- 73.Baloch Z, Lv L, Yi L, et al. Emergence of almost identical f36:A-:B32 plasmids carrying blandm-5 and qepa in escherichia coli from both Pakistan and Canada. Infect Drug Resist 2019; 12:3981–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamer DH, Rizwan A, Freedman DO, et al. GeoSentinel: past, present and future†. J Travel Med 2020; 27:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karp BE, Tate H, Plumblee JR, et al. National antimicrobial resistance monitoring system: two decades of advancing public health through integrated surveillance of antimicrobial resistance. Foodborne Pathog Dis 2017; 14:545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gladstone RA, Lo SW, Goater R, et al. Visualizing variation within global pneumococcal sequence clusters (GPSCS) and country population snapshots to contextualize pneumococcal isolates. Microb Genom 2020; 6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Garcia S, Perez-Arguello A, Henares D, et al. Rapid identification, capsular typing and molecular characterization of Streptococcus pneumoniae by using whole genome nanopore sequencing. BMC Microbiol 2020; 20:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y, Hu Y, Yu Y, et al. Positive result of Sars-Cov-2 in faeces and sputum from discharged patients with COVID-19 in Yiwu, China. J Med Virol 2020; 92:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Foladori P, Cutrupi F, Segata N, et al. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci Total Environ 2020; 743:140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Izquierdo-Lara R, Elsinga G, Heijnen L, et al. Monitoring SARS-CoV-2 circulation and diversity through community wastewater sequencing, the Netherlands and Belgium. Emerg Infect Dis 2021; 27:1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81▪.Peng Y, Liang S, Poonsuk K, et al. Role of gut microbiota in travel-related acquisition of extended spectrum b-lactamase-producing Enterobacteriaceae. J Travel Med 2021; 28. [DOI] [PubMed] [Google Scholar]; This recent study profiles the gut microbiota of a Hong Kong cohort of international travelers using 16S rDNA amplicon sequencing, finding that Actinobacteria richness pretravel is a risk factor for ESBL-PE acquisition.
- 82.Vilkman K, Lääveri T, Pakkanen SH, Kantele A. Stand-by antibiotics encourage unwarranted use of antibiotics for travelers’ diarrhea: a prospective study. Travel Med Infect Dis 2019; 27:64–71. [DOI] [PubMed] [Google Scholar]
- 83.Lorme F, Maataoui N, Rondinaud E, et al. Acquisition of plasmid-mediated cephalosporinase producing Enterobacteriaceae after a travel to the tropics. Plos One 2018; 13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]