Abstract
BACKGROUND
The role of the positive end-expiratory pressure (PEEP) and lung recruitment manoeuvre (LRM) combination (termed open-lung strategy, OLS) during intra-operative mechanical ventilation is not clear.
OBJECTIVE
To determine whether an open-lung strategy constituting medium PEEP (6–8 cmH2O) and repeated LRMs protects against postoperative complications in at-risk patients undergoing laparoscopic colorectal cancer resection under low-tidal-volume ventilation.
DESIGN
A prospective, assessor-blinded, randomised controlled trial.
SETTING
Single university-affiliated hospital, conducted from January 2017 to October 2018.
PATIENTS
A total of 280 patients at risk of pulmonary complications, scheduled for laparoscopic colorectal cancer resection under general anaesthesia and low-tidal-volume (6–8 ml kg−1 predicted body weight) ventilation.
INTERVENTION
The patients were randomly assigned (1 : 1) to a PEEP of 6–8 cmH2O with LRMs repeated every 30 min (OLS group) or a zero PEEP without LRMs (non-OLS group).
MAIN OUTCOME MEASURES
The primary outcome was a composite of major pulmonary and extrapulmonary complications occurring within 7 days after surgery. The secondary outcomes included intra-operative potentially harmful hypotension and the need for vasopressors.
RESULTS
A total of 130 patients from each group were included in the primary outcome analysis. Primary outcome events occurred in 24 patients (18.5%) in the OLS group and 43 patients (33.1%) in the non-OLS group [relative risk, 0.46; 95% confidence interval (CI), 0.26 to 0.82; P = 0.009). More patients in the OLS group developed potentially harmful hypotension (OLS vs. non-OLS, 15% vs. 4.3%; P = 0.004) and needed vasopressors (25% vs. 8.6%; P < 0.001).
CONCLUSION
Among at-risk patients undergoing laparoscopic colorectal cancer resection under low-tidal-volume ventilation, an open-lung strategy with a PEEP of 6–8 cmH2O and repeated LRMs reduced postoperative complications compared with a strategy using zero PEEP without LRMs. Of note, LRMs should be used with caution in patients with haemodynamic instability.
TRIAL REGISTRATION
Clinicaltrials.gov identifier: NCT03160144.
Introduction
Postoperative pulmonary complications (PPCs) that adversely affect patient outcomes1 are common in patients undergoing abdominal surgery, with an incidence as high as 38 to 48% in at-risk patients.2–4 Previous studies5–11 demonstrated that the optimisation of intra-operative mechanical ventilation was associated with reduced PPCs and better outcomes. As a consequence, protective ventilation for abdominal surgery has become an important issue in peri-operative medicine.
Protective ventilation10 refers to the use of low-tidal-volume ventilation and an open-lung12 strategy (OLS). OLS involves opening collapsed alveoli with a lung recruitment manoeuvre (LRM) and keeping them open with a positive end-expiratory pressure (PEEP). However, although low-tidal-volume ventilation for surgery is currently recommended,8,11 the role of an OLS during ventilation is not clear: the clinical benefits of PEEP are not clearly established2,13,14; LRMs are rarely used15,16 in clinical practice and their role is controversial.17,18 In this context, the use of low-tidal-volume ventilation without an OLS remains common in the operating room.16,19–23 Therefore, it is critical to clarify the role of an OLS during intra-operative low-tidal-volume ventilation, which has become a routine clinical practice. The IMPROVE trial5 found that low-tidal-volume ventilation combined with an OLS (PEEP of 6–8 cmH2O with LRMs repeated every 30 min) had protective effects when compared with large-tidal-volume ventilation. However, as there was no control group with low-tidal-volume ventilation only, it is not clear whether it was the OLS or the low-volume-ventilation that had the protective effect in the IMPROVE trial.
We hypothesised that OLS, as used in the IMPROVE trial,5 would reduce major postoperative complications in at-risk patients undergoing abdominal surgery under anaesthesia with low-tidal-volume ventilation. Colorectal cancer resection is a common type of abdominal surgery and a laparoscopic approach is now the preferred surgical approach.24 We tested our hypothesis in patients who were at risk of developing PPCs and were scheduled for laparoscopic colorectal cancer resection. The primary outcome was a composite of major pulmonary and extrapulmonary complications occurring within 7 days after surgery.
Methods
This study was a prospective, assessor-blinded, randomised controlled trial. The Institutional Ethical Committee of the Sixth Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China approved the study (Chairperson, Professor Wei Miao) on 9 January 2017 (2017ZSLYEC-002), and it was performed from January 2017 to October 2018. The study is registered at clinicaltrials.gov (NCT03160144). Written informed consent was obtained from all participants before the study procedures.
Participants
Patients were eligible for participation in the study if they were aged more than 40 years, were scheduled for laparoscopic colorectal cancer resection with an expected pneumoperitoneum time of at least 1.5 h, had a pulse oxygen saturation (SpO2) of at least 92% in room air and had a risk index5 for PPCs of at least class 2. Patients were ineligible if they were classified with an ASA physical status at least IV, had a history of pneumonia, acute respiratory failure or sepsis within the previous 1 month, had a BMI at least 30 kg m−2, had severe chronic obstructive pulmonary disease or pulmonary bullae, had a progressive neuromuscular illness, or were involved in other interventional studies. Participants were also excluded from the study in the event of conversion to laparotomy within the first hour of surgery, or severe surgical complications on the day of surgery.
Randomisation and masking
We randomly allocated patients (1 : 1) to a PEEP of 6 to 8 cmH2O with LRMs repeated every 30 min (the OLS group) or to a zero PEEP without LRMs (the non-OLS group). An interim analysis was planned after the first 100 patients to assess the effects and safety of the intervention. Following this analysis, we recruited a further 180 cases. A completely randomised design was used in the first 100 patients, and a randomised block design (block size of 10) was used thereafter. The random allocation sequence was generated using SPSS statistical software, version 17.0 (SPSS Inc., Chicago, Illinois, USA). The sequence was sealed and allocated by assigned personnel. All patients and outcome assessors were blinded to the group assignment.
Interventions and respiratory management
All patients received volume-controlled ventilation using a tidal volume of 6 to 8 ml kg−1 predicted body weight (PBW).5 After tracheal intubation, PEEP was set at 6 to 8 cmH2O in the OLS group and 0 cmH2O in the non-OLS group. An LRM was performed immediately after tracheal intubation and repeated every 30 min in patients in the OLS group. No LRMs were administered to patients in the non-OLS group. A stepwise increment of tidal volume was used for each LRM, as previously described.25
Other respiratory settings were the same in both groups. The fraction of inspired oxygen (FIO2) was set at 100% during anaesthesia induction and at 40 to 50% during mechanical ventilation. If a patient had an SpO2 less than 92% during ventilation, rescue therapy with stepwise increases (10%) in FIO2 was administered. The respiratory rate was adjusted to maintain end-tidal carbon dioxide within 30 to 50 mmHg, and a plateau pressure of 30 cmH2O or less was the target in both groups.
Intra-operative and postoperative management
Before anaesthesia induction, 500 to 700 ml of Ringer's lactate solution was infused over 1 h, invasive arterial blood pressure was monitored and an epidural catheter was placed at the T12–L1 or L1–L2 level. Mixed (volatile and intravenous) anaesthesia was used in accordance with routine clinical practice. Fluid infusion was administered intra-operatively at 10 to 12 ml kg−1 h−1 to ensure haemodynamic stability. Before skin incision, morphine (2 mg in 5 ml of 0.9% saline) was injected into the epidural space for preemptive analgesia. Approximately 0.5 h before the end of surgery, 7 to 10 ml of ropivacaine (0.1%) was injected into the epidural space as a loading dose for postoperative analgesia. Postoperatively, patient-controlled epidural analgesia with a mixture of 0.1% ropivacaine and 0.01% morphine was administered (basal rate of 1 ml h−1, bolus dose of 1.5 ml, lockout interval 15 min) during the first three postoperative days (PODs). Prophylactic antibiotics and the inhalation of nebulized ambroxol were routinely administered for the first 3 days after surgery.
Postoperative observations
One investigator, who was unaware of the patient allocations, assessed the patients and obtained postoperative data. Intensive follow-up was performed twice daily on the first three PODs (PODs 1, 2 and 3) and once daily on POD 5 and POD 7. The follow-up items included SpO2 (Masimo Rad-5, Masimo Corporation, Irvine, California, USA) in room air (if the patients were receiving oxygen therapy, data were collected at least 10 min after the cessation of oxygen therapy), heart rate, axillary temperature, breathing rate, amount and colour of sputum, presence or absence of dry and wet rashes and numerical rating scores for abdominal pain, cough and dyspnoea. If an episode of hypoxaemia (SpO2 ≤92%) lasting more than 1 min was observed during follow-up, the patient would then be followed up every day in the first 7 postoperative days. A chest X-ray was obtained on POD 3, and laboratory tests (e.g. albumin and blood tests) were performed on PODs 1, 3 and 5. If patients had symptoms of myocardial ischaemia, an ECG was performed, and myocardial enzyme (e.g. troponin I, creatine phosphate kinase MB) levels were examined. Drainage, urine and infusion volumes within 3 days after surgery and information on surgical complications were obtained from clinical data and the Hospital Information System.
Outcomes
Two investigators, who were blinded to the group assignment, independently scored the predefined outcomes according to the intra-operative or postoperative observations. If their judgments were inconsistent, a final conclusion was reached via discussion.
The primary outcome was a composite of major pulmonary and extrapulmonary complications, which was defined as positive when any component occurred within the first 7 days after surgery. Major PPCs included acute respiratory failure, suspected pneumonia and sustained hypoxaemia. Major extrapulmonary complications (see the Appendix for definitions) included sepsis, severe sepsis, septic shock26 and death from any cause.
The following major PPCs were defined. Acute respiratory failure: during a follow-up visit when the patient was awake and breathing room air, SpO2 less than 90% lasting at least 1 min, or PaO2 less than 8 kPa whenever awake.27,28 Sustained hypoxaemia: during a follow-up visit when the patient was awake and breathing room air, SpO2 less than 92% lasting at least 1 min on any three consecutive days. Suspected pneumonia was defined as: patient received their prophylactic antibiotics and met one of the following criteria: firstly, on chest X-ray, the presence of new and/or progressive pulmonary infiltrates with two or more of the following; (a) core temperature (axillary temperature + 0.5 °C) at least 38.5 °C; (b) leucocytosis with a white blood cell count of at least 12 × 109 l−1 or neutrophils greater than 80%; (c) purulent sputum; (d) new onset or worsening cough or dyspnoea; secondly, without chest X-ray, and in the absence of another infectious focus (e.g. urinary or biliary tract infection, intestinal obstruction, intra-abdominal abscess or anastomotic leakage), the presence of three or more of the above-mentioned conditions (a, b, c and d).2,25
Secondary outcomes included: firstly, components of major postoperative complications, systemic inflammatory response syndrome (SIRS), acute myocardial infarction (AMI), intra-abdominal abscess or anastomotic leakage within 7 days after surgery, admission to the ICU and death within 30 days after surgery; secondly, intra-operative OLS-related complications, including pneumothorax,27 rescue therapy for desaturation, potentially harmful hypotension [mean arterial pressure (MAP) ≤55 mmHg lasting ≥1 min), need for vasopressors (MAP ≤55 mmHg or the need for a vasopressor, as assessed by an anaesthesiologist, when MAP <65 mmHg) and thirdly, the length of postoperative hospital stay (LOS).
Post hoc outcomes included modified acute respiratory failure, severe respiratory failure, the number of patients who met all of the defined major PPCs and modified LOS (see the Appendix for definitions).
Statistical analysis
We assumed that the incidence of the primary outcome would be 10.5% in the OLS group and 25% in the non-OLS group based on a previous study.5 Considering the population differences from the previous study,5 at a two-sided α level of 0.05, a power of 0.8 and a dropout rate of 10%, we calculated and finally decided a sample size of 280 patients (140 per group). One interim analysis was performed after enrolment of the first 100 patients, according to the a priori statistical analysis plan. The results showed that OLS had a protective trend, and there were no significant differences in OLS-related complications. The data and safety monitoring group recommended continuing the trial in 280 patients.
The primary outcome analysis was a modified intention-to-treat analysis (not including patients who were excluded from the study after randomisation). All randomised patients were included in the analysis of the OLS-related complications. Post hoc analysis was performed to assess whether different definitions of outcomes affected the trend of the main results.
An unadjusted χ2 test was used for the primary outcome analysis. Multiple logistic regression analysis was performed to correct potential confounders (listed below the outcome results table) associated with binary outcomes. A χ2 test or Fisher's exact test was used for other binary outcomes or parameters. For the primary outcome, a Kaplan–Meier curve was generated to assess the cumulative probability of postoperative complications, and the P value was reported based on the log-rank test. Continuous data were compared using an independent t test or Mann–Whitney U test, as appropriate. A two-sided P value less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS statistical software, version 17.0.
Results
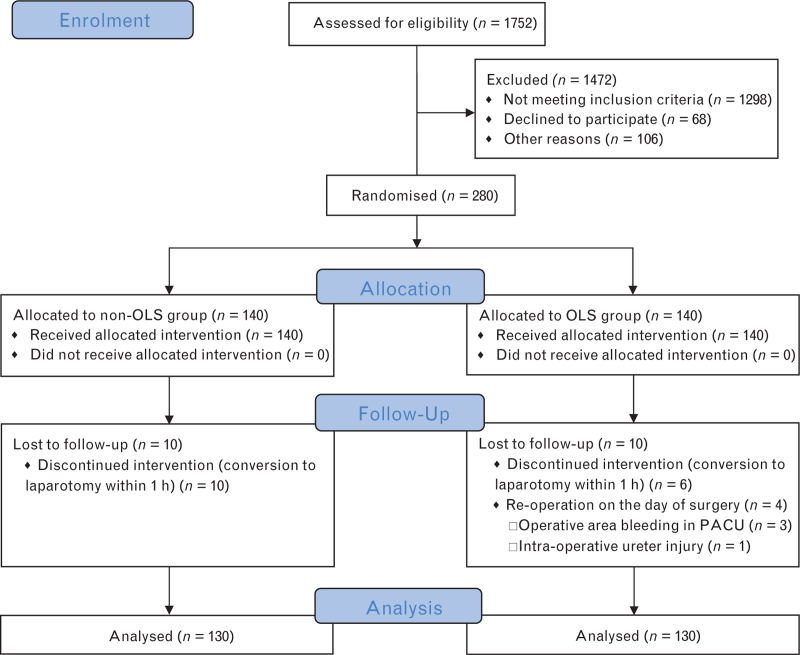
Of the 1752 patients screened for eligibility, 280 were enrolled. All of the participants had a risk class for PPCs of 2 or 3, and an overall mean age of 70 years. Baseline characteristics did not differ significantly between the groups (Table 1). Ten patients in each group were excluded from the study after randomisation and 130 patients per group were included in the final analysis (see Fig. 1 for further details).
Table 1.
Patient characteristics at baseline
| Non-OLS (n = 140) | OLS (n = 140) | |
| Age (years) | 70.8 ± 5.8 | 69.7 ± 5.8 |
| BMI (kg m−2) | 22.3 ± 2.8 | 23.0 ± 2.7 |
| Predicted body weight (kg) | 58.1 ± 8.1 | 59.3 ± 8.7 |
| Sex (male) | 98 (70.0) | 102 (72.9) |
| ASA physical status classification (II/III) | 116/24 | 110/30 |
| New York Heart Association classification (I/II) | 5/135 | 5/135 |
| Preoperative PPC risk classification (2/3) | 132/8 | 135/5 |
| Preoperative PPC risk score | 19.5 ± 3.5 | 19.2 ± 3.2 |
| PaO2 (kPa) | 10.56 ± 1.17 | 10.59 ± 1.19 |
| Current smokers | 39 (27.9) | 32 (22.9) |
| Patients drinking alcohol within 2 weeks | 4 (2.86) | 3 (2.14) |
| Physical functional status (independent) | 140 (100) | 140 (100) |
| Chronic obstructive pulmonary disease | 2 (1.4) | 3 (2.1) |
| Diabetes mellitus | 14 (10.0) | 20 (14.3) |
| Chemotherapy | 22 (15.7) | 19 (13.6) |
| Radiotherapy | 12 (8.6) | 10 (7.1) |
| Use of statins | 6 (4.3) | 4 (2.9) |
| Loss of body weight >10% in the last 6 months | 32 (22.9) | 30 (21.4) |
| Use of systemic steroids | 1 (0.7) | 0 (0.0) |
| Cardiac or cerebral vascular diseases | 20 (14.3) | 15 (10.7) |
| Abnormalities on chest radiograph | 47 (33.6) | 43 (30.7) |
| Abnormalities on pulmonary function tests | 27 (27.8) | 29 (28.2) |
| Haemoglobin (g dl−1) | 12.2 ± 1.8 | 12.0 ± 2.2 |
| Albumin (g dl−1) | 3.94 ± 0.36 | 3.92 ± 0.39 |
Data are the number (%), or mean ± SD. OLS, open-lung strategy; PPC, postoperative pulmonary complication.
Fig. 1.
Trial profile.
OLS, open-lung strategy; PACU, postanaesthesia care unit.
The intra-operative and postoperative characteristics are provided in Tables 2 and 3. The median [IQR] level of PEEP was 8 [8 to 8] cmH2O in the OLS group, and the median [IQR] number of LRMs was 7 [6 to 8]. The values for each of these factors were zero in the non-OLS group. Postoperative chest X-rays were obtained in 56.2% and 67.7% of patients in the OLS and non-OLS groups, respectively (Table 3).
Table 2.
Intra-operative characteristics
| Non-OLS (n = 130) | OLS (n = 130) | P value | |
| Immediately before surgery | |||
| PEEP (cmH2O) | 0 [0 to 0] | 8 [8 to 8] | <0.001 |
| Fraction of inspired oxygen (%) | 45 [44 to 45] | 45 [44 to 45] | 0.679 |
| Tidal volume (ml kg−1 PBW) | 7.6 ± 0.7 | 7.5 ± 0.6 | 0.343 |
| Respiratory rate (breaths min−1) | 12 [12 to 12] | 12 [12 to 12] | 0.346 |
| Peak pressure (cmH2O) | 14 ± 3 | 17 ± 2 | <0.001 |
| Plateau pressure (cmH2O) | 11 ± 2 | 14 ± 2 | <0.001 |
| Driving pressure (cmH2O) | 11 ± 2 | 6 ± 2 | <0.001 |
| Respiratory compliance (ml cmH2O−1) | 34 ± 8 | 50 ± 13 | <0.001 |
| 1.5 h after pneumoperitoneum | |||
| PEEP (cmH2O) | 0 [0 to 0] | 8 [8 to 8] | <0.001 |
| Fraction of inspired oxygen (%) | 45 [44 to 45] | 45 [45 to 45] | 0.279 |
| Tidal volume (ml kg−1 PBW) | 7.1 ± 0.8 | 7.0 ± 0.7 | 0.179 |
| Respiratory rate (breaths min−1) | 16 [15 to 18] | 16 [15 to 18] | 0.283 |
| Peak pressure (cmH2O) | 21 ± 4 | 24 ± 3 | <0.001 |
| Plateau pressure (cmH2O) | 17 ± 4 | 20 ± 3 | <0.001 |
| Driving pressure (cmH2O) | 17 ± 4 | 12 ± 3 | <0.001 |
| Respiratory compliance (ml cmH2O−1) | 21 ± 6 | 27 ± 8 | <0.001 |
| PaO2/FiO2 (kPa) | 49.7 ± 14.7 | 58.8 ± 12.1 | <0.001 |
| Lung recruitment manoeuvres (times) | 0 [0 to 0] | 7 [6 to 8] | <0.001 |
| Duration of surgery (min) | 214 ± 80 | 212 ± 81 | 0.817 |
| Duration of mechanical ventilation (min) | 230 ± 82 | 229 ± 81 | 0.951 |
| Trendelenburg position | 109 (83.8) | 98 (75.4) | 0.123 |
| Pneumoperitoneum pressure (mmHg) | 12 [11 to 12] | 12 [11 to 12] | 0.105 |
| Duration of pneumoperitoneum (min) | 164 ± 73 | 158 ± 74 | 0.512 |
| Muscle relaxant antagonist | 121 (96.0) | 122 (94.6) | 0.769 |
| Epidural analgesia | 129 (99.2) | 129 (99.2) | 1.000 |
| Urine output (ml) | 500 [300 to 712] | 500 [300 to 700] | 0.334 |
| Blood loss (ml) | 50 [50 to 100] | 50 [50 to 100] | 0.290 |
| Volume of fluids administered (ml) | 3161 ± 695 | 3227 ± 663 | 0.436 |
| Crystalloid (ml) | 2039 ± 488 | 2025 ± 505 | 0.831 |
| Red blood cell infusion | 5 (3.8) | 6 (4.6) | 1.000 |
Data are number (%), mean ± SD or median [IQR]. Ten patients in each group were excluded from the study. OLS, open-lung strategy; PBW, predicted body weight; PEEP, positive end-expiratory pressure. Respiratory compliance = tidal volume/(peak pressure − PEEP); driving pressure = plateau pressure − PEEP.
Table 3.
Postoperative characteristics
| Non-OLS (n = 130) | OLS (n = 130) | P value | |
| Postoperative day 1 | |||
| Fluid infusion volume (ml) | 2860 ± 429 | 2981 ± 501 | 0.04 |
| Drainage volume (ml) | 100 [50 to 200] | 100 [50 to 170] | 0.83 |
| Urine output (ml) | 2110 [1500 to 2800] | 2400 [1722 to 3325] | 0.03 |
| NRS for abdominal pain ≥ 3 | 1 (0.8) | 0 (0.0) | 1.00 |
| Albumin (g dl−1) | 2.97 ± 0.39 | 2.95 ± 0.39 | 0.75 |
| Postoperative day 2 | |||
| Fluid infusion volume (ml) | 2856 ± 431 | 2900 ± 473 | 0.43 |
| Drainage volume (ml) | 80 [25 to 160] | 70 [20 to 120] | 0.25 |
| Urine output (ml) | 2800 [1987 to 3525] | 3000 [2150 to 3900] | 0.13 |
| NRS for abdominal pain ≥ 3 | 1 (0.8) | 2 (1.5) | 1.00 |
| Postoperative day 3 | |||
| Fluid infusion volume (ml) | 2811 ± 416 | 2840 ± 495 | 0.61 |
| Drainage volume (ml) | 50 [15 to 150] | 50 [10 to 132] | 0.85 |
| Urine output (ml) | 3115 [2150 to 3787] | 3525 [2487 to 4302] | 0.05 |
| NRS for abdominal pain ≥ 3 | 0 (0.0) | 0 (0.0) | 1.00 |
| Albumin (g dl−1) | 3.23 ± 0.35 | 3.16 ± 0.42 | 0.20 |
| Chest X-ray | 88 (67.7) | 73 (56.2) | 0.07 |
| Blood product transfusion | 1 (0.8) | 2 (1.5) | 1.00 |
| Prophylactic antibiotics | 129 (99.2) | 130 (100.0) | 1.00 |
| Nebulised ambroxol | 129 (99.2) | 129 (99.2) | 1.00 |
| Early ambulationa | 35 (26.9) | 36 (27.7) | 1.00 |
Values are the number (%), mean ± SD or median [IQR]. Ten patients in each group were excluded from the study. NRS, numerical rating score; OLS, open-lung strategy.
Up and moving within 2 days after surgery.
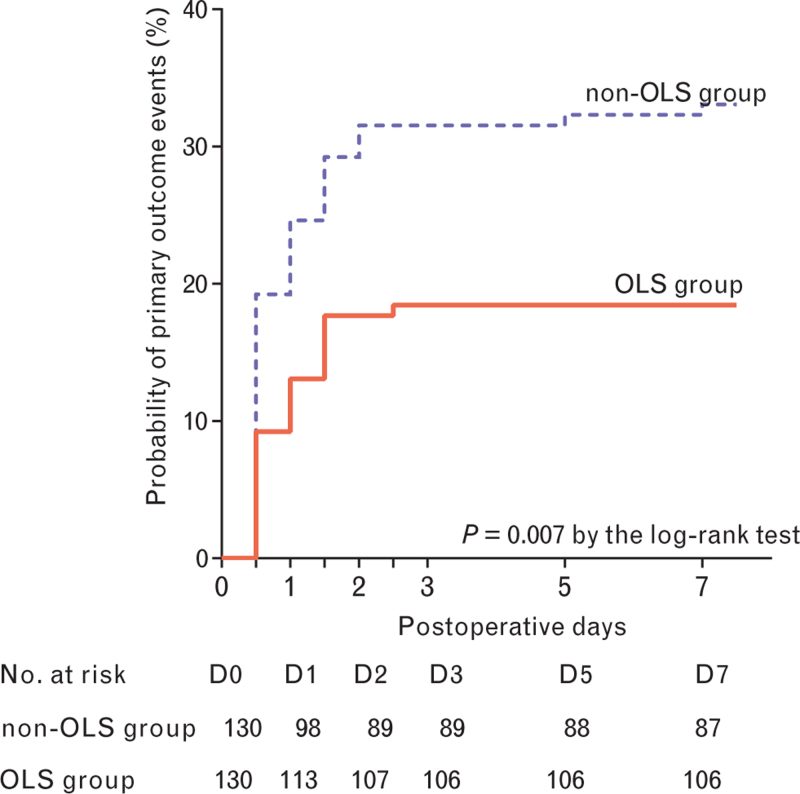
Primary outcome events occurred in 24 patients (18.5%) assigned to the OLS group and 43 patients (33.1%) assigned to the non-OLS group (P = 0.009) (Table 4). The cumulative 7-day probability of primary outcome events was lower in the OLS group than the non-OLS group (P = 0.007) (Fig. 2).
Table 4.
Outcome analysis
| Non-OLS (n = 130) | OLS (n = 130) | Unadjusted relative risk (95% CI) | P value | Adjusted relative risk (95% CI)a | P valuea | |
| Primary outcomeb | ||||||
| Major postoperative pulmonary and extrapulmonary complications | 43 (33.1) | 24 (18.5) | 0.46 (0.26 to 0.81) | 0.010 | 0.46 (0.26 to 0.82) | 0.009 |
| Secondary and post hoc outcomes | ||||||
| Complications within 7 days | ||||||
| Major pulmonary complications | 41 (31.5) | 24 (18.5) | 0.49 (0.28 to 0.88) | 0.022 | 0.49 (0.28 to 0.88) | 0.016 |
| Major extrapulmonary complications | 6 (4.6) | 2 (1.5) | 0.32 (0.06 to 1.63) | 0.281 | 0.32 (0.06 to 1.63) | 0.171 |
| Acute respiratory failure | 39 (30.0) | 24 (18.5) | 0.53 (0.30 to 0.94) | 0.042 | 0.53 (0.30 to 0.94) | 0.031 |
| Modified acute respiratory failurec,f | 30 (23.1) | 17 (13.1) | 0.50 (0.26 to 0.96) | 0.052 | 0.50 (0.26 to 0.96) | 0.038 |
| Suspected pneumoniag | 16 (12.3) | 3 (2.3) | 0.17 (0.05 to 0.59) | 0.003 | 0.18 (0.05 to 0.62) | 0.007 |
| Sustained hypoxaemia | 24 (18.5) | 15 (11.5) | 0.58 (0.29 to 1.16) | 0.164 | 0.58 (0.29 to 1.16) | 0.121 |
| Severe respiratory failured,f | 0 (0.0) | 0 (0.0) | 1.000 | |||
| All defined major PPCs in one patientf | 11 (8.5) | 3 (2.3) | 0.26 (0.07 to 0.94) | 0.051 | 0.26 (0.07 to 0.98) | 0.047 |
| Sepsis | 6 (4.6) | 2 (1.5) | 0.32 (0.06 to 1.63) | 0.281 | 0.32 (0.06 to 1.63) | 0.171 |
| Severe sepsis | 0 (0.0) | 0 (0.0) | 1.000 | |||
| Septic shock | 0 (0.0) | 0 (0.0) | 1.000 | |||
| SIRS | 36 (27.7) | 30 (23.1) | 0.78 (0.48 to 1.37) | 0.476 | 0.79 (0.44 to 1.41) | 0.420 |
| Acute myocardial infarction | 0 (0.0) | 0 (0.0) | 1.000 | |||
| Intra-abdominal abscess or anastomotic leakage | 5 (3.8) | 2 (1.5) | 0.39 (0.07 to 2.05) | 0.447 | 0.39 (0.07 to 2.05) | 0.267 |
| Death within 30 days | 0 (0.0) | 0 (0.0) | 1.000 | |||
| Admission to the ICU within 30 days | 0 (0.0) | 1 (0.8) | 1.01 (0.99 to 1.02) | 1.000 | ||
| Postoperative hospital stay (days) | 9 [8 to 13] | 10 [8 to 12] | 0.943 | |||
| Modified postoperative hospital staye,f | 9 [8 to 10] | 8 [7 to 9] | 0.130 | |||
| Intra-operative complications (n = 140) | ||||||
| Pneumothorax | 0 (0.0) | 0 (0.0) | 1.000 | |||
| Rescue therapy for desaturation | 0 (0.0) | 0 (0.0) | 1.000 | |||
| Potentially harmful hypotension | 6 (4.3) | 21 (15.0) | 3.94 (1.54 to 10.09) | 0.004 | 3.32 (1.27 to 8.66) | 0.014 |
| Need for vasopressors | 12 (8.6) | 35 (25.0) | 3.56 (1.76 to 7.19) | <0.001 | 2.98 (1.45 to 6.13) | 0.003 |
Data are number (%) or median [IQR] as appropriate. Ten patients in each group were excluded from the study. All postoperative complications were defined according to consensus criteria (see the main text or the Supplementary Appendix). ICU, intensive care unit; OLS, open-lung strategy; PPCs, postoperative pulmonary complications; SIRS, systemic inflammatory response syndrome.
Adjustment was performed for preoperative risk index for PPCs, duration of mechanical ventilation, ASA physical status, abnormalities on chest radiography (and/or having COPD) and intra-operative red blood cells transfusion (and/or intra-operative blood loss ≥300 ml, and/or preoperative haemoglobin <8 g dl−1).
The primary outcome was a composite of major pulmonary complications (acute respiratory failure, suspected pneumonia and sustained hypoxaemia) and extrapulmonary complications (sepsis, severe sepsis, septic shock and death from any cause), defined as positive if any component occurred within the first 7 days after surgery.
Met the criterion of acute respiratory failure in twice follow-up or acute respiratory failure with sustained hypoxaemia.
Experienced an invasive or noninvasive ventilator therapy, or PaO2 less than 60 mmHg or SpO2 less than 90% when administering oxygen via a nasal catheter at 3 l min−1 or more.
On the basis of the final analysis population (130 patients per group), this analysis excluded the patients who had none of the primary outcome events but had a postoperative hospital stay more than 10 days.
Post hoc outcomes.
All of these patients had a postoperative chest X-ray except for three in the non-OLS group.
Fig. 2.
Kaplan–Meier curve showing the probability of primary outcome events by postoperative day 7.
The primary outcome was a composite of major pulmonary complications (acute respiratory failure, suspected pneumonia and sustained hypoxaemia) and extrapulmonary complications (sepsis, severe sepsis, septic shock and death from any cause) occurring within 7 days after surgery. OLS, open-lung strategy.
Fewer patients in the OLS group had major PPCs [OLS vs. non-OLS, 24 (18.5%) vs. 41 (31.5%); P = 0.016], acute respiratory failure [24 (18.5%) vs. 39 (30.0%); P = 0.031], suspected pneumonia [3 (2.3%) vs. 16 (12.3%); P = 0.007] and all major PPCs [3 (2.3%) vs. 11 (8.5%); P = 0.047] within 7 days after surgery than patients in the non-OLS group. More patients in the OLS group than the non-OLS group developed potentially harmful hypotension [OLS vs. non-OLS, 21 (15%) vs. 6 (4.3%); P = 0.004] and needed vasopressors [35 (25%) vs. 12 (8.6%); P < 0.001] intra-operatively.
Fewer patients in the OLS group developed major extrapulmonary complications [OLS vs. non-OLS, 2 (1.5%) vs. 6 (4.6%), P = 0.171], sustained hypoxaemia [15 (11.5%) vs. 24 (18.5%), P = 0.121], sepsis [2 (1.5%) vs. 6 (4.6%), P = 0.171], intra-abdominal abscess or anastomotic leakage [2 (1.5%) vs. 5 (3.8%), P = 0.267] and SIRS [30 (23.1%) vs. 36 (27.7%), P = 0.420] within 7 days after surgery than patients in the non-OLS group but the differences were not statistically significant.
Acute respiratory failure was the most common complication observed in this study. Sixty-three (94.0%) of the 67 patients with the primary outcome had acute respiratory failure. Modified acute respiratory failure, which was defined using stricter criteria, remained statistically significantly different between the groups [OLS vs. non-OLS, 17 (13.1%) vs. 30 (23.1%); P = 0.038] in post hoc analysis.
The median [IQR] LOS was similar between the two groups (OLS vs. non-OLS, 10 [8 to 12] vs. 9 [8 to 13] days; P = 0.943). Post hoc analysis revealed that the median [IQR] modified LOS in the OLS group was shorter than the non-OLS group (8 [7 to 9] vs. 9 [8 to 10] days; P = 0.130), but the difference was not statistically significant. None of the enrolled patients died within 30 days or were diagnosed with AMI, severe respiratory failure, severe sepsis, or septic shock within 7 days after surgery. Only one patient in the OLS group was admitted to the ICU immediately after surgery at the request of a surgeon because of his old age (81 years) and the presence of cardiovascular disease. However, the patient did not develop primary outcome events.
Discussion
This investigation differs from the previous studies3,5,29–31 as it distinguishes between the role of an OLS from that of a low-tidal volume alone in protective ventilation. This study showed that an OLS constituting a PEEP of 6–8 cmH2O and repeated LRMs led to a marked decrease in postoperative complications compared with a strategy using zero PEEP without LRMs in patients undergoing laparoscopic colorectal cancer resection under low-tidal-volume ventilation. However, more intra-operative hypotension may develop when OLS is implemented.
In the current study, the incidence of postoperative complications were reduced significantly in the OLS group compared with the non-OLS group, suggesting that the use of OLS led to a considerable reduction in low-tidal-volume ventilation-associated atelectrauma and secondary barotrauma.32 We observed a marked decrease in major PPCs and a decreasing trend in all postoperative complications in the OLS group compared with the non-OLS group, which further confirms the conclusion. Acute respiratory failure was the most common PPC observed in this study, and it was significantly reduced with the use of OLS. A stricter definition (modified acute respiratory failure) did not change the results. We found that 94% of the major postoperative complications were accompanied by respiratory failure, suggesting that we should strengthen the detection and treatment of postoperative respiratory failure.
The incidence of postoperative complications in this study was higher than the IMPROVE trial,5 which may be because of the relatively mild components of major PPCs. However, even minor PPCs are clinically relevant and lead to undesirable outcomes.33,34 The defined PPCs would not fail to identify a significant PPC as all moderate-to-severe PPCs should involve at least one of the defined PPCs. The IMPROVE trial5 found that lung-protective ventilation reduced postoperative complications and LOS, and the current study revealed a reduction in postoperative complications but no reduction in LOS with the use of the OLS. We reasoned that the following conditions contributed to the differences: the enrolled patients were relatively healthy (PPC risk of 2–3 and ASA physical status of II–III); the type of surgery that we observed may have reduced the occurrence of systemic inflammation and interference with the respiratory system; and prophylactic antibiotics and nebulised ambroxol were routinely administered after surgery. These conditions may alleviate the degree of postoperative complications and reduce the between-group difference in LOS.
These results expand our understanding of intra-operative protective ventilation. Previous studies4,7,35 found that low-tidal-volume ventilation with low PEEP (0–5 cmH2O) did not protect against PPCs and led to increased lung inflammation and 30-day mortality compared with large-tidal-volume ventilation with or without PEEP, which is considered to be harmful.8,11 Compared with medium PEEP (6–10 cmH2O), low PEEP led to decreased PaO2/FiO2, and increased shunt fraction, driving pressure and area of atelectasis.31,36,37 Our results suggest that low-tidal-volume ventilation with low PEEP is not a protective ventilation scheme. Previous studies2,29 showed that high PEEP (≥12 cmH2O) with LRMs, compared with low PEEP, improved intra-operative or immediate postoperative physiological parameters (oxygenation, lung compliance, driving pressure), but did not protect the lungs from postoperative complications. These results suggest that the improved physiological parameters resulting from high PEEP do not necessarily mean a reduction in PPCs. We believe that high PEEP itself may result in sustained lung overdistension, thus leading to barotrauma/volutrauma32 and progression to PPCs. Individualised PEEP3,30,38,39 showed similar effects to high PEEP, that is, it improves physiological rather than clinical outcomes.3 The titrated PEEP also showed no advantage over empirical high PEEP in patients with ARDS.40 These studies suggest that the optimal scheme for individualised PEEP needs to be further investigated, and low-tidal-volume ventilation with high PEEP and LRMs may not be a protective ventilation scheme. Unlike the above-mentioned studies, other previous studies5,6 and the current study showed that low-tidal-volume ventilation with medium PEEP and repeated LRMs protected patients from postoperative complications. We speculate that the short-term high pressure of repeated LRMs opened the collapsed alveoli and the sustained medium PEEP kept the alveoli open without lung overdistension, thus conferring a protective effect. In summary, it appears that protective ventilation should strike a balance between the absence of atelectrauma and avoidance of barotrauma/volutrauma.
In this study, more intra-operative hypotension-requiring vasopressors developed when OLS was implemented. We inferred that the hypotension was caused by the repeated LRMs as an LRM reduces venous return to the heart, thereby reducing cardiac output and leading to hypotension.41 However, the haemodynamic fluctuation in this trial (15–25%) was smaller than previous studies (32–62%).2,29 Advantages in the current study that may reduce haemodynamic fluctuation included the use of a medium level of PEEP, which may have less haemodynamic impact,31 and fluid preloading before anaesthesia induction, which may partially correct the hypovolemic state induced by anaesthesia induction and preoperative fasting. One disadvantage in this study that may increase haemodynamic fluctuation was the use of local anaesthetics in the epidural space at the end of surgery. During follow-up, none of the patients suffered serious complications, such as AMI or death, suggesting that the hypotensive events were transient or were treated in a timely and effective manner. Nevertheless, an LRM should be used with caution, especially in patients with haemodynamic instability.5,41
The current study had several limitations. First, we could not identify whether medium PEEP or repeated LRMs contributed the most to the protective effect, and this warrants further investigation. Second, postoperative atelectasis, a very common PPC,42 was not included in the defined major PPCs. However, when diagnosed using a chest X-ray, the incidence of postoperative atelectasis varied greatly in previous studies,4,29 and its accurate diagnosis requires computed tomography scans: this is not economical for a large sample size study. Moreover, postoperative atelectasis is not clearly defined in terms of the percentage area of atelectasis, and it generally has no symptoms.43 Nevertheless, a larger area of atelectasis should be included in the defined PPCs as it inevitably leads to respiratory failure or sustained hypoxaemia. Third, the findings need to be further validated in patients undergoing laparotomy. However, according to the two-hit theory of ventilator-induced lung injury, the inflammatory response to surgery is the first hit, and the injurious mechanical ventilation is the other hit. OLS may confer a more obvious protective effect in laparotomy, which has greater surgical trauma than laparoscopic surgery. Finally, postrandomisation exclusions may cause information loss. However, the causes of case loss were clear, and the number of lost cases in both groups was equal. Therefore, this loss should not have led to bias.
In conclusion, an OLS constituting a PEEP of 6 to 8 cm H2O and repeated LRMs reduced postoperative complications compared with a ventilation strategy using zero PEEP without LRMs in at-risk patients undergoing low-tidal-volume ventilation for laparoscopic colorectal cancer resection.
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: the authors thank Jialing Huang, PhD, at Biostatistics, the School of Public Health, Sun Yat-Sen University, Guangzhou, China, for her valuable advice on the statistical design and analysis. The authors also thank all of the surgeons at the Department of Colorectal Surgery, the Sixth Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China, for their cooperation in postoperative management.
Financial support and sponsorship: this study was supported by the Medical Scientific Research Foundation of Guangdong Province (A2017045, China) and institutional support for the Department of Anaesthesia from the Sixth Affiliated Hospital of Sun Yat-sen University.
Conflicts of interest: none.
Presentation: none.
Hong Li and Zhi-Nan Zheng contributed equally to this study.
Published online 6 August 2021
Supplemental digital content is available for this article.
References
- 1.International Surgical Outcomes Study group. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth 2016; 117:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemmes SN, Gama de Abreu M, et al. PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014; 384:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrando C, Soro M, Unzueta C, et al. Individualized PeRioperative Open-lung VEntilation (iPROVE) Network. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet Respir Med 2018; 6:193–203. [DOI] [PubMed] [Google Scholar]
- 4.Karalapillai D, Weinberg L, Peyton P, et al. Effect of intraoperative low tidal volume vs conventional tidal volume on postoperative pulmonary complications in patients undergoing major surgery: a randomized clinical trial. JAMA 2020; 324:848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Futier E, Constantin JM, Paugam-Burtz C, et al. IMPROVE Study Group. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013; 369:428–437. [DOI] [PubMed] [Google Scholar]
- 6.Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 2013; 118:1307–1321. [DOI] [PubMed] [Google Scholar]
- 7.Levin MA, McCormick PJ, Lin HM, et al. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br J Anaesth 2014; 113:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guay J, Ochroch EA, Kopp S. Intraoperative use of low volume ventilation to decrease postoperative mortality, mechanical ventilation, lengths of stay and lung injury in adults without acute lung injury. Cochrane Database Syst Rev 2018; 7:CD011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marret E, Cinotti R, Berard L, et al. the PPV study group. Protective ventilation during anaesthesia reduces major postoperative complications after lung cancer surgery: a double-blind randomised controlled trial. Eur J Anaesthesiol 2018; 35:727–735. [DOI] [PubMed] [Google Scholar]
- 10.Odor PM, Bampoe S, Gilhooly D, et al. Perioperative interventions for prevention of postoperative pulmonary complications: systematic review and meta-analysis. BMJ 2020; 368:m540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012; 308:1651–1659. [DOI] [PubMed] [Google Scholar]
- 12.Lachmann B. Open up the lung and keep the lung open. Intensive Care Med 1992; 18:319–321. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa FT, Castro AA, de Sousa-Rodrigues CF. Positive end-expiratory pressure (PEEP) during anaesthesia for prevention of mortality and postoperative pulmonary complications. Cochrane Database Syst Rev 2014; 6:CD007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treschan TA, Schaefer M, Kemper J, et al. PROVE Network Investigators. Ventilation with high versus low peep levels during general anaesthesia for open abdominal surgery does not affect postoperative spirometry: a randomised clinical trial. Eur J Anaesthesiol 2017; 34:534–543. [DOI] [PubMed] [Google Scholar]
- 15.Ruszkai Z, Kiss E, Molnar Z. Perioperative lung protective ventilatory management during major abdominal surgery: a hungarian nationwide survey. J Crit Care Med (Targu Mures) 2019; 5:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson X, Chereshneva M, Odor PM, et al. Adoption of lung protective ventilation in patients undergoing emergency laparotomy: the ALPINE study. A prospective multicentre observational study. Br J Anaesth 2018; 121:909–917. [DOI] [PubMed] [Google Scholar]
- 17.Treschan TA, Beiderlinden M. Role of recruitment maneuvers for lung-protective ventilation in the operating room remains unclear. Anesthesiology 2015; 122:472–473. [DOI] [PubMed] [Google Scholar]
- 18.Hartland BL, Newell TJ, Damico N. Alveolar recruitment maneuvers under general anesthesia: a systematic review of the literature. Respir Care 2015; 60:609–620. [DOI] [PubMed] [Google Scholar]
- 19.Patel JM, Baker R, Yeung J, et al. Intra-operative adherence to lung-protective ventilation: a prospective observational study. Perioper Med (Lond) 2016; 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanderer JP, Ehrenfeld JM, Epstein RH, et al. Temporal trends and current practice patterns for intraoperative ventilation at U.S. academic medical centers: a retrospective study. BMC Anesthesiol 2015; 15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karalapillai D, Weinberg L, Galtieri J, et al. Current ventilation practice during general anaesthesia: a prospective audit in Melbourne, Australia. BMC Anesthesiol 2014; 14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bender SP, Paganelli WC, Gerety LP, et al. Intraoperative lung-protective ventilation trends and practice patterns: a report from the multicenter perioperative outcomes group. Anesth Analg 2015; 121:1231–1239. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Na S, Lee WK, et al. Application of intraoperative lung-protective ventilation varies in accordance with the knowledge of anaesthesiologists: a single-centre questionnaire study and a retrospective observational study. BMC Anesthesiol 2018; 18:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hida K, Okamura R, Sakai Y, et al. Japan Society of Laparoscopic Colorectal Surgery. Open versus laparoscopic surgery for advanced low rectal cancer: a large, multicenter, propensity score matched cohort study in Japan. Ann Surg 2018; 268:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemmes SN, Severgnini P, Jaber S, et al. Rationale and study design of PROVHILO - a worldwide multicenter randomized controlled trial on protective ventilation during general anesthesia for open abdominal surgery. Trials 2011; 12:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen IL. Definitions for sepsis and organ failure. The ACCP/SCCM consensus conference committee report. Chest 1993; 103:656. [DOI] [PubMed] [Google Scholar]
- 27.O’Gara B, Talmor D. Perioperative lung protective ventilation. BMJ 2018; 362:k3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jammer I, Wickboldt N, Sander M, et al. European Society of Anaesthesiology (ESA) and the European Society of Intensive Care Medicine (ESICM), European Society of Anaesthesiology, European Society of Intensive Care Medicine. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015; 32:88–105. [DOI] [PubMed] [Google Scholar]
- 29.Bluth T, Serpa Neto A, et al. Writing Committee for the Probese Collaborative Group of the PROtective VEntilation Network for the Clinical Trial Network of the European Society of Anaesthesiology. Effect of intraoperative high positive end-expiratory pressure (PEEP) with recruitment maneuvers vs low PEEP on postoperative pulmonary complications in obese patients: a randomized clinical trial. JAMA 2019; 321:2292–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira SM, Tucci MR, Morais CCA, et al. Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology 2018; 129:1070–1081. [DOI] [PubMed] [Google Scholar]
- 31.Ostberg E, Thorisson A, Enlund M, et al. Positive end-expiratory pressure alone minimizes atelectasis formation in nonabdominal surgery: a randomized controlled trial. Anesthesiology 2018; 128:1117–1124. [DOI] [PubMed] [Google Scholar]
- 32.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013; 369:2126–2136. [DOI] [PubMed] [Google Scholar]
- 33.Bartels K, Kaizer A, Jameson L, et al. Hypoxemia within the first 3 postoperative days is associated with increased 1-year postoperative mortality after adjusting for perioperative opioids and other confounders. Anesth Analg 2020; 131:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg 2017; 152:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato H, Nakamura K, Baba Y, et al. Low tidal volume ventilation with low PEEP during surgery may induce lung inflammation. BMC Anesthesiol 2016; 16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spadaro S, Grasso S, Karbing DS, et al. Physiologic evaluation of ventilation perfusion mismatch and respiratory mechanics at different positive end-expiratory pressure in patients undergoing protective one-lung ventilation. Anesthesiology 2018; 128:531–538. [DOI] [PubMed] [Google Scholar]
- 37.Neumann P, Rothen HU, Berglund JE, et al. Positive end-expiratory pressure prevents atelectasis during general anaesthesia even in the presence of a high inspired oxygen concentration. Acta Anaesthesiol Scand 1999; 43:295–301. [DOI] [PubMed] [Google Scholar]
- 38.Girrbach F, Petroff D, Schulz S, et al. Individualised positive end-expiratory pressure guided by electrical impedance tomography for robot-assisted laparoscopic radical prostatectomy: a prospective, randomised controlled clinical trial. Br J Anaesth 2020; 125:373–382. [DOI] [PubMed] [Google Scholar]
- 39.Piriyapatsom A, Phetkampang S. Effects of intra-operative positive end-expiratory pressure setting guided by oesophageal pressure measurement on oxygenation and respiratory mechanics during laparoscopic gynaecological surgery: a randomised controlled trial. Eur J Anaesthesiol 2020; 37:1032–1039. [DOI] [PubMed] [Google Scholar]
- 40.Beitler JR, Sarge T, Banner-Goodspeed VM, et al. EPVent-2 Study Group. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2019; 321:846–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lovas A, Szakmany T. Haemodynamic effects of lung recruitment manoeuvres. Biomed Res Int 2015; 2015:478970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brueckmann B, Villa-Uribe JL, Bateman BT, et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology 2013; 118:1276–1285. [DOI] [PubMed] [Google Scholar]
- 43.Lindberg P, Gunnarsson L, Tokics L, et al. Atelectasis and lung function in the postoperative period. Acta Anaesthesiol Scand 1992; 36:546–553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.