Abstract
BACKGROUND
In Germany, hypotension induced by spinal anaesthesia is commonly treated with a combination of cafedrine hydrochloride (C, 200 mg) and theodrenaline hydrochloride (T, 10 mg) in 2 ml. We compared the effectiveness of C/T with ephedrine.
OBJECTIVES
The primary objectives were to assess the speed of onset and the ability to restore blood pressure without an increase in heart rate. Secondary objectives were to evaluate maternal/foetal outcomes and the number of required additional boluses or other additional measures.
DESIGN
HYPOTENS was a national, multicentre, prospective, open-label, two-armed, noninterventional study comparing C/T with ephedrine in two prospectively defined cohorts. This study relates to the cohort of patients receiving spinal anaesthesia for caesarean section.
SETTING
German hospitals using either C/T or ephedrine in their routine clinical practice.
PATIENTS
Women aged at least 18 years receiving spinal anaesthesia for caesarean section.
INTERVENTIONS
Bolus administration of C/T or ephedrine at the discretion of the attending anaesthesiologist.
MAIN OUTCOME MEASURES
Endpoints within 15 min after initial administration of C/T or ephedrine were area under the curve between the observed SBP and the minimum target SBP; and incidence of newly occurring heart rate of at least 100 beats min−1.
RESULTS
Although effective blood pressure stabilisation was achieved with both treatments, this effect was faster and more pronounced with C/T (P < 0.0001). The incidence of tachycardia and changes in heart rate were higher with ephedrine (P < 0.01). Fewer additional boluses (P < 0.01) were required with C/T. Although favourable neonatal outcomes were reported in both groups, base deficit and lactate values were greater with ephedrine (P < 0.01). Physician satisfaction was higher with C/T.
CONCLUSIONS
After C/T, tachycardia was not a problem, providing an advantage over ephedrine. Fewer additional boluses were required with C/T, suggesting greater effectiveness. An increased base deficit with ephedrine suggests reduced oxygen supply or increased demands in foetal circulation.
TRIALS REGISTRATION
Clinicaltrials.gov: NCT02893241, German Clinical Trials Register: DRKS00010740.
Introduction
Spinal anaesthesia-induced hypotension (SAH) occurs frequently during caesarean section and is primarily caused by sympathetic nerve blockade. SAH has a negative impact on patient wellbeing, and in the awake parturient, it causes nausea, dizziness and vomiting.1 Maternal hypotension can also have adverse effects on the foetus by decreasing uteroplacental blood flow, negatively affecting Apgar scores as well as causing foetal acidosis and other signs of distress such as tachypnoea.2,3 Rapid and precise restoration of blood pressure is therefore essential to prevent negative maternal and neonatal outcomes.
Preventive measures such as fluid pre- or co-loading can be used for the management of maternal hypotension. However, pharmacological treatment with vasoactive substances has become the method of choice in recent years.4 Phenylephrine and ephedrine are both commonly used during caesarean section and are recommended by international guidelines.2 However, due to effects on the foetal circulation, the choice of vasopressor can be controversial, with the use of ephedrine being associated with negative effects on foetal blood base excess and pH.5,6 Furthermore, phenylephrine has been shown to affect maternal cardiac output by significantly reducing heart rate.7 In recent treatment algorithms, a stratified approach has been suggested where phenylephrine or ephedrine is selected depending on the heart rate of the parturient. Results from clinical practice surveys indicate there has been a shift to the use of phenylephrine as the first-line choice for treatment and prevention of maternal hypotension.2,8,9 Investigation of other agents is a focus of current research and there is particular interest in the use of norepinephrine as an alternative.10 However, the dosing strategy for norepinephrine may depend on repetitive boluses that require additional input, or the use of smart infusion pumps.11,12
In Germany, a 20 : 1 mixture of cafedrine/theodrenaline (C/T, Akrinor, ratiopharm GmbH, Ulm, Germany), which can be administered via bolus injection, is an established alternative to treat hypotension. A nationwide survey conducted in 2011 found that C/T was the vasopressor of choice in 86% of surveyed anaesthesiology departments.13 The α1 and β1 adrenoceptor activity of C/T produces a rapid and sustained increase in blood pressure without negative effects on umbilical cord arterial pH or Apgar scores.14,15 In addition, maternal heart rate appears to remain stable during treatment with C/T.16,17 In a recent retrospective study, the efficacy and safety of C/T was demonstrated to be comparable with ephedrine and phenylephrine for the treatment of hypotension in patients undergoing caesarean section.14
Here, we report results comparing C/T with ephedrine in a prospectively defined cohort of parturients from the HYPOTENS study who received spinal anaesthesia for caesarean section.18 The results for a cohort of patients undergoing general anaesthesia for surgical procedures with an increased risk of developing intra-operative hypotension, in patients aged ≥50 years with comorbidities, have been published previously.19 The primary objective was to investigate the speed of onset and the ability of each drug to restore blood pressure without a clinically relevant increase in heart rate. Secondary objectives included maternal and foetal outcome parameters and assessments of the number of additional boluses of drug or additional measures required to treat hypotension.
Materials and methods
HYPOTENS (clinicaltrials.gov: NCT02893241, German Clinical Trials Register: DRKS00010740) was a national, multicentre, prospective, open-label, two-armed, noninterventional study, designed to compare the effectiveness of C/T with ephedrine for treatment of intra-operative hypotension in two prospectively defined cohorts. A detailed description of the HYPOTENS study design, recruitment, patient population and endpoints has been published previously.18
The ethics committee of the Philipps University of Marburg approved the study on 15 April 2016 (Az.14/16; chairman: Professor Dr G. Richter). Confirmation of this approval was provided to participating physicians so the study could be approved by their local ethics committees. Written informed consent was obtained from all patients. The study complied with the latest version of the Declaration of Helsinki.
Patient population
Patients were recruited between July 2016 and February 2018 from 34 participating centres that used either C/T or ephedrine in their routine clinical practice. A computer-aided matching process was used to pair similar departments for comparison based on prespecified criteria.18 Women at least 18 years of age who received spinal anaesthesia for elective (or nonemergency) caesarean section and who experienced hypotension requiring drug treatment were included in this cohort. Patients with contraindications to the use of C/T or ephedrine, those who received prophylactic treatment with either study drug or other antihypertensive agents, or patients with sepsis, septic shock or systemic inflammatory response syndrome were excluded from the study. Patients with high-risk pregnancies, severe foetal malformation, multigravid pregnancies, chorioamnionitis or those who underwent emergency caesarean section were also excluded.
Definition of hypotension
Hypotension was defined as a SBP less than 100 mmHg and/or a drop of more than 10% compared with the baseline pre-operative SBP.
Study design
Patients were treated with either C/T or ephedrine depending on the drug routinely used in the participating institution. According to the noninterventional nature of the study, the attending anaesthesiologist made the decision whether to treat a patient for hypotension and therefore include them in the study. For each patient, an individually defined minimum target SBP (SBPmin) was determined by the anaesthetist, based on comorbidities and the clinical situation, and documented before the initial bolus was administered. This target SBPmin served as a benchmark for all measurements taken after the initial treatment. The observation period was for 15 min after the first dose of drug. Any additional boluses or additional measures to control blood pressure were also recorded.
Study medication
C/T (cafedrine hydrochloride 200 mg, theodrenaline hydrochloride 10 mg, per 2 ml solution for injection, ratiopharm GmbH, Ulm, Germany) and ephedrine (ephedrine hydrochloride 10 mg per 1 ml solution for injection, Sintetica GmbH, Münster, Germany) were used in this study. Throughout the manuscript, the dose of C/T administered is expressed in terms of cafedrine only. The attending anaesthesiologist determined the bolus dose. Anaesthesiologists were permitted to alter or initiate use of concomitant medications at any time.
Trial endpoints
Two primary endpoints were assessed for the observation period: The area under the curve (AUC) between the observed SBP and the SBPmin and the incidence of newly occurring heart rate of at least 100 beats min−1. For the area under the curve, the differences between the measured blood pressure and SBPmin were squared so that high deviations were over-proportionally weighted.18
To confirm superiority of either study medication, at least one of the two primary endpoints needed to be demonstrated. A multiple testing situation was taken into account by adjusting the alpha level (0.025). Changes from baseline in SBP and heart rate after the initial injection of drug were investigated during post hoc analyses. Secondary endpoints analysed during the trial included neonatal outcome parameters: Apgar score, umbilical arterial pH, base deficit and lactate. Other endpoints included the number of additional boluses of drug or accompanying measures to treat the hypotension (e.g. fluid infusion, maternal position changes, norepinephrine and so on) within the first 15 min after the initial injection of the study drug. See Supplemental Data 1 for a list of all secondary endpoints. Physicians were prompted to assess treatment satisfaction and the effectiveness of the study drug for each patient in terms of speed of onset and precision with a numerical rating scale from one (very good) to six (very poor).
Safety
An adverse drug reaction (ADR) was defined as ‘any noxious and unintended response to a medicinal product, which occurs either following approved administration of the medicinal product at the normal dosage or following incorrect use as a result of medication errors, overdose, misuse (off-label use) and abuse’.
Statistical analysis
Analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). Details on the statistical analyses performed in this study have been published previously.18,19 The results presented are the estimated differences between treatment arms according to the mixed model analyses (direction: C/T compared to ephedrine (E)).
Results
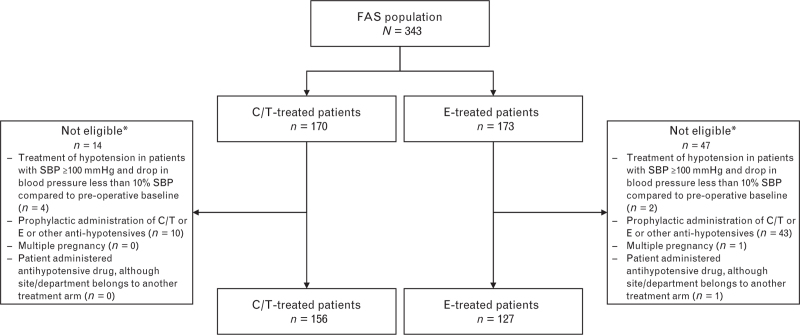
A total of 343 patients (C/T, n = 170; E, n = 173) were enrolled in this cohort (Fig. 1). Of these patients, 283 patients (C/T, n = 156; E, n = 127; 82.5%) were treated according to the monitoring plan (per-protocol analysis set). The most common reason for exclusion was ‘Prophylactic administration of C/T or ephedrine or other antihypotensives’ (C/T, n = 10; E, n = 43; P < 0.0001).
Fig. 1.
Flow chart showing patients analysed as part of the HYPOTENS caesarean section cohort
∗Patients may be included in more than one exclusion category. C/T, cafedrine/theodrenaline; E, ephedrine; FAS, full analysis set.
Maternal characteristics were comparable between treatment groups (Tables 1 and 2). The proportion of patients with cardiovascular comorbidities was slightly higher in the C/T arm, but not significantly so (P = 0.31). The most common cardiovascular comorbidity was hypertension: C/T, n = 8/12 patients (66.7%); E, n = 4/6 patients (66.7%).
Table 1.
Maternal characteristics
| Parameter | C/T (n = 156) | E (n = 127) | P |
| Age (years) | 34 ± 5 | 33 ± 5 | 0.887 |
| Weight (kg) | 88 ± 20 | 88 ± 20 | 0.891 |
| Height (cm) | 166 ± 7 | 166 ± 7 | 0.987 |
| ASA physical status | |||
| I | 49 (31) | 47 (37) | 0.323 |
| II | 105 (67) | 77 (61) | 0.244 |
| IIII | 2 (1) | 3 (2) | 0.493 |
| Comorbidities | 66 (42) | 53 (42) | 0.922 |
| Cardiovascular comorbidities | 12 (8) | 6 (5) | 0.309 |
| Left-side positioning | |||
| 0° to 10° | 98 (63) | 69 (54) | 0.149 |
| 10° to 20° | 55 (35) | 53 (42) | 0.265 |
| 20° to 30° | 3 (2) | 5 (4) | 0.309 |
| Local anaesthetics | |||
| Bupivacaine hyperbaric | 104 (67) | 57 (45) | <0.001 |
| Bupivacaine isobaric | 45 (29) | 62 (49) | <0.001 |
| Other (Hyperbaric or Isobaric) | 7 (5) | 8 (6) | 0.499 |
Data are mean ± SD or n (%).
ASA, American Society of Anesthesiologists; C/T, cafedrine/theodrenaline; E, ephedrine.
Table 2.
Maternal haemodynamic parameters: pre-operative and at the time hypotension was diagnosed
| Parameter | C/T (n = 156) | E (n = 127) | P |
| Pre-operative | |||
| SBP (mmHg) | 139.0 ± 19.1 | 137.6 ± 17.7 | 0.8112 |
| DBP (mmHg) | 81.5 ± 13.5 | 81.9 ± 13.5 | 0.8677 |
| HR (beats min−1) | 92.6 ± 14.9 | 92.9 ± 15.8 | 0.9139 |
| At time of diagnosis | |||
| SBP (mmHg) | 92 ± 16 | 92 ± 13 | 0.6142 |
| DBP (mmHg) | 50 ± 13 | 50 ± 11 | 0.5837 |
| HR (beats min−1) | 86 ± 22 | 87 ± 23 | 0.6153 |
| MAP (mmHg) | 64 ± 13 | 64 ± 10 | 0.5367 |
| Targeted SBP increase (mmHg) | 25 ± 12 | 20 ± 10 | <0.0001 |
Data are mean ± SD.
C/T, cafedrine/theodrenaline; E, ephedrine; HR, heart rate; MAP, mean arterial pressure.
The diagnosis of hypotension and administration of the initial C/T or E dose was made on average 8.5 min (C/T) and 7.9 min (E) after the start of anaesthesia (Table 3).
Table 3.
Baseline characteristics for the caesarean section and the newborns
| C/T (n = 156) | E (n = 127) | ||||
| Parameter | n | n | P | ||
| Baseline caesarean section characteristics | |||||
| Gestation (weeks) | 156 | 38.5 ± 1.4 | 127 | 38.4 ± 1.5 | 0.7355 |
| Primary/secondary caesarean section (%) | 156 | 92 / 8 | 127 | 90 / 10 | 0.5815 |
| Start of anaesthesia to | |||||
| Diagnosis of hypotension (min) | 156 | 8.5 ± 6.4 | 127 | 7.9 ± 5.6 | 0.5619 |
| Incision (min) | 156 | 13.7 ± 5.8 | 127 | 13.2 ± 5.1 | 0.8075 |
| Uterotomy (min) | 156 | 18.5 ± 7.8 | 127 | 17.6 ± 5.9 | 0.7206 |
| Cord clamping (min) | 156 | 20.2 ± 8.0 | 127 | 19.2 ± 6.1 | 0.5404 |
| Neonatal characteristics and outcome | |||||
| Umbilical arterial pH | 142 | 7.32 ± 0.05 | 117 | 7.31 ± 0.07 | 0.3371 |
| Umbilical venous pH | 86 | 7.36 ± 0.05 | 73 | 7.34 ± 0.07 | 0.1134 |
| Foetal acidosis (UA pH < 7.2) (%) | 142 | 2.8 | 117 | 7.7 | 0.0737 |
| UA base deficit | 132 | –1.11 ± 2.15 | 103 | –1.98 ± 2.35 | 0.0093 |
| Weight (g) | 156 | 3421 ± 534 | 127 | 3312 ± 536 | 0.1970 |
| Male/Female (%) | 156 | 58 / 42 | 127 | 54 / 47 | 0.4192 |
| Lactate (mg dl−1) | 70 | 18.1 ± 6.2 | 25 | 25.8 ± 13.5 | 0.0060 |
Data are mean ± SD, or %.
C/T, cafedrine/theodrenaline; E, ephedrine; UA, umbilical cord arterial blood.
Maternal haemodynamic parameters are summarised in Table 2. SBP (mean ± SD) at the time of diagnosis of hypotension was 92 ± 16 mmHg in the C/T arm and 92 ± 13 mmHg in the ephedrine arm. Mean targeted SBP increase, defined as the difference between the minimum target SBP value assigned by the attending anaesthetist and the SBP value taken at diagnosis of hypotension, was notably higher in the C/T arm: C/T, 25 ± 12 mmHg; E, 20 ± 10 mmHg; P < 0.0001.
The mean initial dose of study drug was 0.83 ± 0.43 mg kg−1 (70 ± 34 mg) in the C/T arm and 0.19 ± 0.08 mg kg−1 (16 ± 7 mg) in the ephedrine arm. The mean total cumulative dose within the observation period was 1.36 ± 0.88 mg kg−1 (113.8 ± 70.7 mg) for C/T and 0.37 ± 0.25 mg kg−1 (31.1 ± 20.0 mg) for ephedrine.
Hypotension was diagnosed before the skin incision in the majority of cases. Before the incision: C/T, 80.8% (n = 126) and E, 85.8% (n = 109); at the time of the incision: C/T, 5.1% (n = 8) and E, 2.4% (n = 3): after the incision: C/T, 14.1% (n = 22) and E, 11.8% (n = 15). On average, the time between the first administration of the study drug and the incision was 5.0 ± 6.3 min in the C/T arm and 5.1 ± 5.8 min in the E arm.
No differences were observed regarding the total volumes of fluid used during the procedure (Supplemental Data 2). There was also no difference in the volume of blood lost during caesarean section between treatment arms (Supplemental Data 3), although this was lost mostly after the observation period.
Effectiveness endpoints
Area under curve between observed SBP and SBPmin
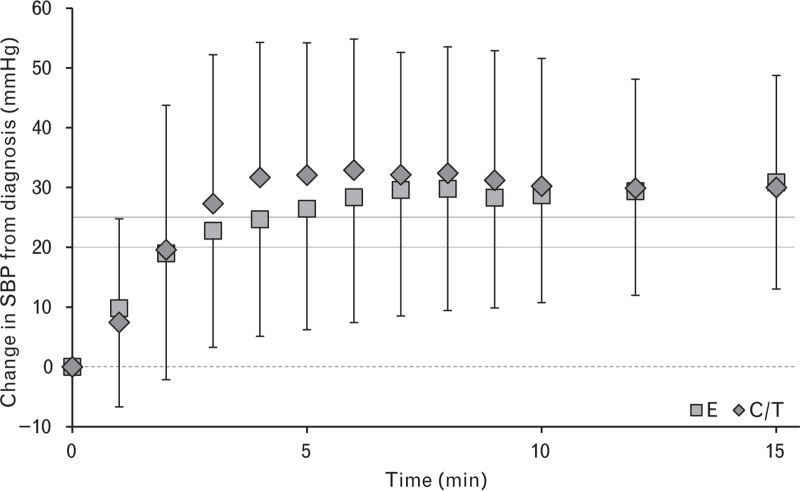
Mean AUC values were not significantly different between treatment arms: AUC values (mmHg2 ∗ min) were C/T, 12.36; E, 10.68; difference estimate (97.5% CI), 0.64 (–0.21 to 1.50); P = 0.0903 (Box-Cox transformation was performed in an effort to normalise skewed AUC data). Despite this, post hoc analysis showed that for the combined total of all SBP measurements during the 15-min observation period the mean SBP increase from diagnosis was faster and more pronounced in the C/T arm (difference estimate (95% CI), 3.46 mmHg [2.12 to 4.81]; P < 0.0001; Fig. 2).
Fig. 2.
Change in SBP during the first 15 min after the diagnosis of hypotension and the administration of C/T or E
Data are mean ± SD. The dotted horizontal line indicates the blood pressure level at diagnosis. Dark and light grey lines represent SBPmin for both C/T and E, respectively. C/T, cafedrine/theodrenaline; E, ephedrine.
New occurrence tachycardia (heart rate ≥100 beats min−1)
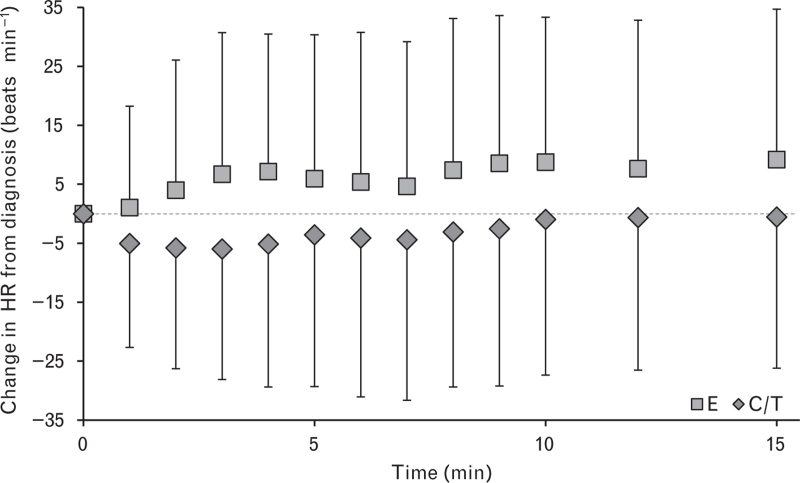
In the per-protocol analysis set, 139 (49.1%) patients experienced a new occurrence of tachycardia during the first 15 min after drug administration. A significantly higher incidence of newly occurring tachycardia was observed in E-treated patients [C/T, 40.4% (n = 63); E, 59.8% (n = 76)]. The overall treatment effect was found to be statistically significant [odds ratio (OR) estimate (97.5% CI): 0.45 (0.25 to 0.80), P = 0.0021]. A treatment-dependent effect was shown on absolute change in heart rate (beats min−1) from diagnosis, with C/T showing lower values during the first 15 min after administration (Fig. 3). The overall difference was statistically significant [difference estimate (95% CI): –8.9 beats min−1 (–10.5 to –7.3), P < 0.0001]. A transient decrease in heart rate from baseline levels was observed in the C/T arm but heart rate was increased in the E arm.
Fig. 3.
Change in heart rate during the first 15 min after the diagnosis of hypotension and the administration of C/T or E
Data are mean ± SD. The dotted horizontal line indicates the HR at diagnosis. C/T, cafedrine/theodrenaline; E, ephedrine; HR, heart rate.
Treatment with C/T required fewer additional boluses and accompanying measures
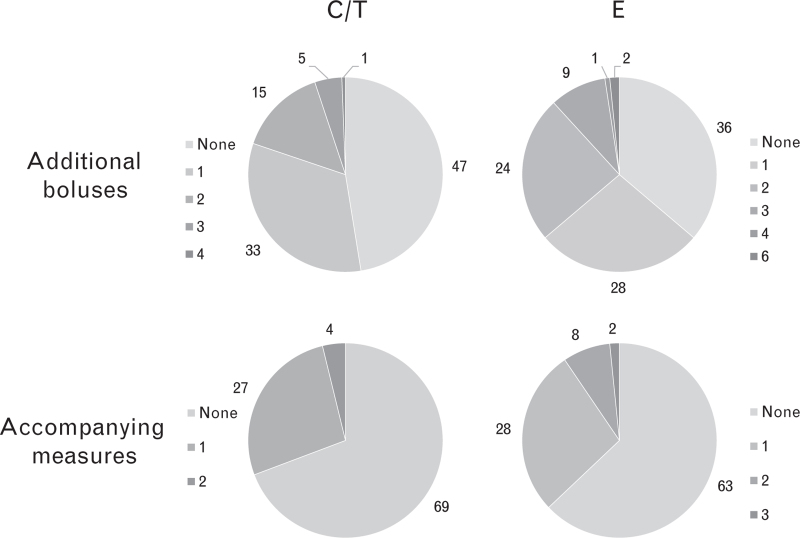
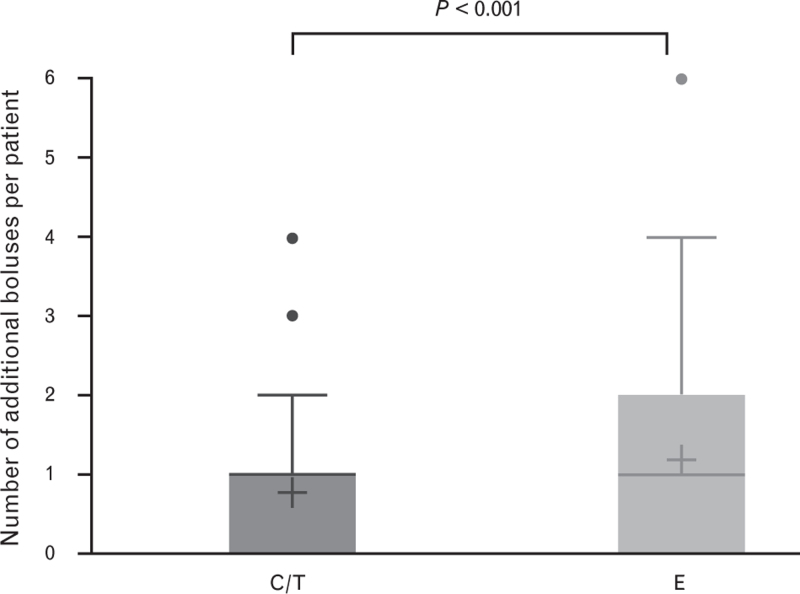
The proportion of patients requiring no additional boluses was numerically higher in the C/T arm, but not significantly so: C/T, 47.4%; E, 36.2%; P = 0.269 (Fig. 4). The mean number of additional boluses was significantly lower in the C/T arm: C/T, 0.8; E, 1.2; P < 0.01 (Fig. 5). Although the proportion of patients in the C/T group requiring no additional accompanying measures was lower than in the E group, this was not statistically significant: C/T, 69.2%; E, 63.0%; P = 0.0576 (Fig. 4). The mean number of accompanying measures was slightly lower in the C/T arm, but not significantly so: C/T, 0.35; E, 0.49; P = 0.174. The types of accompanying measures required for haemodynamic stabilisation included volume loading (C/T, 19.2%; E, 26.0%), positional adjustment to improve venous return (C/T, 3.2%; E, 5.5%), other drug therapy (C/T, 5.8%; E, 4.7%), and other actions within the first 15 min after the first bolus dose (C/T, 2.6%; E, 0.0%). Antiemetics, which can be used to treat the side effects of hypotension, were used more frequently in E-treated patients (C/T, 2.6%; E, 9.5%; Fisher's exact test, P = 0.0179).
Fig. 4.
Frequencies of additional drug boluses or accompanying measures used to stabilise blood pressure during the 15-min observation period in patients treated with C/T (n = 156) or E (n = 127)
The proportions of patients requiring no additional boluses or accompanying measures were not significantly different between treatment arms (P = 0.0576 and P = 0.269, respectively). C/T, cafedrine/theodrenaline; E, ephedrine.
Fig. 5.
Number of additional boluses with C/T or E up to 15 min after initial administration
Data are mean (cross), median (horizontal line), quartiles (top and bottom of the box), minimum and maximum (whisker ends) and outliers (points). Please note that the median for C/T is one. C/T, cafedrine/theodrenaline; E, ephedrine.
Newborn outcomes
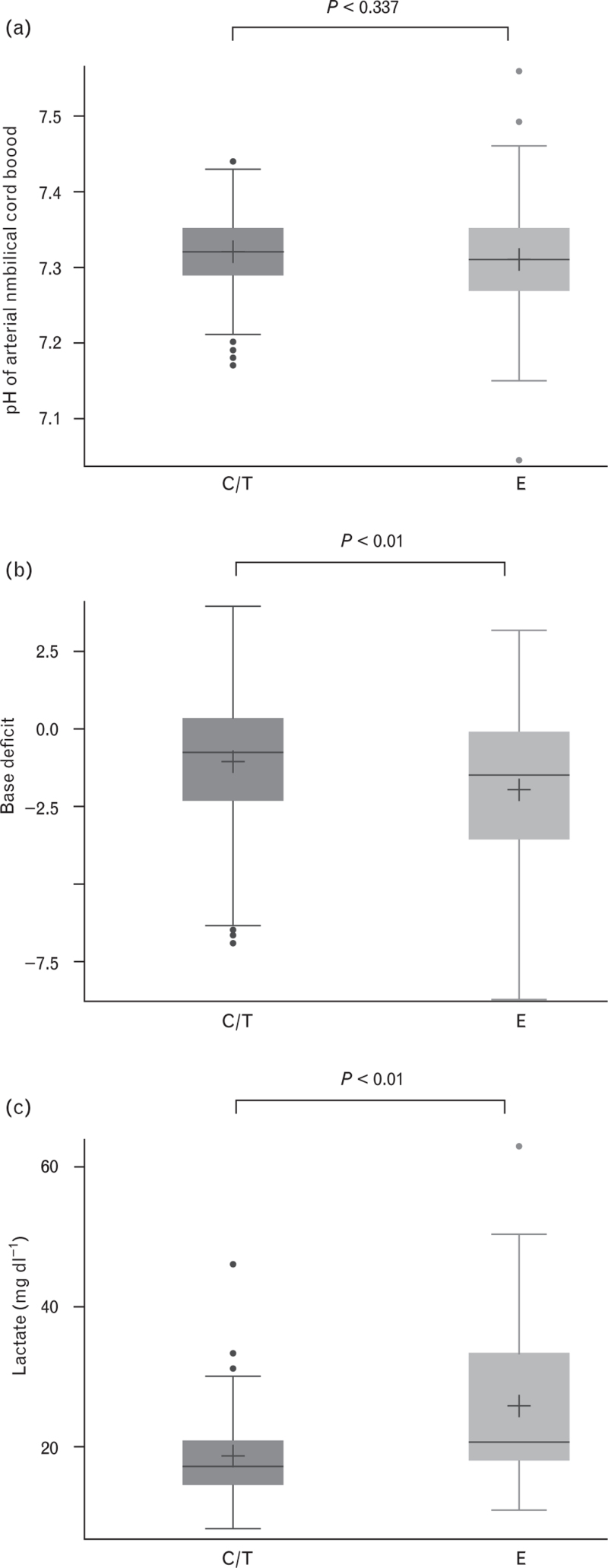
Neonatal and outcome parameters are summarised in Table 3. There were no significant differences in Apgar scores (mean ± SD) at 1 min (C/T, 9 ± 0.7; E, 9 ± 0.9; P = 0.58), 5 min (C/T, 10 ± 0.5; E, 10 ± 0.5; P = 0.75) and 10 min (C/T, 10 ± 0.3; E, 10 ± 0.4; P = 0.90). The pH of arterial umbilical cord blood was comparable in both treatment arms (Table 3 and Fig. 6a). The incidence of foetal acidosis, defined as pH less than 7.2, was numerically higher in the E arm, but not significantly different (Table 3). There was a significant increase in arterial base deficit (Table 3 and Fig. 6b) and lactate values in the E arm (Table 3 and Fig. 6c).
Fig. 6.
Neonatal outcomes, including pH of arterial umbilical cord blood (a), base deficit (b) and lactate (c)
Data are mean (cross), median (horizontal line), quartiles (top and bottom of the box), minimum and maximum (whisker ends) and outliers (points). C/T, cafedrine/theodrenaline; E, ephedrine.
Physician satisfaction and experience with C/T versus E
A mixed model analysis was used to examine endpoints relating to physician satisfaction. Speed of onset was evaluated as better in the C/T arm: odds ratio [OR (95% CI), 2.32 (1.30 to 4.12); P = 0.0045] (Table 4). Speed of onset was evaluated as being very good or good by 80% of treating anaesthesiologists in the C/T arm versus 67% in the E arm. Treatment precision was evaluated as better in the C/T arm [OR (95% CI), 2.19 (1.30 to 3.68); P = 0.0031] with treatment evaluated as very good or good by 73% of anaesthesiologists in the C/T group versus 57% in the E group. The anaesthetic proficiency of the attending anaesthetist and their familiarity with either study drug was comparable in both arms (Supplemental Data 4).
Table 4.
Physician satisfaction with cafedrine/theodrenaline or ephedrine
| Speed | Precision | |||||
| Score | C/T (n = 156) | E (n = 127) | P | C/T (n = 156) | E (n = 127) | P |
| 1 + 2 | 80% | 67% | 0.0116 | 73% | 57% | 0.0039 |
| 3 + 4 | 17% | 28% | 0.0264 | 26% | 39% | 0.0136 |
| 5 + 6 | 3% | 5% | 0.3276 | 1% | 4% | 0.1526 |
Physicians were asked to score satisfaction on a scale between 1 (very good) and 6 (very bad).
C/T, cafedrine/theodrenaline; E, ephedrine.
Safety
In the full analysis set (FAS) population (C/T, n = 170; E, n = 173), the number of patients with documented ADRs was seven in the C/T arm (4.1%) and 19 in the E arm (11.0%), Fisher's exact test: P = 0.0233. The most common ADRs were listed under the individually defined categories of ‘Cardiac General’ (C/T: 5 [2.9%]; E: 10 [5.8%]) and ‘Cardiac Arrhythmia’ (C/T: 2 [1.2%]; E: 8 [4.0%]). No severe ADRs were reported in this cohort. The number of patients experiencing adverse events was 13 (7.7%) in the C/T arm compared with 20 (11.6%) in the E arm (P = 0.219).
Discussion
The aim of this study was to compare the effectiveness of C/T with ephedrine in a cohort of patients receiving spinal anaesthesia for caesarean section. Although both phenylephrine and ephedrine are currently recommended by international guidelines for use during caesarean section,2 and low-dose norepinephrine is suggested in the literature,4 phenylephrine was not regularly available in Germany at the time this study was conceived. Phenylephrine is currently considered to be the first-line choice for treatment and prevention of SAH.2
Effective blood pressure stabilisation was achieved with both C/T and E in this cohort. Although AUC values were observed to be numerically higher in the C/T arm, the difference between treatment arms was not statistically significant. AUC values reported in the HYPOTENS study reflect both the change in SBP and the minimum target SBP defined by the treating physician. Although SBP at diagnosis of hypotension was comparable between both treatment arms, the targeted increase in SBP was significantly higher with C/T, which affected the analysis of this primary endpoint. This effect was also recently described for the general anaesthesia cohort analysed as part of the HYPOTENS study.19 The reason for this higher targeted increase in SBP with C/T is not obvious. In accordance with the noninterventional nature of the study, the determination of the target SBP, before the initial bolus was administered, was at the discretion of the anaesthetist, and differences will reflect the lack of a consensus regarding optimal blood pressure targets. Furthermore, data suggest that blood pressure targets should be individualised to reduce the risk of postoperative organ dysfunction following major surgery.20 The study protocol also endeavoured to appropriately match surgical departments based on predefined criteria, patient characteristics as well as experience of the attending anaesthetists with their preferred study drug. As discussed previously,19 one explanation could be that physicians routinely observe a stronger effect (i.e. a higher SBP) with C/T than E, and this higher SBP becomes their ‘norm’ resulting in higher targeted SBP values in the C/T arm. This is supported by secondary data showing a more pronounced SBP increase from pre-operative baseline with C/T along with the use of fewer additional interventions. In addition, physicians could be afraid of certain dose-dependent adverse effects (e.g. elevations in heart rate), avoiding high doses of ephedrine by setting lower SBP target values and preferring early intervention to counteract arterial hypotension. Indeed, a high proportion of patients in the E arm were excluded from the per-protocol analyses due to ‘prophylactic administration of C/T or E or other antihypotensives’.
The proportions of patients who received hyperbaric or isobaric bupivacaine differed significantly between treatment arms. It has been suggested in the literature that use of isobaric bupivacaine results in increased occurrence of hypotension. For example, a recent prospective cohort study showed that parturients receiving isobaric bupivacaine for elective caesarean section had a significantly higher incidence of hypotension when compared with the hyperbaric bupivacaine group (82 versus 60%, respectively).21 However, a recent systematic review showed no evidence for any differences in the use or dosing of ephedrine with hyperbaric or isobaric bupivacaine treatment groups.22 Although more patients in the ephedrine arm received isobaric bupivacaine in this study, which could be seen to affect blood pressure values, SBP at diagnosis was comparable.
The incidence of newly occurring tachycardia after initial drug administration was considerably higher in patients during caesarean section (139/283 patients, 49.1%) when compared with the general anaesthesia cohort (75/1496 patients, 5.0%).19 An elevated heart rate at the time of diagnosis of hypotension may have contributed to this marked difference. In this cohort, a 20% higher incidence of tachycardia was observed in the ephedrine arm. C/T may be more advantageous when compared with ephedrine in this respect, as tachycardia during caesarean section is an undesired side effect of vasopressor treatment. In addition, use of C/T does not require differential blood pressure management (e.g. ephedrine is currently recommended instead of phenylephrine for patients with bradycardia). Unlike pure alpha agonists, differential blood pressure management is not needed with C/T, as cardiac index even increases with C/T treatment.17 Heart rate values were shown to increase under ephedrine, while heart rate decreased transiently under C/T. This is in accordance with the results of other studies showing that heart rate is stimulated with ephedrine treatment,5,23 and ephedrine is recommended for maternal hypotension during caesarean section in the presence of bradycardia.2 No clinically relevant effect on heart rate was seen with C/T.6
Our results show that fewer additional boluses of drug and fewer accompanying measures were required to restore haemodynamic stability (e.g. volume loading, other drug treatments and so on) in the C/T arm. This implies a favourable effect of C/T, as treatment of SAH seems to be less demanding.16 More antiemetics were administered in the ephedrine arm compared with the C/T arm, which suggests a higher incidence of maternal nausea/vomiting as a presentation of symptomatic hypotension in the ephedrine arm. It is important to note that maternal nausea/vomiting can be prevented through adequate management of circulatory parameters, and the use of antiemetics should be restricted to high-risk patients.1 In addition, the antiemetic ondansetron can potentially be administered to attenuate spinal anaesthesia-induced hypotension and brachycardia.24
Apgar scores and arterial pH values indicated good neonatal outcomes in this study. These data confirm previous results showing that C/T treatment did not have a negative impact on umbilical cord pH and Apgar scores.15 However, mean arterial base deficit values were significantly more negative in the ephedrine arm, and lactate levels higher, suggesting a reduction in oxygen supply or increased metabolic demands in foetal circulation. This is in accordance with a previous study, in which ephedrine-treated patients were found to have significantly more negative umbilical (arterial and venous) base excess values than phenylephrine treated patients.6
The subjective evaluation of treatment satisfaction by the attending anaesthetist was superior in the C/T arm. This result is not surprising given that fewer boluses and additional measures were required in this treatment arm. These observations and the interpretation of these findings may be even more striking, considering that the attending anaesthesiologists had comparable levels of experience with the study drug used routinely in their department. This tends to circumvent one of the limitations of randomised controlled trials; medical staff may be much more familiar with one intervention compared with another intervention. In addition, because C/T does not require a continuous infusion, physicians may prefer this treatment to other options, such as norepinephrine, for which administration with syringe pumps is recommended. Bolus application of norepinephrine requires repetitive dosing due to a relatively short half-life, with a median of nine boluses (interquartile range: 6 to 14) being reported between induction of spinal anaesthesia and delivery in a previous study.25
There are some limitations in our study. Although anaesthetists were advised to act appropriately to avoid hypotension, we did not collect data on specific prophylactic preloads and co-loads. As with any noninterventional study, there are concerns about certain biases when compared with randomised controlled trials. In particular, the physicians’ experience with one or both study drugs varied, which could impact the results when compared with a randomised trial. In addition, the use of nonequivalent doses between treatment arms could have an effect on the number of additional measures required; if one study drug is under-dosed compared with the other, then additional measures and/or additional boluses may be required. As equivalent doses of C/T and ephedrine have not been described in the literature, these results should be interpreted with caution. Further research is warranted with a direct comparison of equivalent doses in a controlled clinical setting. However, on the basis of the experience each anaesthetist has with the drug used in their centre, it can be assumed that the most efficient dosing regimen was used to achieve the desired blood pressure in a short time, without leading to hypertensive reactions. Thus, in view of these considerations, nonblinded and nonrandomised trials may also be associated with some unique features that actually represent strengths. Furthermore, the estimates of equipotent drug doses obtained during this trial may guide the design of subsequent randomised controlled trials.
Conclusion
Both study treatments stabilised blood pressure effectively. However, there was a more pronounced increase in SBP in the C/T arm and physicians using C/T also required fewer additional boluses, suggesting a better efficacy of C/T therapy. C/T also conferred benefits with regard to maintenance of a stable heart rate. Apgar scores and umbilical cord blood pH were comparable between treatment arms. However, base deficit was greater in the ephedrine arm, which suggests reduced oxygen supply or increased foetal demands.
Supplementary Material
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: the authors acknowledge the medical writing assistance of Physicians World Europe GmbH, Mannheim, Germany, funded by ratiopharm GmbH.
Financial support and sponsorship: funding was provided by ratiopharm GmbH, Ulm, Germany.
Conflicts of interest: P. Kranke has received lecturing fees from ratiopharm GmbH (part of the Teva Group) as well as from Sintetica and supports ongoing research from ratiopharm. G. Geldner has received travel reimbursements and a fee for his support of ratiopharm GmbH and supports ongoing research from ratiopharm. D. Chappell has received lecturing fees from ratiopharm GmbH and Sintetica. S. Huljic is an employee of ratiopharm GmbH. T. Koch has received consulting fees from ratiopharm GmbH and supports ongoing research from ratiopharm. T. Keller and S. Weber were subcontractors of the CRO commissioned by the sponsor (Mediveritas GmbH, Munich). L. Eberhart has received consultancy and lecturing fees from ratiopharm GmbH and supports ongoing research from ratiopharm. P. Kienbaum has been consulting for Baxter Gmbh, orion Pharma, Air Liquide and received lecturing fees from ratiopharm GmbH. O. Kunitz, U. Linstedt, J. Wallenborn and H. J. Gerbershagen declare no conflicts of interest.
Presentation: data from this cohort have been presented in a poster at the Deutscher Anästhesiecongress (DAC), 9–11 May 2019, Leipzig.
Data sharing: qualified researchers may request access to patient level data and related study documents, including the study protocol and the statistical analysis plan. Requests will be reviewed for scientific merit, product approval status, and conflicts of interest. Patient level data will be de-identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please E-Mail USMedInfo@tevapharm.com to make your request.
HYPOTENS study group:
Footnotes
Published online 20 February 2021
Supplemental digital content is available for this article.
References
- 1.Jelting Y, Klein C, Harlander T, et al. Preventing nausea and vomiting in women undergoing regional anesthesia for cesarean section: challenges and solutions. Local Reg Anesth 2017; 10:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinsella SM, Carvalho B, Dyer RA, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia 2018; 73:71–92. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Lumbreras-Marquez MI, Farber MK, et al. Transient tachypnea of newborns is associated with maternal spinal hypotension during elective cesarean delivery: a retrospective cohort study. Anesth Analg 2019; 129:162–167. [DOI] [PubMed] [Google Scholar]
- 4.Ngan Kee WD. The use of vasopressors during spinal anaesthesia for caesarean section. Curr Opin Anaesthesiol 2017; 30:319–325. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DW, Carpenter M, Mowbray P, et al. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology 2002; 97:1582–1590. [DOI] [PubMed] [Google Scholar]
- 6.Ngan Kee WD, Khaw KS, Tan PE, et al. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology 2009; 111:506–512. [DOI] [PubMed] [Google Scholar]
- 7.Dyer RA, Reed AR, van Dyk D, et al. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology 2009; 111:753–765. [DOI] [PubMed] [Google Scholar]
- 8.Burns SM, Cowan CM, Wilkes RG. Prevention and management of hypotension during spinal anaesthesia for elective Caesarean section: a survey of practice. Anaesthesia 2001; 56:794–798. [DOI] [PubMed] [Google Scholar]
- 9. Webster L, Allman L, Iqbal S, Carling A. Obstetric Anaesthetists’ Association. Survey 109 2013. https://www.oaa-anaes.ac.uk/ui/content/content.aspx?ID=118. [Accessed 20 October 2020]. [Google Scholar]
- 10.Ngan Kee WD, Lee SW, Ng FF, et al. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology 2015; 122:736–745. [DOI] [PubMed] [Google Scholar]
- 11. Sintetica GmbH. Prescribing information - Sinora 2019 [in German]. https://sintetica.de/wp-content/uploads/2019/08/FI-Sinora-1mgml-Konz-z-Herst-einer-Infus-DE-Apr-2019.pdf. [Accessed 23 January 2020]. [Google Scholar]
- 12.Wang X, Shen X, Liu S, et al. The efficacy and safety of norepinephrine and its feasibility as a replacement for phenylephrine to manage maternal hypotension during elective cesarean delivery under spinal anesthesia. Biomed Res Int 2018; 2018:1869189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus HE, Behrend A, Schier R, et al. Anesthesiological management of Caesarean sections : nationwide survey in Germany. Anaesthesist 2011; 60:916–928. German. [DOI] [PubMed] [Google Scholar]
- 14.Chappell D, Helf A, Gayer J, et al. Antihypotensive drugs in cesarean sections : Treatment of arterial hypotension with ephedrine, phenylephrine and Akrinor® (cafedrine/theodrenaline) during cesarean sections with spinal anesthesia. Anaesthesist 2019; 68:228–238. German. [DOI] [PubMed] [Google Scholar]
- 15.Clemens KE, Quednau I, Heller AR, Klaschik E. Impact of cafedrine/theodrenaline (Akrinor() on therapy of maternal hypotension during spinal anesthesia for cesarean delivery: a retrospective study. Minerva Ginecol 2010; 62:515–524. [PubMed] [Google Scholar]
- 16.Bein B, Christ T, Eberhart LH. Cafedrine/Theodrenaline (20:1) is an established alternative for the management of arterial hypotension in Germany-a review based on a systematic literature search. Front Pharmacol 2017; 8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitzel M, Hammels P, Schorer C, et al. Hemodynamic effects of cafedrine/theodrenaline on anesthesia-induced hypotension. Anaesthesist 2018; 67:766–772. German. [DOI] [PubMed] [Google Scholar]
- 18.Eberhart L, Geldner G, Huljic S, et al. A noninterventional comparative study of the 20:1 combination of cafedrine/theodrenaline versus ephedrine for the treatment of intra-operative arterial hypotension: the ‘HYPOTENS’ study design and rationale. Curr Med Res Opin 2018; 34:953–961. [DOI] [PubMed] [Google Scholar]
- 19.Eberhart E, Geldner G, Kowark A, et al. Treatment of intraoperative hypotension with cafedrine/theodrenaline versus ephedrine in patients receiving general anesthesia in HYPOTENS, a prospective, national, multicenter, noninterventional study. Der Anaesthesist 2020; doi: 10.1007/s00101-020-00877-5. [Online ahead of print]. [Google Scholar]
- 20.Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318:1346–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helill SE, Sahile WA, Abdo RA, et al. The effects of isobaric and hyperbaric bupivacaine on maternal hemodynamic changes post spinal anesthesia for elective cesarean delivery: a prospective cohort study. PLoS One 2019; 14:e0226030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sng BL, Han NLR, Leong WL, et al. Hyperbaric vs. isobaric bupivacaine for spinal anaesthesia for elective caesarean section: a Cochrane systematic review. Anaesthesia 2018; 73:499–511. [DOI] [PubMed] [Google Scholar]
- 23.Cleary-Goldman J, Negron M, Scott J, et al. Prophylactic ephedrine and combined spinal epidural: maternal blood pressure and fetal heart rate patterns. Obstet Gynecol 2005; 106:466–472. [DOI] [PubMed] [Google Scholar]
- 24.Tubog TD, Kane TD, Pugh MA. Effects of ondansetron on attenuating spinal anesthesia-induced hypotension and bradycardia in obstetric and nonobstetric subjects: a systematic review and meta-analysis. AANA J 2017; 85:113–122. [PubMed] [Google Scholar]
- 25.Sharkey AM, Siddiqui N, Downey K, et al. Comparison of intermittent intravenous boluses of phenylephrine and norepinephrine to prevent and treat spinal-induced hypotension in cesarean deliveries: randomized controlled trial. Anesth Analg 2019; 129:1312–1318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.