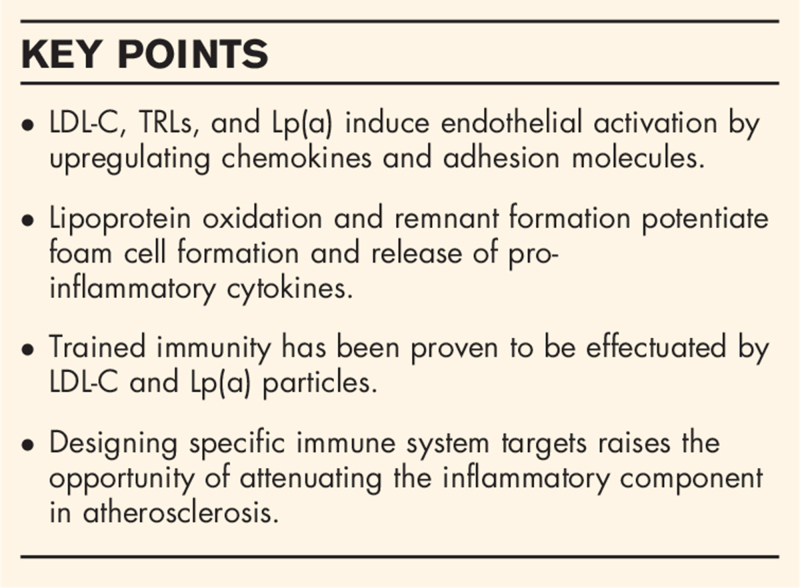
Abstract
Purpose of review
Lipid-mediated atherogenesis is hallmarked by a chronic inflammatory state. Low-density lipoprotein cholesterol (LDL-C), triglyceride rich lipoproteins (TRLs), and lipoprotein(a) [Lp(a)] are causally related to atherosclerosis. Within the paradigm of endothelial activation and subendothelial lipid deposition, these lipoproteins induce numerous pro-inflammatory pathways. In this review, we will outline the effects of lipoproteins on systemic inflammatory pathways in atherosclerosis.
Recent findings
Apolipoprotein B-containing lipoproteins exert a variety of pro-inflammatory effects, ranging from the local artery to systemic immune cell activation. LDL-C, TRLs, and Lp(a) induce endothelial dysfunction with concomitant activation of circulating monocytes through enhanced lipid accumulation. The process of trained immunity of the innate immune system, predominantly induced by LDL-C particles, hallmarks the propagation of the low-grade inflammatory response. In concert, bone marrow activation induces myeloid skewing, further contributing to immune cell mobilization and plaque progression.
Summary
Lipoproteins and inflammation are intertwined in atherogenesis. Elucidating the inflammatory pathways will provide new opportunities for therapeutic agents.
Keywords: atherosclerosis, cardiovascular disease, immune system, inflammation, lipoproteins
INTRODUCTION
Atherosclerosis is a multifactorial process, and lipid accumulation and subsequent inflammation have been shown to play a central permissive role [1]. In the course of further progression of the atherosclerotic lesion, many other inflammatory cells come into play, resulting in a local and systemic chronic low-grade inflammatory state.
Apolipoprotein B (apoB)-containing lipoproteins are causally related with atherosclerotic cardiovascular disease (ASCVD). These comprise low-density lipoprotein (LDL), triglyceride rich lipoproteins (TLRs), very-low-density lipoprotein (VLDL), remnant particles (remnants particles (remnants of VLDL and chylomicrons), lipoprotein(a) [Lp(a)] and intermediate-density lipoprotein (IDL). The number of apoB particles, which mostly are LDL particles, is loglinearly associated with ASCVD risk [2]. Therefore, the number of apoB containing TRLs is similarly associated [3]. In addition, Lp(a) emerges as a third causal and independent risk factor [4]. These lipoproteins promote inflammation by upregulating multiple pathways, both locally and systemically. Many patients continue to suffer from CVD events, despite reaching guideline-recommended lipid target levels. Part of this residual risk is attributed to inflammation, and contemporary studies have shown that this inflammatory residual risk, as defined by C-reactive protein (CRP) plasma levels of 2 mg/L or above, is present in almost half of the patients [5–8]. Therefore, attenuating both lipoproteins and inflammation may be pivotal in further targeting ASCVD.
In this review, we will describe how LDL cholesterol (LDL-C), TRLs, and Lp(a) contributes to the inflammatory state observed at the subendothelial level, within the circulation and in immune cell production in atherogenesis.
Box 1.
no caption available
Lipids in endothelial dysfunction: the first step toward an inflammatory environment
The endothelium constitutes the first-line defense mechanism shielding the subendothelial space from entering of harmful blood-born components. A low or disturbed blood flow pattern can lead to endothelial dysfunction [9,10]. By disintegration of the glycocalyx, the surface layer of the endothelial surface, increased vesicular trafficking and open endothelial junctions, lipoproteins up to 70 nm in diameter are able to migrate and to be retained in the intimal space [11]. Once retained in a highly oxidative environment, the LDL particles are modified by exposure to oxidizing agents and enzymes forming minimally modified LDL (mmLDL). The oxidized form of Lp(a), oxidized Lp(a) [oxLp(a)], is mainly formed within the bloodstream prior to migration.
Within the early process of atherogenesis, the key inflammatory process is monocyte recruitment and accumulation, instigated by endothelial activation. This increased monocyte recruitment is mediated via increased expression of endothelial adhesion molecules (ICAM-1, VCAM-1, E-selectin and P-selectin), cytokines (MCP-1 and IL-8) and pro-inflammatory receptors (toll-like receptor 2 [TRL2]) [12–14] on either endothelial cells and/or circulating monocytes.
Low-density lipoprotein cholesterol
Subendothelial retained mmLDL contributes to monocyte chemotaxis by its binding and stimulation of endothelial secretion of MCP-1, one of the key chemoattracting proteins for monocytes [15,16]. Other chemokines, including IL-8, are upregulated by mmLDL in human aortic endothelial cells (HAEC) [17]. In addition, the endothelial adhesion molecules E-selectin, P-selectin, ICAM-1 and VCAM-1 are upregulated by mmLDL, contributing to monocyte adhesion [18,19]. In addition, the CD40/CD40L signaling pathway, which plays an important role in atherogenesis including the expression of adhesion molecules, is upregulated by mmLDL [20].
Triglyceride rich lipoproteins and remnants
Triglycerides and TRLs promote endothelial activation by upregulation of both chemo- and adhesion cytokines. The role of TRLs in MCP-1 regulation is not fully elucidated. Although some in vitro studies showed that MCP-1 expression in monocytes and macrophages was downregulated upon TRL incubation, other studies have reported MCP-1 upregulation [21–24]. Chylomicron remnants prime monocytes toward the endothelium by a direct stimulation to secrete IL-8 [21].
Activation of the endothelium was observed in human endothelial cells upon incubation with TRLs, where the mRNA expression of VCAM-1, ICAM-1, P-selectin and E-selectin was increased [25]. Stimulation of isolated human monocytes with lipolysis products of VLDL, particularly free fatty acids, resulted in monocyte adhesion and transmigration with coincides with increased expression of integrin complexes including Mac-1 and VLA-4 (ligand of VCAM-1) [26]. In addition, the expression of pro-inflammatory cytokines TNF-α, IL-1β and IL-8 was increased.
Lipoprotein(a)
For Lp(a), it has been shown that, similar to mmLDL, it is able to bind MCP-1 [15]. Also, Lp(a) increases IL-8 expression in macrophages and human endothelial cells respectively [27,28]. Similar findings for several adhesion molecules were found in cultured human endothelial cells, where Lp(a) was demonstrated to stimulate the surface and mRNA expression of ICAM-1, VCAM-1 and E-selectin [29,30]. In addition, a Mac-1 dependent transendothelial migration (TEM) pathway was also shown to be upregulated by Lp(a), activating the pro-inflammatory transcription factor NF-κB [31].
Recently, the OxPL impact on arterial endothelial cells has been examined. Lp(a) stimulated human endothelial cells had a 2-fold increase in monocyte adhesion and a concomitant 5-fold increase in TEM compared to unstimulated endothelial cells [32▪]. This was accompanied by an increased expression of chemokines and cytokines which was linked to enhancement of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase (PFKFB3)–mediated glycolysis. These data illustrate the capacity of Lp(a), and in particular the oxidized phospholipids bound to apo(a), to provoke the activation of the vascular endothelium.
Plaque development by monocyte foam cell formation and cholesterol deposition
With endothelial dysfunction, monocytes are primed for adhesion and migration. Whilst retained, monocyte differentiate into macrophages and internalize the various lipoproteins to become foam cells. Subsequently, these macrophages reprogram in a pro-inflammatory phenotype [33].
Low-density lipoprotein cholesterol
mmLDL is the main source of lipid accumulation and foam cell formation [34]. One of the adhesion receptors controlling lipid uptake, CD146, intriguingly also has been shown to trigger macrophage retention [35].
Upon stimulation of the activated endothelium and lipid accumulation, macrophages are mainly polarized into two main phenotypes, a pro-inflammatory phenotype mainly characterized by CD11c and CD80/CD86 expression (referred to as classically activated or M1 macrophages) or anti-inflammatory phenotypes which may express CD206 (alternatively activated or M2a-d, M4, Mox, Mhem macrophages) [36]. The pro-inflammatory macrophages aggravate plaque progression as has been observed in murine and human models. These macrophages produce pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α and predominate in unstable plaques [36–38]. Anti-inflammatory macrophages on the other hand, can elicit plaque regression and produce anti-inflammatory cytokines [39].
Conversely, it has been demonstrated that pro-inflammatory macrophages are more prone to mmLDL for the foam cell formation in comparison with anti-inflammatory macrophages [40]. In addition, the degree of LDL oxidation influences the macrophage differentiation; a lower oxidation level was found to shift toward a more inflammatory macrophage phenotype [41]. For both phenotypes however, mmLDL does increase expression of pro-inflammatory cytokines, suggesting an overall pro-inflammatory effect on macrophages [42].
Circulating lipoproteins can serve as a source for intracellular cholesterol crystals [43]. Following an overload of intracellular cholesterol, cholesterol crystals are formed which trigger the NLRP3 inflammasome [44]. NLRP3 plays a central role in inflammation as it induces release of IL-1β and IL-18 and downstream cytokine IL-6. This complex is central in promoting a prolonged inflammatory response in atherogenesis [45]. Regarding endothelial activation, IL-1β has been shown to stimulate ICAM-1, VCAM-1 and MCP-1 [46,47].
Furthermore, it has been demonstrated that mmLDL can activate the NLRP3 inflammasome independently of cholesterol crystals [48]. In macrophages, CD36-dependent absorption of mmLDL resulted in cholesterol crystallization and release of IL-1β.
Triglyceride rich lipoproteins and remnants
Lipoprotein lipase (LPL) hydrolyzes TGL, which results in the formation of TRL remnants and fatty acids. Remnants, although larger than LDL particles, are much smaller compared to VLDL and can cross the endothelium. One remnant particle can carry up to 4 times more cholesterol compared to one LDL-C particle, which explains why up to 30% of the postprandial cholesterol load in the apoB fraction is transported by remnant particles. Once migrated into the subendothelial space, remnants have been shown to have a lower preponderance to egress compared to LDL particles, resulting in longer retention [49,50].
Apart from receptor mediated uptake, TRLs may also be taken up by macrophages in a receptor-independent manner suggesting a potent ability of TRLs in enhancing foam cell formation [51,52].
Remnants do not require oxidation or other modifications to be phagocytized by macrophages [53]. These combined characteristics allow TRLs and remnants, despite their lower plasma concentration compared with LDL particles, to potentially contribute more cholesterol to the vessel wall than LDL can [50,54].
In addition to the direct role, TRL also contributes to atherogenesis by the products of lipolysis, such as fatty acids [55]. Upon TLR stimulation, reactive oxygen species (ROS) and pro-inflammatory proteins are produced [56,57]. LPL is not only present on the endothelium but also resides within the atherosclerotic lesions itself, which induces a very pro-atherogenic milieu locally [58].
Lipoprotein(a)
In a rabbit model, it was shown that Lp(a) particles do migrate more easily into the subendothelial space compared to LDL particles [59]. This could in part be explained by the longer plasma residence time of Lp(a), which results in a more oxidized state compared to LDL particles [60]. Lp(a) and OxPLs orchestrate a sustained pro-inflammatory and pro-atherogenic response. It has been shown that Lp(a) promotes macrophages into a pro-inflammatory phenotype and induce the production of pro-inflammatory cytokines such as IL-β and IL-6 [61,62].
In addition, the OxPL content of Lp(a) contributes to macrophage apoptosis by upregulating TRL2 and CD36-dependent signaling pathways, which results in necrotic core formation and inflammation [63].
Lipids in circulating immune cells
Lipid loading in circulating monocytes
In the previous section we described the monocyte activating role of subendothelial cholesterol. However, recent studies have shown that activation of monocytes also takes place in the bloodstream by interacting with lipoproteins. High plasma serum LDL-C levels have a profound effect on circulating monocytes by being able to enrich them with cholesterol. These monocytes are more potent to adhere to and migrate through the endothelium [64,65]. In patients with elevated LDL-C levels, monocytes displayed greater intracellular lipid content as compared to healthy controls with lower LDL-C levels [66]. Furthermore, CCR2 expression, the MCP-1 ligand, was increased which coincided with a 1.6-fold increased migratory capacity.
Monocytes have also been shown to incorporate lipid particles in response to elevated triglyceride levels, especially during the postprandial rise [67,68]. During this hypertriglyceridemia, monocytes internalized TRLs through the LPR-1 receptor. This was accompanied by CD11c and VCAM-1 upregulation, further contributing to monocyte recruitment [64,68]. In addition, fatty acids, produced by local lipolysis, can increase lipid droplet accumulation [69].
Innate immune system – trained immunity
The innate immune system has been widely considered to lack immunological memory. However, recent studies suggest that triggering the innate immune system can induce a sustained pro-inflammatory response which is known as ‘trained immunity’ [70,71]. Driven by epigenetic and metabolic reprogramming, it contributes to atherosclerosis by inducing pro-inflammatory cytokines and a monocyte phenotype.
In atherosclerosis, this phenomenon has been observed for both mmLDL and Lp(a) on a cellular level [62,72,73]. Brief exposure of isolated human monocytes to mmLDL induced prolonged expression of pro-inflammatory proteins including IL-6, IL-18 and MCP-1 [72]. In two recent studies, mmLDL priming of human monocytes and endothelium cells promoted metabolic reprogramming and epigenetic modifications to increase an innate immune cell phenotype, characterized by increased production of pro-inflammatory cytokines such as TNF-α and IL-6 [74,75▪].
The relevance of lipid-induced trained immunity was shown in a murine model [76]. LDL-receptor knock-out mice received a Western-type diet which resulted in monocyte activation as measured by CD86 expression. Mice were subsequently fed with a chow diet and after four weeks, monocytes were still found to be activated.
In line with these results are the observations of a study conducted in patients with high Lp(a) levels [62]. In these patients, isolated monocytes showed increased production of TNF-α and IL-6 upon TLR stimulation which persisted up to 7 days ex vivo and was particularly effectuated by OxPLs carried on Lp(a).
Adaptive immune system
As shown in human and murine studies, T-cell are the predominant leukocyte cells in plaque lesions [77,78]. A wide variety of T-cell subsets modulate the inflammatory response by producing either atherogenic (IL-17, IFN-γ and TNF-α) or atheroprotective (IL-10) cytokines [79]. Distinct roles for pro-atherosclerotic Th1 cells and anti-atherogenic regulatory T (Treg) cells have been observed [80▪]. T-cells are activated by peptides presenting on all nucleated cells or antigen-presenting cells (APC) [81]. Peptide epitopes of mmLDL and apoB are the main driver of a T-cell mediated response [80▪]. T-cells derived from human atherosclerotic plaques are capable of recognizing mmLDL presented by APCs [82]. ApoB peptides were shown to be another recognizing signal in murine and human cells, mainly in Tregs [83]. This indicates LDL as a self-antigen and a potential driver of an autoimmune response. However, whether lipoproteins have an overall stimulating or attenuating inflammatory effect remains to be elucidated.
Increased production of immune cells
Bone marrow
Despite significant immune cell signaling and deposition at the local level of the endothelium, a substantial role in atherogenesis is attributable to a systemic pro-inflammatory state [84–86].
In experimental models, acute events such as stroke and myocardial infarction elicit elevated monocyte and other immune cell levels [85,87]. Accordingly, in humans, an acute myocardial infarction can induce both bone marrow and remote atherosclerotic activity [84].
However, since the circulation of immune cell ranges up to only a few days, a pro-atherogenic effect of elevated monocytes implies persistent systemic immune cell mobilization. Support for this notion has been found in mice, where after induction of myocardial infarction, an increased monocyte recruitment from bone marrow niches persisted for several months [85].
In addition, increased production and mobilization of immune cells in bone marrow sites are associated with the progression rate of atherosclerosis [88–90]. It is also established that circulating monocyte levels are an independent risk factor for ASCVD [91].
In regard to dyslipidemia, hypercholesterolemia in mice promotes mobilization of bone marrow monocytes by induction of extramedullary hematopoiesis [92–94]. Both mmLDL and LDL are able to stimulate myeloid proliferation [95,96].
CONCLUSION AND CLINICAL IMPLICATIONS
Lipids and inflammation are key components in atherogenesis [97,98]. Here, we show the profound effects of dyslipidemia on a plethora of inflammatory pathways. Initiating at prone endothelial sites, the immune response extends from atherosclerotic plaques to a systemic response.
Although effective lipid lowering therapies significantly lower the ASCVD burden, current therapies do not attenuate the residual inflammatory risk. In posthoc analyses of the FOURIER trial, it was shown that patients with a pro-inflammatory phenotype, characterized by high CRP levels, were at increased risk for future ASCVD events compared to participants with normal CRP levels, despite having achieved their LDL target levels [99]. In large clinical trials it has been shown that anti-inflammatory agents lower the risk for CVD. In the Canakinumab Anti-inflammatory Thrombosis Outcome study (CANTOS) trial, the monoclonal antibody directed against IL-β canakinumab significantly lowered major adverse cardiovascular events [100]. This beneficial effect came at a price of increased risk for (fatal) infections and canakinumab has not been further developed for widespread use. In contrast, the LoDoCo2 trial which investigated colchicine in patients with chronic coronary disease, showed a beneficial effect without an increase in fatal infections [101▪▪].
Despite its challenges, targeting inflammation appears to be successful in reducing CVD risk. By combining lipid-lowering and anti-inflammatory therapy, specific modulation should benefit patients with a residual inflammatory risk in the future.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
J.M.K. declares no conflicts of interest.
G.K.H. reports research grants from the Netherlands Organization for Scientific Research (vidi 016.156.445), CardioVascular Research Initiative, European Union and the Klinkerpad fonds, institutional research support from Aegerion, Amgen, AstraZeneca, Eli Lilly, Genzyme, Ionis, Kowa, Pfizer, Regeneron, Roche, Sanofi, and The Medicines Company; speaker's bureau and consulting fees from Amgen, Aegerion, Sanofi, and Regeneron until April 2019 (fees paid to the academic institution); and part-time employment at Novo Nordisk A/S, Denmark since April 2019.
E.S.G.S. reports personal fees from Novartis, personal fees from Amgen, personal fees from Sanofi-Regeneron, personal fees from Mylan, personal fees from Esperion. All fees were paid to the institution.
J.K. has received a research grant from Oxitope Pharma BV.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Borén J, Williams KJ. The central role of arterial retention of cholesterolrich apolipoprotein-b-containing lipoproteins in the pathogenesis of atherosclerosis: A Triumph of Simplicity. Curr Opin Lipidol 2016; 27:473–483. [DOI] [PubMed] [Google Scholar]
- 2.Sniderman AD, Thanassoulis G, Glavinovic T, et al. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol 2019; 4:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ference BA, Kastelein JJP, Ray KK, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA 2019; 321:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nave AH, Lange KS, Leonards CO, et al. Lipoprotein (a) as a risk factor for ischemic stroke: a meta-analysis. Atherosclerosis 2015; 242:496–503. [DOI] [PubMed] [Google Scholar]
- 5.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report From the American Heart Association. Circulation 2021; 143:254–743. [DOI] [PubMed] [Google Scholar]
- 6.Pradhan AD, Aday AW, Rose LM, Ridker PM. Residual inflammatory risk on treatment with PCSK9 inhibition and statin therapy. Circulation 2018; 138:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker PM. Clinician's guide to reducing inflammation to reduce atherothrombotic risk: JACC review topic of the week. J Am Coll Cardiol 2018; 72:3320–3331. [DOI] [PubMed] [Google Scholar]
- 8.Riaz H, Khan SU, Lateef N, et al. Residual inflammatory risk after contemporary lipid lowering therapy. Eur Heart J Qual Care Clin Outcomes 2020; 6:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 1998; 18:677–85. [DOI] [PubMed] [Google Scholar]
- 10.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol 2004; 24: [DOI] [PubMed] [Google Scholar]
- 11.Mundi S, Massaro M, Scoditti E, Carluccio MA, et al. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res 2018; 114:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamik A, Lin Z, Kumar A, et al. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem 2007; 282:13769–13779. [DOI] [PubMed] [Google Scholar]
- 13.SenBanerjee S, Lin Z, Atkins GB, et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med 2004; 199:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yurdagul A, Chen J, Funk SD, et al. Altered nitric oxide production mediates matrix-specific PAK2 and NF-kB activation by flow. Mol Biol Cell 2013; 24:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiesner P, Tafelmeier M, Chittka D, et al. MCP-1 binds to oxidized LDL and is carried by Lipoprotein(a) in human plasma. J Lipid Res 2013; 54:1877–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy S, Hama S, Grijalva V, et al. Mitogen-activated protein kinase phosphatase 1 activity is necessary for oxidized phospholipids to induce monocyte chemotactic activity in human aortic endothelial cells. J Biol Chem 2001; 276: [DOI] [PubMed] [Google Scholar]
- 17.Mattaliano MD, Huard C, Cao W, et al. LOX-1-dependent transcriptional regulation in response to oxidized LDL treatment of human aortic endothelial cells. Am J Physiol Cell Physiol 2009; 296: [DOI] [PubMed] [Google Scholar]
- 18.Li D, Chen H, Romeo F, et al. Statins modulate oxidized low-density kipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: role of LOX-1. J Pharmacol Exp Ther 2002; 302: [DOI] [PubMed] [Google Scholar]
- 19.Pirillo A, Reduzzi A, Ferri N, et al. Upregulation of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) by 15-lipoxygenase-modified LDL in endothelial cells. Atherosclerosis 2011; 214:331–337. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Liu L, Chen H, et al. LOX-1, an oxidized LDL endothelial receptor, induces CD40/CD40L signaling in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol 2003; 23:816–821. [DOI] [PubMed] [Google Scholar]
- 21.Bentley C, Hathaway N, Widdows J, et al. Influence of chylomicron remnants on human monocyte activation in vitro. Nutr Metab Cardiovasc Dis 2011; 21:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YS, Sung HJ, Son SJ, et al. Triglyceride (TG) down-regulates expression of MCP-1 and CCR2 in PMA-derived THP-1 Macrophages. Genes Genom 2013; 35:125–130. [Google Scholar]
- 23.Armengol Lopez S, Botham KM, Lawson C. The oxidative state of chylomicron remnants influences their modulation of human monocyte activation. Int J Vasc Med 2012; 2012:942512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Napolitano M, Botham KM, Bravo E. Postprandial human triglyceride-rich lipoproteins increase chemoattractant protein secretion in human macrophages. Cytokine 2013; 63:18–26. [DOI] [PubMed] [Google Scholar]
- 25.Norata GD, Grigore L, Raselli S, et al. Postprandial endothelial dysfunction in hypertriglyceridemic subjects: molecular mechanisms and gene expression studies. Atherosclerosis 2007; 193:321–327. [DOI] [PubMed] [Google Scholar]
- 26.den Hartigh LJ, Altman R, Norman JE, Rutledge JC. Postprandial VLDL lipolysis products increase monocyte adhesion and lipid droplet formation via activation of ERK2 and NFκB. Am J Physiol Heart Circ Physiol 2014; 306: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scipione CA, Sayegh SE, Romagnuolo R, et al. Mechanistic Insights into Lp(a)-induced IL-8 expression: a role for oxidized phospholipid modification of apo(a). J Lipid Res 2015; 56:2273–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haque NS, Zhang X, French DL, et al. CC chemokine I-309 is the principal monocyte chemoattractant induced by Apolipoprotein(a) in human vascular endothelial cells. Circulation 2000; 102:786–792. [DOI] [PubMed] [Google Scholar]
- 29.Takami S, Yamashita S, Kihara S, et al. Lipoprotein(a) enhances the expression of intercellular adhesion molecule-1 in cultured human umbilical vein endothelial cells. Circulation 1998; 97:721–728. [DOI] [PubMed] [Google Scholar]
- 30.Allen S, Khan S, Tam S-P, et al. Expression of adhesion molecules by Lp(a): a potential novel mechanism for its atherogenicity. FASEB J 1998; 12: [DOI] [PubMed] [Google Scholar]
- 31.Sotiriou SN, Orlova VV, Al-Fakhri N, et al. Lipoprotein(a) in atherosclerotic plaques recruits inflammatory cells through interaction with Mac-1 Integrin. FASEB J 2006; 20:559–561. [DOI] [PubMed] [Google Scholar]
- 32▪.Schnitzler JG, Hoogeveen RM, Ali L, et al. Atherogenic Lipoprotein(a) increases vascular glycolysis, thereby facilitating inflammation and leukocyte extravasation. Circ Res 2020; 126:1346–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]; Research showing the OxPLs of Lp(a) effectuating an inflammatory response. Upon incubating human aortic endothelial cells with Lp(a), monocytes showed a 5-fold increase in TEM. In addition, in this model and in human carotid plaques comparing individuals with normal and elevated Lp(a) levels, an increased PFKFB3-mediated glycolysis expression was observed.
- 33.Idzkowska E, Eljaszewicz A, Miklasz P, et al. The role of different monocyte subsets in the pathogenesis of atherosclerosis and acute coronary syndromes. Scand J Immunol 2015; 82:163–173. [DOI] [PubMed] [Google Scholar]
- 34.Maguire EM, Pearce SWA, Xiao Q. Foam cell formation: a new target for fighting atherosclerosis and cardiovascular disease. Vasc Pharmacol 2019; 112:54–71. [DOI] [PubMed] [Google Scholar]
- 35.Luo Y, Duan H, Qian Y, et al. Macrophagic CD146 promotes foam cell formation and retention during atherosclerosis. Cell Res 2017; 27:352–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liberale L, Dallegri F, Montecucco F, Carbone F. Pathophysiological relevance of macrophage subsets in atherogenesis. Thromb Haemost 2017; 117:7–18. [DOI] [PubMed] [Google Scholar]
- 37.Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and T H1-TH17 responses. Nat Immunol 2011; 12:231–238. [DOI] [PubMed] [Google Scholar]
- 38.Boytard L, Spear R, Chinetti-Gbaguidi G, et al. Role of proinflammatory CD68+ mannose receptor-macrophages in peroxiredoxin-1 expression and in abdominal aortic aneurysms in humans. Arterioscler Thromb Vasc Biol 2013; 33:431–438. [DOI] [PubMed] [Google Scholar]
- 39.Peled M, Fisher EA. Dynamic aspects of macrophage polarization during atherosclerosis progression and regression. Front Immunol 2014; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Tits LJH, Stienstra R, van Lent PL, et al. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Krüppel-like factor 2. Atherosclerosis 2011; 214:345–349. [DOI] [PubMed] [Google Scholar]
- 41.Seo JW, Yang EJ, Yoo KH, Choi IH. Macrophage differentiation from monocytes is influenced by the lipid oxidation degree of low density lipoprotein. Mediators Inflamm 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De La Paz Sánchez-Martínez M, Blanco-Favela F, Mora-Ruiz MD, et al. IL-17-differentiated macrophages secrete pro-inflammatory cytokines in response to oxidized low-density lipoprotein. Lipids Health Dis 2017; 16:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumer Y, McCurdy S, Weatherby TM, Mehta NNH, et al. Hyperlipidemia-induced cholesterol crystal production by endothelial cells promotes atherogenesis. Nat Commun 2017; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajam̈aki K, Lappalainen J, Öörni G K., et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE 2010; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grebe A, Hoss F, Latz E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res 2018; 122:1722–1740. [DOI] [PubMed] [Google Scholar]
- 46.Bevilacqua MP, Pober JS, Wheeler ME. Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol 1985; 121: [PMC free article] [PubMed] [Google Scholar]
- 47.Libby P, Ordovas JM, Auger KR, et al. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol 1986; 124: [PMC free article] [PubMed] [Google Scholar]
- 48.Sheedy FJ, Grebe A, Rayner KJ, et al. Ligands in Sterile Inflammation. Nat Immunol 2013; 14:812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Proctor SD, Vine DF, Mamo JCL. Arterial permeability and efflux of apolipoprotein b-containing lipoproteins assessed by in situ perfusion and three-dimensional quantitative confocal microscopy. Arterioscler Thromb Vasc Biol 2004; 24: [DOI] [PubMed] [Google Scholar]
- 50.Elsegood CL, Pal S, Roach PD, Mamo JCL. Binding and uptake of chylomicron remnants by primary and THP-1 human monocyte-derived macrophages: determination of binding proteins. Clin Sci 2001; 101: [PubMed] [Google Scholar]
- 51.Van Lenten BJ, Fogelman AM, Jackson RL, et al. Receptor-mediated uptake of remnant lipoproteins by cholesterol-loaded human monocyte-macrophages∗. J Biol Chem 1985; 260: [PubMed] [Google Scholar]
- 52.Whitman SC, Miller DB, Wolfe BM, et al. Uptake of type III hypertriglyceridemic VLDL by macrophages is enhanced by oxidation, especially after remnant formation. Arterioscler Thromb Vasc Biol 1997; 17:1707–1715. [DOI] [PubMed] [Google Scholar]
- 53.Batt KV, Patel L, Botham KM, Suckling KE. Chylomicron remnants and oxidised low density lipoprotein have differential effects on the expression of mRNA for genes involved in human macrophage foam cell formation. J Mol Med 2004; 82: [DOI] [PubMed] [Google Scholar]
- 54.Proctor SD, Mamo JCL. Retention of fluorescent-labelled chylomicron remnants within the intima of the arterial wall - evidence that plaque cholesterol may be derived from post-prandial lipoproteins. Eur J Clin Investig 1998; 28: [DOI] [PubMed] [Google Scholar]
- 55.Schwartz EA, Reaven PD. Lipolysis of triglyceride-rich lipoproteins, vascular inflammation, and atherosclerosis. Biochim Biophys Acta - Mol Cell Biol Lipids 2012; 1821:858–866. [DOI] [PubMed] [Google Scholar]
- 56.Ono-Moore KD, Snodgrass RG, Huang S, et al. Postprandial inflammatory responses and free fatty acids in plasma of adults who consumed a moderately high-fat breakfast with and without blueberry powder in a randomized placebo-controlled trial. J Nutr 2016; 146: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Higgins LJ, Rutledge JC. Inflammation associated with the postprandial lipolysis of triglyceriderich lipoproteins by lipoprotein lipase. Curr Atheroscler Rep 2009; 11:199–205. [DOI] [PubMed] [Google Scholar]
- 58.Mas S, Martínez-Pinna R, Martín-Ventura JL, et al. Local non-esterified fatty acids correlate with inflammation in atheroma plaques of patients with type 2 diabetes. Diabetes 2010; 59: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nielsen LB, Stender S, Kjeldsen K, Nordestgaard BG. Specific accumulation of Lipoprotein(a) in balloon-injured rabbit aorta in vivo. Circ Res 1996; 78: [DOI] [PubMed] [Google Scholar]
- 60.Tsimikas S. A test in context: Lipoprotein(a) diagnosis, prognosis. Controv Emerg Ther 2017; 692–711. [DOI] [PubMed] [Google Scholar]
- 61.Orsó E, Schmitz G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl 2017; 12:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Der Valk FM, Bekkering S, Kroon J, et al. Oxidized phospholipids on Lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 2016; 134:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab 2010; 12:467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mhatre V. Ho, Ji-Ann Lee KCM. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to VCAM-1. Bone 2012; 23:1–7. [Google Scholar]
- 65.Xu L, Dai Perrard X, Perrard JL, et al. Foamy monocytes form early and contribute to nascent atherosclerosis in mice with hypercholesterolemia. Arterioscler Thromb Vasc Biol 2015; 35:1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bernelot Moens SJ, Neele AE, Kroon J, et al. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J 2017; 38:1584–1593. [DOI] [PubMed] [Google Scholar]
- 67.Alipour A, Van Oostrom AJHHM, Izraeljan A, et al. Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol 2008; 28:792–797. [DOI] [PubMed] [Google Scholar]
- 68.Wu H, Gower RM, Wang H, et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation 2009; 119:2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.den Hartigh LJ, Connolly-Rohrbach JE, Fore S, et al. Fatty acids from very low-density lipoprotein lipolysis products induce lipid droplet accumulation in human monocytes. J Immunol 2010; 184: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cell Host and Microbe Elsevier Inc, Netea MG, Quintin J, Van Der Meer JWM. Trained immunity: a memory for innate host defense. 2011; p. 355-61. [DOI] [PubMed] [Google Scholar]
- 71.Christ A, Bekkering S, Latz E, Riksen NP. Long-term activation of the innate immune system in atherosclerosis. Semi Immunol 2016; 384–393. [DOI] [PubMed] [Google Scholar]
- 72.Bekkering S, Quintin J, Joosten LAB, et al. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol 2014; 34: [DOI] [PubMed] [Google Scholar]
- 73.Drummer C, Saaoud F, Shao Y, et al. Trained immunity and reactivity of macrophages and endothelial cells. Arterioscler Thromb Vasc Biol 2021; 1032–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sohrabi Y, Lagache SMM, Voges VC, et al. OxLDL-mediated immunologic memory in endothelial cells. J Mol Cell Cardiol 2020; 146. [DOI] [PubMed] [Google Scholar]
- 75▪.Keating ST, Groh L, Thiem KB, et al. Rewiring of glucose metabolism defines trained immunity induced by oxidized low-density lipoprotein. J Mol Med 2020; 98:819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]; Trained immunity was observed in human primary monocytes with oxLDL incubation. An upregulation of glycolytic activity and oxygen consumption was observed and was dependent on glycolytic enzymes PFKFB3 and PFKP, marking the imporance of glucose metabolism in trained immunity.
- 76.Christ A, Günther P, Lauterbach MAR, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 2018; 172: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fernandez DM, Rahman AH, Fernandez NF, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med 2019; 25: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winkels H, Ehinger E, Vassallo M, et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res 2018; 122: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev 2006; 86: [DOI] [PubMed] [Google Scholar]
- 80▪.Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol 2020; 17: [DOI] [PMC free article] [PubMed] [Google Scholar]; Extensive review on the complex role of the various T-cells in atherosclerosis.
- 81.Steinman RM. Decisions about dendritic cells: past, present, and future. Ann Rev Immunol 2012; 30: [DOI] [PubMed] [Google Scholar]
- 82.Stemme S, Faber B, Holm J, et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A 1995; 92:3893–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gillentine MA, Berry LN, Goin-Kochel RP, et al. Regulatory CD4+ T cells recognize MHC-II-restricted peptide epitopes of apolipoprotein B. J Autism Dev Disord 2017; 47:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emami H, Singh P, MacNabb M, et al. Splenic metabolic activity predicts risk of future cardiovascular events demonstration of a cardiosplenic axis in humans. XXXX 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature 2012; 487:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zimmer S, Grebe A, Bakke SS, et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci Transl Med 2016; 8:333ra50-1333ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Courties G, Herisson F, Sager HB, et al. Ischemic stroke activates hematopoietic bone marrow stem cells. Circ Res 2014; 116:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol 2004; 44:1945–1956. [DOI] [PubMed] [Google Scholar]
- 89.Taqueti VR, Di Carli MF, Jerosch-Herold M, et al. Increased microvascularization and vessel permeability associate with active inflammation in human atheromata. Circ Cardiovasc Imaging 2014; 7:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010; 7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giugliano G, Brevetti G, Lanero S, et al. Leukocyte count in peripheral arterial disease: a simple, reliable, inexpensive approach to cardiovascular risk prediction. Atherosclerosis 2010; 210:288–293. [DOI] [PubMed] [Google Scholar]
- 92.Rahman MS, Murphy AJ, Woollard KJ. Effects of dyslipidaemia on monocyte production and function in cardiovascular disease. Nat Rev Cardiol 2017; 14:387–400. [DOI] [PubMed] [Google Scholar]
- 93.Combadière C, Potteaux S, Rodero M, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 2008; 117:1649–1657. [DOI] [PubMed] [Google Scholar]
- 94.Robbins CS, Chudnovskiy A, Rauch PJ, et al. Extramedullary hematopoiesis generates Ly-6C high monocytes that infiltrate atherosclerotic lesions. Circulation 2012; 125:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feng Y, Schouteden S, Geenens R, et al. Hematopoietic stem/progenitor cell proliferation and differentiation is differentially regulated by high-density and low-density lipoproteins in mice. PLoS ONE 2012; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tolani S, Pagler TA, Murphy AJ, et al. Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: studies in mice and FH children. Atherosclerosis 2013; 229: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ridker PM, Cushman M, Meir MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently health men. J Cardiopulm Rehabil 1997; 17:280. [DOI] [PubMed] [Google Scholar]
- 98.Libby P. Inflammation in Atherosclerosis. Nature 2002; 420:868–874. [DOI] [PubMed] [Google Scholar]
- 99.Bohula EA, Giugliano RP, Leiter LA, et al. Inflammatory and cholesterol risk in the FOURIER trial. Circulation 2018; 138:131–140. [DOI] [PubMed] [Google Scholar]
- 100.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 101▪▪.Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020; 383:1838–1847. [DOI] [PubMed] [Google Scholar]; Randomized controlled trial in patients with chronic coronary disease showing a reduced risk of cardiovascular events with colchicine treatment compared to placebo. After a median follow-up of 2.5 years, the composite primary endpoint of cardiovascular death, spontaneous (nonprocedural) myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization was lower in the treatment group (6.8% vs 9.6%) with a hazard ratio of 0.69 (confidence interval 0.57 to 0.83; P < 0.001).