Abstract
Purpose of review
Artemisinin-based combination therapies (ACTs) are globally the first-line treatment for uncomplicated falciparum malaria and new compounds will not be available within the next few years. Artemisinin-resistant Plasmodium falciparum emerged over a decade ago in the Greater Mekong Subregion (GMS) and, compounded by ACT partner drug resistance, has caused significant ACT treatment failure. This review provides an update on the epidemiology, and mechanisms of artemisinin resistance and approaches to counter multidrug-resistant falciparum malaria.
Recent findings
An aggressive malaria elimination programme in the GMS has helped prevent the spread of drug resistance to neighbouring countries. However, parasites carrying artemisinin resistance-associated mutations in the P. falciparum Kelch13 gene (pfk13) have now emerged independently in multiple locations elsewhere in Asia, Africa and South America. Notably, artemisinin-resistant infections with parasites carrying the pfk13 R561H mutation have emerged and spread in Rwanda.
Summary
Enhancing the geographic coverage of surveillance for resistance will be key to ensure prompt detection of emerging resistance in order to implement effective countermeasures without delay. Treatment strategies designed to prevent the emergence and spread of multidrug resistance must be considered, including deployment of triple drug combination therapies and multiple first-line therapies.
Keywords: artemisinin, epidemiology, Plasmodium. falciparum, resistance, treatment
INTRODUCTION
Deployment of artemisinin-based combination therapies (ACTs) along with vector control interventions have contributed to an important reduction in the global burden of Plasmodium falciparum malaria [1]. ACTs consist of a highly potent, fast-acting artemisinin derivative (artemether, artesunate or dihydroartemisinin) with a short plasma half-life, and a slower acting partner drug (lumefantrine, amodiaquine, mefloquine, piperaquine, sulphadoxine-pyrimethamine or pyronaridine) that remains in the blood for longer periods. Antimalarial treatment with artemisinins results in a 10 000-fold reduction in parasite load per 48-h erythrocytic life cycle. After a 3-day ACT course, any surviving parasites are then cleared by the partner drug [2]. Artesunate plus mefloquine (ASMQ) was the first ACT to be introduced over 25 years ago in north-western Thailand along the border with Myanmar in a context where multidrug-resistant falciparum malaria had become difficult to treat [3]. Increasing resistance to chloroquine and sulphadoxine-pyrimethamine in sub-Saharan Africa, bearing more than 90% of the world's malaria burden, eventually led to ACTs becoming the treatment of choice for uncomplicated P. falciparum malaria in all endemic countries, as recommended by the WHO since 2006 [4,5]. In 2010, parenteral artesunate was recommended as the first-line treatment for both paediatric and adult severe malaria after large clinical trials showed the significantly reduced mortality in patients receiving artesunate compared with those receiving quinine [6–8].
Combining two drugs with different mechanisms of parasiticidal action provide mutual protection against the emergence of drug resistance in parasite populations [2]. However, artemisinin derivatives were used as monotherapies in South-East Asia (SEA) for decades until their use was discouraged in 2006 and is likely to have continued in the years thereafter [4,9]. Use of artemisinin monotherapies and of partner drugs at subtherapeutic doses and/or in substandard formulations has likely contributed to the emergence of artemisinin resistance (ART-R) in SEA, first described in western Cambodia in 2008 [10–12]. ART-R is characterized by reduced killing of ring-stage P. falciparum, resulting in delayed parasite clearance down to 100-fold per 48-h parasite life cycle [13]. In the Greater Mekong Subregion (GMS), ART-R has been compounded by ACT partner drug resistance, causing high treatment failure of ACTs. Dihydroartemisinin-piperaquine failure rates reached high levels in Cambodia and southern Vietnam, whereas ASMQ failed in the Myanmar-Thai border region [14,15]. In the GMS, all of the six available ACTs have shown efficacy below 90% at some point of time.
In this review, we will describe recent advances in the understanding of ART-R, its evolving epidemiology and strategies to treat multidrug-resistant falciparum malaria in order to combat its further emergence and spread.
Box 1.
no caption available
CLINICAL PHENOTYPE OF ARTEMISININ RESISTANCE
An early study of ART-R demonstrated that peripheral blood parasitaemia was cleared more slowly in patients with recrudescent infections after ACT treatment [12]. The parasite clearance time or the proportion of patients that are still parasitaemic by light microscopy 72 h after start of treatment is used for surveillance of ART-R, but is confounded by multiple factors including the starting parasitaemia [16]. A slower parasite clearance rate provides a more accurate measure of ART-R, as it is largely independent from the ACT partner drug and initial parasitaemia [17]. The parasite clearance rate is estimated from the slope of the linear part of the log-linear parasite clearance curve, assessed by frequent light microscopy assessments of the peripheral blood parasite densities, and is described by the parasite clearance half-life (PC½) [18]. A PC½ more than 5 h has been used to define ART-R in Southeast Asia, though a threshold of more than 5.5 h has also been proposed [19,20▪]. In regions of high malaria transmission such as in many parts of sub-Saharan Africa, human host immunity will accelerate parasite clearance [21], and the threshold is likely lower. An alternative threshold for these settings, however, has not yet been validated.
MECHANISMS OF ARTEMISININ RESISTANCE
Although several specific parasite targets for artemisinins have been described, it is likely that the parasiticidal effect of artemisinins is mediated through alkylation of multiple cellular proteins and lipids. Given the broad scope of attack on the parasite and the diversity of ART-R parasite populations, it has proven to be difficult to define with precision a unifying mechanism of resistance to artemisinins. However, a few common interwoven themes have emerged from the extensive laboratory and omics-based research conducted to decipher the mechanism of ART-R (reviewed in [22▪▪,23]). First, the progression through the asexual intracellular development cycle of ART-R parasites appears to be altered. This property of ART-R parasites was first identified as quiescence in cultured parasites exposed to increasing doses of artemisinin and eventually aided the discovery of mutations in the propeller domains of P. falciparum Kelch13 gene (pfk13) as genetic markers for ART-R [24,25]. Close to 100 pfk13 mutations have since been described, of which 10 are currently listed as validated markers for artemisinin resistance, whereas another 11 are associated or candidate markers [26]. A corollary from this work was the observation that early ring stages of ART-R parasites had an increased ability to survive the transient attack from the short-lived artemisinins [27]. This is corroborated by the inverse relationship between PC½ and increasing parasite age at treatment observed in infections with parasites carrying pfk13 mutations known to confer ART-R [28]. Second, although transfection of P. falciparum with a number of pfk13 mutations has proven a causal relationship between these mutations and ART-R in vitro[29], establishing these mutations within the parasite populations in the field likely requires a genetic backbone of supportive mutations, possibly to compensate for a loss in fitness of pfk13 mutant parasites [30]. This genetic backbone was observed in ART-R parasites in the GMS, but the same background mutations were not detected in the more recently described ART-R parasite population in Rwanda [31▪]. Further, there can be significant divergence in the genomic, transcriptomic and proteomic profiles even within ART-R parasite populations with validated pfk13 mutations [32,33]. However, these profiles, reflecting the upregulation and downregulation of different cellular pathways, do converge into a distinct number of cellular responses to artemisinin exposure. These responses include an enhanced unfolded protein or cellular stress response and degradation of damaged protein in proteasomes as well as diminished or arrested transcription, translation and metabolism in ring-stage parasites, delaying progression through the asexual life cycle. The reduced trafficking and metabolism of haemoglobin in particular may in itself reduce the activation, and hence effectiveness, of artemisinins [22▪▪]. Finally, although some studies suggest putative drugs for coadministration with ACTs to reverse the effects of ART-R, none have so far been shown to be effective in treatment of patients with multidrug-resistant malaria [33,34].
EPIDEMIOLOGY OF ARTEMISININ RESISTANCE
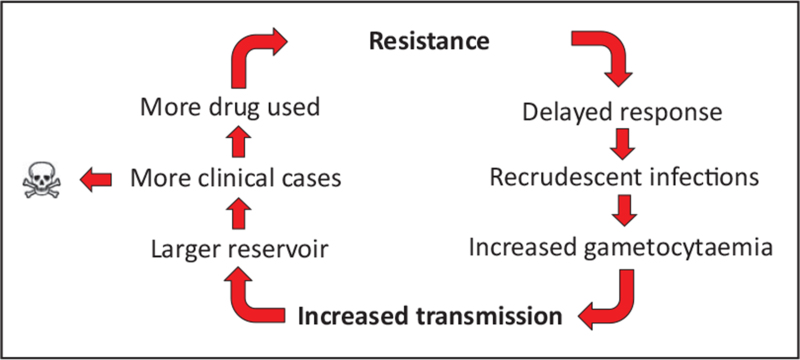
In areas with P. falciparum resistant to both the artemisinin and ACT partner drug, recrudescence rates will be very high, as observed in Cambodia and Vietnam after dihydroartemisinin-piperaquine treatment [14,35,36]. This caused a very rapid spread throughout Cambodia towards Vietnam of a single parasite lineage resistant to both artemisinins and piperaquine [37,38]. In the absence of partner drug resistance, the majority of ART-R infections can still be cleared adequately. However, in these infections, a much higher remaining parasite biomass after 3 days of ACT therapy will be exposed to only the ACT partner drug, because of the very short plasma half-life of the artemisinins. This facilitates the emergence of resistance to the partner drug. Such sequential acquisition of multidrug resistance has been observed in SEA with ASMQ [15], and dihydroartemisinin-piperaquine [14], although piperaquine resistance might have been present in Cambodia at the moment of dihydroartemisinin-piperaquine deployment. Further, whereas artemisinins are gametocytocidal for stage I to early-stage V gametocytes in susceptible parasite strains, ART-R causes gametocytes to become less susceptible and possibly also promotes gametocytogenesis [19,39,40]. In general, gametocyte carriage is increased in recrudescent P. falciparum infections, amplifying the transmission of resistant parasites when ACT efficacy starts to dwindle (Fig. 1) [40]. These factors confer a selective advantage for ART-R and in particular multidrug-resistant parasites, expanding their prevalence in the parasite population, especially in regions with low malaria transmission [37].
FIGURE 1.
Circular cascade of events leading to increased transmission, cases and deaths due to antimalarial drug resistance.
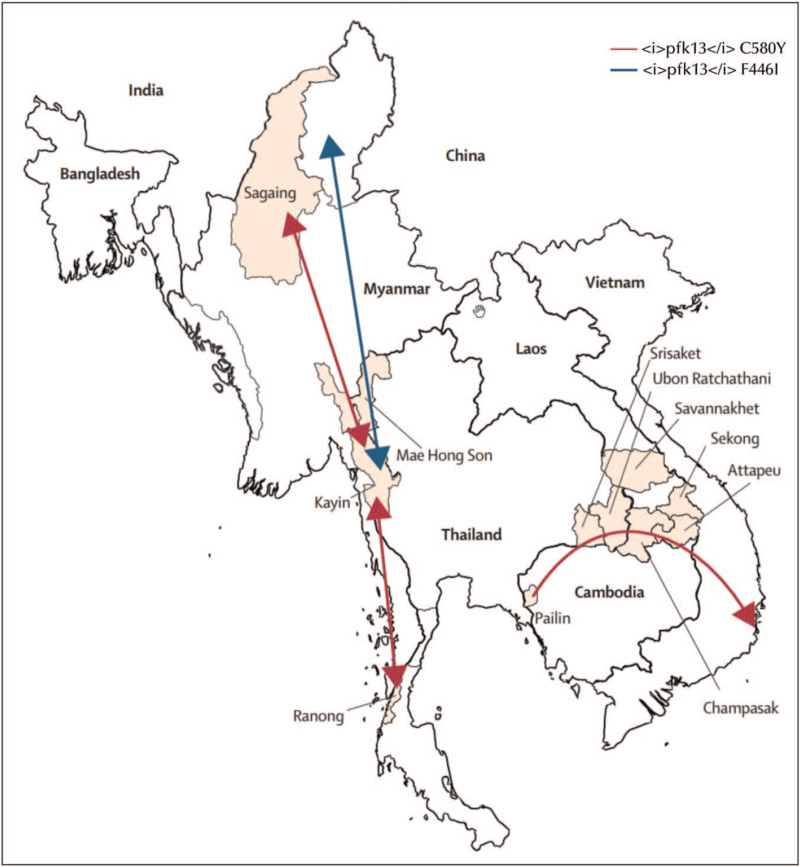
Mutations in the propeller region of the pfk13 gene, which confer ART-R have emerged in multiple locations within and outside of the GMS (Fig. 2) [41▪▪,42▪▪,43▪,44▪]. In the eastern GMS, several pfk13 mutations associated with increased PC½ emerged on a common genetic background [19,30] and converged to a single lineage of parasites carrying the pfk13 C580Y mutation. This allele has become predominant in Cambodia, Laos and Vietnam, assisted by the acquisition of mutations conferring piperaquine resistance (P. falciparum plasmepsin 2/3 gene amplification and novel pfcrt mutations). The predominance of the C580Y mutation occurred despite the relatively higher fitness cost of the C580Y mutation in vitro as compared to some other pfk13 mutants [45], and was likely facilitated by continued drug pressure from dihydroartemisinin-piperaquine as first-line antimalarial treatment providing a selective advantage for these parasites [37]. In Myanmar, multiple pfk13 mutants, including an independently emergent C580Y allele, have been detected but parasites with the F446I allele are now the most widespread [41▪▪]. The increased prevalence of this validated marker for ART-R, which confers an intermediate phenotype of moderately prolonged parasite clearance but potentially increased transmissibility, has currently not led to higher treatment failure rates with artemether-lumefantrine, which is the first-line treatment in Myanmar. The F446I allele appears to have emerged in north-western Myanmar before spreading throughout Myanmar and also into India [46]. A study from West Bengal in India reported pfk13 mutations identified from locally acquired or imported cases, but these findings have not been confirmed in later studies [47,48▪]. However, vigilant surveillance of antimalarial drug resistance markers in India remains important as it has in the past been the corridor of spread of resistance to chloroquine and sulphadoxine-pyrimethamine from the GMS to Africa.
FIGURE 2.
Spread of pfk13 haplotypes across the Greater Mekong Subregion. The long <i>pfk13</i> C580Y haplotype that emerged in 2008 spread from its origin in western Cambodia to Thailand, Laos and Viet Nam. The blue arrows depict a single <i>pfk13</i> F446I haplotype that probably originated in northern Myanmar. Adapted from [41▪▪].
Although there is no further evidence thus far that pfk13-mediated ART-R has spread beyond the GMS, de-novo emergence of ART-R has been clearly documented more recently. Most worryingly, P. falciparum carrying a validated marker of ART-R, pfk13 R561H, has emerged and expanded in Rwanda [31▪]. Analysis of the microsatellites in the flanking regions of the pfk13 gene showed a common origin for this parasite population, different from the R561H haplotype identified in the GMS. This Rwandan ART-R parasite lineage shows the phenotypic hallmarks of ART-R, that is delayed parasite clearance in vivo along with increased survival after in vitro exposure to dihydroartemisinin [49▪▪], but has fortunately not as yet affected the efficacy of artemether-lumefantrine, the first-line ACT in Rwanda. ART-R associated pfk13 mutations, which have emerged independently from the GMS have also been observed in Guyana [50], New Guinea [51] and Uganda [52,53]. The pfk13 C580Y mutation in Guyana were found in surveys between 2010 and 2017, but its prevalence has decreased. In Uganda, an increasing prevalence of the A675 V mutation was observed over time. A low prevalence, typically less than 3%, of pfk13 mutant P. falciparum has been described in multiple studies from sub-Saharan Africa, without evidence of selection, likely representing the background mutation rate in the pfk13 gene and also mutations not associated with ART-R such as the pfk13 A578S mutation [30]. In addition, there have been recent reports of decreasing artemether-lumefantrine efficacy detected in Angola and Burkina Faso that however appear to be unrelated to ART-R [54,55]. All this emphasizes the need for continued and sufficiently granular surveillance for ART-R and ACT partner drug resistance in the African setting [42▪▪,46].
TREATMENT OF ARTEMISININ-RESISTANT MALARIA
ACTs continue to be the only widely deployable treatment option to treat uncomplicated falciparum malaria, even in the GMS context wherein ART-R is highly prevalent. Five ACTs are currently recommended by the WHO; artemether-lumefantrine, ASMQ, dihydroartemisinin-piperaquine, artesunate–amodiaquine and artesunate–sulphadoxine-pyrimethamine [8]. In consideration of recent positive scientific opinion regarding its safety and efficacy, the WHO now also recommends the ACT artesunate-pyronaridine [56]. The current strategy to address confirmed ACT failure of more than 10% as assessed in therapeutic efficacy studies is to cycle through these ACTs based on the prevalent sensitivity of parasites to partner drugs [26]. In Cambodia, first-line antimalarial treatment shifted from ASMQ in 2000 to dihydroartemisinin-piperaquine in 2008 back to ASMQ in 2016 [14,36,57]. This strategy has however been logistically challenging. Delays in implementation led to patients being treated with an inferior ACT and spread of multidrug-resistant parasites, threatening region-wide malaria elimination efforts. New strategies to treat ART-R malaria are needed to circumvent these challenges.
A number of promising new antimalarials are in clinical development [58▪]. Some of these including, cipargamine (KAE609), a spiroindolone that acts even more rapidly than artemisinin and ganaplacide (KAF156), an imidazolopiperazine acting on different stages of the parasite life cycle, are new classes of compounds, whereas others, including the 4-aminoquinoline ferroquine, are not. These new drugs are in Phase II clinical trials and are unlikely to become available within the next few years for rapid deployment in regions where ART-R is prevalent or emerging [59–61].
Alternative strategies involving the innovative use of existing ACTs have been proposed. Prolonging the duration of therapy, either to 5 days with the same ACT or sequential 3-day treatments with two different ACTs, are likely to be efficacious and well tolerated, but the longer regimen may compromise adherence to the full course [62]. Using multiple ACTs as first-line treatment at the same time in an area or sequential deployment at fixed intervals could reduce the selective pressure on individual partner drugs while sustaining efficacy [63]. Whether under-resourced countries are able to manage these novel strategies effectively remains unclear. Triple artemisinin-based combination therapies (TACTs) provide another promising option that could be rapidly deployed. TACTs are combinations of an artemisinin derivative and two partner drugs selected based on their elimination half-lives and observed parasite resistance profiles to ensure mutual protection [60,64,65▪▪,66]. Two such TACTs, artemether-lumefantrine with amodiaquine and dihydroartemisinin-piperaquine with mefloquine have been shown to be well tolerated and highly efficacious in Thailand, Cambodia and Vietnam where dihydroartemisinin-piperaquine failed in nearly 50% of patients [48▪]. Another triple combination containing arterolane-piperaquine and mefloquine was also recently found to be safe, well tolerated and efficacious in Kenyan children with uncomplicated falciparum malaria [67]. Finally, administering a single gametocytocidal low dose of primaquine (0.25 mg/kg) along with ACTs or TACTs as is currently recommended by the WHO in low-transmission areas is important to reduce the transmission of multidrug-resistant P. falciparum[8].
Treatment of severe malaria
Artemisinin resistance is a threat to the life-saving effect of parenteral artesunate in the treatment of severe falciparum malaria. The reduction in case fatality in patients treated with artesunate compared with quinine is in particularly prominent in adult and paediatric patients presenting with ring-stage hyperparasitaemia [6,7]. This suggests that the rapid ring-stage parasiticidal effect of artesunate, which is absent with quinine treatment, is important in saving lives. In ART-R P. falciparum, artemisinin sensitivity of ring-stage parasites is compromised, which would allow continued parasite maturation to the trophozoite and schizont stages that sequester in the microcirculation of vital organs contributing to organ failure. Case reports from the GMS show dangerously delayed parasite clearance after intravenous artesunate in artemisinin-resistant severe malaria, resulting in death or requiring rescue treatment with quinine [68]. However, a recent observational study from Vietnam did not report a high case fatality after intravenous artesunate treatment in patients with severe malaria acquired in areas of artemisinin resistance [69]. The WHO guidelines on the management of severe malaria suggest a combination of intravenous artesunate and quinine in infections originating from areas with artemisinin resistance [70]. This combination has proven to be well tolerated [71], but evidence from randomized controlled trials of its benefit above treatment with artesunate alone in ART-R severe malaria is currently still lacking.
FUTURE PERSPECTIVES AND CONCLUSION
Artemisinin resistance in falciparum malaria causes delayed parasite clearance, and increases reliance on the ACT partner drug to cure the infection. However, more than a decade after its first description, the ART-R phenotype has not evolved into a further increase in the PC½ above approximately 7 h or an extension of resistance to the more mature asexual parasite stages [14,20▪]. In the presence of an effective partner drug, ACTs are thus still efficacious. The possibility that other and/or pfk13-independent forms of ART-R may emerge cannot be eliminated, and comprehensive and systematic surveillance for ART-R with wide geographic coverage is essential, using molecular or genomic epidemiology to target in vitro and/or in vivo phenotypic assessments wherever molecular evidence of ART-R is found.
In the GMS, artemisinin resistance is compounded by partner drug resistance, rendering the infection increasingly difficult to treat. This concern, and the fear of resistant parasites spreading to other malaria endemic regions, in particular sub-Saharan Africa, has prompted a concerted effort to eliminate malaria from the GMS. This effort is now well underway and the number of P. falciparum infections has dropped to under 20 000 cases in 2020 [72]. ART-R has to date not spread from the GMS, but has emerged independently in several countries. In particular, the recent reports from Rwanda are concerning, and jeopardises the efficacy of artemether-lumefantrine in the country.
New drugs and strategies to protect existing drugs from falling to resistance will be critical countermeasures against ART-R and multidrug-resistant falciparum malaria. There are several promising drugs in the development pipeline, but none are likely to be available in the next few years. In the interim, judicious use of antimalarials already on the market to prolong their utility by combining them as TACTs or finding practical ways to implement multiple first-line therapies will be important strategies to ensure continued efficacious antimalarial treatment [63,65▪▪,73].
In conclusion, ART-R and ACT partner drug resistance are expanding and constitute a major threat to malaria control and elimination. Close surveillance of drug resistance and implementation of strategies to treat or delay drug-resistant malaria are paramount. Investments to support the systematic and proactive deployment of surveillance and treatment strategies could avert many deaths from multidrug resistant-falciparum malaria in the future.
Acknowledgements
None.
Financial support and sponsorship
A.D. receives salary support from the Wellcome Trust of Great Britain.
Conflicts of interest
M.D., C.A. are coordinators and A.D. is the PI of the Development of Triple Artemisinin-based Combination Therapies (DeTACT) project, funded by the Foreign, Commonwealth and Development Office of the UK Government. A.D. chaired the Novartis Malaria Advisory Council from January until May 2021.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Bhatt S, Weiss DJ, Cameron E, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015; 526:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg 2007; 77: 6 Suppl: 181–192. [PubMed] [Google Scholar]
- 3.Nosten F, Luxemburger C, ter Kuile FO, et al. Treatment of multidrug-resistant Plasmodium falciparum malaria with 3-day artesunate-mefloquine combination. J Infect Dis 1994; 170:971–977. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Guidelines for the treatment of malaria. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 5.White NJ, Hien TT, Nosten FH. A brief history of qinghaosu. Trends Parasitol 2015; 31:607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dondorp A, Nosten F, Stepniewska K, et al. South East Asian Quinine Artesunate Malaria Trial g. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005; 366:717–725. [DOI] [PubMed] [Google Scholar]
- 7.Dondorp AM, Fanello CI, Hendriksen IC, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 2010; 376:1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organisation. Guidelines for the treatment of malaria. 3rd edGeneva, Switzerland: World Health Organisation; 2015. [Google Scholar]
- 9.Yeung S, Van Damme W, Socheat D, et al. Access to artemisinin combination therapy for malaria in remote areas of Cambodia. Malar J 2008; 7:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers WO, Sem R, Tero T, et al. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar J 2009; 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009; 361:455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noedl H, Se Y, Schaecher K, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 2008; 359:2619–2620. [DOI] [PubMed] [Google Scholar]
- 13.Dondorp AM, Fairhurst RM, Slutsker L, et al. The threat of artemisinin-resistant malaria. N Engl J Med 2011; 365:1073–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Pluijm RW, Imwong M, Chau NH, et al. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis 2019; 19:952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phyo AP, Ashley EA, Anderson TJC, et al. Declining efficacy of artemisinin combination therapy against P. Falciparum malaria on the Thai-Myanmar Border (2003-2013): the role of parasite genetic factors. Clin Infect Dis 2016; 63:784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WWARN Artemisinin based Combination Therapy Africa Baseline Study Group. Clinical determinants of early parasitological response to ACTs in African patients with uncomplicated falciparum malaria: a literature review and meta-analysis of individual patient data. BMC Med 2015; 13:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White NJ. The parasite clearance curve. Malar J 2011; 10:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flegg JA, Guerin PJ, White NJ, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J 2011; 10:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashley EA, Dhorda M, Fairhurst RM, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2014; 371:411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.WWARN K13 Genotype-Phenotype Study Group. Association of mutations in the Plasmodium falciparum Kelch13 gene (Pf3D7_1343700) with parasite clearance rates after artemisinin-based treatments-a WWARN individual patient data meta-analysis. BMC Med 2019; 17:1. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive meta-analysis of individual patient-level data on pfk13 mutations and the strength of their association with the delayed parasite clearance phenotype.
- 21.Lopera-Mesa TM, Doumbia S, Chiang S, et al. Plasmodium falciparum clearance rates in response to artesunate in Malian children with malaria: effect of acquired immunity. J Infect Dis 2013; 207:1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪▪.Talman AM, Clain J, Duval R, et al. Artemisinin bioactivity and resistance in malaria parasites. Trends Parasitol 2019; 35:953–963. [DOI] [PubMed] [Google Scholar]; Detailed review of the current knowledge on the mechanisms of action of artemisinins and resistance.
- 23.Sutherland CJ, Henrici RC, Artavanis-Tsakonas K. Artemisinin susceptibility in the malaria parasite Plasmodium falciparum: propellers, adaptor proteins and the need for cellular healing. FEMS Microbiol Rev 2021; 45:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witkowski B, Lelievre J, Barragan MJ, et al. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother 2010; 54:1872–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ariey F, Witkowski B, Amaratunga C, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014; 505:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Report on antimalarial drug efficacy, resistance and response: 10 years of surveillance. Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 27.Witkowski B, Khim N, Chim P, et al. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob Agents Chemother 2013; 57:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Intharabut B, Kingston HW, Srinamon K, et al. Artemisinin resistance and stage dependency of parasite clearance in falciparum malaria. J Infect Dis 2019; 219:1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straimer J, Gnädig NF, Witkowski B, et al. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 2015; 347:428–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miotto O, Amato R, Ashley EA, et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet 2015; 47:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪.Uwimana A, Legrand E, Stokes BH, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med 2020; 26:1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report of the emergence of artemsinin-resistant P. falciparum in Rwanda.
- 32.Zhu L, Tripathi J, Rocamora FM, et al. The origins of malaria artemisinin resistance defined by a genetic and transcriptomic background. Nat Commun 2018; 9:5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mok S, Stokes BH, Gnadig NF, et al. Artemisinin-resistant K13 mutations rewire Plasmodium falciparum's intra-erythrocytic metabolic program to enhance survival. Nat Commun 2021; 12:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dogovski C, Xie SC, Burgio G, et al. Targeting the cell stress response of Plasmodium falciparum to overcome artemisinin resistance. PLoS Biol 2015; 13:e1002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saunders DL, Vanachayangkul P, Lon C. Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med 2014; 371:484–485. [DOI] [PubMed] [Google Scholar]
- 36.Amaratunga C, Lim P, Suon S, et al. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis 2016; 16:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imwong M, Suwannasin K, Kunasol C, et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong Subregion: a molecular epidemiology observational study. Lancet Infect Dis 2017; 17:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amato R, Pearson RD, Almagro-Garcia J, et al. Origins of the current outbreak of multidrug-resistant malaria in southeast Asia: a retrospective genetic study. Lancet Infect Dis 2018; 18:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lozano S, Gamallo P, González-Cortés C, et al. Gametocytes from K13 propeller mutant Plasmodium falciparum clinical isolates demonstrate reduced susceptibility to dihydroartemisinin in the male gamete exflagellation inhibition assay. Antimicrob Agents Chemother 2018; 62:e01426-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witmer K, Dahalan FA, Delves MJ, et al. Transmission of artemisinin-resistant malaria parasites to mosquitoes under antimalarial drug pressure. Antimicrob Agents Chemother 2020; 65:e00898-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪▪.Imwong M, Dhorda M, Myo Tun K, et al. Molecular epidemiology of resistance to antimalarial drugs in the Greater Mekong subregion: an observational study. Lancet Infect Dis 2020; 20:1470–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]; A report on molecular epidemiology studies of antimalarial drug resistance conducted over a 10-year period in the Greater Mekong Subregion.
- 42▪▪.Kayiba NK, Yobi DM, Tshibangu-Kabamba E, et al. Spatial and molecular mapping of Pfkelch13 gene polymorphism in Africa in the era of emerging Plasmodium falciparum resistance to artemisinin: a systematic review. Lancet Infect Dis 2021; 21:e82–e92. [DOI] [PubMed] [Google Scholar]; A systematic review of the molecular evidence of artemisinin resistance from Africa.
- 43▪. WorldWide Antimalarial Resistance Network. Artemisinin Molecular Surveyor 2021. https://www.wwarn.org/tracking-resistance/artemisinin-molecular-surveyor. [Accessed 7 July 2021]. [Google Scholar]; A regularly updated map-based visualization of published and unpublished molecular data on artemisinin-resistance.
- 44▪. World Health Organization. Malaria threats map Geneva, Switzerland. World Health Organization; 2021. https://apps.who.int/malaria/maps/threats/. [Accessed 7 July 2021]. [Google Scholar]; A regularly updated map-based visualization of molecular data on artemisinin-resistance from publications and therapeutic efficacy studies.
- 45.Nair S, Li X, Arya GA, et al. Fitness costs and the rapid spread of kelch13-C580Y substitutions conferring artemisinin resistance. Antimicrob Agents Chemother 2018; 62:e00605-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chhibber-Goel J, Sharma A. Profiles of Kelch mutations in Plasmodium falciparum across South Asia and their implications for tracking drug resistance. Int J Parasitol Drugs Drug Resist 2019; 11:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Das S, Manna S, Saha B, et al. Novel pfkelch13 gene polymorphism associates with artemisinin resistance in Eastern India. Clin Infect Dis 2019; 69:1144–1152. [DOI] [PubMed] [Google Scholar]
- 48▪.van der Pluijm RW, Tripura R, Hoglund RM, et al. Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial. Lancet 2020; 395:1345–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of a large multicentre trial assessing the efficacy, safety, tolerability of triple artemisinin-based combination therapies in contexts with high prevalence of multidrug-resistant P. falciparum.
- 49▪▪.Uwimana A, Umulisa N, Venkatesan M, et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis 2021; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Phenotypic confirmation of the emergence of artemisinin resistance in Rwanda.
- 50.Mathieu LC, Cox H, Early AM, et al. Local emergence in Amazonia of Plasmodium falciparum k13 C580Y mutants associated with in vitro artemisinin resistance. Elife 2020; 9:e51015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miotto O, Sekihara M, Tachibana SI, et al. Emergence of artemisinin-resistant Plasmodium falciparum with kelch13 C580Y mutations on the island of New Guinea. PLoS Pathog 2020; 16:e1009133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikeda M, Kaneko M, Tachibana SI, et al. Artemisinin-resistant Plasmodium falciparum with high survival rates, Uganda, 2014-2016. Emerg Infect Dis 2018; 24:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asua V, Conrad MD, Aydemir O, et al. Changing prevalence of potential mediators of aminoquinoline, antifolate, and artemisinin resistance across Uganda. J Infect Dis 2021; 223:985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dimbu PR, Horth R, Candido ALM, et al. Continued low efficacy of artemether-lumefantrine in Angola in 2019. Antimicrob Agents Chemother 2021; 65:e01949-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gansané A, Moriarty LF, Ménard D, et al. Antimalarial efficacy and resistance monitoring of artemether-lumefantrine and dihydroartemisinin-piperaquine shows inadequate efficacy in children in Burkina Faso, 2017-2018. Malar J 2021; 20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization. The use of artesunate-pyronaridine for the treatment of uncomplicated malaria. Geneva, Switzerland: World Health Organization, Program GM; 2019. [Google Scholar]
- 57.Lim P, Dek D, Try V, et al. Decreasing pfmdr1 copy number suggests that Plasmodium falciparum in Western Cambodia is regaining in vitro susceptibility to mefloquine. Antimicrob Agents Chemother 2015; 59:2934–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58▪. Medicines for Malaria Venture. Medicines for malaria venture: global portfolio of antimalarial medicines Geneva, Switzerland: medicines for malaria venture; 2021. https://www.mmv.org/research-development/mmv-supported-projects. [Accessed 7 July 2021]. [Google Scholar]; A description of the antimalarial drug development pipeline.
- 59.Ashley EA, Phyo AP. Drugs in development for malaria. Drugs 2018; 78:861–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanboonkunupakarn B, White NJ. Advances and roadblocks in the treatment of malaria. Br J Clin Pharmacol 2020; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tse EG, Korsik M, Todd MH. The past, present and future of antimalarial medicines. Malar J 2019; 18:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tun KM, Jeeyapant A, Myint AH, et al. Effectiveness and safety of 3 and 5 day courses of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in an area of emerging artemisinin resistance in Myanmar. Malar J 2018; 17:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boni MF, White NJ, Baird JK. The community as the patient in malaria-endemic areas: preempting Ddug resistance with multiple first-line therapies. PLoS Med 2016; 13:e1001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kunkel A, White M, Piola P. Novel antimalarial drug strategies to prevent artemisinin partner drug resistance: a model-based analysis. PLoS Comput Biol 2021; 17:e1008850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65▪▪.van der Pluijm RW, Amaratunga C, Dhorda M, Dondorp AM. Triple artemisinin-based combination therapies for malaria: a new paradigm? Trends Parasitol 2021; 37:15–24. [DOI] [PubMed] [Google Scholar]; A comprehensive review of triple artemisinin-based combination therapies: rationale, current knowledge, potential barriers and outstanding questions.
- 66.Thu AM, Phyo AP, Landier J, et al. Combating multidrug-resistant Plasmodium falciparum malaria. Febs J 2017; 284:2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamaluba M, van der Pluijm RW, Weya J, et al. Arterolane-piperaquine-mefloquine versus arterolane-piperaquine and artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children: a single-centre, open-label, randomised, noninferiority trial. Lancet Infect Dis 2021; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phyo AP, Win KK, Thu AM, et al. Poor response to artesunate treatment in two patients with severe malaria on the Thai-Myanmar border. Malar J 2018; 17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duong MC, Pham OKN, Nguyen PT, et al. Predictors of treatment failures of plasmodium falciparum malaria in Vietnam: a 4-year single-centre retrospective study. Malar J 2021; 20:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization. Severe malaria. Trop Med Int Health 2014; 19:7–131. [DOI] [PubMed] [Google Scholar]
- 71.Newton PN, Chierakul W, Ruangveerayuth R, et al. A comparison of artesunate alone with combined artesunate and quinine in the parenteral treatment of acute falciparum malaria. Trans R Soc Trop Med Hyg 2001; 95:519–523. [DOI] [PubMed] [Google Scholar]
- 72.World Health Organization. World malaria report. Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 73.Okell LC, Reiter LM, Ebbe LS, et al. Emerging implications of policies on malaria treatment: genetic changes in the Pfmdr-1 gene affecting susceptibility to artemether-lumefantrine and artesunate-amodiaquine in Africa. BMJ global health 2018; 3:e000999. [DOI] [PMC free article] [PubMed] [Google Scholar]