Abstract
Policy Points.
This article describes a strategic combination of research, advocacy, corporate campaigns, communications, grassroots mobilization, legislation, regulatory actions, and litigation against companies and government to secure a national policy to remove artificial trans fat from the US food system.
Sharing lessons we learned can help inform policymakers, academics, policy practitioners, and students across disciplines. Some of our lessons are that system change means that all consumers benefit without the need for individual behavior change; research can both identify opportunities to improve health and support policy adoption; policy efforts can serve as public education campaigns; policy campaigns can drive marketplace changes; and engaging forward‐thinking companies can diffuse opposition to passing a policy.
Context
For many decades, partially hydrogenated vegetable oil (PHO), the primary source of artificial trans fat in the American diet, was used widely in processed and restaurant foods. In the early 1990s, studies linked the consumption of artificial trans fat with heart disease. This article details how research and advocacy led to eliminating artificial trans fat from the US food supply.
Methods
We synthesized published studies of the health impact of trans fat, the legislative history of state and local trans fat bills, the Food and Drug Administration's (FDA) regulatory docket on trans fat labeling and its declaration that PHOs are no longer Generally Recognized as Safe (GRAS), and our own files, which included strategy documents, notes from meetings with the FDA staff, correspondence between advocates and the FDA, fact sheets, press releases, news clips, and other materials.
Findings
This history of trans fat provides insights into policy strategy and advocacy best practices that resulted in the removal of trans fat from food in the United States, preventing an estimated 50,000 premature deaths a year. The lessons we learned are that system change benefits all consumers without the need for individual behavior change; research can both identify opportunities to improve health through policy and support policy adoption; policy campaigns can serve as public education campaigns; policy can drive changes to products and the marketplace; and engaging forward‐thinking companies can help diffuse opposition to passing a policy. Securing this policy required the persistence of scientists and health advocates in first discovering the risks and then using the science to secure policies to mitigate the identified harm.
Conclusions
An understanding of the tactics used to help attain the targeted policies and how challenges were addressed (such as through communications, leveraging an expanding research base and expert reports, showing that a national policy was feasible through voluntary corporate changes and state and local policy, and litigation against companies and government agencies) may provide a model for scientists, students, advocates, and policymakers. We hope this account will inform efforts to address other public health challenges, such as the current threats of excessive exposure to sodium and added sugars, which persist in the US food system.
Keywords: health policy, nutritional sciences, public health, history
For more than a century, from 1911 through 2018, Americans were exposed to a significant man‐made hazard added to food: artificial trans fat. This article describes how this novel ingredient was developed and achieved widespread use and how the first scientific efforts to understand its impact on health were met with skepticism. It details the persistence of scientists and health advocates in first seeking to fully characterize the risks and then wielding the science to compel the US Food and Drug Administration (FDA) to mitigate the harm (Figure 1).
Figure 1.
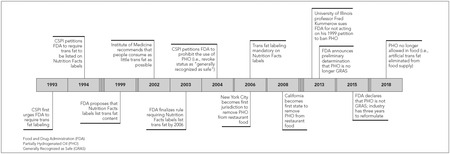
Key Policy Milestones in 25‐Year Effort to Remove Artificial Trans Fat from the US Food Supply
Artificial trans fat, which is created when oil is partially hydrogenated, was ubiquitous until recently in cookies, pies, and other baked goods; margarine; fried potatoes and chicken; microwave popcorn; and other processed and restaurant foods.1 Trans fat also occurs naturally in small amounts in ruminant animal products, such as beef, butter, cheese, and lamb.
It is instructive to examine this successful example of how risks uncovered by research were used to secure policy changes to protect the public's health. In the United States, artificial trans fat in food was estimated in 2006 to cause one in five (up to 250,000) heart attacks and 50,000 deaths a year.2 In 2018, after more than 25 years of advocacy, the FDA's ban on the use of partially hydrogenated oil (PHO) as a food ingredient went into effect.
An understanding of the tactics used to help attain the targeted policies and how challenges were addressed (such as through creative communications, leveraging an expanding research base and expert reports, showing that a national policy was feasible through securing corporate voluntary changes and state and local policy, and litigation against companies and the government agency) may provide a model for scientists, students, advocates, and policymakers. This history also may inform efforts to address other health challenges, including the current threats of excessive exposure to sodium and added sugars, which persist in the US food system.
Several of us (Wootan, Jacobson, Willett) actively participated in the research and advocacy described here. One strength of that lived history is that we have firsthand, extensive knowledge of the topic and the events that led to the removal of trans fat from the US food supply. But because this also raises a risk of potential bias, we have tried to mitigate it by extensively referencing source materials to verify the events described. We have also included coauthors and reviewers who did not actively participate in most of the activities we reference, as well as authors with extensive experience in and perspective from both trans fat research and advocacy.
The Introduction of Artificial Trans Fat Into the Food System
Produced in the rumen of cows and sheep, naturally occurring trans fat is found in foods such as beef, butter, and cream, generally making up no more than 4% of their total fat content. Artificial trans fat was developed in 1901 when the chemist Wilhelm Normann designed a process, hydrogenation, to transform liquid oil into a solid fat.3
When oils are partially hydrogenated in a commercial or laboratory setting, some mono‐ and polyunsaturated fatty acids are converted to saturated fatty acids and others are converted to trans fatty acids (or trans fat) (Figure 2). The resultant partially hydrogenated oils (PHO) were widely used in the US food supply owing to their semisolid physical property that mimicked butter, lard, and other animal fats and tropical oils. In addition, by destroying the essential fatty acids in natural vegetable oils that are sensitive to oxidation, PHO contributed to the shelf life of processed foods and the number of times that oils could be reused in deep‐fat fryers.
Figure 2.
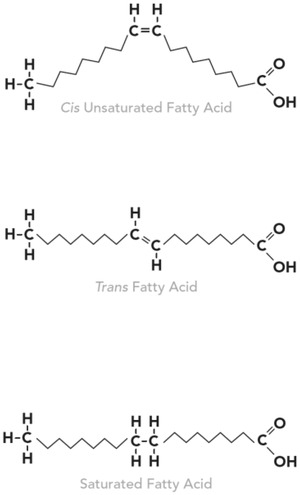
The Structure of Trans Fat Is More Like Saturated Fat Than Unsaturated Fat
When oils are partially hydrogenated in a commercial or laboratory setting, some mono‐ and polyunsaturated fatty acids are converted to saturated fatty acids and others are converted to trans fatty acids (or trans fat).
Margarine was invented in France in 1870 in response to a challenge issued by Emperor Louis Napoleon III to create a substitute for butter.4 In 1910, partial hydrogenation was incorporated into margarine's manufacturing process, thereby making liquid vegetable oil spreadable. By 1920, margarine production in the United Stated had reached 3.4 pounds per person annually, peaking at 11.9 pounds in 1976.5 In 1911, Crisco, the first shortening made entirely of PHO, was introduced to US stores,6 and by 2002, the United States was producing 32.8 pounds per capita per year of shortening.5
Growing Doubts About the Safety of Trans Fat
In the 1950s, clinical studies provided the first evidence that partially hydrogenated fats raised blood lipid levels.7, 8, 9 It was unclear, however, whether the increases were caused by the lower proportion of unsaturated to saturated fats or the introduction of trans fats, both of which occur when vegetable oil is partially hydrogenated.
Although further animal and clinical studies were carried out in the 1970s and 1980s, the negative effects of trans fat remained in doubt. For example, a review by the Federation of American Societies for Experimental Biology in 1976, conducted at the FDA's request, concluded that there was “no evidence” to show that partially hydrogenated soybean oil posed a “hazard” to the public.10 Moreover, a subsequent evaluation commissioned by the FDA concluded that PHO is “no more, or little more, cholesterolemic than oleic acid [a natural cis‐monounsaturated fatty acid].”11 Both a report by the US Surgeon General12 in 1988 and a report by the Institute of Medicine (now called the National Academies of Sciences, Engineering and Medicine [NASEM])13 in 1989 concluded that trans fat was similar to unsaturated fat in its contribution to blood cholesterol levels.
Lingering concerns about the safety of trans fat motivated epidemiologists at the Harvard University School of Public Health, led by Dr. Walter Willett, to include trans fat in a long‐term study designed to identify the dietary causes of heart disease.14 This study was made possible, in part, by researchers at the US Department of Agriculture (USDA), who established an analytic laboratory and developed a comprehensive account of the trans fat content of commonly consumed foods.
In 1980, a questionnaire designed to assess the major sources of trans fat in the diet was sent to a prospective cohort of more than 90,000 women, the Nurses’ Health Study. A decade later, the researchers found that women who consumed the most trans fat (5.7 grams/day) had a 35% higher risk of coronary heart disease (CHD) than did women with the lowest intake (2.4 grams/day).14
Throughout the 1990s, evidence of the harm of trans fat grew. Three additional prospective cohort studies also linked higher trans fat consumption to an increased risk of CHD.15, 16, 17 Longer follow‐up data from the Nurses’ Health Study confirmed the initial findings, demonstrating that the participants’ higher intake of trans fat, ascertained through food frequency questionnaires18, 19 and blood samples,20 predicted a higher risk of CHD. Together, these studies found a 23% higher risk of CHD for each 2% increase in energy intake from trans fat.2 An additional analysis of prospective cohort studies concluded that consumption of trans fats was linked to significantly elevated risks for all‐cause mortality, CHD mortality, and total CHD incidence.21
In addition to the epidemiologic evidence, in 1990 a carefully controlled feeding study conducted in the Netherlands showed that trans fat had uniquely adverse effects on blood lipids: LDL cholesterol was raised and HDL cholesterol was reduced.22 This was followed by a flurry of other controlled feeding trials confirming trans fat's adverse effects on blood lipids.23, 24, 25, 26, 27, 28 A study conducted by Joseph Judd and colleagues was particularly influential, given that they worked at the US Department of Agriculture and the study was partially funded by the food and edible oil industry through the Institute of Shortening and Edible Oils.29
Translating Science Into Public Health Policy
Trans Fat Labeling Paves the Way
In 1990, as the result of a decade‐long strategic advocacy effort, Congress enacted the Nutrition Labeling and Education Act of 1990 (NLEA; 9 U.S.C. §301), which brought needed transparency in the form of the Nutrition Facts label, standard serving sizes, and definitions for health and nutrition claims on packaged foods and beverages. The final requirements for the Nutrition Facts label were issued by the FDA in 1993 and did not include a requirement to disclose the levels of trans fat in foods, although the grams of total fat and saturated fat were required.30 The FDA, however, acknowledging a possible difference between the healthfulness of these types of unsaturated fats, did not allow trans fats to be included in the voluntary disclosures of unsaturated fats.
Concerned about the growing evidence for the harms of trans fat and the fact that consumers had no way to know how much was in food, the Center for Science in the Public Interest (CSPI) urged the FDA in 1993 to list the quantities of trans fat together with saturated fat on packaged foods. In the following year, CSPI formally petitioned the FDA to require trans fat labeling and to revise its rules for label claims so that foods high in trans fat could not make claims like “saturated‐fat free,” “made with vegetable oil,” “no cholesterol,” or claims related to heart health.
In the 1990s and 2000s, to support the adoption of its petition, to encourage companies to reformulate their products, and to educate the public about the health risks of trans fat, CSPI publicized the high levels of trans fat in certain foods.31, 32 Scientists working on trans fat held numerous interviews with journalists about the harms of trans fat, thereby providing a cost‐free way to disseminate this information to a wide audience. Consequently, as public awareness and pressure grew, many manufacturers and restaurants began reducing or removing PHO from their products.33
This momentum, described in more detail later, and the growing strength of the evidence eventually persuaded the agency to act. In 1999, the FDA proposed a regulation for labeling trans fat and provided the public with an opportunity to comment on the proposal.34 It received more than 1,650 letters, including a sign‐on letter from scientists and letters from the public.1 The agency reopened the comment period twice: first, to consider the definitions for reduced trans fat claims and, second, in 2002, to respond to new recommendations from the National Academies of Sciences, Engineering and Medicine to lower the intake of trans fat.35
Unlikely allies have played a role in securing a number of health policies, including healthier school food and menu labeling.36 Trans fat advocates found such an ally in John Graham, the administrator of the Office of Information and Regulatory Affairs in the White House Office of Management and Budget under the George W. Bush administration. Graham had regularly been on the opposite side of consumer and environmental advocates on issues such as pesticides, plastics, tobacco, workplace safety, and clean air.37 Before going to the White House, Graham was a faculty member at the Harvard School of Public Health and was familiar with the issue given Harvard's research and labeling of trans fat in the school's cafeteria. Graham sent his first “prompt letter” to Tommy Thompson, secretary of the US Department of Health and Human Services, urging the FDA to finalize the trans fat–labeling rule, citing its potential to avert coronary heart disease (CHD) cases, deaths, and costs.38
The FDA issued a final labeling rule (68 Fed. Reg. at 41433) in July 2003 and gave companies until January 1, 2006, to comply—seven years after labeling was first proposed by the FDA and 13 years after advocates first requested labeling. The FDA based its labeling requirement on studies establishing a relationship between trans fat and an increased risk for CHD, projecting that by 2009, trans fat labeling would prevent 600 to 1,200 cases of CHD as well as 250 to 500 CHD‐related deaths per year.39 This relatively low number was based on the assumption that trans fat acted only by increasing blood cholesterol levels, that consumers would make only modest changes in their purchasing in response to food labels, and that no processed foods would be reformulated. In addition, the FDA allowed companies to label foods as having zero grams of trans fat if they contained less than 0.5 grams of it. Thus, some foods labeled as having no trans fat actually contained PHO or low levels of trans fat.
Building Momentum for Limits on Trans Fat Through State and Local Policy
On the heels of this new transparency, in 2006, New York City became the first major US jurisdiction to eliminate artificial trans fat from food service establishments after unsuccessfully encouraging restaurants and bakeries voluntarily to stop using PHO.40
Over the next three years, the state of California and 20 localities (including Philadelphia, PA; Montgomery County, MD; Seattle/King County, WA; Boston, MA; and Baltimore, MD) adopted policies to eliminate PHO from food service establishments. While not every effort was successful, a sufficient number of jurisdictions enacted measures to engage the attention of national policymakers and companies. Accordingly, instead of reformulating foods specifically for California, New York City, and other jurisdictions, many restaurant chains replaced PHO nationwide, leading to important reductions of trans fat in the food supply and demonstrating the feasibility of large‐scale preparation of foods that did not contain PHO. For example, a study of the New York City policy found that this resulted in a 2.4 gram decrease of trans fat per purchase in restaurant foods, and purchases with zero grams of trans fat rose from 32% to 59%.41 Three years after New York City and other counties in the state banned the use of PHO in restaurants, hospital admissions for heart attacks and strokes dropped by 6.2% more in counties with bans than in counties without bans.42 An earlier study attributed a 4.5% reduction in deaths from heart disease to these counties’ bans.43
Working Toward a National Ban on Artificial Trans Fat
Responding to strong advocacy from its nutrition research community, Denmark effectively banned artificial trans fat from its food supply in 2003 (before US cities and states passed policies to eliminate trans fats from food service establishments). This demonstrated that companies could replace PHO with other ingredients without consumers noticing a change.44
In 2004, CSPI petitioned the FDA to revoke PHO's “generally recognized as safe” (GRAS) status.45 If PHO was no longer considered GRAS, companies would have to petition the FDA to approve each use of it as a food additive, based on a scientific demonstration of a “reasonable certainty of no harm.” The growing scientific consensus that trans fat was linked to CHD risk cited in CSPI's petition was reflected in the 2005 edition of the federal government's Dietary Guidelines for Americans (DGA), which for the first time recommended that people “keep trans fatty acid consumption as low as possible.”46
In response to the CSPI's petition, in 2005, the FDA reviewed the scientific evidence regarding trans fat and CHD. The review included 12 short‐term dietary intervention studies that showed adverse effects on blood cholesterol fractions and 8 observational studies that showed increased risks of heart disease (the FDA concluded that the observational studies supported the intervention studies). The FDA concluded its review noting, “It is apparent that trans fatty acids in the food supply represent a potential health risk to humans.”39
In addition, evidence regarding plausible substitutions for PHO demonstrated that the removal of trans fat was feasible. One study found that the average trans fat content of 360 randomly chosen brand‐name foods that contained trans fat in 2007 had declined by half by 2011.33 Another study demonstrated that trans fat could be removed while reducing the total amount of unhealthy fats in food. With few exceptions, the sum of saturated plus trans fat decreased in those foods that replaced PHO with other fats.47
The FDA was slowly evaluating the research and preparing to respond to the 2004 GRAS petition when, in 2009, Fred Kummerow, a University of Illinois faculty member who had conducted research on trans fat since the 1960s, filed a petition similar to CSPI's 2004 petition to ban PHO. This petition, alongside CSPI's, triggered another review of the scientific literature by the FDA's Toxicology Group.
The FDA's 2010 review was substantially more comprehensive than the one in 2005. It included 50 human observational studies and clinical trials, a nonhuman primate study, 33 reviews of health effects or consumption, and some in vitro studies.48 The review concluded that “both controlled trials and observational human studies … provide consistent evidence” for the effects of trans fat on intermediary risk factors, such as serum lipoproteins, and CHD. It described “unanimity” among dietary experts from the Dietary Guidelines for Americans to the World Health Organization that trans fat caused “dose‐dependent increases in CHD events in humans.” The report suggested mechanisms, biomarkers, or risk factors for the clinical outcomes and cited expert panel recommendations that “intake of industrially‐produced” trans fat “should be as low as possible.” Because naturally occurring levels in food were already close to the FDA's recommended limit for trans fat of less than 1% of daily calories, the FDA noted that “this would leave virtually no room for industrially manufactured” trans fat in the diet.
In 2013, Kummerow sued the FDA for the delay in responding to his citizen petition to ban trans fat, obliging the agency to set a deadline for taking action.49 In November 2013, the FDA published its “tentative” determination that PHOs were no longer GRAS because there was “not a consensus that PHOs” “are safe for use in food” (78 Fed. Reg. at 67169, 67171). The FDA noted that “as new scientific data and information develop about a substance” that “expert opinion regarding the safety of a substance for a particular use may change such that there is no longer a consensus that the specific use is safe” (78 Fed. Reg. at 67170).
In 2015, following a period for comment on its proposal, the FDA revoked the GRAS status of PHO, giving industry three years to eliminate it.50 When the Grocery Manufacturers Association (GMA; now called the Consumer Brands Association), a trade association that represents many large food manufacturers, subsequently filed a food additive petition asking for approval to retain certain minor uses of PHO, the FDA concluded that the petition lacked “convincing evidence to support the conclusion that the proposed uses of [trans fat] are safe” and denied the petition (85 Fed. Reg. at 23382). Thus, as of June 2018, industrially produced trans fat was effectively banned in the United States.
Trans Fat Remains a Problem in Many Countries
The 2003 ban on trans fat in Denmark was followed by restrictions in Switzerland, Iceland, Canada, Hungary, Norway, Sweden, and other countries between 2003 and 2018.51 Troublingly, most restrictions on the use of trans fat have been in the industrialized world. The World Health Organization is currently encouraging low‐ and middle‐income countries to eliminate it, and Resolve to Save Lives is providing technical assistance to countries to do so.52
Health Advocates and Researchers Created an Atmosphere for Change
Communications as an Essential Health Policy Tool
CSPI held its first press conference on trans fat in 1993 to publicize results from its study of the trans fat content of packaged and restaurant foods. The group's messages, including “French fries will never be a health food, but they don't have to raise your cholesterol” and fast‐food restaurants need to “get an oil change,” were widely reported in news outlets around the country. CSPI subsequently conducted several other widely publicized studies on the trans fat content of popular restaurant and packaged foods to support both its labeling and GRAS petitions.
The policy campaign served as a public education campaign. National and local newspapers, radio stations, television networks, and magazines published hundreds of stories about trans fat. Later in the campaign, social media platforms helped educate and mobilize the public to pressure restaurants and food manufacturers to remove trans fat and urge the FDA to act. The publicity campaign made PHO a substance that late‐night comedians lampooned and consumers avoided, encouraging companies to eliminate PHO and label their products “trans fat free,” thereby creating additional awareness that it was to be avoided.
CSPI's work specifically called out companies and foods by name, driving news coverage and garnering corporate attention. For instance, in 2013, lab testing revealed that Long John Silver's Big Catch meal contained 33 grams of trans fat—16 times the American Heart Association's recommended daily limit—in addition to 19 grams of saturated fat (and nearly 3,700 mg of sodium). CSPI dubbed the meal, which consisted of fish, hush puppies, and onion rings, all fried in PHO, the “Worst Restaurant Meal in America.” Company sales immediately plummeted, top officials met with CSPI, and, within two months, the company discontinued the Big Catch and transitioned to trans fat–free frying oil. Similarly, the ongoing academic studies on the health effects of trans fats, described earlier, garnered press attention as well.
The food industry also harnessed the power of the media and used its influence to cultivate doubt about the strength of the evidence and the need for action on trans fat. A 1996 press release from the Grocery Manufacturers Association argued that the research on trans fat was inconclusive and that “partial science and media theatrics” should not drive the labeling decision for trans fat.53 In 1996, the American Council on Science and Health, an organization largely funded by food and other industries, issued a press release, “Trans Fatty Acids: Just the Latest Scare du Jour Served Up by CSPI.”54 As late as 2002, a GMA lobbyist criticized the FDA's proposed labeling rule, stating that the food label's purpose is “not to provide nutritional counseling.”55
Mobilizing Support Among Scientists and Health Groups
In the early 1990s, few organizations were aware of trans fat, and some health and professional groups opposed trans fat labeling (although they eventually supported it).56 For example, a 1995 joint letter to the FDA from the American Society for Clinical Nutrition (ASCN), American Institute for Nutrition (later the AIN, the ASCN, and other groups merged to form the American Society for Nutrition), and the Institute of Food Technologists argued that the evidence for trans fat labeling was insufficient because of the decline in heart disease mortality during the historical period in which the consumption of trans fat increased, and it warned of “unwarranted food scares.”
Given the lack of support from health and professional organizations, early advocacy efforts relied on support from academic researchers. For example, in 1994, one of us (Walter Willett) sent a letter to the FDA commissioner urging the agency to address the relationship between trans fat and the risk of CHD. To facilitate participation by scientists, health departments, and national, state, and local organizations, CSPI provided information to them on how to submit comments to the FDA and circulated sign‐on letters that were sent to food companies and the FDA.
Food Industry Split on Trans Fat
Considerable opposition from powerful companies and trade associations, first to labeling and then to removing PHO from food, was a key reason why national policymaking regarding trans fat took more than two decades. Industry groups largely opposed the 1994 trans fat labeling petition. The Institute of Shortening and Edible Oils told the FDA that “since age‐adjusted mortality rates due to cardiovascular disease … have significantly decreased during the last 30 years, a time period when hydrogenated fats have been at their highest level of consumption, an association between trans fatty acids and an increased risk of heart disease does not appear plausible.”57 This conclusion, however, ignored that many other changes were taking place during that period, and it committed the cardinal sin in epidemiology: correlation should not be mistaken for causation.
Some companies, however, recognized the health (or perhaps the marketplace) implications of trans fat consumption and began reformulating their products before the FDA took any action. Cargill marketed a trans‐free shortening as early as 1993. Unilever was the first to offer a zero‐trans margarine. Working with Willett, Legal Sea Foods, a regional seafood restaurant chain, became trans fat–free by 2008, showing that reformulation was feasible despite skepticism by the restaurant industry in general. Reformulation required a supply‐chain shift; that is, trans‐free foods meant that manufacturers needed healthier oils, and oil processers needed crops that could provide oils that could replace PHOs, thereby increasing the demand for high‐oleic soybean and canola oil.
By the time the FDA proposed its labeling rule in 1999, many companies supported labeling, though some, like the Snack Food Association, Kraft, and Nabisco, sought delays and modifications. Numerous industry groups, including the National Food Processors Association (which later merged with the GMA), argued that promulgating the labeling rule would be premature.
The mandatory labeling of trans fat, local policies to remove trans from restaurant foods, and campaigns to urge individual companies to remove trans fat spurred major reformulations across the industry (Table 1). According to the FDA, the intake of trans fat fell by 78% from 2003 to 2012, from 4.6g to 1.0g/person/day (80 Fed. Reg. at 34650).
Table 1.
Examples of Product Reformulations
| Trans Fat Contenta | ||
|---|---|---|
| Product | Pre‐mandatory Labeling (1996) | Post‐mandatory Labeling (2019) |
| Crisco shortening (1 tablespoon) | 1.5 g | 0 g |
| Land O Lakes margarine, stick (1 tablespoon) | 2.5 g | 0 g |
| Wendy's French Fries (large) | 7 g | 0 g |
| KFC Chicken Pot Pie | 8 g | 0 g |
| Van de Kamp's Breaded Fish Sticks (6) | 5 g | 0 g |
| Red Lobster Admiral's Feast | 22.5 g | 1 g |
The mandatory labeling of trans fat, local policies to remove trans fat from restaurant foods, and campaigns to urge individual companies to remove trans fat spurred major reformulations across the industry.
Product information is from CSPI analysis or collected from manufacturers.
Some companies that resisted or delayed reformulation came under legal scrutiny. Stephen Joseph, a California attorney, started an organization called BanTransFat.com. In May 2003, he sued Kraft Foods for selling Oreo cookies on the grounds that they contained trans fat but did not disclose that fact on labels (the FDA's labeling rule had not yet taken effect). The lawsuit generated enormous publicity, and Kraft agreed to replace PHO by January 1, 2006.58, 59
In 2003, Joseph sued McDonald's for reneging on its 2002 pledge to eliminate trans fat from its cooking oil. In 2004, CSPI added fuel to the fire by sponsoring a full‐page ad in the New York Times calling out McDonald's for “a broken McPromise.”60 Joseph's two lawsuits resulted in a settlement that required McDonald's to give $7 million to the American Heart Association for a trans fat education and advocacy program and to spend $1.5 million communicating its switch to oil that was not partially hydrogenated.61, 62 In 2006, CSPI sued KFC63 and Burger King64 for not disclosing to customers that their products contained trans fat. Although both cases were dismissed, the companies eliminated PHO soon thereafter.
One negative consequence of banning PHO was the industry's increased use of palm oil, an inexpensive, semisolid (and highly saturated) fat that can substitute for PHO in many products.65 The expansion of oil palm plantations, especially in Indonesia and Malaysia, has resulted in the destruction of the rainforest habitat occupied by white rhinoceroses, orangutans, and other endangered species unique to the area. Advocates have repeatedly urged companies to minimize the use of palm oil or to use sustainable sources.
Discussion
The campaign to remove artificial trans fat from the US food supply used sound science, dogged advocacy, creative communications, lawsuits, state and local policies, and changes by restaurants, food manufacturers, vegetable oil processors, seed companies, and farmers.
Trans fat's history provides many lessons—and indeed a model—that may be useful for addressing other public health problems (Figure 3). It is a policy and systems approach to improve the health of the entire population that in the end did not require individual behavior change. Food policy and system changes support people's efforts to eat well, for example, by making healthy food more available, affordable, or the default option. Food policy and systems changes’ advantages over educational and program approaches include a broader reach and longer lasting change. In addition, access to educational resources and programs can be difficult for lower‐income families with less‐flexible work hours, limited transportation or child care, and multiple hardships that compete for their time and attention. Even so, lower‐income people and people of color are at higher risk for health inequities and diet‐related diseases, such as diabetes,66 high blood pressure,67 and obesity.68 A systems approach also might be effective for reducing sodium intake, as more than 70% of the sodium consumed in the United States is processed into food by food manufacturers and restaurants,69 thus making it harder for individuals to reduce their intake.
Figure 3.

Lessons Learned from the Trans Fat Movement
While policy and systems changes can be an equitable and sustainable approach, it can take time. The FDA did not ban PHO until more than 25 years after the first solid evidence that artificial trans fat increased the risk of CHD appeared. Similarly, the effort to get sodas and unhealthy snack foods out of schools took more than 25 years, from the early 1990s when research indicated that school snacks and beverages were largely unhealthy to the early 2000s when states and localities were passing school food policies, to 2006 when the first national bill was introduced to remove sodas and unhealthy snacks from schools until the national bill passed in 2010 and was implemented in 2014.36 The first state bill to require calorie labeling on chain restaurant menus was introduced in 2003; the national bill was passed in 2010 and was implemented by the FDA in 2018.
Industry opposition often slows the pace of food policy change. Many food manufacturers, restaurants, and their trade associations tried to cultivate doubt that trans fat was a threat to health and that labeling trans fat and reformulating products would do irreparable harm to their businesses. They lobbied the FDA and state legislatures in opposition to trans fat policies, funded their own research, sponsored pro–trans scientific reports and conference sessions, attended and rebutted advocates’ press conferences, and issued press releases. But industry is not monolithic. Companies like Cargill invested in the development of a trans‐free shortening that was available as early as 1993. Others reformulated their products only after being threatened with litigation. Early adopters of trans‐free fats, including companies in Denmark where trans fat was strictly limited in 2003, showed other companies and policymakers that reformulation to remove trans fat was functionally and economically feasible. Similarly, the restaurant industry aggressively lobbied against menu labeling in the early to mid‐2000s. Then as more policies were passed, industry opposition lessened to the point that a number of national chains, such as McDonald's and Panera, voluntarily posted calories on their menus nationally. Eventually, they, other restaurants, and the National Restaurant Association supported passage of the national menu labeling bill (Public Law 111–148; Sec. 4205), primarily to avoid having to comply with a diversity of local or state laws. More recently, some companies supported added‐sugars labeling on the Nutrition Facts Panel and the FDA voluntary sodium guidelines, while others actively opposed those policies.70
The work on trans fat policy drove changes to products and the marketplace. Even before the FDA banned the use of PHO, national labeling, local bans on trans fat in food service, and campaigns directed at individual major companies led to significant product reformulation and reductions in trans fat in the food supply, with the intake of trans fat dropping by 78% from 2003 to 2012 (80 Fed. Reg. at 34650). Menu labeling,71 school nutrition standards,72 and sodium‐reduction73 efforts also have resulted in nutritional improvements to many foods and meals.
Policy campaigns also serve as a way to educate the public on issues. Nonprofit organizations generally have limited resources for advertising or public education. Press coverage of new studies showing the negative health effects of trans fat attracted significant attention through newspapers, magazines, television, and other media outlets. Advocates also generated awareness that trans fat should be avoided and that there was a need for policy solutions through press conferences, press releases, exposés of high levels of trans fat in popular products, outreach to reporters, and ligation against food companies and the FDA. Efforts to pass local sugary drink taxes have generated not only local but also national press coverage, drawing national attention to the health harms of soda consumption. (Likewise, public education campaigns facilitate policy actions.)
The road to successful policy adoption can differ depending on the issue, the vehicle (e.g., legislation, regulation), the level of government (national, state, or local), the magnitude and certainty of the health problem, and the amount of opposition. Notably, the initial successes in smoking and trans fat bans occurred at the local level, probably in part because powerful national lobbies were less effective in obstructing progress. These actions were critical in documenting the feasibility of these policies.
Conclusion
Securing a national policy to remove artificial trans fat from the US food system ultimately involved a strategic combination of research, advocacy, corporate campaigns, communications, grassroots mobilization, legislation, regulatory actions, and litigation against companies and government. These policy changes have saved billions of dollars and tens of thousands of lives per year. They also provide lessons that could help inform future public health policy efforts.
Funding/Support: None.
Acknowledgments: The authors thank Laura MacCleery, Bonnie Liebman, Jessi Silverman, and Peter Lurie for their thoughtful review of the manuscript.
Conflict of Interest Disclosures: All authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest. No conflicts were reported.
References
- 1.Food labeling: trans. Federal Register. 2003;21(101):41433‐41506. [PubMed] [Google Scholar]
- 2.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601‐1613. [DOI] [PubMed] [Google Scholar]
- 3.Normann W, von Schuckman G. Hydrogenation of Higher Fatty Acids. US Patent 2,127,367. August 16, 1938. [Google Scholar]
- 4.van Stuyvenberg JH. Margarine: An Economic, Social, and Scientific History, 1869–1969. Liverpool, England: Liverpool University Press; 1969. https://openlibrary.org/works/OL5545009W/Margarine_an_economic_social_and_scientific_history_1869-1969. Accessed April 20, 2021. [Google Scholar]
- 5.US Department of Agriculture, Economic Research Service . Food availability (per capita) data system, fats and oils (added). https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/. Published July 31, 2019. Accessed September 11, 2019. [Google Scholar]
- 6.Our Heritage . Crisco. https://www.crisco.com/our‐heritage. Accessed December 20, 2019.
- 7.Anderson JT, Grande F, Keys A. Hydrogenated fats in the diet and lipids in the serum of man. J Nutr. 1961;75:388‐394. [DOI] [PubMed] [Google Scholar]
- 8.Bronte‐Stewart B, Antonis A, Eales L, Brock JF. Effects of feeding different fats on serum cholesterol level. Lancet. 1956;270:521‐526. [DOI] [PubMed] [Google Scholar]
- 9.Ahrens EH, Insull W, Blomstrand R, Hirsch J, Tsaltas TT, Peterson ML. The influence of dietary fats on serum‐lipid levels in man. Lancet. 1957;272:943‐953. [DOI] [PubMed] [Google Scholar]
- 10.Senti FR, ed. Evaluation of the Health Aspects of Hydrogenated Soybean Oil as a Food Ingredient. Bethesda, MD: Federation of American Societies for Experimental Biology; 1976. https://faseb.org/Portals/2/PDFs/LSRO_Legacy_Reports/1976_SCOGS-70%20Hydrogenated%20SoyBean%20Oil.pdf. Accessed April 20, 2021. [Google Scholar]
- 11.Senti FR, ed. Health Aspects of Dietary Trans Fatty Acids. Bethesda, MD: Federation of American Societies for Experimental Biology; 1985. [Google Scholar]
- 12.US Public Health Service, Office of the Surgeon General . The Surgeon General's Report on Nutrition and Health. Washington, DC: US Government Printing Office; 1988. [Google Scholar]
- 13.National Research Council (US), Committee on Diet and Health . Diet and Health: Implications for Reducing Chronic Disease Risk. Washington, DC: National Academies Press; 1989. [PubMed] [Google Scholar]
- 14.Willett WC, Stampfer MJ, Manson JE, et al. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet. 1993;341(8845):581‐585. [DOI] [PubMed] [Google Scholar]
- 15.Ascherio A, Rimm EB, Giovannucci EL, Spiegelman D, Stampfer M, Willett WC. Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ. 1996;313(7049):84‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piertinen P, Ascherio A, Korhonen P, et al. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The alpha‐tocopherol, beta‐carotene cancer prevention study. Am J Epidemiol. 1997;145(10):876‐887. [DOI] [PubMed] [Google Scholar]
- 17.Oomen CM, Ocké MC, Feskens EJM, van Erp‐Baart MJ, Kok F, Kromhout D. Association between trans fatty acid intake and 10‐year risk of coronary heart disease in the Zutphen elderly study: a prospective population‐based study. Lancet. 2001;357(9258):746‐751. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337(21):1491‐1499. [DOI] [PubMed] [Google Scholar]
- 19.Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC. Dietary fat and risk of coronary heart disease in women: 20 years of follow‐up to the nurses’ health study. Am J Epidemiol. 2005;161(7):672‐679. [DOI] [PubMed] [Google Scholar]
- 20.Sun Q, Ma J, Campos H, et al. A prospective study of trans fatty acids in erythrocytes and risk of coronary heart disease. Circulation. 2007;115(14):1858‐1865. [DOI] [PubMed] [Google Scholar]
- 21.de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta‐analysis of observational studies. BMJ. 2015;351:h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mensink RP, Katan MB. Effect of dietary trans fatty acids on high‐density and low‐density lipoprotein cholesterol levels in healthy subjects. N Engl J Med. 1990;323:439‐445. [DOI] [PubMed] [Google Scholar]
- 23.Wood R, Kubena K, O'Brien B, Tseng S, Martin G. Effect of butter, mono‐ and polyunsaturated fatty acid‐enriched butter, trans fatty acid margarine, and zero trans fatty acid margarine on serum lipids and lipoproteins in healthy men. J Lipid Res. 1993;34(1):1‐11. [PubMed] [Google Scholar]
- 24.Lichtenstein AH, Ausman LM, Carrasco W, Jenner JL, Ordovas JM, Schaefer EJ. Hydrogenation impairs the hypolipidemic effect of corn oil in humans. Atheroscl Thromb. 1993;13(2):154‐161. [DOI] [PubMed] [Google Scholar]
- 25.Zock PL, Katan MB. Hydrogenation alternatives: effects of trans fatty acids and stearic acid versus linoleic acid on serum lipids and lipoproteins in humans. J Lipid Res. 1993;33(3):399‐410. [PubMed] [Google Scholar]
- 26.Nestel P, Noakes M, Belling B, et al. Plasma lipoprotein lipid and lp[a] changes with substitution of elaidic acid for oleic acid in the diet. J Lipid Res. 1992;33(7):1029‐1036. [PubMed] [Google Scholar]
- 27.Nestel PJ, Noakes M, Belling GB, McArthur R, Clifton RM, Abbey M. Plasma cholesterol‐lowering potential of edible oil blends suitable for commercial use. Am J Clin Nutr. 1992;55(1):46‐50. [DOI] [PubMed] [Google Scholar]
- 28.Mensink RP, Zock PL, Katan MB, Hornstra G. Effect of dietary cis and trans fatty acids on serum lipoprotein[a] levels in humans. J Lipid Res. 33(1992):1493‐1501. [PubMed] [Google Scholar]
- 29.Judd JT, Clevidence BA, Muesing RA, Wittes J, Sunkin ME, Podczasy JJ. Dietary trans fatty acids: effects on plasma lipids and lipoproteins of healthy men and women. American J Clin Nutr. 1994;33(10):1493‐1501. [DOI] [PubMed] [Google Scholar]
- 30.Food labeling: mandatory status of nutrition labeling and nutrient content revision, format for nutrition label. Federal Register. 1993;58(3)2079‐2205. [Google Scholar]
- 31.Wootan MG, Liebman B, Rosofsky W. Trans: the phantom fat. Nutr Action Healthletter. 1996;23:1,10‐13. [Google Scholar]
- 32.Liebman B, Wootan MG. Trans fat. Nutr Action Healthletter. 1999;26:9‐11. [Google Scholar]
- 33.Otite FO, Jacobson MF, Dahmubed A, Mozaffarian D. Trends in trans fatty acids reformulations of US supermarket and brand‐name foods from 2007 through 2011. Prev Chronic Dis. 2013;10:120198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Food labeling: trans fatty acids in nutrition labeling, nutrient content claims, and health claims. Federal Register. 1999;64:62746‐62825. [PubMed] [Google Scholar]
- 35.Institute of Medicine [now National Academies of Sciences, Engineering and Medicine] . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, DC: National Academies Press; 2005. https://www.nap.edu/catalog/10490/dietary-reference-intakes-for-energy-carbohydrate-fiber-fat-fatty-acids-cholesterol-protein-and-amino-acids. Accessed April 20, 2021. [Google Scholar]
- 36.Schwartz C, Wootan MG. How a public health goal became a national law. Nutr Today. 2019;54(2)67‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Public Citizen. Safeguards at risk: John Graham and corporate America's back door to the Bush white house. Washington, DC: Public Citizen; 2001. [DOI] [PubMed] [Google Scholar]
- 38.Graham JD to Thompson TG. [letter] September 18, 2001. https://www.reginfo.gov/public/prompt/hhs_prompt_letter.html. Accessed December 20, 2019.
- 39.Division of Petition Review , Toxicology Group I (HFS‐265) to Isabel Chen. FAP 4Z4757 (petition for rulemaking to revoke the authority for industry to use partially hydrogenated vegetable oils in foods): literature review on health impact of trans fatty acids since the July 2003 FDA nutrition labeling regulation [memorandum]. August 10, 2005. https://www.regulations.gov/document?D=FDA‐2013‐N‐1317‐0231. Accessed December 20, 2019.
- 40.New York City Department of Health and Mental Hygiene . The regulation to phase out artificial trans fat in New York City food service establishments. 2006. https://www1.nyc.gov/assets/doh/downloads/pdf/cardio/cardio-transfat-bro.pdf. Accessed December 20, 2019. [Google Scholar]
- 41.Angell SY, Cobb LK, Curtis CJ, Konty KJ, Silver LD. Change in trans fatty acid content of fast‐food purchases associated with New York City's restaurant regulation. Ann Intern Med. 2012;157(2):81‐86. [DOI] [PubMed] [Google Scholar]
- 42.Brandt EJ, Myerson R, Perraillon MC, Polonsky TS. Hospital admissions for myocardial infarction and stroke before and after the trans‐fatty acid restrictions in New York. JAMA Cardiol. 2017;2(6):627‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Restrepo BJ, Rieger M. Trans fat and cardiovascular disease mortality: evidence from bans in restaurants in New York. J Health Econ. 2016;45:176‐196. [DOI] [PubMed] [Google Scholar]
- 44.Stender S, Dyerberg J. Influence of trans fatty acids on health. Ann Nutr Metab. 2004;48(2):61‐66. 10.1159/000075591. [DOI] [PubMed] [Google Scholar]
- 45.Center for Science in the Public Interest to US Food and Drug Administration . Petition for rulemaking to revoke the authority for industry to use partially hydrogenated vegetable oils in foods. May 18, 2004. https://cspinet.org/sites/default/files/attachment/trans_fat_petition_may_18.pdf. Accessed March 9, 2021.
- 46.US Department of Health and Human Services and US Department of Agriculture. Dietary Guidelines for Americans, 2005. 2005. 6th ed.https://www.dietaryguidelines.gov/about‐dietary‐guidelines/previous‐editions/2005‐dietary‐guidelines‐americans. Accessed July 19, 2020. [Google Scholar]
- 47.Mozaffarian D, Jacobson MF, Greenstein JS. Food reformulations to reduce trans fatty acids. N Engl J Med. 2010;362(21):2037‐2039. [DOI] [PubMed] [Google Scholar]
- 48.Division of Petition Review, Toxicology Group (HFS‐265) to Mical Honigfort. Citizen petition on trans fatty acids: literature review on health impact [memorandum]. August 19, 2010. https://www.regulations.gov/document?D=FDA‐2013‐N‐1317‐0232. Accessed December 20, 2019.
- 49.Fred Kummerow to US Food and Drug Administration . Citizen petition to ban partially hydrogenated fat from the American diet. August 4, 2009. https://www.regulations.gov/document/FDA-2009-P-0382-0001. Accessed December 20, 2019. [Google Scholar]
- 50.Final determination regarding partially hydrogenated oils. Federal Register. 2015;80(116):34650‐34670. [PubMed] [Google Scholar]
- 51.World Health Organization (WHO) . Policies to eliminate industrially produced trans fat consumption. 2018. https://www.who.int/docs/default‐source/documents/replace‐transfats/replace‐act‐information‐sheet.pdf?Status=Temp&sfvrsn=9e5806a6_8. Accessed December 20, 2019. [Google Scholar]
- 52.World Health Organization (WHO) . REPLACE trans fat. https://www.who.int/nutrition/topics/replace‐transfat. Accessed December 20, 2019.
- 53.Grocery Manufacturers Association . No news in CSPI's calls for mandatory trans fat labeling. August 7, 1996. [Google Scholar]
- 54.American Council on Science and Health. Trans fatty acids: just the latest scare du jour served up by CSPI. https://www.acsh.org/news/1996/08/07/trans-fatty-acids-just-the-latest-scare-du-jour-served-up-by-cspi. August 7, 1996. Accessed December 20, 2019. [Google Scholar]
- 55.Abboud L, Callahan P. Food industry gags at proposed label rule for trans fats. Wall Street Journal. December 27, 2002. [Google Scholar]
- 56.American Dietetic Association . Nation's nutrition experts agree “jury is still out” on trans fatty acids issue. 1996.
- 57.Institute of Shortening and Edible Oils to the Food and Drug Administration (HFA‐305). Re: Docket No. 94P‐0036 [comment]. April 14, 2000 .
- 58.Severson, K. Lawsuit seeks to ban sale of Oreos to children in California/Nabisco taken to task over trans fat's effects. San Francisco Chronicle. May 12, 2003. https://www.sfgate.com/health/article/Lawsuit‐seeks‐to‐ban‐sale‐of‐Oreos‐to‐children‐in‐2617337.php. Accessed December 20, 2019. [Google Scholar]
- 59.Ban Trans Fats. The Oreo case . http://bantransfats.com/theoreocase.html. Accessed December 20, 2019.
- 60.Center for Science in the Public Interest. A broken mcpromise advertisement. February 11, 2005. https://cspinet.org/resource/broken‐mcpromise‐ad. Accessed December 20, 2019.
- 61.Ban trans fats. The McDonald's settlement. http://bantransfats.com/mcdonalds.html. Accessed December 20, 2019.
- 62.Ackman D. McDonald's plaintiff not your average mcfatso. Forbes. July 12, 2004. https://www.forbes.com/2004/07/12/cx_da_0712topnews.html#4325dbc6190a. Accessed December 20, 2019. [Google Scholar]
- 63.Center for Science in the Public Interest . KFC sued for fouling chicken with partially hydrogenated oil. June 12, 2006. https://cspinet.org/news/kfc‐sued‐fouling‐chicken‐partially‐hydrogenated‐oil‐20060612. Accessed December 20, 2019.
- 64.Center for Science in the Public Interest . Burger king hit with trans fat lawsuit. May 16, 2007. https://cspinet.org/news/burger‐king‐hit‐trans‐fat‐lawsuit‐20070516. Accessed December 20, 2019. [Google Scholar]
- 65.Center for Science in the Public Interest . Crude oil: how palm oil harms health, rainforest & wildlife. May 1, 2005. https://cspinet.org/resource/cruel‐oil. Accessed December 20, 2019. [Google Scholar]
- 66.Centers for Disease Control and Prevention . Table 14. Diabetes prevalence and glycemic control among adults aged 20 and over, by sex, age, and race and Hispanic origin: United States, selected years 1988–1994 through 2013–2016. https://www.cdc.gov/nchs/data/hus/2018/014.pdf. Accessed October 30, 2020.
- 67.Centers for Disease Control and Prevention . Facts about hypertension. https://www.cdc.gov/bloodpressure/facts.htm. Accessed October 30, 2020.
- 68.Centers for Disease Control and Prevention. Table 26 . Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States, selected years 1988–1994 through 2013–2016. https://www.cdc.gov/nchs/data/hus/2018/026.pdf. Accessed October 30, 2020.
- 69.Harnack LJ, Cogswell ME, Shikany JM, et al. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135(19):1775‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sustainable Food Policy Alliance . Nutrition. https://foodpolicyalliance.org/issue/nutrition/. Accessed October 30, 2020.
- 71.Bruemmer B, Krieger J, Saelens BE, Chan N. Energy, saturated fat, and sodium were lower in entrees at chain restaurants at 18 months compared with 6 months following the implementation of mandatory menu labeling regulation in King County, Washington. J Acad Nutr Diet. 2012;112(8):1169‐1176. [DOI] [PubMed] [Google Scholar]
- 72.Mathematica Policy Research , Abt Associates Inc. , US Department of Agriculture , School Nutrition and Meal Cost Study. April 2019. Washington, DC: US Department of Agriculture; 2019: https://www.fns.usda.gov/school‐nutrition‐and‐meal‐cost‐study. Accessed October 30, 2020. [Google Scholar]
- 73.Jacobson MF. Salt Wars: The Battle Over the Biggest Killer in the American Diet. Cambridge, MA: MIT Press; 2020. [Google Scholar]


