Abstract
Follicular helper CD4+ T (TFH) cells are a specialized subset of effector T cells that play a central role in orchestrating adaptive immunity. TFH cells mainly promote germinal center (GC) formation, provide help to B cells for immunoglobulin affinity maturation and class-switch recombination of B cells, and facilitate production of long-lived plasma cells and memory B cells. TFH cells express the nuclear transcriptional repressor B cell lymphoma 6 (Bcl-6), the chemokine (C-X-C motif) receptor 5 (CXCR5), the CD28 family members programmed cell death protein-1 (PD-1) and inducible costimulator (ICOS) and are also responsible for the secretion of interleukin-21 (IL-21) and IL-4. Follicular regulatory CD4+ T (TFR) cells, as a regulatory counterpart of TFH cells, participate in the regulation of GC reactions. TFR cells not only express markers of TFH cells but also express markers of regulatory T (Treg) cells containing FOXP3, glucocorticoid-induced tumor necrosis factor receptor (GITR), cytotoxic T lymphocyte antigen 4 (CTLA-4), and IL-10, hence owing to the dual characteristic of TFH cells and Treg cells. ICOS, expressed on activated CD4+ effector T cells, participates in T cell activation, differentiation, and effector process. The expression of ICOS is highest on TFH and TFR cells, indicating it as a key regulator of humoral immunity. Multiple sclerosis (MS) is a severe autoimmune disease that affects the central nervous system and results in disability, mediated by autoreactive T cells with evolving evidence of a remarkable contribution from humoral responses. This review summarizes recent advances regarding TFH cells, TFR cells, and ICOS, as well as their functional characteristics in relation to MS.
1. Follicular Helper CD4+ T Cells
Follicular helper CD4+ T (TFH) cells, recognized as a distinct lineage of helper CD4+ T cells in tonsils of humans more than a decade ago, are primarily located in germinal center (GC) and provide help for B cells to promote immunoglobulin affinity maturation, class-switch recombination, and production of memory B cells and long-lived plasma cells [1]. TFH cells specially produce high levels of interleukin-21 (IL-21) and IL-4 and self-specific transcription factor the nuclear transcriptional repressor B cell lymphoma 6 (Bcl-6) and express a unique combination of surface molecules, which include chemokine (C-X-C motif) receptor 5 (CXCR5), programmed cell death protein-1 (PD-1), and the costimulatory molecule inducible costimulator (ICOS) [2]. A great deal of recent researches have been conducted to examine the exact process of TFH cell differentiation, the cellular biology of TFH cell-mediated selection of GC B cells, and the crucial roles of TFH cells in health and disease, especially in chronic autoimmune diseases (such as multiple sclerosis and systemic lupus erythematosus), primary and acquired immunodeficiencies (for example, X-linked lymphoproliferative disease and autosomal dominant hyper IgE syndrome), infections (for instance, viral infection and bacterial infection), allergy (such as asthma and pollen allergy), cancers (for example, breast cancer and follicular lymphoma), and so on [3–8] (Figure 1).
Figure 1.
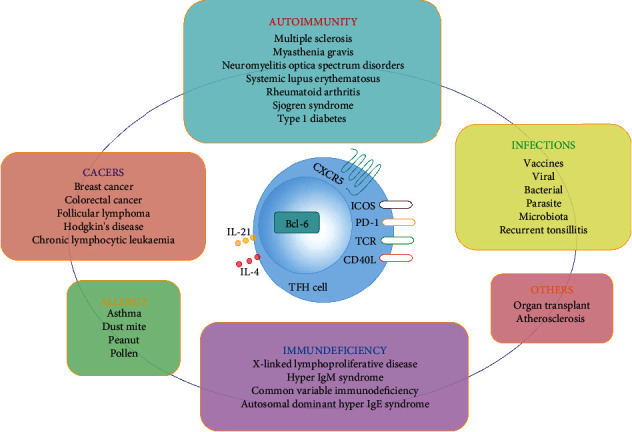
Relationships of TFH cells to diseases. TFH cells have been associated with various diseases, such as autoimmunity, cancers, infections, allergy, and immunodeficiency, with the TFH cells contributing either protective or pathogenic roles in different contexts.
1.1. Canonical TFH Cell Differentiation Pathway
Upon different stimulation, naive CD4+ T cell differentiation into various subsets of T helper (Th) cells relies on distinct cytokines and transcription factors (TFs) [9]. Th cells are comprised of Th1, Th2, Th17, Th22, TFH, and regulatory T (Treg) cells [10]. The differentiation of TFH cells from naive CD4+ T cells is a multifactorial and multistep process. In the T cell zone of secondary lymphoid tissues (SLOs), after engagement of peptide/MHC II on dendritic cells (DC) with TCR on naive CD4+ T cells, these T cells prime initially acquired TFH cell characteristic, including the induction of Bcl-6 expression, upregulating CXCR5 expression and downregulating chemokine (C-C motif) receptor (CCR) 7 expression [11]. Bcl-6, accompanied by other TFs (c-Maf, IRF4, and so on), induces the expression of CXCR5, ICOS, and PD-1 [12]. Simultaneously, IL-21 secreted by these naive CD4+ T cells, coupled with IL-6 and IL-27 produced by DC, enhances Bcl-6 and c-Maf expression in naive CD4+ T cells [13]. Then, these naive CD4+ T cells become pre-TFH cells and migrate toward the T cell-B cell border, the location of the second stage of TFH cell differentiation [14]. There, pre-TFH cells initially interact with cognate B cells to induce a high expression of Bcl-6 and CXCR5 [15]. ICOS on pre-TFH cells combines with ICOSL on B cells, thus inducing the directional migration of pre-TFH cells [2]. This process also requires the adapter signaling lymphocytic activation molecule- (SLAM-) associated protein (SAP) and SLAM family receptors Ly108 and CD84 [16, 17]. Pre-TFH and B cells together form motile conjugates which keep steady contact for minutes to hours [1]. This ensures stable localization of the cells in follicles and maintains mature TFH cell differentiation [18]. Pre-TFH cells promote B cells either differentiating into short-lived extrafollicular plasmablasts or migrating into follicles [19]. The third stage of TFH cell differentiation happens at GC, which is a particular structure comprised of GC TFH cells, GC B cells, follicular dendritic cells, macrophages, and stroma [20]. In GC, pre-TFH cells differentiate into final TFH cells also named GC TFH cells, which are CXCR5hiPD-1hiBcl-6hiMafhiSAPhi and secrete C-X-C motif chemokine 13 (CXCL13), IL-21, and IL-4 [7]. Adhesion molecules are central to GC TFH cells, because they can regulate their localization and their interaction with CC B cells [7]. GC TFH cells not only regulate the selection of high-affinity GC B cells but also regulate the development of long-term humoral immunity [3]. It is shown that even TFH cells in the GC are not terminally differentiated [21]. They are able to shuttle between the GC and the follicle and can even eventually enter the circulation [21].
1.2. Different Subsets of TFH Cells
1.2.1. Circulating TFH Cells
Circulating TFH (cTFH) cells, a group of CXCR5+CD4+ T cells with “TFH-like” characteristics, have been identified in peripheral blood of humans and mice [1]. cTFH cells express lower ICOS and PD-1 but do not express Bcl-6 compared with GC TFH cells [22]. Newly generated cTFH cells express low amounts of CCR7, which have an effector memory phenotype. After 1-2 weeks in the absence of antigen stimulation, antigen-specific effector memory cTFH cells can gradually transform into central memory cTFH cells with the CXCR5+CCR7hiPD-1−ICOS− phenotype [23]. In health, approximately 80% of cTFH is the central memory phenotype, showing the low degree of active TFH cell differentiation which exists in a homeostatic state [24].
1.2.2. Memory TFH Cells
Nearly 20% of human central memory CD4+ T cells are CXCR5+, indicating that memory TFH cells occupy an essential part of human T cell memory [7]. TFH cells become memory TFH cells and downregulate Bcl-6 after migrating from GC [25]. Memory TFH cells, majorly residing in the spleen, lymph node (LN), and bone marrow, exhibit a central memory phenotype and have the capacity to recirculate in blood [26]. Resting memory TFH cells express low amounts of PD-1. Upon reactivation, memory TFH cells preferentially become GC TFH cells and TFH cells [27].
1.2.3. TFH1, TFH2, and TFH17 Cells
TFH cells are composed of three subsets according to the expression of chemokine receptors: CXCR3+CCR6− TFH (TFH1) cells, CXCR3−CCR6− TFH (TFH2) cells, and CXCR3−CCR6+ TFH (TFH17) cells [28]. The three subsets share identical signature transcription factors and cytokines of their equivalent Th cell subsets: T-bet and IFN-γ for Th1 and TFH1 cells; GATA3, IL-4, IL-5, and IL-13 for Th2 and TFH2 cells; and RORγt, IL-17, and IL-22 for Th17 and TFH17 cells [28]. TFH2 and TFH17 efficiently induce naive B cells to produce immunoglobulins through IL-21 [28]. TFH2 promotes the production of IgG and IgE, while TFH17 induces the production of IgG and IgA [15]. In contrast, TFH1 cells lack the capacity to help B cells [28]. Recently, a CXCR3+CCR6+ TFH cell subpopulation named TFH17.1 cells was found, which produced both IFN-γ and IL-17 [29].
1.2.4. NKTFH Cells
NKTFH cells, recently recognized in GC, express Bcl-6, CXCR5, and PD-1 and promote B cell responses [30]. They have potential to induce memory B cell responses to T-dependent antigens [31]. NKTFH cells can provide help to promote the B cell differentiation program with features of GC formation, extrafollicular plasmablasts, affinity maturation, and IgG antibody response [31]. However, NKTFH cells do not induce the production of long-lived plasma cells or memory B cells [31].
1.2.5. γδTFH Cells
Human γδTFH cells, the same as conventional TFH cells, express CXCR5 and are located in follicles and GCs [22]. γδT cells are activated after they recognize nonpeptidic phosphoantigens which are derived from microbial metabolites; they subsequently differentiate into γδTFH cells with requirement of exogenous IL-21 [32].
2. Follicular Regulatory CD4+ T Cells
Follicular regulatory CD4+ T (TFR) cells, a regulatory counterpart for TFH cells, are a subset of regulatory T (Treg) cells which have been found in the spleen, lymph nodes (LN), or other lymphoid tissues such as Peyer's patches and also in blood [33–37]. TFR cells not only share numerous TFH-related molecules including CXCR5, PD-1, Bcl-6, and ICOS but also express large amounts of Treg-related factors, such as glucocorticoid-induced tumor necrosis factor receptor (GITR), cytotoxic T-lymphocyte antigen 4 (CTLA-4), IL-10, CD25, and FOXP3, so they are different from both Treg and TFH cells [38, 39]. TFR cells lack expression of CD40L, IL-4, and IL-21 [40]. On the one hand, TFR cells control excessive GC responses through acting on TFH and GC B cells; on the other hand, their suppression of B cells occurs at diverse stages of B cell differentiation, from activation to class-switched B cells and plasma cells [41]. TFR cells not only secrete immunosuppressive cytokines IL-10 and TGF-β but also suppress TFH cells through CTLA-4 and control GC B cells by suppression of CD28 ligands [42]. In addition, there are cTFR (CXCR5+FOXP3+ T) cells in the peripheral blood, which own a reduced capacity to regulate humoral immunity in contrast with tissue-resident TFR cells and circulating Treg cells [43]. In conclusion, TFR cells lead to the selection of the highest affinity antigen-specific antibody and memory B cells [41].
2.1. TFR Cell Differentiation Pathway
It was initially proposed that TFR cells are primarily derived from thymic Treg cells [37]. In addition, TFR cells can also derive from naive Th cells in a PD-L1-dependent manner using certain adjuvants [44]. TFR cells appear to experience a multistage Bcl-6-dependent differentiation process similar to TFH cells [45]. First, the differentiation of TFR cells is initiated after interaction with activated DC in the T cell zone [41]. After that, a part of TFR cells leaves the LN, enters the peripheral circulation, and becomes a memory-like TFR cell in blood [41]. The circulating TFR cells retain a reduced suppressive function and lower expression of ICOS contrasted with TFR cells in LN [43]. Alternatively, another part of TFR cells migrates toward the T-B border and enters follicles to interact with B cells and then becomes intermediate TFR cells. The final maturation step is in the GC where TFR cells acquire a phenotype of effector TFR cells upon interaction with B cells and TFH cells [41]. Effector TFR cells which have lower expression of CXCR5, ICOS, and PD-1 than GC TFH cells suppress TFH and GC B cells and GC formation [33]. Like TFH cells, TFR cell differentiation requires TCR stimulation and costimulatory molecules CD28 and ICOS which are important for TFR differentiation in both the T cell zone and follicle/GC [33]. IL21 has a negative role in TFR cells while it promotes TFH cell differentiation [46].
Although TFH and TFR cells are characterized by their anatomical location in secondary lymphoid tissues, an increasing number of experiments have shown putative circulating counterparts of these cells in peripheral blood especially for human samples. It is limiting to have access to secondary lymphoid tissues. A recent study showed that circulating TFR cells were increased in Sjögren syndrome (SS), a systemic autoimmune disease characterized by ongoing GC reactions [43]. In addition, there was a substantial increase in the TFR/TFH ratio in SS patients, which was associated with serum autoantibodies of SS patients [43]. Another study indicated that the circulating TFR/TFH ratio and activated TFH cells were associated with pathological lymphocytic infiltration in the SS target organ minor salivary gland (MSG) and disease activity, respectively, in primary SS [47]. On account of the regulatory role of TFR cells, the predictive value of TFR cells and TFR/TFH ratio in SS patients may appear controversial [48]. Fonseca et al. showed that human blood TFR cells remain immature, generated prior to T-B interactions, lack full B cell-suppressive capacity, and have a naïve-like phenotype, although they correlated with humoral responses [43]. Nevertheless, the frequency of blood TFR cell and the TFR/TFH ratio were reduced in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) patients [49, 50]. The TFR/TFH ratio and the frequency of TFR cells were negatively correlated with disease activity and serum anti-dsDNA antibody level in SLE [49]. Moreover, the TFR/TFH ratio was negatively correlated with values of ESR, RF, CRP, and DAS28 scores while it was positively correlated with serum TGF-β level [50]. In conclusion, the TFH, TFR, and TFR/TFH ratio may be responsible for the immunopathogenesis of diseases.
3. Inducible Costimulator (ICOS)
According to the two-signal model for T cell activation, T cells first require an antigen-specific signal and then a costimulatory signal in order to induce an optimal adaptive immunity [51] . The costimulatory signals are essential for T cell proliferation, lymphokine secretion, and effector function [52]. ICOS, as a member of the CD28 family of costimulatory molecules, is expressed at low levels on resting naive T cells and is rapidly induced following TCR ligation and CD28 costimulation [53]. Then, ICOS has a high expression on activated T cells and delivers a positive costimulatory signal [54]. ICOS signal is initiated upon ligation with the ICOS ligand (ICOSL) which is expressed on antigen-presenting cells, including B cells, DCs, macrophages, certain endothelial cells, and lung epithelium [55]. ICOS ligation induces phosphatidylinositol 3-kinase (PI3K) signaling which contributes to the production of membrane-bound phosphatidylinositol 3,4,5-trisphosphate (PIP3), followed by the activation of Akt—a kinase that promotes cellular proliferation and survival [56, 57]. So, the engagement of ICOS promotes cell differentiation, proliferation, and survival [58]. ICOS can induce the differentiation of Th1, Th2, Th17, TFH, Treg, and TFR cells depending on different contexts of the inflammatory circumstances, because ICOS coinduces the production of IFN-γ, TNF-α, IL-4, IL-5, IL-6, IL-10, IL-21, and so on [53] (Figure 2). Activation of ICOS upregulates transcription factors NFATc2 and T-bet in Th1 cells and promotes the secretion of IFN-γ, whereas in Th2 cells, binding of ICOS to ICOSL upregulates transcription factors NFATc1 and c-Maf and promotes GATA-3 transcription and IL-4 secretion [53, 59] (Figure 2). ICOS-ICOSL interaction promotes FOXP3 transcription, favors NFAT binding to FOXP3, and promotes the production of IL-10 in Treg cells [60] (Figure 2). Moreover, ICOS stimulation induces RORC2 and c-Maf expression in Th17 cells and Bcl-6 expression in TFH cells, leading to increased secretion of IL-17 and IL-21, respectively [56, 61]. What is more, ICOS ligation upregulates transcription factors FOXP3 and Bcl-6 and promotes IL-10 and TGF-β production in TFR cells [62] (Figure 2).
Figure 2.
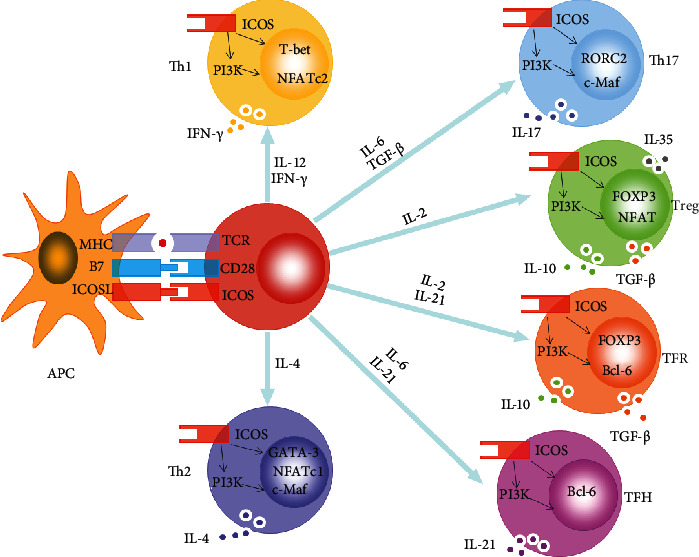
ICOS induces the differentiation of CD4+ T cell subsets. For Th1 cells, ICOS ligation upregulates transcription factors NFATc2 and T-bet and promotes IFN-γ production. For Th2 cells, ICOS engagement upregulates transcription factors NFATc1 and c-Maf and promotes GATA-3 transcription and the production of IL-4. Moreover, binding of ICOS to ICOSL promotes FOXP3 transcription, favoring NFAT binding to FOXP3 in Treg cells, and promotes IL-10, IL-35, and TGF-β secretion. In addition, ICOS-ICOSL interaction induces RORC2 and c-Maf expression and promotes IL-17 production in Th17 cells, whereas binding of ICOS to ICOSL induces Bcl-6 expression in TFH cells, leading to increased secretion of IL-21. What is more, ICOS ligation upregulates transcription factors FOXP3 and Bcl-6 and promotes IL-10 and TGF-β production in TFR cells.
3.1. ICOS and TFH Cells
ICOS was first found on the cell surface of GC T cells, indicating its relevant role in T cell-B cell interactions [56]. Signals through ICOS-ICOSL are crucial for each stage of TFH cell differentiation and function [63]. For the differentiation and maintenance of TFH cells, ICOS can recruit PI3K which has a dual role in transcriptional regulation of genes [64] (Figure 3). On the one hand, activation of Akt through the p110δ catalytic subunit of PI3K phosphorylates the transcription factor Foxo1, which is accordingly retained in the cytoplasm [65] (Figure 3). This timely inhibition of Foxo1 upregulates Bcl-6 and downregulates KLF2, which both are vital transcriptional regulators controlling the expression of TFH signature genes involved in TFH migration and function [66]. On the other hand, the PI3K regulatory subunit p85α forms complexes with the intracellular forms of osteopontin (OPNi) which migrates into the nucleus and binds to Bcl-6 in order to protect Bcl-6 from ubiquitin-dependent degradation [67] (Figure 3). Besides, ICOS signaling can also affect IL-21 production via c-Maf, thereby regulating TFH cell differentiation [63].
Figure 3.
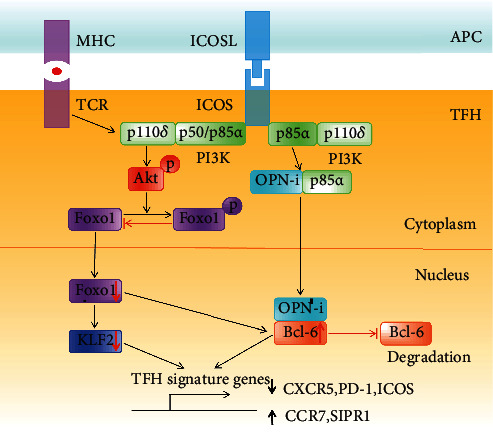
Signaling pathway for ICOS costimulation in TFH cell differentiation. For TFH cell differentiation, ICOS-mediated recruitment of PI3K has a dual role in transcriptional regulation of genes. On the one hand, the activation of PI3K causes activation of Akt and subsequently inhibits the transcription factor Foxo1 function through phosphorylation-mediated cytosolic retention. The inhibition of Foxo1 upregulates Bcl-6 and downregulates KLF2, which both are crucial transcriptional regulators controlling the expression of TFH signature genes. On the other hand, the regulatory subunit p85α of PI3K can bind with the intracellular form of osteopontin (OPNi), whereafter OPNi migrates into the nucleus and binds to Bcl-6 which can protect Bcl-6 from ubiquitin-dependent degradation and regulate the expression of TFH signature genes.
ICOS-mediated signals are not only necessary for TFH differentiation but also required for TFH migration and T-B collaboration [64]. At the T-B border, the ligation of ICOS on pre-TFH cells and ICOSL on bystander B cells that do not present cognate antigens promotes T cell motility allowing cognate T-B conjugation and GC enter [64]. This process depends on the ICOS-PI3K signaling and presumably activation of small GTPases directly and/or indirectly by ICOS [68]. In the context of high-affinity interactions between the TCR and peptide-MHC complexes, ICOS ligation allows focused delivery of T cell “help” to cognate GC B cells within the GC. The ICOS-PI3K-mTOR signaling has been shown to facilitate IL-4 production from existing mRNA by inducing polysome formation [69]. Meanwhile, ICOS-mediated calcium flux promotes CD40L externalization providing CD40 costimulation to the differentiation of GC B cells [70]. In turn, CD40 signaling elevates ICOSL expression level. This feedforward cross-talk promotes selection of high-affinity GC B cell clones [64].
ICOS is regarded as a marker of TFH cells in human [71]. Increased frequency of ICOS+ or ICOShigh cTFH cells standing for an “activated” phenotype has been found in patients with autoimmunity such as RA [72], SS [73], myasthenia gravis (MG) [74], SLE [75], autoimmune thyroid diseases [76], and all autoimmune pathologies partially caused by autoantibodies [1]. Interestingly, increased frequency of TFH cells was positively correlated with tissue damage, high titers of autoantibodies, IL-21 levels, and frequencies of circulating GC B cells and plasma cells in these pathologies [4]. Blockade of ICOS-ICOSL signaling can ameliorate disease through disrupting TFH cell responses in SLE, MG, collagen-induced arthritis, allergic asthma, and pemphigus vulgaris [77–79]. Thus, TFH and ICOS, involved in pathogenic mechanisms, may be potential therapeutic targets in autoimmune diseases.
3.2. ICOS and TFR Cells
ICOS is essential for TFR differentiation and function [64]. First, ICOS-ICOSL interaction induces FOXP3 transcription, favoring the transcription factor NFAT combining with FOXP3 and upregulating FOXP3 downstream regulatory genes [60]. Second, elevation of PI3K- mammalian target of rapamycin (mTOR) after ICOS ligation induces Treg conversion to the TFR lineage in Roquin-deficient mice [80]. In addition, the ICOS-calcium pathway plays a crucial role in the development of TFR cells since the calcium-dependent transcription factor NFAT2 is essential to upregulate CXCR5 in TFR cells [81]. What is more, impaired calcium signaling caused by Stim1 and Stim 2 deletion resulted in reduced TFR cell differentiation and spontaneous autoantibody production [82]. Thus, these data indicate that ICOS signaling contribute to TFR generation.
4. TFH Cells, TFR Cells, and ICOS in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis
Multiple sclerosis (MS), one of the most common chronic immune-mediated diseases of the central nervous system (CNS), is affecting more than 2.5 million people worldwide [83]. MS is characterized by inflammation of CNS and demyelination of neurons and causes collateral damage in the neighboring CNS system [84]. There are three crucial disease phenotypes of MS which are traditionally recognized: relapsing-remitting MS (RRMS), secondary progressive MS (SPMS), and primary progressive (PPMS) [85]. MS majorly affects individuals in their early adult life, so it has an enormous impact functionally and financially on the quality of life [86]. Experimental autoimmune encephalomyelitis (EAE), one of the most widely used MS animal models, is used to explore the pathogenesis of MS.
Although the exact etiology remains unknown, current researches have suggested that genetic and environmental factors, including low vitamin D levels, cigarette smoking, and obesity, have causal roles in MS [86]. Previously, autoreactive T cells are regarded as the central drivers of MS pathogenesis and progression [87]. Nevertheless, recent studies have shown that the interplay between B and T cells is an essential player in immune responses involved in MS [88]. According to the researches, B cells not only present antigen to T cells and promote autoproliferation of brain-homing T cells but also produce soluble toxic factor and proinflammatory cytokines and chemokines in MS [89]. In addition, there are intrathecal antibodies and B cell-rich aggregates in the meninges surrounding the brain and spinal cord, which are essential contributors of CNS compartmentalized inflammation [89]. Moreover, CD19/20 monoclonals depleting B cells are effective for MS patients [85].
Thus, understanding the role of TFH cells, TFR cells, and surface molecule ICOS which were involved in the interaction between B and T cells is important in looking for new therapeutic strategies in MS and EAE.
4.1. TFH Cells, TFR Cells, and ICOS in Multiple Sclerosis
Guo et al. found that the proportion of circulating TFH cells (CD3+CD4+CXCR5+PD-1+) was higher in relapsing RRMS patients than in remitting RRMS and healthy controls (HCs) [90]. The level of circulating TFH cells was positively correlated with the level of serum IL21 and B cells [90]. Another study showed that the numbers of circulating ICOS+, CCR7+, and CCR7+ICOS+ memory TFH cells, as well as plasma IL21 level, were higher in MS patients than in HCs [91]. In addition, the frequency of circulating TFH1 cells is decreased in MS patients compared to HCs, and the frequency of circulating TFH17 cells was reduced in PPMS [92]. A proinflammatory bias presented higher frequencies of circulating TFH17.1 cells and lower frequencies of TFH2 cells, which was found in RRMS patients compared to HCs [93]. In addition, IL21 and IL21R are involved in the immunopathogenesis of MS [94]. An experiment showed that the levels of serum IL21 and IL-21 mRNA were significantly increased in MS patients compared with HCs and were positively correlated with EDSS scores and progression index of MS patients [95]. Moreover, the increased frequencies of IL21-producing TFH cells were positively related to MS severity and progression [95]. Another study reported that the frequencies of circulating TFR cells in MS patients were significantly lower than HCs [96]. Furthermore, TFR cells in MS patients owned an obviously reduced suppressive function compared with HCs, indicating prominent TFR cell impairment in MS [96]. Similarly, another study also indicated lower TFR frequencies and higher TFH/TFR ratio in RRMS patients at the clinical onset compared with HCs [97]. The TFH/TFR ratio, as a matter of fact, is positively associated with abnormal IgG synthesis in serum and CSF in RRMS patients, indicating that autoreactive B cell clones, derived from deregulated peripheral GC reaction, may colonize the CNS [97]. TFH cells can migrate to the CNS in MS patients [98]. There is evidence that the levels of CXCL13 and the chemokine ligand for CXCR5 on TFH cells and T cells were increased in the CSF of MS patients, suggesting that TFH cells migrate and stay in the CSF because of elevated CXCL13 expression [98]. A recent study showed a cluster-independent increase in TFH cells potentially driving the MS-specific B cell expansion in the CSF of MS through cell set enrichment analysis [99]. They found an obviously increased percentage of TFH, PD-1+ TFH, and PD-1+ICOS+ TFH cells in the CSF of MS patients by flow cytometry, while the proportion of PD-1+ TFH cells had a positive relation to the percentage of CSF plasma cells and disease progression [99]. Another study indicated that the increased frequency of memory TFH cells in CD4+ T cells in the CSF of RRMS has a significant correlation with the percentage of antibody-secreting B cells [100].
A recent study showed that abatacept, a CTLA-4-Ig fusion protein inhibiting T cell activation and function, reduced the relative frequencies of circulating CD45RO+ TFH cells expressing the activation markers CD38 and ICOS in patients with RRMS [101]. Dimethyl fumarate (DMF), an oral fumaric acid ester, is a disease-modifying therapy for RRMS. MS patients had reduced proportions of TFH cells and antigen-experienced B cells, accompanied by increased TFR cells in DMF-treated MS patients compared to untreated patients [102]. Another study showed that DMF treatment resulted in a progressive increase in cTFH2 cells, together with a decrease in cTFH1 and the pathogenic cTFH17.1 cells, indicating a possibly pathogenic cTFH proinflammatory profile of RRMS [93].
4.2. TFH Cells, TFR Cells, and ICOS in Experimental Autoimmune Encephalomyelitis
It is reported that the frequency of PD-1+ and ICOS+ TFH cells in the spleen and draining lymph nodes increased before the manifestation of clinical symptoms, attained a maximum during the peak stage, and declined at the remission stage of EAE [90]. The percentage of these TFH cells elevated slightly in the chronic phase [90]. What is more, the protein levels of Bcl-6, CXCR5, and IL21, together with the serum IL-21 level, presented the similar changes as those of TFH cells [90]. Both the frequencies of TFH cells and the serum level of IL21 had positive correlations with the disease scores of EAE [90]. In Th17-induced EAE, there are a massive number of PD-1+ TFH cells in the CNS tissue which was correlated with the number of infiltrating B cells at the peak of disease [103]. TFH cells induce B cell infiltration into the CNS and locally drive B cell response in the CNS of EAE [99]. The proportion of PD-1+ TFH cells in the spinal cord and brain reached a maximum at the peak phase of EAE and had a positive correlation with the disease score [90]. A number of ICOS+ TFH and PD-1+ TFH cells were also found within the ectopic lymphoid structures of spinal cords in EAE. Moreover, TFH cells had a great potential in boosting anti-MOG antibody production of B cells via IL-21 and CD40 ligand (CD40L) and the synergy effect of the STAT3 and noncanonical NF-κB signaling pathway inside B cells [90]. Besides, adoptive transfer of TFH cells could enhance the disease severity and delay disease remission [90]. Interestingly, mice with a FOXP3-specific deletion of Blimp1 develop severe EAE and increased TFH-B-Ab response, while having increased frequency of TFR cells [104]. Most TFH cells were TFH17cells and expressed more IL-17A and GM-CSF, as well as CXCL13 in Prdm1fl/flFOXP3Cre EAE mice [104]. In contrary to WT TFR cells, Blimp1-deleted TFR cells were more encephalitogenic, reflecting dysregulated Ab response and EAE progression [104].
A previous study showed that blockade of the ICOS/ICOSL pathway during the efferent immune response (9–20 days after immunization) alleviated the disease, but ICOS blockade during the antigen priming stage (1–10 days after immunization) aggravated the disease in proteolipid protein- (PLP-) induced EAE [105]. For the former, ICOS blockage inhibited the activation and proliferation of the encephalitogenic ICOS+ T cells and restrained the following recruitment of nonantigen-specific leukocytes in the efferent immune stage [105]. In the latter however, blockade of the ICOS/ICOSL pathway in antigen priming response resulted in an increase in the ratio of Th1 : Th2 cells and amplified the activation of monocytes or macrophages and microglia [105]. In another study, the researchers used an antagonistic antibody against CXCL13, the chemokine ligand for CXCR5 on TFH cells to treat Th17-EAE in order to block TFH trafficking, and found that it can significantly reduce TH17-EAE disease [106]. A recent study showed that laquinimod suppressed TFH (PD-1+CXCR5+BCL-6+ TFH) and B cell aggregates and reduced disease progression in EAE [107].
In summary, TFH cells, TFR cells, and ICOS are all involved in the pathological process of MS and EAE and are regarded as markers of disease severity, progression, and prognosis. Hence, TFH cells, TFR cells, and ICOS may be beneficial therapeutic targets of the disease. Monitoring TFH cells and TFR cells in the treatment process of MS is supposed to become a biological marker of drug efficacy.
5. Conclusion
TFH cells, TFR cells, and ICOS play significant roles in the pathogenesis of various diseases. TFH cells, TFR cells, and ICOS might be the potentially key targets for novel therapeutic selections in diseases. Further studies are still needed to better understand the precise roles of them in diseases, which will open a new avenue to explore the mechanisms of the pathogenic process in diseases.
Acknowledgments
This work was supported by the following grants: National Natural Science Foundation of China (grant numbers 81701192 and 81901380); Shandong Provincial Natural Science Foundation, China (grant numbers ZR2017BH078 and ZR2017BC047); and Scientific Research Foundation of Binzhou Medical University (grant numbers BY2017KYQD15 and BY2016KYQD21).
Contributor Information
Jinbo Chen, Email: chenjinbo6720@126.com.
Xueli Fan, Email: xuelifan@yeah.net.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contributions
Xue Zhang, Ruli Ge, and Hongliang Chen contributed equally to this work. Jinbo Chen and Xueli Fan contributed equally to this work.
References
- 1.Song W., Craft J. T follicular helper cell heterogeneity: time, space, and function. Immunological Reviews. 2019;288(1):85–96. doi: 10.1111/imr.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crotty S. Follicular helper CD4 T cells (TFH) Annual Review of Immunology. 2011;29(1):621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 3.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50(5):1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gensous N., Charrier M., Duluc D., et al. T follicular helper cells in autoimmune disorders. Frontiers in Immunology. 2018;9:p. 1637. doi: 10.3389/fimmu.2018.01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherm M. G., Ott V. B., Daniel C. Follicular helper T cells in autoimmunity. Current Diabetes Reports. 2016;16(8):p. 75. doi: 10.1007/s11892-016-0770-2. [DOI] [PubMed] [Google Scholar]
- 6.Ma C. S., Deenick E. K. Human T follicular helper (Tfh) cells and disease. Immunology and Cell Biology. 2014;92(1):64–71. doi: 10.1038/icb.2013.55. [DOI] [PubMed] [Google Scholar]
- 7.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu-Trantien C., Migliori E., Buisseret L., et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017;2(11) doi: 10.1172/jci.insight.91487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jogdand G. M., Mohanty S., Devadas S. Regulators of Tfh cell differentiation. Frontiers in Immunology. 2016;7:p. 520. doi: 10.3389/fimmu.2016.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatzileontiadou D. S. M., Sloane H., Nguyen A. T., Gras S., Grant E. J. The many faces of CD4(+) T cells: immunological and structural characteristics. International Journal of Molecular Sciences. 2021;22 doi: 10.3390/ijms22010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannons J. L., Lu K. T., Schwartzberg P. L. T follicular helper cell diversity and plasticity. Trends in Immunology. 2013;34(5):200–207. doi: 10.1016/j.it.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andris F., Denanglaire S., Anciaux M., Hercor M., Hussein H., Leo O. The transcription factor c-Maf promotes the differentiation of follicular helper T cells. Frontiers in Immunology. 2017;8:p. 480. doi: 10.3389/fimmu.2017.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan X., Lin C., Han J., Jiang X., Zhu J., Jin T. Follicular helper CD4+ T cells in human neuroautoimmune diseases and their animal models. Mediators of Inflammation. 2015;2015:11. doi: 10.1155/2015/638968.638968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varricchi G., Harker J., Borriello F., Marone G., Durham S. R., Shamji M. H. T follicular helper (Tfh) cells in normal immune responses and in allergic disorders. Allergy. 2016;71(8):1086–1094. doi: 10.1111/all.12878. [DOI] [PubMed] [Google Scholar]
- 15.Kim S. J., Lee K., Diamond B. Follicular helper T cells in systemic lupus erythematosus. Frontiers in Immunology. 2018;9:p. 1793. doi: 10.3389/fimmu.2018.01793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi H., Cannons J. L., Klauschen F., Schwartzberg P. L., Germain R. N. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455(7214):764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannons J. L., Qi H., Lu K. T., et al. Optimal Germinal Center Responses Require a Multistage T Cell:B Cell Adhesion Process Involving Integrins, SLAM-Associated Protein, and CD84. Immunity. 2010;32(2):253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston R. J., Poholek A. C., DiToro D., et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma C. S., Deenick E. K., Batten M., Tangye S. G. The origins, function, and regulation of T follicular helper cells. The Journal of Experimental Medicine. 2012;209:1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke M. A., Eto D., Locci M., et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. Journal of Immunology. 2012;188(8):3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi H. T follicular helper cells in space-time. Nature Reviews. Immunology. 2016;16(10):612–625. doi: 10.1038/nri.2016.94. [DOI] [PubMed] [Google Scholar]
- 22.Tangye S. G., Ma C. S., Brink R., Deenick E. K. The good, the bad and the ugly -- TFH cells in human health and disease. Nature Reviews Immunology. 2013;13(6):412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 23.He J., Tsai L. M., Leong Y. A., et al. Circulating Precursor CCR7loPD-1hi CXCR5+ CD4+ T Cells Indicate Tfh Cell Activity and Promote Antibody Responses upon Antigen Reexposure. Immunity. 2013;39(4):770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Deng J., Wei Y., Fonseca V. R., Graca L., Yu D. T follicular helper cells and T follicular regulatory cells in rheumatic diseases. Nature Reviews Rheumatology. 2019;15(8):475–490. doi: 10.1038/s41584-019-0254-2. [DOI] [PubMed] [Google Scholar]
- 25.Dong L., He Y., Cao Y., et al. Functional differentiation and regulation of follicular T helper cells in inflammation and autoimmunity. Immunology. 2020;163(1):19–32. doi: 10.1111/imm.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chevalier N., Jarrossay D., Ho E., et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. Journal of Immunology. 2011;186(10):5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 27.Hale J. S., Youngblood B., Latner D. R., et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38(4):805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita R., Schmitt N., Bentebibel S. E., et al. Human Blood CXCR5+CD4+ T Cells Are Counterparts of T Follicular Cells and Contain Specific Subsets that Differentially Support Antibody Secretion. Immunity. 2011;34(1):108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramstein J., Broos C. E., Simpson L. J., et al. IFN-γ–Producing T-helper 17.1 cells are increased in sarcoidosis and are more prevalent than T-helper type 1 cells. American Journal of Respiratory and Critical Care Medicine. 2016;193(11):1281–1291. doi: 10.1164/rccm.201507-1499OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rampuria P., Lang M. L. CD1d-dependent expansion of NKT follicular helper cells in vivo and in vitro is a product of cellular proliferation and differentiation. International Immunology. 2015;27(5):253–263. doi: 10.1093/intimm/dxv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang M. L. The influence of invariant natural killer T cells on humoral immunity to T-dependent and -independent antigens. Frontiers in Immunology. 2018;9:p. 305. doi: 10.3389/fimmu.2018.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vantourout P., Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nature Reviews. Immunology. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sage P. T., Francisco L. M., Carman C. V., Sharpe A. H. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nature Immunology. 2013;14(2):152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sage P. T., Tan C. L., Freeman G. J., Haigis M., Sharpe A. H. Defective TFH cell function and increased TFR cells contribute to defective antibody production in aging. Cell Reports. 2015;12(2):163–171. doi: 10.1016/j.celrep.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderleyden I., Linterman M. A., Smith K. G. Regulatory T cells and control of the germinal centre response. Arthritis Research & Therapy. 2014;16(5):p. 471. doi: 10.1186/s13075-014-0471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y., Zou L., Liu Y. C. T follicular helper cells, T follicular regulatory cells and autoimmunity. International Immunology. 2016;28(4):173–179. doi: 10.1093/intimm/dxv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y., Chen Z., Wang H., et al. Follicular regulatory T cells: a novel target for immunotherapy? Clinical & translational immunology. 2020;9(2):p. e1106. doi: 10.1002/cti2.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung Y., Tanaka S., Chu F., et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature Medicine. 2011;17(8):983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sage P. T., Sharpe A. H. T follicular regulatory cells in the regulation of B cell responses. Trends in Immunology. 2015;36(7):410–418. doi: 10.1016/j.it.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pratama A., Vinuesa C. G. Control of TFH cell numbers: why and how? Immunology and Cell Biology. 2014;92(1):40–48. doi: 10.1038/icb.2013.69. [DOI] [PubMed] [Google Scholar]
- 41.Sage P. T., Sharpe A. H. T follicular regulatory cells. Immunological Reviews. 2016;271(1):246–259. doi: 10.1111/imr.12411. [DOI] [PubMed] [Google Scholar]
- 42.Sage P. T., Paterson A. M., Lovitch S. B., Sharpe A. H. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41(6):1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fonseca V. R., Agua-Doce A., Maceiras A. R., et al. Human blood Tfrcells are indicators of ongoing humoral activity not fully licensed with suppressive function. Science Immunology. 2017;2(14):p. eaan1487. doi: 10.1126/sciimmunol.aan1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aloulou M., Carr E. J., Gador M., et al. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nature communications. 2016;7(1) doi: 10.1038/ncomms10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fonseca V. R., Ribeiro F., Graca L. T follicular regulatory (Tfr) cells: dissecting the complexity of Tfr-cell compartments. Immunological Reviews. 2019;288(1):112–127. doi: 10.1111/imr.12739. [DOI] [PubMed] [Google Scholar]
- 46.Jandl C., Liu S. M., Canete P. F., et al. IL-21 restricts T follicular regulatory T cell proliferation through Bcl-6 mediated inhibition of responsiveness to IL-2. Nature communications. 2017;8(1) doi: 10.1038/ncomms14647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fonseca V. R., Romao V. C., Agua-Doce A., et al. The ratio of blood T follicular regulatory cells to T follicular helper cells marks ectopic lymphoid structure formation while activated follicular helper T cells indicate disease activity in primary Sjögren's syndrome. Arthritis & Rhematology. 2018;70(5):774–784. doi: 10.1002/art.40424. [DOI] [PubMed] [Google Scholar]
- 48.Maceiras A. R., Fonseca V. R., Agua-Doce A., Graca L. T follicular regulatory cells in mice and men. Immunology. 2017;152(1):25–35. doi: 10.1111/imm.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu B., Wang S., Zhou M., et al. The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus. Clinical Immunology. 2017;183:46–53. doi: 10.1016/j.clim.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao G., Wang P., Cui Z., et al. An imbalance between blood CD4+CXCR5+Foxp3+ Tfr cells and CD4+CXCR5+Tfh cells may contribute to the immunopathogenesis of rheumatoid arthritis. Molecular Immunology. 2020;125:1–8. doi: 10.1016/j.molimm.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Mages H. W., Hutloff A., Heuck C., et al. Molecular cloning and characterization of murine ICOS and identification of B7h as ICOS ligand. European Journal of Immunology. 2000;30(4):1040–1047. doi: 10.1002/(SICI)1521-4141(200004)30:4<1040::AID-IMMU1040>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 52.McAdam A. J., Greenwald R. J., Levin M. A., et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409(6816):102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 53.Simpson T. R., Quezada S. A., Allison J. P. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS) Current Opinion in Immunology. 2010;22(3):326–332. doi: 10.1016/j.coi.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Sharpe A. H., Freeman G. J. The B7-CD28 superfamily. Nature Reviews. Immunology. 2002;2(2):116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 55.Wilcox R. A. A three-signal model of T-cell lymphoma pathogenesis. American Journal of Hematology. 2016;91(1):113–122. doi: 10.1002/ajh.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wikenheiser D. J., Stumhofer J. S. ICOS co-stimulation: friend or foe? Frontiers in Immunology. 2016;7:p. 304. doi: 10.3389/fimmu.2016.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slomovitz B. M., Coleman R. L. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clinical Cancer Research. 2012;18(21):5856–5864. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 58.Sanmamed M. F., Pastor F., Rodriguez A., et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Seminars in Oncology. 2015;42(4):640–655. doi: 10.1053/j.seminoncol.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Tan A. H., Goh S. Y., Wong S. C., Lam K. P. T Helper Cell-specific Regulation of Inducible Costimulator Expression via Distinct Mechanisms Mediated by T-bet and GATA-3. The Journal of Biological Chemistry. 2008;283(1):128–136. doi: 10.1074/jbc.M707693200. [DOI] [PubMed] [Google Scholar]
- 60.Li D. Y., Xiong X. Z. ICOS(+) Tregs: a functional subset of Tregs in immune diseases. Frontiers in Immunology. 2020;11:p. 2104. doi: 10.3389/fimmu.2020.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paulos C. M., Carpenito C., Plesa G., et al. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Science translational medicine. 2010;2(55):p. 55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hao H., Nakayamada S., Tanaka Y. Differentiation, functions, and roles of T follicular regulatory cells in autoimmune diseases. Inflammation and Regeneration. 2021;41(1):p. 14. doi: 10.1186/s41232-021-00164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu H., Deng Y., Zhao M., et al. Molecular control of follicular helper T cell development and differentiation. Frontiers in Immunology. 2018;9:p. 2470. doi: 10.3389/fimmu.2018.02470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panneton V., Chang J., Witalis M., Li J., Suh W. K. Inducible T-cell co-stimulator: signaling mechanisms in T follicular helper cells and beyond. Immunological Reviews. 2019;291(1):91–103. doi: 10.1111/imr.12771. [DOI] [PubMed] [Google Scholar]
- 65.Hutloff A. Regulation of T follicular helper cells by ICOS. Oncotarget. 2015;6(26):21785–21786. doi: 10.18632/oncotarget.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stone E. L., Pepper M., Katayama C. D., et al. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity. 2015;42(2):239–251. doi: 10.1016/j.immuni.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leavenworth J. W., Verbinnen B., Yin J., Huang H., Cantor H. A p85α-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nature Immunology. 2015;16(1):96–106. doi: 10.1038/ni.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu H., Li X., Liu D., et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS- driven motility. Nature. 2013;496(7446):523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 69.Gigoux M., Lovato A., Leconte J., Leung J., Sonenberg N., Suh W. K. Inducible costimulator facilitates T-dependent B cell activation by augmenting IL-4 translation. Molecular Immunology. 2014;59(1):46–54. doi: 10.1016/j.molimm.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Liu D., Xu H., Shih C., et al. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 2015;517(7533):214–218. doi: 10.1038/nature13803. [DOI] [PubMed] [Google Scholar]
- 71.Bentebibel S. E., Schmitt N., Banchereau J., Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(33):E488–E497. doi: 10.1073/pnas.1100898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma J., Zhu C., Ma B., et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clinical & Developmental Immunology. 2012;2012, article 827480:7. doi: 10.1155/2012/827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pontarini E., Murray-Brown W. J., Croia C., et al. Unique expansion of IL-21+ Tfh and Tph cells under control of ICOS identifies Sjögren’s syndrome with ectopic germinal centres and MALT lymphoma. Annals of the Rheumatic Diseases. 2020;79(12):1588–1599. doi: 10.1136/annrheumdis-2020-217646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo C., Li Y., Liu W., et al. Expansion of circulating counterparts of follicular helper T cells in patients with myasthenia gravis. Journal of Neuroimmunology. 2013;256(1-2):55–61. doi: 10.1016/j.jneuroim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Simpson N., Gatenby P. A., Wilson A., et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis and Rheumatism. 2010;62(1):234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 76.Zhu C., Ma J., Liu Y., et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. The Journal of Clinical Endocrinology and Metabolism. 2012;97(3):943–950. doi: 10.1210/jc.2011-2003. [DOI] [PubMed] [Google Scholar]
- 77.Vinuesa C. G., Tangye S. G., Moser B., Mackay C. R. Follicular B helper T cells in antibody responses and autoimmunity. Nature Reviews. Immunology. 2005;5(11):853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 78.Uwadiae F. I., Pyle C. J., Walker S. A., Lloyd C. M., Harker J. A. Targeting the ICOS/ICOS-L pathway in a mouse model of established allergic asthma disrupts T follicular helper cell responses and ameliorates disease. Allergy. 2019;74(4):650–662. doi: 10.1111/all.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim A. R., Han D., Choi J. Y., et al. Targeting inducible costimulator expressed on CXCR5+PD-1+ TH cells suppresses the progression of pemphigus vulgaris. Journal of Allergy and Clinical Immunology. 2020;146(5):1070–1079.e8. doi: 10.1016/j.jaci.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 80.Essig K., Hu D., Guimaraes J. C., et al. Roquin suppresses the PI3K-mTOR signaling pathway to inhibit T helper cell differentiation and conversion of Treg to Tfr cells. Immunity. 2017;47(6):1067–1082.e12. doi: 10.1016/j.immuni.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 81.Vaeth M., Muller G., Stauss D., et al. Follicular regulatory T cells control humoral autoimmunity via NFAT2-regulated CXCR5 expression. The Journal of Experimental Medicine. 2014;211(3):545–561. doi: 10.1084/jem.20130604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaeth M., Eckstein M., Shaw P. J., et al. Store-Operated Ca2+ Entry in Follicular T Cells Controls Humoral Immune Responses and Autoimmunity. Immunity. 2016;44(6):1350–1364. doi: 10.1016/j.immuni.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reich D. S., Lucchinetti C. F., Calabresi P. A. Multiple sclerosis. The New England Journal of Medicine. 2018;378(2):169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quinn J. L., Axtell R. C. Emerging role of follicular T helper cells in multiple sclerosis and experimental autoimmune encephalomyelitis. International journal of molecular sciences. 2018;19(10):p. 3233. doi: 10.3390/ijms19103233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Piehl F. Current and emerging disease-modulatory therapies and treatment targets for multiple sclerosis. Journal of Internal Medicine. 2021;289(6):771–791. doi: 10.1111/joim.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thompson A. J., Baranzini S. E., Geurts J., Hemmer B., Ciccarelli O. Multiple sclerosis. Lancet. 2018;391:1622–1636. doi: 10.1016/S0140-6736(18)30481-1. [DOI] [PubMed] [Google Scholar]
- 87.Arneth B. Contributions of T cells in multiple sclerosis: what do we currently know? Journal of Neurology. 2020 doi: 10.1007/s00415-020-10275-x. [DOI] [PubMed] [Google Scholar]
- 88.Comi G., Bar-Or A., Lassmann H., et al. Role of B cells in multiple sclerosis and related disorders. Annals of Neurology. 2021;89(1):13–23. doi: 10.1002/ana.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li R., Patterson K. R., Bar-Or A. Reassessing B cell contributions in multiple sclerosis. Nature Immunology. 2018;19(7):696–707. doi: 10.1038/s41590-018-0135-x. [DOI] [PubMed] [Google Scholar]
- 90.Guo J., Zhao C., Wu F., et al. T follicular helper-like cells are involved in the pathogenesis of experimental autoimmune encephalomyelitis. Frontiers in Immunology. 2018;9:p. 944. doi: 10.3389/fimmu.2018.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fan X., Jin T., Zhao S., et al. Circulating CCR7+ICOS+ memory T follicular helper cells in patients with multiple sclerosis. PLoS One. 2015;10(7):p. e0134523. doi: 10.1371/journal.pone.0134523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Romme Christensen J., Bornsen L., Ratzer R., et al. Correction: Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One. 2013;8(3) doi: 10.1371/annotation/b4e623eb-4950-48d9-8d85-8d70426d95a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cunill V., Massot M., Clemente A., et al. Relapsing-remitting multiple sclerosis is characterized by a T follicular cell pro-inflammatory shift, reverted by dimethyl fumarate treatment. Frontiers in Immunology. 2018;9:p. 1097. doi: 10.3389/fimmu.2018.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghalamfarsa G., Mahmoudi M., Mohammadnia-Afrouzi M., et al. IL-21 and IL-21 receptor in the immunopathogenesis of multiple sclerosis. Journal of Immunotoxicology. 2016;13(3):274–285. doi: 10.3109/1547691X.2015.1089343. [DOI] [PubMed] [Google Scholar]
- 95.Gharibi T., Hosseini A., Marofi F., et al. IL-21 and IL-21-producing T cells are involved in multiple sclerosis severity and progression. Immunology Letters. 2019;216:12–20. doi: 10.1016/j.imlet.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Dhaeze T., Peelen E., Hombrouck A., et al. Circulating follicular regulatory T cells are defective in multiple sclerosis. Journal of Immunology. 2015;195:832–840. doi: 10.4049/jimmunol.1500759. [DOI] [PubMed] [Google Scholar]
- 97.Puthenparampil M., Zito A., Pantano G., et al. Peripheral imbalanced TFH/TFR ratio correlates with intrathecal IgG synthesis in multiple sclerosis at clinical onset. Multiple Sclerosis. 2019;25:918–926. doi: 10.1177/1352458518779951. [DOI] [PubMed] [Google Scholar]
- 98.Qiu H., Wu H., Chan V., Lau C. S., Lu Q. Transcriptional and epigenetic regulation of follicular T-helper cells and their role in autoimmunity. Autoimmunity. 2017;50:71–81. doi: 10.1080/08916934.2017.1284821. [DOI] [PubMed] [Google Scholar]
- 99.Schafflick D., Xu C. A., Hartlehnert M., et al. Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nature communications. 2020;11:p. 247. doi: 10.1038/s41467-019-14118-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Enose-Akahata Y., Azodi S., Smith B. R., et al. Immunophenotypic characterization of CSF B cells in virus-associated neuroinflammatory diseases. PLoS Pathogens. 2018;14(4):p. e1007042. doi: 10.1371/journal.ppat.1007042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Glatigny S., Hollbacher B., Motley S. J., et al. Abatacept targets T follicular helper and regulatory T cells, disrupting molecular pathways that regulate their proliferation and maintenance. Journal of Immunology. 2019;202:1373–1382. doi: 10.4049/jimmunol.1801425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holm Hansen R., Hojsgaard Chow H., Sellebjerg F., Rode von Essen M. Dimethyl fumarate therapy suppresses B cell responses and follicular helper T cells in relapsing-remitting multiple sclerosis. Multiple Sclerosis. 2019;25:1289–1297. doi: 10.1177/1352458518790417. [DOI] [PubMed] [Google Scholar]
- 103.Quinn J. L., Kumar G., Agasing A., Ko R. M., Axtell R. C. Role of TFH cells in promoting T helper 17-induced neuroinflammation. Frontiers in Immunology. 2018;9:p. 382. doi: 10.3389/fimmu.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luo L., Hu X., Dixon M. L., et al. Dysregulated follicular regulatory T cells and antibody responses exacerbate experimental autoimmune encephalomyelitis. Journal of Neuroinflammation. 2021;18(1):p. 27. doi: 10.1186/s12974-021-02076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rottman J. B., Smith T., Tonra J. R., et al. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nature Immunology. 2001;2(7):605–611. doi: 10.1038/89750. [DOI] [PubMed] [Google Scholar]
- 106.Klimatcheva E., Pandina T., Reilly C., et al. CXCL13 antibody for the treatment of autoimmune disorders. BMC immunology. 2015;16(1):p. 6. doi: 10.1186/s12865-015-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Varrin-Doyer M., Pekarek K. L., Spencer C. M., et al. Treatment of spontaneous EAE by laquinimod reduces Tfh, B cell aggregates, and disease progression. Neurology-Neuroimmunology Neuroinflammation. 2016;3(5):p. e272. doi: 10.1212/NXI.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]


